ABSTRACT
Background
Giant cell tumor (GCT) of bone is a benign locally aggressive tumor that constitutes 20% of the body's benign bone tumors. Most of the GCTs exhibit a typical epiphyseal location that shows a tendency for significant bone destruction and local recurrence. We aimed to assess the functional and oncological outcomes of GCT patients treated with bone grafting and cementation with or without gel foam.
Materials and methods
This prospective study included 40 patients presented at El Hadara University Hospital with GCT of bone around the knee from January 2017 to January 2022 treated by bone graft and cementation. Twenty cases were treated with gel foam (Group I) and 20 cases were treated without gel foam (Group II) through random allocation without selection. Recurrence was assessed as progressive lysis of 5 mm at the bone cement interface. Functional outcomes were assessed using the musculoskeletal tumor society score (MSTS) after a period of minimum 30 months.
Results
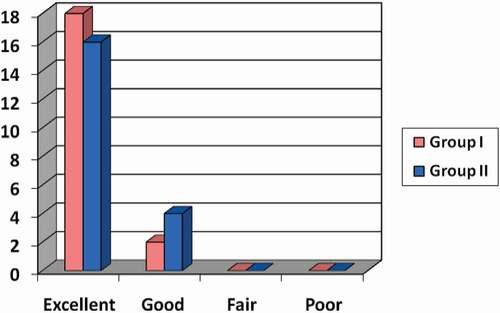
In Group I, 18 patients (90%) had excellent results (range 24 and 30) according to MSTS and two patients (10%) had good results (range 18 and 23), while in Group II, 16 patients (80%) had excellent results and four patients (20%) had good results. No patients were graded as having fair or poor results. Twenty patients (100%) had satisfactory results, and no patients (0%) had unsatisfactory results. The overall recurrence rate was about 15%.
Conclusion
Reconstruction of GCT of bone with sandwich technique offers good option as joint preserving surgery. Most of the patients get benefit in terms of better quality of life and good function regardless of age and gender. Subchondral bone grafting reduces the effect of heat on articular cartilage, but longer follow-up is required. There is no benefit of gel foam addition in terms of function or oncological outcome.
1. Introduction
Giant cell tumor (GCT) of bone is a benign locally aggressive tumor that constitutes 5% of primary bone tumors (benign and malignant). Although benign, GCTs show a tendency for significant bone destruction, with local recurrence [Citation1].
It usually affects young adults between the ages of 20 and 40 years [Citation2]. Fifty percent of GCTs occur at the knee [Citation3]. Approximately 10–25% of patients with GCT may present with pathologic fracture as first presentation. These patients had a high risk of local recurrence [Citation3,Citation4].
Radiologically, GCT of bone is characterized by radiolucent, epiphyseal, eccentric, and geographic appearance without sclerotic margin due to aggressiveness of the tumor [Citation5]. The GCT is classified by Enneking and later graded by Campanacci based on radiographic appearance [Citation5].
Although GCT is a benign tumor, pulmonary metastasis is reported in around 5% of cases. It is usually developed after a couple of years after initial diagnosis of GCT, and it is not lethal [Citation6]. Pulmonary metastasis is more common in those patients with stage 3 lesions, primary tumors in distal radius, and sacrum and recurrent lesions denoting the aggressiveness of the tumor cells [Citation3].
It compromises mainly mononuclear stromal neoplastic cells, multinucleated giant cells, and mononuclear histiocytic cells [Citation3]. Osteoclasts and their precursors are RANKL-dependent. On the other hand, the osteoclasts secrete tumor growth factors, which stimulate growth of GCT creating a vicious circle [Citation7]. Denosumab (monoclonal antibody) treatment of GCT blocks the effect of RANKL on osteoclasts, reducing bone destruction and giant cells component [Citation2].
