ABSTRACT
Objectives
We tested whether the 2012 Briganti nomogram for the risk of pelvic lymph node invasion (PLNI) may represent a predictor of disease progression after surgical management in high-risk (HR) prostate cancer (PCa) patients according to the European Association of Urology.
Methods
Between January 2013 and December 2021, HR PCa patients treated with robot-assisted radical prostatectomy (RARP) and extended pelvic lymph node dissection (ePLND) were identified. The 2012 Briganti nomogram was evaluated as a continuous and categorical variable, which was dichotomized using the median. The risk of disease progression, defined as the event of biochemical recurrence and/or local recurrence/distant metastases was assessed by Cox regression models.
Results
Overall, 204 patients were identified. The median 2012 Briganti nomogram score resulted 12.0% (IQR: 6.0–22.0%). PLNI was detected in 57 (27.9%) cases. Compared to patients who had preoperatively a 2012 Briganti nomogram score ≤12%, those with a score >12% were more likely to present with higher percentage of biopsy positive cores, palpable tumors at digital rectal examination, high-grade cancers at prostate biopsies, and unfavorable pathology in the surgical specimen. At multivariable Cox regression analyses, disease progression, which occurred in 85 (41.7%) patients, was predicted by the 2012 Briganti nomogram score (HR: 1.02; 95%CI: 1.00–1.03; p = 0.012), independently by tumors presenting as palpable (HR: 1.78; 95%CI: 1.10.2.88; p = 0.020) or the presence of PLNI in the surgical specimen (HR: 3.73; 95%CI: 2.10–5.13; p = 0.012).
Conclusions
The 2012 Briganti nomogram represented an independent predictor of adverse prognosis in HR PCa patients treated with RARP and ePLND. As the score increased, so patients were more likely to experience disease progression, independently by the occurrence of PLNI. The association between the nomogram, unfavorable pathology and tumor behavior might turn out to be useful for selecting a subset of patients needing different treatment paradigms in HR disease.
Introduction
Actually, prostate cancer (PCa) is an epidemic issue, as stated by the European Association of Urology (EAU) and the National Comprehensive Cancer Network (NCCN) whose task is to continuously update guidelines in order to address best treatment options thus avoiding overtreatments and regret of patients [Citation1–4]. Accordingly, patients are classified into prognostic risk groups that are heterogenous and often not equivalent for the two systems, which consider management options varying from monitoring to active treatment strategies including radical prostatectomy (RP), eventually associated with extended pelvic lymph node dissection (ePLND), and radiation therapy (RT) [Citation1,Citation2].
Approximately from 17 to 31% of newly-diagnosed PCa patients have a high-risk (HR) localized or locally advanced disease at clinical presentation [Citation5,Citation6]. This risk class represents one of the most challenging for being heterogeneous and not equivalent among inclusion criteria for the EAU and the NCCN [Citation1,Citation2]. Additionally, despite these patients require active treatments, according to life expectancy issues, there is still no consensus regarding the optimal management, and currently different strategies, including both local and systemic treatments as a part of a multi-modal therapy seem to provide better cancer control outcomes [Citation7]. In this context, more prognostic factors might turn useful in order to further stratify this group of patients to identify the most appropriate management.
In HR PCa patients candidates to RP, ePLND is strongly recommended by international guidelines due to the risk of pelvic lymph node invasion (PLNI) which ranges from 15 to 40% according to reports [Citation1,Citation2,Citation7,Citation8]. Several validated nomograms assessing the risk of PLNI are available [Citation9–14]. Of these, the 2012 Briganti nomogram is one of the most used for its facility to compute [Citation9]. Nevertheless, its role as a potential prognostic risk factor in this subset of patients, after surgery, has not yet been evaluated [Citation1,Citation2,Citation5]. Accordingly, the aim of the present study was to test whether the 2012 Briganti nomogram score may also predict disease progression after surgery in a selected cohort of EAU HR PCa patients.
Materials and methods
Selection and evaluation of the EAU high-risk population
The present study was approved by internal Institutional Review Board. From January 2013 to December 2021, data on 204 EAU HR PCa patients treated with robot-assisted radical prostatectomy (RARP) and ePLND at the Department of Urology of the Integrated University Hospital of Verona were retrospectively evaluated. All patients were not under androgen blockade, did not undergo previous active treatments and had available follow-up data.
