Abstract
Despite numerous studies on arbuscular mycorrhizal fungi (AMF) in potato (Solanum tuberosum L.), nothing is known about colonization by dark septate endophytes (DSE). In a study of DSE and AMF associations in a potato field at Meghalaya, northeast India, monthly DSE and AMF colonization ranged from 7.9±2.9–13.2±1.65 and 6.98±3.13–12.4±3.53%, respectively. There were no significant differences in monthly DSE and AMF colonization. AMF hyphal colonization had a significant negative correlation with pH and available phosphorus (p < 0.05). Total AMF colonization was negatively correlated with organic carbon (p < 0.05). DSE colonization showed a significant negative correlation with root hair density (p < 0.05). However, AMF colonization, although negatively correlated with root hairs, was not significant. Sixteen AMF morphotypes were identified from the genera Acaulospora, Gigaspora, Glomus, Pacispora and Scutellospora; a few remain unidentified. The results indicated that DSE and AMF colonization progressed synchronously with the dominance of Glomus tortuosum, Pacispora boliviana and Gigaspora margarita.
1. Introduction
Mycorrhizal symbiosis has been frequently thought to be identical with mutualistic symbiosis, where fungi benefit by acquiring carbohydrates from a host and the host, in turn, gains by improved growth and more efficient nutrient acquisition. Dark septate endophytes (DSE) are a miscellaneous group of root-inhabiting conidial sterile ascomycetous fungi that colonize living plant roots without causing noticeable harmful effects (Jumpponen Citation2001). Arbuscular mycorrhizal fungi (AMF) are involved in plant mineral nutrition, an association which is prevalent in agricultural plants (Smith and Read Citation1997). Both groups are essential for successful low-input agricultural practices. Hence, the formation and functioning of AMF symbiosis will play an important role in sustainable agriculture (Schreiner and Bethlenfalvay Citation1995).
Solanum tuberosum L. (Potato) is a staple food in northeast India and studies on AMF and DSE will assist sustainable agriculture in this region. Studies on AMF associations in potato have shown that phosphate fertilization suppressed AMF colonization (Black and Tinker Citation1977). Bhattarai and Mishra (Citation1984) reported that AMF colonization increased with age in potato plants. Potato plants have a low root density (Pursglove and Sanders Citation1981) and high growth potential; thus, AMF symbiosis may be of particular significance in coping with P deficiency stress in natural ecosystems. This is also true for commercial potato production, since significant yield increases due to AMF have been recorded (McArthur and Knowles Citation1991). A reduction in phosphate fertilizer use can also be achieved in potato by inoculating with AMF (Sieverding Citation1991). Morphological features, such as root hair development, were greater on non-mycorrhizal roots than on AMF roots (McArthur and Knowles Citation1993). Davies et al. (Citation2005) reported an increase in potato yield with AMF inoculation; in addition, flavanoid increased the extraradical hyphae of AMF. A study of the AMF large-subunit (LSU) rRNA gene analyzing the fungal community suggested that potato roots were preferentially colonized by Glomus intraradices Schenck & Smith 1982 (Cesaro et al. Citation2008).
Despite these studies, nothing is known about DSE colonization. The present study investigated the association between DSE and AMF in potato.
2. Methods
2.1 Site description
In September 2007, two potato plots, each 100 × 50 m2 in area, were selected for root and soil sampling in Swer village (latitude 25°25′ N and longitude 91°47′ E) in the East Khasi Hills District of Meghalaya in northeast India. The site is situated about 34 km south of Shillong on the road to Cherrapunjee. Altitude varies between 1910 and 1975 m.a.s.l. The climate is monsoonal with distinct warm-wet and cold-dry seasons. The rainy season starts in May and continues till October.
Potato is grown in two seasons i.e. in summer it extends from February to June/July, while in autumn it extends from July/August to November/December. The local Khasi tribal farmers grow varieties of Kufri Jyoti, a high yielding potato cultivar which covers over 50% of the total potato growing area in the state.