Treatment of GCT of bone is usually managed surgically. Intralesional approach is highly recommended as it preserves the native joint. Various surgical treatment options have been advocated, including curettage, curettage and bone grafting, or curettage and bone cement [Citation8]. Adjuvant therapies like the use of phenol, cryotherapy, hydrogen peroxide, and argon laser have been tried [Citation2].
Wide margin resection is carried out if the tumor is large enough or when the articular cartilage is largely damaged or when there is inadequate bone stock post-curettage and resection results in no significant morbidity such as proximal fibula and distal ulna [Citation8]. It has a lower recurrence risk and functional outcome in comparison with intralesional curettage. [Citation8]
The aim of the study was to prospectively assess the functional outcome, recurrence rates, metastasis, and complications of GCT patients around the knee treated with the Sandwich technique.
2. Materials and methods
This is a randomized prospective study that included 40 patients presented at El Hadara University Hospital with giant cell tumors of bone around the knee from January 2017 to January 2022. Patients with primary GCT of bone at the knee are included in the study. Subjects with arthritic joint changes, extensive lesion with more than two-thirds of the cortical destruction, and subchondral bone stock (>5 mm) after extended curettage are excluded. Twenty cases were treated by bone grafting and cementation with gel foam (Group I), and 20 cases were treated by bone grafting and cementation without gel foam (Group II) through random allocation without selection. The patients were informed about the aim of the study and signed informed consent to be included. All patients were evaluated by clinical examination, plain X-ray, chest X-ray, and magnetic resonance imaging.
2.1. Surgical technique and postoperative protocol
The patients were operated under appropriate anesthesia. Adequate exposure was achieved by making a large cortical window to access the tumor. Multiple angled curettes with different sizes were used to identify and access small pockets of residual disease. The remaining cristae and septa in the cavity were denuded.
A high power burr was used to break the bony ridges. A pulsatile jet lavage system was used after curettage to bare the raw cancellous bone and physically wash out tumor cells. Adjuvant such as hydrogen peroxide was used routinely.
Iliac crest was prepared for tricortical corticocancellous structural autografts. In Group I, the defect after extended curettage was repaired by subchondral bone grafting and cementation with gel foam in between (Sandwich technique). The cement was then used to fill the entire cavity in order to restore the anatomical shape of the bone. While in Group II, the reconstruction was performed by bone grafting and cementation without gel foam.
Internal fixation (screws or Kirschner wires) was used intra-operatively to fix the pathological fracture or to stabilize the graft position. Closure of the soft tissue, subcutaneous tissue, and skin was done in layers. Histopathologic examination was performed to confirm the diagnosis.
Post-operatively, the patient was instructed for bracing a fully extended knee and initiating knee range-of-motion as soon as the pain subsides. The brace was kept for 4 to 6 weeks, to allow soft tissue healing. Patients were allowed partial protected weight-bearing walking using crutches with “brace on.” Full weight-bearing was delayed in all patients until 12 weeks to allow reconstitution of the subchondral stiffness.
At the end of follow-up (minimum 30 months), functional outcomes were assessed using MSTS. Recurrence was assessed as progressive lysis of 5 mm at the bone cement interface. Adjacent joint arthritic changes were assessed using Kellgren-Lawrence grading.
Statistical analysis of the data was analyzed using IBM SPSS software package version 20.0. (Armonk, NY: IBM Corp). Qualitative data were described using number and percent. The Kolmogorov–Smirnov test was used to verify the normality of distribution. Quantitative data were described using range (minimum and maximum), mean, standard deviation, median, and interquartile range. Significance of the obtained results was judged at the 5% level. The chi-square test was used for categorical variables to compare between different groups. Fisher’s Exact or Monte Carlo correction was used for correction for chi-square when more than 20% of the cells have expected count less than 5. Student's t-test was used for normally distributed quantitative variables to compare between the two studied groups.
3. Results
We found that the mean age of all the studied subjects was 31. From all 40 patients, 21 were females and 19 were males, distal femur was involved by the tumor in 22 patients, and proximal tibia was involved in 18 patients. Twenty-two GCT cases were graded according to Campanacci as grade II, and 18 cases were grade III. Only six patients were presented with pathological fracture.