Patients were clinically evaluated for age (years), body mass index (BMI; kg/m2), physical status according to the American Society of Anesthesiologist classification system [Citation15], prostate specific antigen (PSA; ng/mL), prostate volume (PV, mL), biopsy positive cores (BPC; percentage), International Society of Urological Pathology (ISUP) grade group at prostate biopsy [Citation16], and tumor stage according to the Tumor Node Metastasis (TNM) system (8th edition, 2017 version) [Citation17]. Surgery, which was performed by five skilled surgeons, included RARP and ePLND with a template including external iliac, obturator, Cloquet’s and Marcille’s regions [Citation18,Citation19]. Two dedicated uro-pathologists assessed surgical specimens for tumor grade, stage, as well as for cancer invasion of surgical margins [Citation20] and of counted pelvic lymph nodes; accordingly, tumors were graded according to the ISUP system and staged by the TNM system [Citation16,Citation17].
After surgery, patients were followed up according to guidelines and decisions of further treatments (adjuvant or at disease progression) were discussed in a multidisciplinary setting including urologists, radiation oncologists, and medical oncologists to optimize recommendations with patients’ personal issues.
The outcome of interest was disease progression that was defined as the event of biochemical recurrence and/or PSA persistence and/or local recurrence and/or distant metastases.
Statistical analysis
Descriptive statistics included frequencies and proportions for categorical variables. Medians and interquartile ranges (IQR) were used for continuous variables. The 2012 Briganti nomogram score (%) was evaluated both as a continuous and categorical variable, which was dichotomized at the median. Associations of categorized 2012 Briganti nomogram with clinical and pathological factors were assessed by the logistic regression model (univariable and multivariable analysis).
The length of time between surgery and PCa progression or the last available follow-up was measured as time to event occurrence. Accordingly, non-adjusted Kaplan–Meier estimator curves were generated. The association of clinical and pathological factors with the risk of PCa progression was evaluated by the Cox proportional hazard regression models including univariable and multivariable analysis, which was performed according to the Wald’s forward method for collinearity of the nomogram. Odds ratios (ORs), hazard ratios (HRs), and relative 95% confidence intervals (CIs) were computed. IBM-SPSS version 26.0 (IBM Corp., Armonk, NY, USA) was used for all analyses. All tests were two-sided with p < 0.05 considered to indicate statistical significance.
Results
Characteristics of the study population
The main characteristics of 204 HR PCa patients treated with RARP and ePLND are listed in . Median age was 67 (61–71) years. Preoperative ASA physical status was 1 in 15 (7.4%) patients, 2 in 163 (79.9%) patients, and 3 in 26 (12.7%) patients. Median 2012 Briganti nomogram score was 12.0% (6.0–22.0%). Anatomical staging of pelvic lymph nodes was performed in all cases and the median number of counted lymph nodes was 25 (19–32). PLNI was detected in 57 (27.9%) cases. Compared to patients who had preoperatively a 2012 Briganti nomogram score ≤12%, those who had a score >12% were more likely to present with higher percentage of BPC (58.8% vs. 28.5%), palpable tumors at digital rectal examination (78.8% vs. 54.3%), and high-grade cancers at prostate biopsies (70.7% vs. 51.4%). Furthermore, they were also more likely to harbor unfavorable pathology in the surgical specimen including tumors with ISUP grade group 4–5 (73.7% vs. 45.7%), extracapsular extension (18.2% vs. 11.4%), seminal vesicle invasion (43.4% vs. 16.2%), as well as PLNI (39.4% vs. 17.1%).
Table 1. Descriptive statistics of the study population stratified according to the 2102 Briganti nomogram score (≤12% vs. >12%) and logistic regression models testing the association of clinical and pathological factors with the 2012 Briganti nomogram score.
The prognostic impact of the 2012 Briganti nomogram on disease progression
Median follow-up was 61.0 (54.0–67.9) months. Disease progression occurred in 85 (41.7%) patients (). Patients who did experience disease progression presented a higher median 2012 Briganti nomogram score compared to those who did not experience progression (16.0% vs. 10.0%; ). Kaplan–Meier plots depicted PCa progression free-survival according to the 2012 Briganti nomogram score; here, median progression free survival was higher in patients with a nomogram score ≤12% compared to those with a nomogram score >12% (67.0 vs 52.0 months, p < 0.001; ). At multivariable Cox proportional hazards regression analysis, PCa progression was independently predicted by the 2012 Briganti nomogram (HR: 1.02; 95%CI: 1.00–1.03; p = 0.012), independently by tumors presenting as palpable (HR: 1.78; 95%CI: 1.10.2.88; p = 0.020) or by the presence of PLNI in the surgical specimen (HR: 3.73; 95%CI: 2.10–5.13; p = 0.012; ). The distribution of the median 2012 Briganti nomogram score according to clinical tumor stage (palpable vs. not palpable tumors) and stratified by the occurrence of disease progression is shown in .
