Abstract
Diffuse large B-cell lymphomas are fairly common adult haematolymphoid malignancies. Approximately 40% of such tumours may present in an extranodal site. These lymphomas are, however, infrequently identified in the female genital tract and even more rarely, identified in the endometrium. The histopathological features and molecular findings of endometrial diffuse large B-cell lymphoma are discussed herein.
A 36-year old female presented with per vaginal bleeding. She underwent an endometrial curettage, which demonstrated morphological and immunophenotypical features of a diffuse large B-cell lymphoma. Amplification of the immunoglobulin heavy chain (IgH) gene by polymerase chain reaction (PCR) confirmed B-cell clonality. Epstein–Barr encoding region (EBER) in-situ hybridisation was positive in tumour cells. Fluorescent in-situ hybridisation (FISH) for the detection of BCL6 and MYC gene rearrangement as well as for the detection of the t(14;18) (q32;q21) translocation was performed, all of which yielded negative results. Unfortunately, the patient was lost to follow-up.
Whilst diffuse large B-cell lymphoma is not a commonly identified tumour within the uterine cavity, it should be included in the differential diagnosis of endometrial neoplastic infiltrates so as not to misdiagnose this tumour. This facilitates rapid commencement of further management for the patient.
Clinical history
A 36-year-old Para 3 female, whose HIV status was unknown, presented with vaginal bleeding. On examination she demonstrated mucosal pallor but did not have any lymphadenopathy on palpation. Gynaecological examination revealed no tenderness or abdominal masses. Cervical examination showed a small, normal appearing cervical os with minimal blood. An endometrial curettage was performed, and the patient was discharged. The specimen was submitted for histopathological examination. As the patient was seen at a peripheral hospital, she did not undergo ultrasound examination. Unfortunately, she failed to return for her follow-appointment and for transfer to a tertiary institution for additional treatment. Attempts by the histopathologist to contact the treating clinician to inform them of the histopathology results and to obtain clarity on the nature of the vaginal bleeding were unsuccessful despite numerous attempts. The same applies to ascertaining why the patient was discharged despite the low haemoglobin level.
Her full blood count showed the following:
White cell count: 7.67 ×109/L
Red cell count: 2.61 ×1012/L
Haemoglobin: 5.8 g/dl
Platelets: 488 ×109/L
Pathology
Macroscopically, the specimen submitted for examination consisted of moderate curettings, which were processed in their entirety. Microscopic examination demonstrated endometrial glands and stroma together with fresh haemorrhage. The endometrial glands were separated by a diffuse, sheet-like proliferation of large lymphoid cells. The cells had round to ovoid nuclei with vesicular chromatin and one to multiple nucleoli. The surrounding cytoplasm in some cells was scanty and amphophilic, whilst other cells had increased cytoplasm that was basophilic. Brisk mitotic activity was noted, and numerous apoptotic cells were identified. The endometrial glands were lined by cytologically bland columnar cells displaying evenly dispersed chromatin and pinpoint nucleoli.
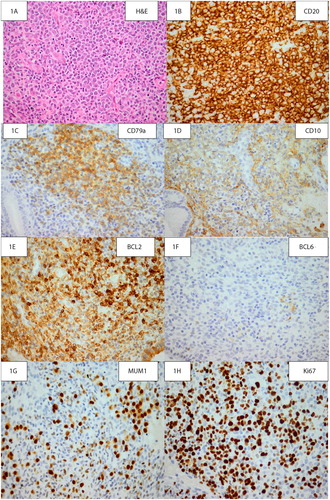
Immunohistochemistry confirmed B-cell lineage of the neoplastic cells with positive staining on CD20 and CD79a. CD10 was very focally positive in < 30% of the atypical lymphocytes. BCL2 was positive in > 50% of the neoplastic B-cells, whilst BCL6 was negative in the tumour cells. MUM1 was positive in > 30% of neoplastic cells. A Ki-67 proliferative index of approximately 80% was obtained. The panel of stains indicated that the DLBCL was of activated B-cell subtype, which has a more aggressive course. Distinguishing activated B-cell subtype from germinal centre cell subtype is necessary as addition of certain drugs has shown improvement in patients with the activated B-cell type.Citation1 Epstein–Barr encoding region (EBER) in-situ hybridisation was positive in neoplastic cells, which has been associated with a more aggressive clinical course and worse overall prognosis in diffuse large B-cell lymphoma .Citation2