The mean age of 31.90 ± 8.06 years in Group I (with gel foam) while in Group II (without gel foam) the mean age of 30.80 ± 12.06 years. Campanacci grading, pathological fracture, and other demographic data were noted ().
Table 1. Distribution of the studied groups regarding demographic data.
There was no statistically significant relation between patients’ age and final score for group I (P = 1.0) and group II (p = 0.326). There was no statistically significant difference between gender and the final clinical score for group I (P = 1.0) and group II (P = 1.0).
According to the MSTS, 18 patients (90%) had excellent results (range 24 and 30) and two patients (10%) had good results (range 18 and 23) in Group I, while in Group II, 16 patients (80%) had excellent results (range 24 and 30) and four patients (20%) had good results (range 18 and 23). No patients were graded as having fair or poor results. Twenty patients (100%) had satisfactory results, and no patients (0%) had unsatisfactory results ().
Graph 1. Distribution of the studied patients regarding the functional outcome.
The mean final MSTS score for Group I was 27.35 ± 2.16, while in Group II, it was 26.60 ± 2.68 (); however, this was not statistically significant.
Table 2. Relations between MSTS parameters at the end of the follow-up period between both groups.
The overall recurrence rate in our study was 15%. Four cases (20%) were recognized in group I, and 2 cases (10%) were in group II within 18 months after surgery.
No metastases were diagnosed, and no infection was developed. One case with pathological fracture that was fixed with 2 k wires developed pain after 18 months that require removal of the prominent k wire.
Two patients (10%) developed arthritic changes in Group I. While in Group II, three patients (15%) developed arthritis radiologically. The two arthritic cases in group I and the three arthritic cases in group II had excellent results (P = 1.00). This difference was statistically insignificant () ().
Figure 1. A 43-year-old male patient with Rt GCT distal femur (group I). (a and b) AP and lateral X-ray of Rt knee showing osteolytic lesion affecting medial femoral condyle. (c and d) Coronal & axial T1 weight MRI showing hypointense lesion with cortical thinning without soft tissue extension. (e−g) Intraoperative photos showing the reconstruction of the medial femoral condyle after extended curettage with subchondral iliac bone graft followed by gel foam and cement. (h and i) AP and lateral x-ray of Rt knee immediately postoperative. (j and k) AP and lateral X-ray of Rt knee at the end of follow-up period showing full incorporation of bone graft and no evidence of recurrence.
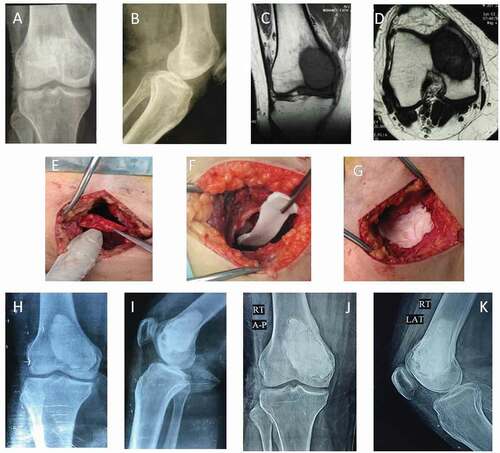
Figure 2. A 24-year-old female patient with Lt GCT distal femur (group II) with pathological fracture. (a and b) AP and lateral X-ray of Rt knee showing osteolytic lesion affecting medial femoral condyle with pathological fracture. (c and d) Coronal and axial CT delineating the fracture pattern and cortical thinning of the condyle. (e and f) Coronal and axial T2-weighted MRI showing isointense lesion with intralesional fluid signals of hemorrhage without soft tissue extension. (g and h) Intraoperative photos showing extended curettage. (i-k) Intraoperative photos showing fixation of fracture with 2 k-wires with subchondral iliac bone graft and cement. (l and m) AP and lateral x-ray of Rt knee immediately postoperative. (n and o) AP and lateral X-ray of Rt knee at the end of follow-up period showing full incorporation of bone graft and no evidence of recurrence. (p and q) AP and lateral x-ray of Rt knee; the prominent k wire was painful, which requires its removal.