Figure 1. Box and whisker plots illustrating the distribution of the 2012 Briganti nomogram score predicting lymph node invasion stratified according to the occurrence of disease progression in 204 EAU high-risk patients treated with radical prostatectomy and extended pelvic lymph node dissection. Median risk score was significantly higher in patients experiencing disease progression compared to those who did not progress (16.0, IQR: 7.0–29.5 vs. 10.0, IQR 5.0–19.0; OR: 1.03; 95%CI: 1.01–1.04; p <0.001).
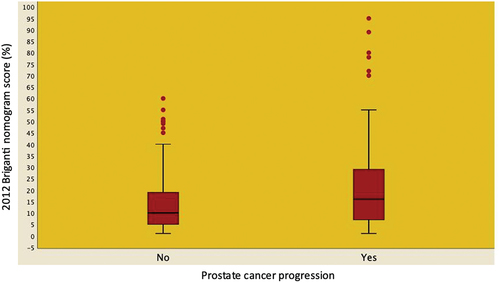
Figure 2. Kaplan-Meier plots depicting prostate cancer (PCa) progression – free survival in 204 EAU high – risk patients treated with robot-assisted radical prostatectomy and extended pelvic lymph node dissection according to the 2012 Briganti nomogram score (up to the median vs. above the median). Median PCa progression free survival was higher in patients exhibiting a score ≤ 12% (67.0, IQR: 59.1–4.3 months) compared to those exhibiting a score > 12% (52.0, IQR: 44.6–59.3 months) with the difference being statistically significant (Mantel-Cox log rank test: p < 0.001; univariable hazard ratio: 1.87, 95% CI: 1.21–2.87; p = 0.005).
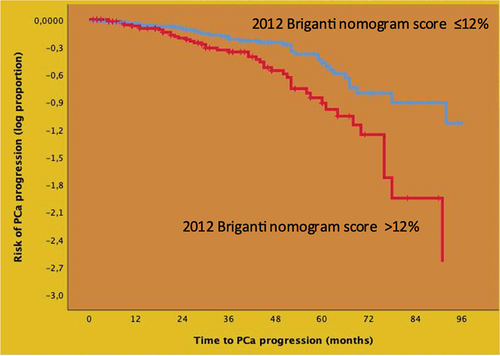
Figure 3. Distribution of median 2012 Briganti nomogram score predicting pelvic lymph node invasion stratified according to clinical tumor stage (palpable vs. not palpable tumors) and disease progression in 204 EAU high-risk prostate cancer patients treated with robot-assisted radical prostatectomy and extended pelvic lymph node invasion. Median 2012 Briganti nomogram score was higher in progressing patients, independently by clinical tumor stage.
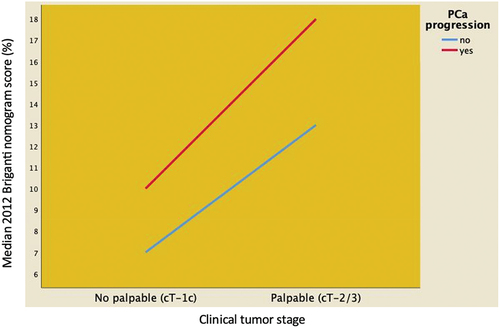
Table 2. Cox regression models testing the 2012 Briganti nomogram as a predictor of disease progression after surgery in 204 EAU high-risk prostate cancer (PCa) patients treated with robot-assisted radical prostatectomy and extended pelvic lymph node dissection.
The prognostic impact of the 2012 Briganti nomogram on PCa progression is summarized in . Accordingly, as the nomogram score increased, so patients were more likely to experience progression independently by presenting with unfavorable clinical factors, as well as harboring unfavorable pathology in the surgical specimen.
Table 3. Summary of the prognostic impact of the 2012 Briganti nomogram score on prostate cancer progression in 204 EAU high-risk prostate cancer patients treated with robot-assisted radical prostatectomy and extended pelvic lymph node.