Figure 1: A is an H&E photomicrograph of the patient’s endometrial curettage showing high magnification of the haematolymphoid neoplasm. B shows diffuse, strong positive CD20 staining of neoplastic B-cells. C shows positive CD79a staining of neoplastic B-cells. D shows focal membranous CD10 staining of neoplastic B-cells. E shows diffuse positive staining of tumour cells on a BCL2 stain. F shows negative staining of neoplastic cells with a BCL6 stain. G shows positive MUM1 staining of neoplastic cells. H demonstrates a high proliferative index on a Ki-67 stain (original magnification A-H: x400). The panel of stains seen in figures B–H allows for a pathological diagnosis of diffuse large B-cell lymphoma to be reached. The panel of stains indicates that the DLBCL is of activated B-cell subtype, which has a more aggressive course.Citation1 Distinguishing activated B-cell subtype from germinal centre cell subtype is necessary as addition of certain therapeutic drugs has shown improvement in patients with the activated B-cell type.Citation1
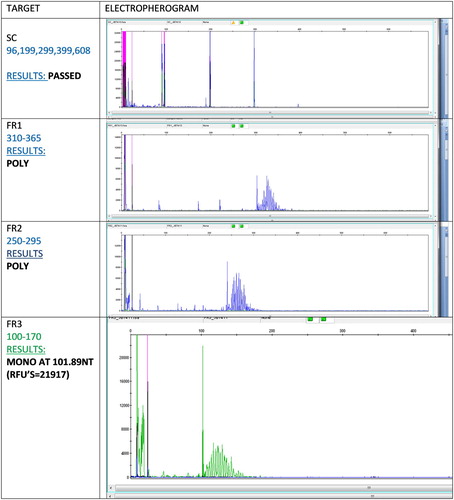
Amplification of the immunoglobulin heavy chain (IgH) gene by polymerase chain reaction (PCR) confirmed B-cell clonality with FR3 primers. This is indicative of a proliferation of genetically identical cells. A reproducible monoclonal band was detected, which suggested that a pseudomonoclone was unlikely ().Citation3,Citation4 The constellation of morphological features, immunohistochemical profile and PCR results thus indicated a neoplastic proliferation, which would then have required further management by way of immunochemotherapy, radiation and/or surgery.Citation5,Citation6
Figure 2: Electropherogram: immunoglobulin heavy chain (IgH) gene amplification by polymerase chain reaction demonstrated monoclonality with FR3 primers.
Fluorescent in-situ hybridisation (FISH) for the detection of BCL6 and MYC gene rearrangement as well as for the detection of the t(14;18) (q32;q21) translocation was performed, all of which yielded negative results. These results thus excluded a diagnoses of high-grade B-cell lymphoma with MYC and BCL6-rearrangements and thus a diagnosis of diffuse large B-cell lymphoma not otherwise specified (NOS), non-germinal centre/activated B-cell subtype, was rendered.Citation1,Citation7 Such patients may be offered treatment for diffuse large B-cell lymphoma.
Discussion
Diffuse large B-cell lymphomas (DLBCLs) are haematolymphoid malignancies that account for up to 35% of non-Hodgkin lymphomas in the adult population in Western societies, with a higher incidence rate in developing nations.Citation1 Up to 40% of patients diagnosed with DLBCLs may present with tumour at an extranodal site, with the gastrointestinal tract being the most common of these locations.Citation1,Citation5,Citation8 Lymphomas primarily arising in the uterus are however, extremely rare but, relatively speaking, the female genital tract may be more often secondarily affected by lymphoma.Citation5,Citation9,Citation10 The cervix has been identified as the site within the female genital tract that is most often primarily effected by a lymphoma.Citation5,Citation11,Citation12 Primary endometrial lymphomas usually arise in post-menopausal females.Citation8,Citation10 Patients tend to present with abnormal vaginal bleeding such as menorrhagia, post-menopausal bleeding or discharge, and as such histopathological evaluation is required for a definitive diagnosis.Citation8,Citation10,Citation13
Criteria that must be fulfilled for a diagnosis of a primary endometrial lymphoma, as proposed by Fox et al.,Citation14 include the following: confinement of the disease to the uterus at the time of first diagnosis, no identifiable leukaemia on a full blood count, no evidence of disease at other sites in the body, and a period of a number of months must pass between the identification of a secondary site of involvement and the initial tumour site.Citation5,Citation8,Citation14 In cases where the disease is at an advanced stage, it may be challenging to identify the primary and secondary sites of involvement.Citation5,Citation8 In the current case, the tumour was identified within the uterine cavity and the patient did not have evidence of leukaemia on her full blood count. However, as she failed to return for follow-up, she did not undergo a thorough investigative work-up to detect other sites of tumour involvement. Furthermore, it is not possible to know if currently there is involvement of an additional anatomical site.