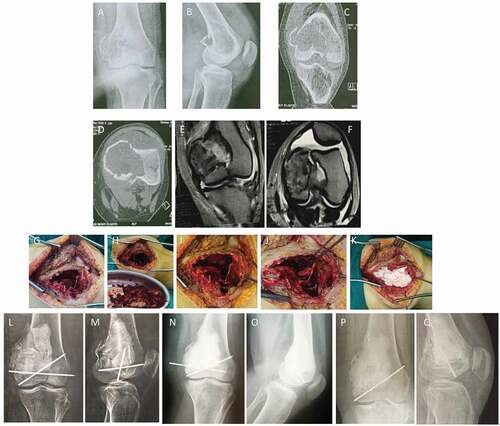
Table 3. Distribution of the studied patients regarding Kellgren-Lawrence grading for osteoarthritis.
4. Discussion
GCT of bone is a benign primary bone tumor with aggressive biological behavior [Citation8]. It usually affects the epiphyseal area of the distal femur, proximal tibia, distal radius, and sacrum in a descending order. GCT is characterized by bone erosion and destruction [Citation9].
GCT usually affects middle age group between 20 and 40 [Citation3,Citation10]. The mean age of the overall cohort was 31.20 ± 10.06 years in line with other studies [Citation5,Citation11,Citation12]. Meena et al. found that the function and quality of life are equal in all affected age groups [Citation10].
In our study, among the GCT patients, 19 were male and 21 were female with a female-to-male ratio of 1.1:1. Most studies in western countries had female predilection.
Balke et al. found a female-to-male ratio of 1.2:1 [Citation11], Saibaba et al. found a F:M of 1.57:1 [Citation5], and Meena et al. found a F:M of 1.25:1 [Citation10].
There was no statistically significant difference between males and females as regards the final clinical score. This coincides with the results of Chen et al. [Citation13] and Meena et al. [Citation10]
We found the rate of pathological fracture in the presented GCT cases in our study to be four cases (20%) in group I and two cases (10%) in group II. In Group I, one recurrent case had pathological fracture and the other three recurrent cases did not have a fracture, while in Group II, the two recurrent cases did not have any fracture. It does not show any relation between the recurrence and the preoperative pathological fracture.
There are limited reports in determining the local recurrence in relation to pathological fracture. It seems that there is no relation between recurrence and the cases presented with pathological fracture [Citation14].
Deheshi et al. reported no increase in risk of recurrence with pathological fracture, which, however, makes the curettage more difficult to be completely accomplished with a higher risk in arthrofibrosis postoperatively [Citation15].
However, O’Donnell et al. found that pathological fracture has a direct relation with the increase in local recurrence rate due to fracture hematoma and dissemination to the soft tissue circumference [Citation8]. Dreinhofer et al. reported an increased recurrence rate in cases with pathological fracture, but this was not a contraindication for intralesional curettage [Citation16].
In Group I, three recurrent cases are classified as grade II and one recurrent case is classified as grade III. While in Group II, the two recurrent cases are classified as grade III. This difference between both groups was statistically insignificant. It does not show any relation between the recurrence and the Campanacci grading preoperatively.
Campanacci et al. concluded that the tumor grade was not associated with local recurrence [Citation16]. Gitelis et al. found that the recurrence rate is not related to tumor grading [Citation10].
On the contrary, other authors suggested that the recurrence rate increases with grade 3 tumors [Citation11]. Hasan et al. reported 17% recurrence rate after curettage and adjuvant treatment and concluded that the recurrence rate is higher in advanced-grade tumors as well as tumors with soft tissue extension [Citation17].