Discussion
The EAU HR PCa class is a heterogenous and controversial category requiring primary and often secondary treatments for the risk of disease recurrence and progression [Citation5,Citation7,Citation21]. Likewise, surgically treated HR PCa patients have mortality rates that vary from 5.8% to 13.5% at 10 years, according to class 4 and 5 of the Cambridge Prognostic Group Classification, respectively [Citation22,Citation23]. However, not all HR PCa patients will experience disease progression, thus stressing the issue of identifying more prognostic factors. Accordingly, it has been shown that factors associated with disease progression include early biochemical recurrence, unfavorable tumor grades, and PSA doubling time; nevertheless, multilevel nomograms could improve the accuracy of such prognostic factors [Citation24,Citation25]. The 2012 Briganti nomogram, which accounts for PSA, clinical tumor stage, primary and secondary Gleason Grade Group at prostate biopsies, and percentage of BPC, still stand as one of the most effective tools for predicting PLNI [Citation9], for those including multiparametric magnetic resonance imaging (mpMRI) findings are not always reproducible [Citation11,Citation26].
In our study, we have shown that the 2012 Briganti nomogram score was an independent predictor of PCa progression in a cohort of patients presenting with EAU HR disease treated at a tertiary referral center. Accordingly, as the nomogram score increased, so patients were more likely to experience disease progression; conversely, patients presenting with unfavorable tumor stage and/or harboring PLNI but having a lower median nomogram score were less likely to undergo disease progression.
The findings of the present study are a novelty and may have important clinical implications. In HR PCa including both localized and locally advanced disease, although surgery is one of the main primary treatments, appropriate selection of patients still remains a challenging task because of implications on paradigm treatments, which include local and systemic treatments within a multidisciplinary integrated approach [Citation7,Citation21,Citation27]. Although blood and tissue novel biomarkers can help for selecting patients, they are still far from everyday clinical practice [Citation28]. Accordingly, our results have shown that EAU HR PCa patients can be stratified according to the 2012 Briganti nomogram score, which was higher in patients who were more likely to experience progression, independently by presenting with palpable tumors and/or harboring PLNI. Specifically, a nomogram score above 12% predicted adverse prognosis after associating with unfavorable pathology in the surgical specimen. However, confirmatory studies are required. Likewise, our results showed that it is possible to cluster EAU HR PCa patients at clinical presentation according to the score of the nomogram.
In our study, we showed that 2012 Briganti nomogram predicted the natural history of PCa after tracing patterns of unfavorable pathology in the surgical specimen. Accordingly, as the risk score increased, so patients not only were more likely to have undifferentiated cancers invading seminal vesicle and metastasizing to pelvic lymph nodes but also to experience disease progression. In our opinion, these findings might be explained by the fact that the nomogram includes several clinical variables, which interact and integrate with each other at a high-dimensional level, thus allowing the identification of cancers that will show a malignant behavior because of the dynamic genetic instability structuring these cancers, as well. Nevertheless, these hypotheses need to be tested by controlled studies.
Despite its novelty, the present study is not devoid of limitations. First, it is a retrospective study. Second, surgical procedures were performed by different surgeons, thus reflecting real-world practice but possibly affecting outcomes assessment. Third, MRI findings were not evaluated for not being available in all cases; therefore, we did not use the updated version of the nomogram, which specifically accounts for clinical stage and Gleason Grade Group based on MRI data, as well as for maximum diameter of the targeted index lesion at MRI, demonstrating higher accuracy compared to other existing tools [Citation11]. Nevertheless, our study has strengths such as anatomical staging of pelvic lymph nodes that was extensive and appropriate for evaluating oncological results of the EAU HR category [Citation29,Citation30].
Conclusions
The 2012 Briganti nomogram represented an independent predictor of disease progression in EAU HR PCa patients treated with RARP and ePLND at a tertiary referral center. Accordingly, as the risk score increased, so patients were more likely to experience disease progression, independently by the occurrence of PLNI. The association of the nomogram with unfavorable pathology and tumor behavior turns out to be useful for selecting subset of patients needing different treatment paradigms in high-risk disease.
Authors’ contributions
Study concept and design: Antonio Benito Porcaro.
Data acquisition: Andrea Panunzio, Rossella Orlando, Sebastian Gallina, Alberto Bianchi, Emanuele Serafin, Giovanni Mazzucato, Francesca Montanaro, Alberto Baielli, Francesco Artoni, Francesco Ditonno, Luca Roggero, Andrea Franceschini, Michele Boldini, Lorenzo Pierangelo Treccani.
Data analysis: Antonio Benito Porcaro.
Drafting of manuscript: Antonio Benito Porcaro, Andrea Panunzio, Rossella Orlando.