2.2 Sampling
Potato tubers were planted in August 2007 and sampling was conducted on the first week of subsequent months, i.e. September, October and November; after the emergence of stalks from the tuber. Three plants from each plot were sampled for root and 500 g soil at a depth of 0–25 cm, and combined into one sample. In addition, six samples were collected randomly, three from each plot, during the last month (November) and again combined into one sample for an AMF diversity study. The roots were fixed in FAA (Johansen Citation1940) and soil samples were air dried after analysis of pH and moisture content for further soil physico-chemical studies.
2.3 Root processing
The fixed roots were washed in tap water, cleared in 10% KOH at 90°C and stained with black Faber Castell stamp pad ink (Das and Kayang Citation2008). Fifty segments of ∼1 cm long stained root samples were mounted on slides in lactoglycerol and examined for AMF and DSE structures under a light microscope (Olympus 41209). Estimations of AMF and DSE colonization were done by the magnified intersection method (McGonigle et al. Citation1990).
2.4 Root hair density measurement
Root hair density measurements were performed on root segments similar to those examined for quantification of fungal colonization. The root segments were counted three-dimensionally by adjusting the microscope's plane of focus (Ma et al. Citation2001).
2.5 AMF spore diversity
The spores were extracted by a modified wet sieving and decanting method (Muthukumar et al. Citation2006). The soil weighing 100 g was dispersed in 1 l of water and decanted through a series of 710- to 38-μm sieves. The residues were filtered through gridded filter papers and all whole spores were counted using a light microscope at 40× magnification. Sporocarps and spore clusters were considered as one unit. The isolated spores were picked up with a needle in polyvinyl alcohol/lactoglycerol under a microscope (Koske and Tessier Citation1983) and also in mixed polyvinyl alcohol/lactoglycerol–Meltzer's reagent (1:1, v/v) for identification. Complete and broken spores were examined using a light microscope. Taxonomic identification of spores to species level was based on sporocarpic size, colour, ornamentation and wall characteristics by matching original descriptions (http://www.invam.caf.wvu.edu; Koske et al. Citation1986; Blaszkowski Citation1989; Almeida and Schenck Citation1990; Wu et al. Citation1995; Oehl and Sieverding Citation2004). Photography of the root segments colonized by fungi and spores of AMF was via a Leica EC 3 camera attached in a Leica dm 1000 microscope. Spore density, relative abundance (RA) and species richness (SR) were all recorded (Zhao and Zhao Citation2007).
2.6 Soil properties
Soil texture was analyzed using the sodium hexametaphosphate method (Allen et al. Citation1974). For moisture content (%), 10 g subsamples of soil were oven-dried and the final weight determined. Measurement of pH was done using a microprocessor-based Pocket pH yester 2 (Eutech Instruments). Available phosphorus in the soil was determined following the molybdenum-blue method (Allen et al. Citation1974). Soil organic carbon was estimated using a colorimetric method (Anderson and Ingram Citation1993).
2.7 Data analysis
A one-sample t-test was conducted to analyse the monthly variation in the soil physico-chemical properties. All colonization variables were submitted to one-way ANOVA and Tukey's HSD test was used for comparison of means. Pearson correlation was done to determine relationships between the soil physico-chemical properties, fungal colonization and spore density. The data were analyzed with Statistica 9.0 software.
3. Results
3.1 Soil properties
The mean values of soil physico-chemical characteristics are presented in , which varies significantly in all three months (p < 0.05). Moisture content of soil samples ranges between 25.38(±3.9) and 31.17(±2.35)%. pH varies between 5.17(±0.06) and 5.43(±0.11). The percentage of organic carbon and available phosphorus ranged from 10.28(±0.26)–12.36(±0.74)% and 4.7(±0.54)–7.8(±0.92) mg/kg, respectively.