The gold standard treatment of GCT is surgical but still debatable and controversial. It is either resection or intralesional curettage. Resection shows lower risk of recurrence but higher risk of complication as infection and revision in case of usage megaprosthesis for reconstruction [Citation18].
Errani et al. reported statistical differences in functional results between intralesional curettage in comparison with wide margin resection [Citation16]. No significant functional difference or MSTS scoring was found with treating GCT patients with intralesional curettage with either cement or bone graft [Citation16,Citation19].
Intralesional curettage allows joint preservation but with a higher recurrence rate. It is a worldwide applied procedure regardless of the grade of tumor [Citation17]. High-speed burr provides extending the marginal curettage of the cavity by 2 mm, and it generates a heat effect that causes tumor cell necrosis as well [Citation20].
Algawahmed performed a comparison study of the recurrence rates using the high-speed burr in extended curettage with versus without using the adjuvant. He reported that extended curettage with high-speed burr without using adjuvants is the crucial step in reducing the local recurrence [Citation21].
The large-sized cavity can be left without filler, but it will take a long time to consolidate. There is a high direct proportion between the size of the cavity and the complications such as fracture or osteoarthritis [Citation20]. It is recommended to fill the cavity of more than 60 cm3 with filler to avoid such complications [Citation22].
Most of the authors suggested filling the cavity with bone graft, PMMA, or both. Bone graft provides stress remodeling and permanent reconstruction once incorporated. Its amount is limited to fill large cavity. While cement provides the thermal effect and cytotoxic effect for tumor cells, it avoids subchondral collapse or fracture and is easier for recurrence detection [Citation23].
Meta-analysis of six studies applied to more than thousand patients treated with extensive curettage and filling with either PMMA or allogenic graft had proved that usage of PMMA reduces the recurrence rate [Citation24]. This is attributed to the cytotoxic effect on tumor cells and thermal heat effect observed by radiolucent area between bone cement interface [Citation25].
Kivioja concluded that there is less risk of recurrence after intralesional curettage and PMMA as filler [Citation26]. However, Turcotte concluded that filler or the adjuvant does not affect the local recurrence significantly [Citation19]. Becker et al. reported 22% recurrence rate after PMMA treatment, while 49% recurrence rate after bone graft treatment [Citation14].
The effect of cement on the articular cartilage has not settled yet. Turcotte et al. reported that the functional outcome of cement or bone graft reconstruction in the subchondral area does not show any statistical significance and suggested that the subchondral bone graft may avoid the undesired effect of cement [Citation19].
Gaston et al. suggested that the application of cement in the treatment of GCT patients had greater probability of joint replacement (18%) independent of local recurrence due to the biomechanical deficiencies and higher modulus of cement to replace the subchondral region, increasing the possibility of articular fracture lines and osteoarthritis [Citation27].
Van der Heijden et al. showed that about 17% of GCT patients treated with curettage and cement developed osteoarthritis radiographically (grade 3 &4) [Citation28]. The lifestyle activity and the function did not differ in relation to those of low-grade arthritis [Citation29].
Radev et al. suggested that a subchondral bone of 2–5 mm is the least thickness to prevent the heat effect and cytotoxic effect of PMMA on the articular cartilage [Citation29].
The sandwich technique allows to get the advantages of cement and to avoid its complication regarding the effect articular damage and arthritis. Once the bone graft is incorporated in the subchondral area or in any cortical defect, it becomes infinite reconstruction and provides good bone stock for subsequent curettage in the case of recurrence [Citation10].
The mean value of the final MSTS score at the end of the follow-up period for group I was 27.35 ± 2.16, while for group II, it was 26.60 ± 2.68. Meena et al. reported that the average final MSTS score was 27.4 [Citation14]. The final average MSTS score reported by Saibaba et al. [Citation7] was 27.7, and by Samik et al. was 26 [Citation10].
Meena et al. reported that the functional outcome was excellent (92%) in GCT patients treated with curettage, burring, and H2O2 as adjuvant followed by the sandwich technique as a method of reconstruction [Citation10].