Critical revision of the manuscript: Alessandro Tafuri, Alessandro Veccia, Riccardo Rizzetto, Matteo Brunelli, Vincenzo De Marco, Salvatore Siracusano, Riccardo Bertolo, Maria Angela Cerruto, Alessandro Antonelli.
All authors read and approved the final version of the manuscript.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. The study was approved by our Institutional Review Board. Data were collected prospectively but evaluated retrospectively; as such, Ethical Committee Approval was not required.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
All data generated or analyzed during this study are included in this article. Further enquires can be directed to the corresponding author.
Additional information
Funding
References
- EAU Guidelines on Prostate Cancer. Edn. presented at the EAU Annual Congress Milan 2023. Arnhem, The Netherland: EAU Guidelines Office; n.d. ISBN 978-94-92671-19-6.
- NCCN. Clinical practice guidelines in oncology, prostate cancer, version 4. 2023 [cited 2023 Nov 4th]. n.d. Available from: https://www.nccn.org/professionals/physician_gls/pdf/prostate.pdf
- Wallis CJD, Zhao Z, Huang L-C, et al. Association of treatment modality, functional outcomes, and baseline characteristics with treatment-related regret among men with localized prostate cancer. JAMA Oncol. 2022;8(1):50–59. doi: 10.1001/jamaoncol.2021.5160
- Hamdy FC, Donovan JL, Lane JA, et al. Fifteen-year outcomes after monitoring, surgery, or radiotherapy for prostate cancer. N Engl J Med. 2023;388(17):1547–1558. doi: 10.1056/NEJMoa2214122
- Cooperberg MR, Cowan J, Broering JM, et al. High-risk prostate cancer in the United States, 1990–2007. World J Urol. 2008;26(3):211–218. doi: 10.1007/s00345-008-0250-7
- Chierigo F, Flammia RS, Sorce G, et al. Racial/Ethnic disparities in the distribution and effect of type and number of high-risk criteria on mortality in prostate cancer patients treated with radiotherapy. Arab J Urol. 2023;21(3):135–141. doi: 10.1080/2090598X.2022.2148867
- Moris L, Cumberbatch MG, Van den Broeck T, et al. Benefits and risks of primary treatments for high-risk localized and locally advanced prostate cancer: an international multidisciplinary systematic review. Eur Urol. 2020;77:614–627. doi: 10.1016/j.eururo.2020.01.033
- Gongora M, Stranne J, Johansson E, et al. Characteristics of patients in SPCG-15-A randomized trial comparing radical prostatectomy with primary radiotherapy plus androgen deprivation therapy in men with locally advanced prostate cancer. Eur Urol Open Sci. 2022;41:63–73. doi: 10.1016/j.euros.2022.04.013
- Briganti A, Larcher A, Abdollah F, et al. Updated nomogram predicting lymph node invasion in patients with prostate cancer undergoing extended pelvic lymph node dissection: the essential importance of percentage of positive cores. Eur Urol. 2012;61:480–487. doi: 10.1016/j.eururo.2011.10.044
- Gandaglia G, Fossati N, Zaffuto E, et al. Development and internal validation of a novel model to identify the candidates for extended pelvic lymph node dissection in prostate cancer. Eur Urol. 2017;72:632–640. doi: 10.1016/j.eururo.2017.03.049
- Gandaglia G, Ploussard G, Valerio M, et al. A novel nomogram to identify candidates for extended pelvic lymph node dissection among patients with clinically localized prostate cancer diagnosed with magnetic resonance imaging-targeted and systematic biopsies. Eur Urol. 2019;75(3):506–514. doi: 10.1016/j.eururo.2018.10.012
- Tosoian JJ, Chappidi M, Feng Z, et al. Prediction of pathological stage based on clinical stage, serum prostate-specific antigen, and biopsy Gleason score: partin tables in the contemporary era. BJU Int. 2017;119(5):676–683. doi: 10.1111/bju.13573
- Memorial Sloan Kettering Cancer Center. Pre-radical prostatectomy tool to predict probability of lymph node involvement in prostate cancer patients. n.d. Available from: https://www.mskcc.org/nomograms/prostate/pre_op
- Yu JB, Makarov DV, Gross C. A new formula for prostate cancer lymph node risk. Int J Radiat Oncol Biol Phys. 2011;80:69–75. doi: 10.1016/j.ijrobp.2010.01.068
