ABSTRACT
Leccinum scabrum (Bull.) Gray (Boletaceae) is an edible mycorrhizal species with potential application interest due to its food and medicinal properties. A field investigation carried out during summer in the Białowieża Primeval Forest, located along the border between Belarus and Poland allowed to collect samples for chemical composition analysis and antibacterial activity evaluation. Mushroom extracts were prepared with microwave-assisted as well as ultrasound-assisted extraction techniques (UAE and MAE). The analysis of a dry sample of L. scabrum showed a significant content of vitamins and minerals and also a remarkable content of carbohydrates, protein, dietary fibre, total sugars, total free amino acids, and polyunsaturated fatty acids. The antibacterial activity of L. scabrum aqueous extracts showed inhibitory activity against all tested bacteria. In general, MAE extract exhibits a higher inhibition activity against Listeria monocytogenes ATCC 19114. As regards the Minimum Inhibitory Concentration (MIC) values, the high antibacterial activity of MAE extract was detected for L. monocytogenes ATCC 19114 and Escherichia coli ATCC 25922. Regarding UAE, high antibacterial activity was detected for Salmonella enterica ATCC 13076 and L. monocytogenes ATCC 19114. Based on data hereby reported, L. scabrum is a culinary-medicinal mushroom with a promising potential use as a high-quality food and nutraceutical mycological resource.
1. Introduction
Among foods, edible mushrooms represent an important source of beneficial components, such as carbohydrates, proteins, vitamins, minerals, etc. Moreover, thanks to the presence of bioactive compounds, some of them have remarkable medicinal properties, such as antiviral, anti-inflammatory, antitumor, anti-obesity, and antimicrobial activity (Vukajlović et al. Citation2021). Leccinum scabrum (Bull.) Gray, is a member of the widely distributed family Boletaceae Chevall. It is an edible, mycorrhizal mushroom of potential application interest for both food and medicinal properties and is traditionally consumed in Scandinavia, Central, and Eastern Europe. Described in 1783 by French naturalist Jean Baptiste Francois (Pierre) Bulliard under the name Boletus scaber Bull. it was in 1821 included in the genus Leccinum by Samuel Frederick Gray. Leccinum scabrum is mainly collected under birch trees, it prefers deciduous woods and is also found under Fagus sylvatica. Leccinum scabrum fructification period extends from early summer to autumn, in grassy areas or with the presence of low bushes, in open spaces, or at the edge of the woods. Overall, the medicinal properties of L. scabrum are poorly investigated. The few studies about L. scabrum medicinal potential reported the presence of health-promoting substances, such as flavonoids, phenolic acids, vitamins and antioxidants, antiulcer, and cytotoxic activity of this edible mushroom (Gąsecka et al. Citation2017). Biological activities of acetone extract of L. scabrum have been evaluated by Vukajlović et al. (Citation2021). In a study on phenolic constituents, antiradical, and antimicrobial activity of some wild edible mushrooms from Poland (Nowacka et al. Citation2014) the mushroom extract of L. scabrum shows a low total phenolic content (3.8 mg GAE/g extract) in comparison with other species of Basidiomycetes, a protocatechuic acid content of 0.23 mg/kg DW, trace of caffeic acid, an IC50 between 20 and 50 mg/mg DPPH and a low antimicrobial activity against Gram-positive and Gram-negative bacteria strains.
Considering the current issue of antibiotic resistance of some human pathogenic bacteria and the urgency of finding new natural bioactive compounds to fight this problem, this paper provides data on the chemical composition of L. scabrum and the antibacterial activity of its aqueous extracts. Moreover, being known that conventional extraction methods of secondary metabolites from natural sources usually require long extraction time as well as large amounts of organic solvents, the innovative ultrasound- and microwave-assisted extraction techniques (UAE and MAE, respectively) were applied to overcome these drawbacks and efficiently extract bioactive compounds from L. scabrum powder. To our knowledge, this is the first study that tests the antibacterial activity of aqueous extract of L. scabrum against some human pathogenic bacteria.
2. Materials and methods
2.1. Sample collection and evaluation of morphological characters
Leccinum scabrum was collected during summer in the subcontinental oak-hornbeam forest belonging to the plant association Tilia cordata – Carpinetum betuli Tracz. 1962 of the Białowieża Forest (87,600 ha) in Podlaskie Voivodeship 53°20’42’’N 22°46’10’’E, situated on the border between Poland and Belarus. Białowieża Forest is the only place in Europe where fragments of primaeval forests have been preserved to this day.
Samples were manually collected in over a hundred-year-old, well-preserved stands in which no forest management was carried out.
The specimen was transferred to the Department of Agricultural, Food and Forest Sciences (SAAF) of the University of Palermo and stored at <4 °C for up to 24 h before morphological characterisation, which was carried out according to den Bakker and Noordeloos (Citation2005). The macro-morphological features (pileus, cuticle, pores, stipe, and context, etc.) were recorded on fresh material, while microscopic features (hyphal system, generative hyphae, basidia, sterigmata, cystidia, cystidioles, and basidiospores) were evaluated by using 3% potassium hydroxide and ammoniacal Red Congo at light microscope (Axioskop; Zeiss, Oberkochen, Germany) coupled to an AxioCam MRc5 (Zeiss, Oberkochen, Germany) digital camera. The images were captured using the software AxioVision 4.6 (Zeiss, Oberkochen, Germany). Voucher collections of L. scabrum were dried at room temperature using a universal dryer (475 Watt) and deposited in the Herbarium of the Department of Agricultural, Food and Forest Sciences, University of Palermo, Palermo, Italy (SAF 545).