Saibaba et al. [Citation7] concluded that the excellent functional result in their study was 92.3%, while the result reported by Gupta et al. was 72% [Citation16]. Abdelrahman et al. reported 93.9% excellent results using the Sandwich technique with liquid nitrogen as adjuvant [Citation30].
5. Conclusion
Reconstruction of GCT of bone with sandwich technique with or without using of gel foam offers good option as joint preserving surgery. Most of the patients get benefit in terms of better quality of life and good function regardless of age and gender. Subchondral bone grafting reduces the effect of heat on articular cartilage, but longer follow-up is required. There is no benefit of gel foam addition in terms of function or oncological outcome. Internal fixation of pathologically fractured patients in addition to cavity-filled cement allows early mobilization and proper holding of the autograft.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Notes on contributors
Ahmed AlaaElDin Ibrahim ElDesouqi
Ahmed eldesouqi is an assistant lecturer of orthopedic and traumatology.
Raafat Kamal Ragab
Raafat ragab is a Professor of orthopedic and traumatology.
Abdel Sabour Abdel Hamid Ghoneim
Abdelsabour ghoneim is a professor of orthopedic and traumatology.
Bassma Mohamed Sabaa
Bassma sabaa is a Professor of pathology.
Awad Abdel Moneim Rafalla
Awad Rafalla is a Professor of orthopedic and traumatology, Alexandria university , Egypt
References
- Ward SWG, Li IIIG. Customized treatment algorithm for giant cell tumor of bone: report of a series. Clin Orthop Relat Res. 2002 Apr 1;397:259–270.
- Skubitz KM. Giant cell tumor of bone: current treatment options. Curr Treat Options Oncol. 2014 Sep 1;15(3):507–518.
- Turcotte RE. Giant cell tumor of bone. Orthop Clin North Am. 2006;37(1):35–51.
- Jeys LM, Suneja R, Chami G, et al. Impending fractures in giant cell tumors of the distal femur: incidence and outcome. Int Orthop. 2006;30(2):135–138.
- Saibaba B, Chouhan DK, Kumar V, et al. Curettage and reconstruction by the sandwich technique for giant cell tumours around the knee. J Orthop Surg. 2014;229(3):351–355.
- Amanatullah DF, Clark TR, Lopez MJ, et al. Giant cell tumor of bone. Orthopedics. 2014;37(2):112–120.
- López-Pousa A, Broto JM, Garrido T, et al. Giant cell tumour of bone: new treatments in development. Clin Transl Oncol. 2015 Jun;17(6):419–430.
- Prosser GH, Baloch KG, Tillman RM, et al. Does curettage without adjuvant therapy provide low recurrence rates in giant-cell tumors of bone? Clin Orthop Relat Res. 2005 Jun;&NA;(435):211–218.
- Omlor GW, Lange J, Streit M, et al. Retrospective analysis of 51 intralesionally treated cases with progressed giant cell tumor of the bone: local adjuvant use of hydrogen peroxide reduces the risk for tumor recurrence. World J Surg Oncol. 2019 Dec;17(1):1.
- Meena AM, Jain P, Dayma RL. Retrospective study of function outcome in giant cell tumor treated by sandwich technique with internal fixation. Int J Orthop. 2017;3(2):817–822.
- Balke M, Ahrens H, Streitbuerger A, et al. Treatment options for recurrent giant cell tumors of bone. J Cancer Res Clin Oncol. 2009 Jan;135(1):149–158.
- Gupta SP, Garg G. Curettage with cement augmentation of large bone defects in giant cell tumors with pathological fractures in lower-extremity long bones. J Orthop Traumatol. 2016 Sep;17(3):239–247.
- Chen TH, Su YP, Chen WM. Giant cell tumors of the knee: subchondral bone integrity affects the outcome. Int Orthop. 2005 Feb;29(1):30–34.