Table 1. Selected physico-chemical properties of soil from a potato field in Meghalaya, India
3.2 Fungal colonization and root hair density
Root hairs and mycorrhizal structures were confirmed, viz. intraradical hyphae, vesicles, arbuscules, microsclerotia and septate hyphae (). The monthly means for fungal colonization and AMF spore density are presented in . There was a minor increase in monthly fungal colonization. AMF and DSE colonization ranged from 6.98(±3.13)–12.4(±3.53)% and 7.9(±2.9)–13.2(±1.65)%, respectively. There were no significant differences in the monthly total AMF and DSE colonization (p < 0.05). However, there was a significant difference in monthly AMF structural colonization i.e. vesicle colonization (p < 0.05). Monthly fungal colonization is shown in . Root hair density/cm of root segment ranged from 42.12(±6.54)–58.86(±6.67).
Figure 1. Dark septate endophyte, arbuscular mycorrhizal colonization and root structure of potato. (a & b) Root segments with root hairs. Bar = 1.2 mm & 1.2 mm, respectively; (c-i) portions of roots showing DSE colonization. Bar = 300μm, 100μm, 100μm, 100μm, 50μm, 50μm, & 150μm, respectively; (j-o) roots showing AMF structures. Bar = 1.2mm, 300μm, 50μm, 75μm, 50 μm & 100μm, whereas; rh-root hairs, ms-microsclerotia, dsh-dark septate hyphae, ar-arbuscules, ich-intracellular hyphae and v-vesicles.
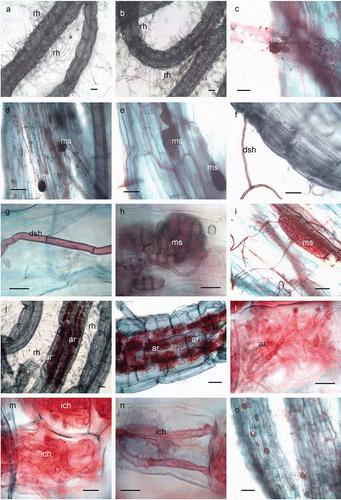
Figure 2. Arbuscular mycorrhizal and dark septate endophyte colonization in the roots of potato collected from three months.
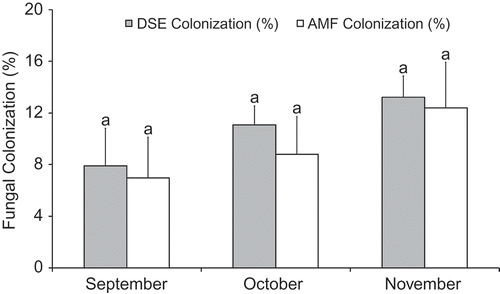
Table 2. AMF and DSE colonization of potato and monthly AMF spore density
Correlation values are given in , where AMF hyphal colonization was negatively correlated with pH and available phosphorus. There was an insignificant negative colonization between root hair density and AMF colonization. However, there was a significant negative correlation between root hair density and DSE. In addition, there was a significant negative correlation between organic carbon and AMF colonization. No significant relation was observed between spore density and AMF colonization.
Table 3. Correlation coefficients between fungal colonization, root hair density and soil physico-chemical properties
3.3 AMF spore diversity
Sixteen morphotypes were identified from soil in the potato field (). There were seven species from genus Glomus, three from Pacispora, four from Acaulospora and one each from Gigaspora and Scutellospora, and a few were unidentified. The dominant AMF species were Glomus tortuosum Schenck & Smith, followed by Pacispora boliviana Sieverd. & Oehl, and Gigaspora margarita Becker & Hall (). The relative abundance of AMF species in the potato field follows the trend Glomus > Pacispora > Gigaspora > Acaulospora > Scutellospora with 52.54, 23.25, 19.23, 4.55 and 0.43%, respectively. There were significant differences (p < 0.05) in relative abundance between genera. The species richness of Glomus, Acaulospora, Pacispora, Gigaspora and Scutellospora was 7.3, 4.7, 2.7, 1.3 and 0.7, respectively. There were no significant differences (p < 0.05) in species richness of the different genera.