The clinical differential diagnosis in a female of reproductive age who presents with abnormal bleeding includes both cervical and endometrial pathology. Cervical causes of bleeding encompass cervicitis including tuberculosis, schistosomiasis and viral warts. In addition, squamous cell carcinoma and adenocarcinoma must be considered. Endometrial causes of bleeding include endometritis, endometrial polyps, submucosal or pedunculated leiomyomas. Despite the patient being of reproductive age, an endometrial carcinoma should be considered in the differential diagnosis. Exogenous hormones in contraceptives or endogenous oestrogen-producing ovarian tumours may also present with abnormal bleeding. Furthermore, gestational trophoblastic neoplasias such hydatidiform mole, choriocarcinoma, placental site trophoblastic tumour and epithelioid trophoblastic tumour must be included in the clinical differential diagnosis.
Microscopically, the differential diagnosis of dyscohesive large cells arranged in sheets included a poorly differentiated carcinoma, other large cell lymphomas such as Burkitt lymphoma, plasmablastic lymphoma, peripheral T-cell lymphoma not otherwise specified and anaplastic large cell lymphoma; in addition, myeloid sarcoma, high-grade endometrial stromal sarcoma, Ewing sarcoma, amelanotic melanoma and reactive lymphoid hyperplasia or a lymphoma-like lesion should be considered.
Morphologically and immunohistochemically, however, the neoplastic cells in this case did not have features of Burkitt, plasmablastic or a peripheral T-cell lymphoma, NOS or anaplastic large cell lymphoma, ALK-positive or negative. In addition, the tumour cells stained positively for haematolymphoid markers, thus excluding an undifferentiated carcinoma, high-grade endometrial sarcoma and melanoma. Moreover, reactive lymphoid hyperplasia was disregarded as PCR demonstrated reproducible IgH clonality. The microscopic features did not suggest the possibility of any of the clinical differential diagnoses, and as such these are not discussed further.
Additional management could not be instituted for the patient in this case report as she was lost to follow-up. As this is an uncommon diagnosis, extensive studies have not been performed.Citation5,Citation13 Cubo et al.Citation15 noted that with the passage of time chemotherapy with or without radiation therapy has become the preferred treatment option in contrast to previous treatment plans in which surgery was the predominant modality.Citation15 Furthermore, the addition of the monoclonal antibody rituximab to chemotherapeutic agents (cyclophosphamide, doxorubicin, oncovin and prednisolone (R-CHOP) has resulted in an increased overall survival for patients with DLBCL.Citation15 Fertility preservation may be a consideration in younger patients and as such radiation therapy may be omitted to prevent premature ovarian failure. Alternatively, ovarian translocation may be carried out to shield the ovaries from the radiation field. Usage of cyclophosphamide results in ovarian failure in more than two-thirds of patients, especially when used in combination with other chemotherapeutic drugs.Citation6 Studies by Horning et al.Citation16 have demonstrated an increased 10-year disease-free survival in patients treated with combined therapy.Citation16 Presently, a combined treatment approach is favoured as immuno-chemotherapeutic agents prevent disease relapse and radiation decreases the likelihood of local recurrence.Citation6,Citation13,Citation16
Conclusion
Lymphomas of the female genital tract are rare, with primary lymphomas arising at this site being even more uncommon. It is important to have a broad differential diagnosis both clinically and histologically for a patient who presents with vaginal bleeding so as not to miss an unusual tumour in this site, such as a lymphoma. This may facilitate early diagnosis and implementation of treatment for the patient.
Conflict of interest and sources of funding
None was declared.
Ethics
Ethical clearance was obtained from the Human Research Ethics Committee (Medical) (clearance number M180297).