- Becker WT, Dohle J, Bernd L, et al. Local recurrence of giant cell tumor of bone after intralesional treatment with and without adjuvant therapy. JBJS. 2008 May 1;90(5):1060–1067.
- Deheshi BM, Jaffer SN, Griffin AM, et al. Joint salvage for pathologic fracture of giant cell tumor of the lower extremity. Clin Orthop Relat Res. 2007 Jun 1;459:96–104.
- Errani C, Ruggieri P, Asenzio MA, et al. Giant cell tumor of the extremity: a review of 349 cases from a single institution. Cancer Treat Rev. 2010 Feb 1;36(1):1–7.
- Hasan O, Ali M, Mustafa M, et al. Treatment and recurrence of giant cell tumors of bone–A retrospective cohort from a developing country. Ann Med Surg. 2019 Dec 1;48:29–34.
- Muscolo DL, Ayerza MA, Aponte-Tinao LA, et al. Use of distal femoral osteoarticular allografts in limb salvage surgery. JBJS. 2005 Nov 1;87(11):2449–2455.
- Turcotte RE, Wunder JS, Isler MH, et al. Giant cell tumor of long bone: a Canadian sarcoma group study. Clin Orthop Relat Res. 2002 Apr 1;397:248–258.
- Kundu ZS, Gogna P, Singla R, et al. Joint salvage using sandwich technique for giant cell tumors around knee. J Knee Surg. 2015 Apr;28(2):157–164.
- Algawahmed H, Turcotte R, Farrokhyar F, et al. High-speed burring with and without the use of surgical adjuvants in the intralesional management of giant cell tumor of bone: a systematic review and meta-analysis. Sarcoma. 2010 Oct;2010:1–5.
- Hirn M, de Silva U, Sidharthan S, et al. Bone defects following curettage do not necessarily need augmentation: a retrospective study of 146 patients. Acta Orthop. 2009 Jan 1;80(1):4–8.
- Wu M, Yao S, Xie Y, et al. A novel subchondral bone-grafting procedure for the treatment of giant-cell tumor around the knee: a retrospective study of 27 cases. Medicine (Baltimore). 2018 Nov;97(45): 1–10.
- Zuo D, Zheng L, Sun W, et al. Contemporary adjuvant polymethyl methacrylate cementation optimally limits recurrence in primary giant cell tumor of bone patients compared to bone grafting: a systematic review and meta-analysis. World J Surg Oncol. 2013 Dec;11(1):1–7.
- Von Steyern FV, Bauer HC, Trovik C, et al. Treatment of local recurrences of giant cell tumour in long bones after curettage and cementing: a Scandinavian sarcoma group study. J Bone Joint Surg. 2006 Apr;88(4):531–535. British volume.
- Kivioja AH, Blomqvist C, Hietaniemi K, et al. Cement is recommended in intralesional surgery of giant cell tumors: a Scandinavian sarcoma group study of 294 patients followed for a median time of 5 years. Acta Orthop. 2008 Jan 1;79(1):86–93.
- Gaston CL, Bhumbra R, Watanuki M, et al. Does the addition of cement improve the rate of local recurrence after curettage of giant cell tumours in bone? J Bone Joint Surg. 2011 Dec;93(12):1665–1669. British volume.
- Van der Heijden L, Van de Sande MA, Heineken AC, et al. Mid-term outcome after curettage with polymethylmethacrylate for giant cell tumor around the knee: higher risk of radiographic osteoarthritis? JBJS. 2013 Nov 6;95(21):e159.
- Mukkamalla SK, Sekhar AC. Joint salvage surgery using sandwich technique for distal femur giant cell tumour in young male patient–A case report. IOSR Journal of Dental and Medical Sciences (IOSR-JDMS). 2019;18(7):36–41.
- Abdelrahman M, Bassiony AA, Shalaby H, et al. Cryosurgery and impaction subchondral bone graft for the treatment of giant cell tumor around the knee. HSS J®. 2009 Sep;5(2):123–128.