Figure 3. Arbuscular mycorrhizal fungi isolated from potato field. (a) Glomus macrocarpum. Bar = 200μm; (b) G. clavisporum. Bar = 150μm; (c) G. fuegianum. Bar = 200μm; (d) peridium [p] surrounding the spores of G. tortuosum. Bar = 200μm; (e) spores of G. aggregatum inside unidentified spore [us]. Bar = 500μm; (f) spores of G. microaggregatum inside cyst nematode [cs]. Bar = 500μm; (g) Glomus sp 1. Bar = 100μm; (h) Acaulospora tuberculata with sporiferous saccule [ss]. Bar = 50μm; (i) A. cavernata with sporiferous saccule. Bar = 50μm; (j) pitted ornamentation on the wall of A. cavernata. Bar = 10μm; (k) ridges on the surface of A. rehmii. Bar = 10μm; (l) wall of Pacispora chimonobambusae with clavate projections. Bar = 10μm; (m) P. bolivianawith ornamented pits. Bar = 100μm; (n) Gigaspora margarita. Bar = 400μm; (o) Scutellospora sp 2. Bar = 300μm; (p) germination shield [gs] of Scutellospora sp 2. Bar = 20μm; (q) auxiliary cells [ac] of Scutellospora sp 2. Bar = 10μm; (r) Pacispora like spore with ornamentation on the wall. Bar = 200μm; (s) hyaline layer [hl] on the wall of unidentified species. Bar = 100μm; (t) unidentified species. Bar = 100μm; (u) thorn like projection [tp] on the wall of unidentified species. Bar = 25μm; (v) wart like projection [wp] on the wall of unidentified species. Bar = 100μm & (w) hyaline layer on the wall of unidentified species. Bar = 200μm.
![Figure 3. Arbuscular mycorrhizal fungi isolated from potato field. (a) Glomus macrocarpum. Bar = 200μm; (b) G. clavisporum. Bar = 150μm; (c) G. fuegianum. Bar = 200μm; (d) peridium [p] surrounding the spores of G. tortuosum. Bar = 200μm; (e) spores of G. aggregatum inside unidentified spore [us]. Bar = 500μm; (f) spores of G. microaggregatum inside cyst nematode [cs]. Bar = 500μm; (g) Glomus sp 1. Bar = 100μm; (h) Acaulospora tuberculata with sporiferous saccule [ss]. Bar = 50μm; (i) A. cavernata with sporiferous saccule. Bar = 50μm; (j) pitted ornamentation on the wall of A. cavernata. Bar = 10μm; (k) ridges on the surface of A. rehmii. Bar = 10μm; (l) wall of Pacispora chimonobambusae with clavate projections. Bar = 10μm; (m) P. bolivianawith ornamented pits. Bar = 100μm; (n) Gigaspora margarita. Bar = 400μm; (o) Scutellospora sp 2. Bar = 300μm; (p) germination shield [gs] of Scutellospora sp 2. Bar = 20μm; (q) auxiliary cells [ac] of Scutellospora sp 2. Bar = 10μm; (r) Pacispora like spore with ornamentation on the wall. Bar = 200μm; (s) hyaline layer [hl] on the wall of unidentified species. Bar = 100μm; (t) unidentified species. Bar = 100μm; (u) thorn like projection [tp] on the wall of unidentified species. Bar = 25μm; (v) wart like projection [wp] on the wall of unidentified species. Bar = 100μm & (w) hyaline layer on the wall of unidentified species. Bar = 200μm.](/cms/asset/599883bd-bbf7-4728-bff7-d5069b14142d/tmyc_a_517787_o_f0003g.jpg)
Table 4. Relative abundance of AMF species in the potato field
4. Discussion
AMF colonization was found to increase slightly from 1-month to 2-month-old plants; Bhattarai and Mishra (Citation1984) reported that AMF colonization increased with the age of the plant. There was also an increase in DSE colonization from the first to last month. The arbuscular peak was observed earlier than the vesicular peak, which supports the observation of Bhattarai and Mishra (Citation1984). DSE colonization has also been observed to occur simultaneously with AMF, as reported previously (Jumpponen and Trappe Citation1998), and the present study suggest that DSE colonization progress in the same trend as AMF.