- Porcaro AB, Rizzetto R, Amigoni N, et al. American Society of Anesthesiologists’ (ASA) physical status system and risk of major clavien-dindo complications after robot-assisted radical prostatectomy at hospital discharge: analysis of 1143 consecutive prostate cancer patients. Indian J Surg Oncol. 2022;13(4):848–857. doi: 10.1007/s13193-022-01577-9
- Epstein JI, Egevad L, Amin MB, et al. The 2014 International Society of Urological Pathology (ISUP) consensus conference on Gleason grading of prostatic carcinoma: definition of grading patterns and proposal for a new grading system. Am J Surg Pathol. 2016;40(2):244–252. doi: 10.1097/PAS.0000000000000530
- Paner GP, Stadler WM, Hansel DE, et al. Updates in the eighth edition of the tumor-node-metastasis staging classification for urologic cancers. Eur Urol. 2018;73(4):560–569. doi: 10.1016/j.eururo.2017.12.018
- Tafuri A, Sebben M, Pirozzi M, et al. Predictive factors of the risk of long-term hospital readmission after primary prostate surgery at a single tertiary referral center: preliminary report. Urol Int. 2020;104(5–6):465–475. doi: 10.1159/000505409
- Porcaro AB, Tafuri A, Panunzio A, et al. Endogenous testosterone density is an independent predictor of pelvic lymph node invasion in high-risk prostate cancer: results in 201 consecutive patients treated with radical prostatectomy and extended pelvic lymph node dissection. Int Urol Nephrol. 2022;54(3):541–550. doi: 10.1007/s11255-022-03103-w
- Panunzio A, Sorce G, Hoeh B, et al. Effect of positive surgical margins at radical prostatectomy on cancer-specific mortality in high/very high-risk prostate cancer patients with Gleason grade group 4–5. Prostate. 2023;83(3):268–276. doi: 10.1002/pros.24458
- Delporte G, Henon F, Ploussard G, et al. Radical prostatectomy for locally advanced and high-risk prostate cancer: a systematic review of the literature. Progrès en Urologie. 2018;28(16):875–889. doi: 10.1016/j.purol.2018.08.007
- Parry MG, Cowling TE, Sujenthiran A, et al. Risk stratification for prostate cancer management: value of the Cambridge prognostic group classification for assessing treatment allocation. BMC Med. 2020;18(1):114. doi: 10.1186/s12916-020-01588-9
- Gnanapragasam VJ, Bratt O, Muir K, et al. The Cambridge prognostic groups for improved prediction of disease mortality at diagnosis in primary non-metastatic prostate cancer: a validation study. BMC Med. 2018;16(1):31. doi: 10.1186/s12916-018-1019-5
- Van den Broeck T, van den Bergh RCN, Arfi N, et al. Prognostic value of biochemical recurrence following treatment with curative intent for prostate cancer: a systematic review. Eur Urol. 2019;75(6):967–987. doi: 10.1016/j.eururo.2018.10.011
- Tilki D, Preisser F, Graefen M, et al. External validation of the European Association of urology biochemical recurrence risk groups to predict metastasis and mortality after radical prostatectomy in a European cohort. Eur Urol. 2019;75(6):896–900. doi: 10.1016/j.eururo.2019.03.016
- Oderda M, Diamand R, Albisinni S, et al. Indications for and complications of pelvic lymph node dissection in prostate cancer: accuracy of available nomograms for the prediction of lymph node invasion. BJU Int. 2021;127(3):318–325. doi: 10.1111/bju.15220
- Greenberger BA, Zaorsky NG, Den RB. Comparison of radical prostatectomy versus radiation and androgen deprivation therapy strategies as primary treatment for high-risk localized prostate cancer: a systematic review and meta-analysis. Eur Urol Focus. 2020;6(2):404–418. doi: 10.1016/j.euf.2019.11.007
- McKay RR, Feng FY, Wang AY, et al. Recent advances in the management of high-risk localized prostate cancer: local therapy, systemic therapy, and biomarkers to guide treatment decisions. Am Soc Clin Oncol Educ Book. 2020;40(40):e241–e252. doi: 10.1200/EDBK_279459
- Prendeville S, van der Kwast TH. Lymph node staging in prostate cancer: perspective for the pathologist. J Clin Pathol. 2016;69(12):1039–1045. doi: 10.1136/jclinpath-2016-203643
- Boscolo-Berto R, Siracusano S, Porzionato A, et al. The underestimated posterior lymphatic drainage of the prostate: an historical overview and preliminary anatomical study on cadaver. Prostate. 2020;80(2):153–161. doi: 10.1002/pros.23927