References
- Gascoyne R, Campo E, Jaffe E, et al. Diffuse large B-cell lymphoma, NOS. In: Swerdlow S, Campo E, Harris N, et al., editors. WHO classification of tumours of the haematopoietic and lymphoid tissues. Revised 4th Edition. Lyon: International Agency for Research on Cancer (IARC); 2017. P. 291–297.
- Gao X, Li J, Wang Y, et al. Clinical characteristics and prognostic significance of EBER positivity in diffuse large B-cell lymphoma: a meta-analysis. PLOS ONE. 2018;13(6):e0199398. doi: 10.1371/journal.pone.0199398
- Elenitoba-Johnson KSJ, Bohling SD, Mitchell RS, et al. PCR analysis of the immunoglobulin heavy Chain gene in polyclonal processes can yield pseudoclonal bands as an artifact of low B cell number. J Mol Diagn JMD. 2000;2(2):92–96. doi: 10.1016/S1525-1578(10)60622-8
- Rittenbach J, Cao JD, Weiss LM, et al. Primary diffuse large B-cell lymphoma of the uterus presenting solely as an endometrial polyp. Int J Gynecol Pathol Off J Int Soc Gynecol Pathol. 2005;24(4):347–351.
- Malempati LM, Nandan N, Babu S. Case of extranodal non-hodgkins lymphoma involving the endometrium and the ovaries: a rare case report. Int J Reprod Contracept Obstet Gynecol. 2017;6(9):4131–4134. doi: 10.18203/2320-1770.ijrcog20174076
- Mandato VD, Palermo R, Falbo A, et al. Primary Diffuse Large B-Cell Lymphoma of the Uterus: Case Report and Review. Anticancer Res. 2014;34(8):4377–4390.
- Kluin PM, Harris NL, Stein H, et al. High-grade B-cell lymphoma. In: WHO classification of tumours of haematopoieetic and lymphoid tissues. 4. Lyon: International Agency for Research on Cancer (IARC); 2017. p. 335–341.
- Yamamoto Y, Chaki O, Nakayama M. Two Cases of Non-Hodgkin’s Lymphoma Involving the Uterus. Gynecol Obstet. 2014;4(3). doi: 10.4172/2332-0672.1000213
- Zou Y, Zhao H, Gao J, et al. Primary diffuse large B-cell lymphoma of endometrium. Adv Mod Oncol Res. 2015;1(1):36–40. doi: 10.18282/amor.v1.i1.33
- Prat J, Ferry J, Oliva E, et al. Lymphoid and myeloid tumours. In: Kurman R, Carcangiu M, Herrington C, Young R, editor. 4th Edition. WHO classification of tumours of female reproductive organs. Lyon: International Agency for Research on Cancer (IARC); 2014. p. 153.
- Kasai M, Ichimura T, Murakami M, et al. Two cases of uterine malignant lymphoma diagnosed by needle biopsy. J Obstet Gynaecol Res. 2015;41(10):1664–1668. doi: 10.1111/jog.12759
- Alvarez A, Ortiz JA, Sacristán F. Large B-cell lymphoma of the uterine corpus: case report with immunohistochemical and molecular study. Gynecol Oncol. 1997;65(3):534–538. doi: 10.1006/gyno.1997.4679
- Frey NV, Svoboda J, Andreadis C, et al. Primary lymphomas of the cervix and uterus: The University of Pennsylvania’s experience and a review of the literature. Leuk Lymphoma. 2006;47(9):1894–1901. doi: 10.1080/10428190600687653
- Fox H, More J. Primary malignant lymphoma of the uterus. J Clin Pathol. 1965;18:723–728. doi: 10.1136/jcp.18.6.723
- Cubo AM, Soto ZM, Cruz MÁ, et al. Primary diffuse large B cell lymphoma of the uterine cervix successfully treated by combined chemotherapy alone. Medicine (Baltimore). 2017;96(19). doi:10.1097/MD.0000000000006846.
- Horning SJ, Weller E, Kim K, et al. Chemotherapy with or without Radiotherapy in limited-stage diffuse aggressive Non-Hodgkin’s Lymphoma: eastern cooperative oncology group study 1484. J Clin Oncol. 2004;22(15):3032–3038. doi: 10.1200/JCO.2004.06.088