Potato plants have been characterized as relatively ineffective at acquiring soil phosphorus (Pursglove and Sanders Citation1981), but are capable of morphological and physiological adaptations during phosphorus deficiency that may substantially improve P acquisition (Cogliatti and Clarkson Citation1983). Morphological changes, such as the presence of root hairs, may help in phosphorus acquisition. In general, colonization is comparatively low, which may be due to the high availability of phosphorus coupled with the presence of root hairs, as decreased fungal colonization in the roots with high levels of phosphorus was reported by Galvez et al. (Citation2001). Moreover, DSE possesses a penetrating potential through root hairs (O'Dell et al. Citation1993). However, the inverse correlation between DSE and root hair density was significantly high, which is in accordance with the study of Muthukumar and Udaiyan (Citation2002). They reported an inverse relationship between root morphology and AMF colonization levels in Cycads, where the length of root colonized by AMF was proportional to the length and density of root hairs. Since mycorrhiza function as extensions of the root system, it is possible that species with fewer and short root hairs are more dependent on AMF than species with more abundant and longer root hairs.
Synchronized colonization by DSE and AMF could possibly provide a support system during periods when mycorrhizal fungi are adversely affected by environmental conditions, as reported previously (Jumpponen and Trappe Citation1998), and, in the present case, to combat host susceptibility to disease. The negative correlation between AMF colonization and soil organic carbon may be due to the input of organic manure, which agrees with the report of Piotrowskia et al. (Citation2008) that AMF hyphal length, inoculum potential and colonization of roots were all suppressed at sites with greatest litter and soil organic matter accumulation. No significant relation was observed between spore density and AMF colonization, which is in accordance with the study of Moreira et al. (Citation2006).
Bharadwaj et al. (Citation2007) showed that G. intraradices and G. mosseae (Nicol. & Gerd.) (Gerd. & Trappe) are the most common fungi in potato roots and soil. Cesaro et al. (Citation2008) confirmed that Glomus species were identified, regardless of some pedological dissimilarity. In addition, they reported that G. intraradices was the preferred AMF species colonizing potato root. Our findings suggest that Glomus spp. were abundant, particularly G. tortuosum. This is the first report of Pacispora spp. in these soils and also to our knowledge from soils of India (Das and Kayang Citation2010). P. boliviana may favour this sub-tropical region, as ubiquitously reported (Oehl and Sieverding Citation2004). However, G. microaggregatum Koske, Gemma & P.D. Olexia Citation1986 was found inside a nematode cyst, whereas the same species was reported to occur inside the dead spores of other AMF species (Koske et al. Citation1986). G. aggregatum Schenck & Smith 1982 was also recovered inside an unknown spore-like structure.
5. Conclusion
In the present investigation, a number of species isolated from the potato field remain unidentified; therefore, appropriate trap culture techniques will be the focus of further studies. For instance, species such as G. tortuosum and P. boliviana, were found in high abundance. A comprehensive study is now required, including the highly abundant AMF species, to examine the growth and nutrition of potato plants. Furthermore, it is unclear what factors are responsible for the suppression of mycorrhizal fungal colonization, although they are likely related to the introduction of inorganic fertilization as normal agriculture practice in this region. However, AMF species were found to be abundant; therefore, a proper awareness program is necessary for local farmers to access the benefits of these biological fertilizers. This work provides a platform to analyse the role of AMF and DSE in plant growth and nutrition in several other crop plants in northeast India.
Acknowledgements
The authors thank the Head of the Botany Department for providing laboratory facilities. The first author is grateful to UGC for financial assistance in the form of a Senior Research Fellowship. We are also thankful to the two anonymous reviewers and an anonymous associate editor for their helpful remarks to improve the manuscript.
Notes
References
- Allen , SE , Grimshaw , HM , Parkinson , JA and Quaramby , C. 1974 . Chemical analysis of ecological materials , Oxford : Blackwell .
- Almeida , RT and Schenck , NC. 1990 . A revision of the genus Sclerocystis (Glomaceae, Glomales) . Mycologia , 82 : 703 – 714 .
- Anderson , JM and Ingram , JSI. 1993 . Tropical soil biology and fertility: A handbook of methods , Wallingford, , UK : CAB International .
- Bharadwaj , DP , Lundquist , P and Alströma , S. 2007 . Impact of plant species grown as monocultures on sporulation and root colonization by native arbuscular mycorrhizal fungi in potato . Appl Soil Ecol. , 35 : 213 – 225 .
- Bhattarai , ID and Mishra , RR. 1984 . Study on the vesicular-arbuscular mycorrhiza of three cultivars of potato (Solanum tuberosum L.) . Plant Soil , 79 : 299 – 303 .
- Black , RLB and Tinker , PB. 1977 . Interaction between effects of vesicular–arbuscular mycorrhiza and fertiliser phosphorus on yields of potatoes in the field . Nature , 267 : 510 – 511 .
- Blaszkowski , J. 1989 . Acaulospora cavernata (Endogonaceae): a new species from Poland with pitted spores . Crypt Bot. , 1 : 204 – 207 .
- Cesaro , P , van Tuinen , D , Copetta , A , Chatagnier , O , Berta , G , Gianinazzi , S and Lingua , G. 2008 . Preferential colonization of Solanum tuberosum L. roots by the fungus Glomus intraradices in arable soil of a potato farming area . Appl Environ Microbiol. , 74 : 5776 – 5783 .
- Cogliatti , DH and Clarkson , DT. 1983 . Physiological changes in, and phosphate uptake by potato plants during development of, and recovery from phosphate deficiency . Physiol Plant. , 58 : 287 – 294 .
- Das , P and Kayang , H. 2008 . Stamp pad ink, an effective stain for observing arbuscular mycorrhizal structure in roots . Wor J Agric Sci. , 4 : 58 – 60 .
- Das , P and Kayang , H. 2010 . Mycorrhizal colonization and distribution of arbuscular mycorrhizal fungi associated with Michelia champaca L . under plantation system in northeast India. J For Res. , 21 : 137 – 142 .
- Davies , FT Jr , Calderón , CM and Huaman , Z. 2005 . Influence of arbuscular mycorrhizae indigenous to Peru and a flavonoid on growth, yield, and leaf elemental concentration of ‘Yungay’ potatoes . Hort Sci. , 40 : 381 – 385 .
- Galvez , L , Douds , DD , Drinkwater , LE and Wagoner , P. 2001 . Effect of tillage and farming system upon VAM fungus populations and mycorrhizas and nutrient uptake of maize . Plant Soil , 228 : 299 – 308 .
- Johansen , DA. 1940 . Plant microtechnique , New Delhi : Tata McGraw-Hill .
- Jumpponen , A and Trappe , JM. 1998 . Dark septate endophytes: a review of facultative biotrophic root-colonizing fungi . New Phytol. , 140 : 295 – 310 .
- Jumpponen , A. 2001 . Dark septate endophytes-are they mycorrhizal? . Mycorrhiza , 11 : 207 – 211 .
- Koske , RE and Tessier , B. 1983 . A convenient, permanent slide mounting medium . Mycol Soc Am News , 34 : 59
- Koske , RE , Gemma , JN and Olexia , PD. 1986 . Glomus microaggregatum, a new species in the Endogonaceae . Mycotaxon , 26 : 125 – 132 .
- Ma , Z , Bielenberg , DG , Brown , KM and Lynch , JP. 2001 . Regulation of root hair density by phosphorus availability in Arabidopsis thaliana . Plant Cell Environ. , 24 : 459 – 467 .
- McArthur , DAJ and Knowles , NR. 1991 . Response of ‘Russet Burbank’ potato to V.A. mycorrhizal fungi . Proceeding of the Prairie Potato Council. ,
- McArthur , DAJ and Knowles , NR. 1993 . Influence of vesicular-arbuscular mycorrhizal fungi on the response of potato to phosphorus deficiency . Plant Physiol. , 101 : 147 – 160 .
- McGonigle , TP , Miller , MH , Evans , DG , Fairchild , GL and Swan , JA. 1990 . A new method which gives an objective measure of colonization of roots by vesicular–arbuscular mycorrhizal fungi . New Phytol. , 115 : 495 – 501 .
- Moreira , M , Baretta , D , Tsai , SM and Cardoso , EJBN. 2006 . Spore density and root colonization by arbuscular mycorrhizal fungi in preserved or disturbed Araucaria angustifolia (Bert.) O . Ktze. ecosystems. Sci Agric. , 63 : 380 – 385 .
- Muthukumar , T and Udaiyan , K. 2002 . Arbuscular mycorrhizas in cycads of southern India . Mycorrhiza , 12 : 213 – 217 .
- Muthukumar , T , Senthilkumar , M , Rajangam , M and Udaiyan , K. 2006 . Arbuscular mycorrhizal morphology and dark septate fungal associations in medicinal and aromatic plants of Western Ghats, Southern India . Mycorrhiza , 17 : 11 – 24 .
- O'Dell , TE , Massicotte , HB and Trappe , JM. 1993 . Root colonization of Lupinus latifolius Agardh . and Pinus contorta Dougl. by Phialocephala fortinii Wang & Wilcox. New Phytol. , 124 : 93 – 100 .
- Oehl , F and Sieverding , E. 2004 . Pacispora, a new vesicular–arbuscular mycorrhizal fungal genus in the Glomeromycetes . J Appl Bot. , 78 : 72 – 82 .
- Piotrowskia , JS , Morford , SL and Rillig , MC. 2008 . Inhibition of colonization by a native arbuscular mycorrhizal fungal community via Populus trichocarpa litter, litter extract, and soluble phenolic compounds . Soil Biol Biochem. , 40 : 709 – 717 .
- Pursglove , JD and Sanders , FE. 1981 . The growth and phosphorus economy of the early potato (Solanum tuberosum) . Commun Soil Sci Plant Anal. , 12 : 1105 – 1121 .
- Schreiner , RP and Bethlenfalvay , GJ. 1995 . Mycorrhizal interactions in sustainable agriculture . Critic Rev Biotechnol. , 15 : 271 – 285 .
- Sieverding , E. 1991 . Vesicular–arbuscular mycorrhizal management in tropical agrosystems. Eschborn , Germany : Deutsche Gesellschaft für Technische Zusammenarbeit .
- Smith , SE and Read , DJ. 1997 . Mycorrhizal symbiosis , London : Academic Press .
- Wu , CG , Liu , YS , Hwuang , YL , Wang , YP and Chao , CC. 1995 . Glomales of Taiwan: V . Glomus chimonobambusae and Entrophospora kentinensis, spp. nov. Mycotaxon , 53 : 283 – 294 .
- Zhao , D and Zhao , Z. 2007 . Biodiversity of arbuscular mycorrhizal fungi in the hot-dry valley of the Jinsha River, southwest China . Appl Soil Ecol. , 37 : 118 – 128 .