2.2. DNA extraction, PCR, and sequencing
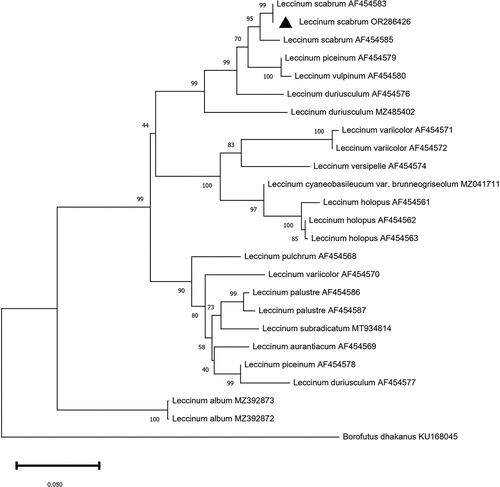
DNA was extracted from the innermost tissue L. scabrum basidioma using the Extract-N-Amp™ kit (Sigma-Aldrich, St. Louis, MO, USA) following the manufacturer’s instructions. DNA concentration and purity were measured at 260/280 nm and 260/230 nm using the NanoDrop ND-1000 spectrophotometer (Thermo Fisher Scientific, Waltham, MA, USA). The internal transcribed spacer region of rDNA (ITS) was amplified using ITS1F and ITS4 primers by polymerase chain reaction (PCR) in a total reaction volume of 20 µL consisting of 4 µL of extracted DNA, 10 µL of the Extract-N-Amp PCR reaction mix (Sigma-Aldrich, St. Louis, USA), 1 µL of each primer at 10 μmol/L and 4 µL of sterilised distilled water. The amplification was carried out in a MultiGene OptiMax thermocycler (Labnet International Inc., Edison, NJ, USA) with an initial denaturation cycle at 94 °C for 3 min, followed by 35 cycles at 94 °C for 30 s, annealing at 55 °C for 30 s, elongation at 72 °C for 45 s, and a final extension at 72 °C for 10 min. The PCR product was separated by electrophoresis in 1.5% agarose gel and detected under a UV transilluminator. The PCR product was purified using Exo I-SAP protocol according to the manufacturer’s instructions (Applied Biosystems, Foster City, CA, USA) and sent to BMR Genomics (Padova, Italy) for sequencing. Only primer ITS1F was used in the sequencing reaction. The obtained sequence was manually adjusted and compared with those reported in GenBank through the BLASTn tool (https://blast.ncbi.nlm.nih.gov). The new sequence was deposited in GenBank. Sequence with the 100% of similarity, as well as Leccinum spp. representative sequences from previous ITS-phylogenetic studies (den Bakker et al. Citation2004; Meng et al. Citation2021) were retrieved from GenBank and aligned with the isolate sequence obtained in this study. Alignments were made using ClustalW software and manually adjusted if needed. To construct the phylogenetic tree, a Neighbour-joining statistical analysis was performed using MEGA11. The evolutionary distances were computed using the Maximum Composite Likelihood method, and the phylogenetic tree was automatically generated by the software. Confidence values for individual branches were determined by bootstrap test (1,000 replicates).
2.3. Evaluation of nutritional value
A dry sample of basidiomes of L. scabrum, suitably pulverised using a mill, was entrusted for chemical analysis to EcamRicert LTD in Monte di Malo (Vicenza, Italy). The results of the test report the number 20LA09820 dated 28 July 2020. The contents of vitamins (D3, B1, B2, B3, B5, B6, B8, and B12), sodium, potassium, iron, calcium, phosphorus, magnesium, zinc, copper, selenium, manganese, nickel, chromium, total sulphur, protein, carbohydrate, dietary fibre content, sugar composition, fatty acid composition, and free amino acid composition were determined. All analyses were carried out according to the analytical methods used for chemical control of food by the Italian National Institute of Health (Baldini et al. Citation1996). Protein, carbohydrate, lipid, moisture, and ash content, expressed as a percentage, were analysed by centesimal analysis. The analyses were carried out in triplicate. By UNI ISO 3534–1:2000, for each value, the measurement uncertainty has been taken (it has the dimensions of a mean square deviation).
2.4. Preparation of the extracts
Leccinum scabrum extracts were prepared by application of two extraction methods: ultrasound-assisted extraction (UAE) (Zhang et al. Citation2013) and microwave-assisted extraction (MAE) (Cavalluzzi et al. Citation2022) with some modifications.
For UAE, 90 mL of distilled water was placed in a beaker containing 3 g of L. scabrum powder and sonicated with Soniprep 150 (MSE, London, UK) for 30 min. To avoid the solution overheating the beaker was placed in an ice-water bath. After sonication, the solution was filtered with grade 1 paper (Whatman, Maidstone, UK), lyophilised, and stored refrigerated (4 °C) until analysis.
For MAE, a closed-system MAE was carried out at a constant temperature, with continuous stirring, in a CEM Discover Bench Mate microwave reactor equipped with Synergy software. The temperature was measured and controlled by a built-in infrared detector. Briefly, 75 mg of L. scabrum powder in 2 mL of water was irradiated with microwaves 30 W at 80 °C for 5 min. The solution was then filtered, and the solvent was evaporated to dryness under reduced pressure to afford a solid which was stored at –20 °C until needed for analysis.
2.5. Determination of antimicrobial activity
Before assays, each extract was resuspended in sterile water at a concentration of 100 mg/mL.
2.5.1. Bacterial strains and growth conditions
Four bacterial strains provided by the American Type Culture Collection (ATCC®) were used for susceptibility testing of L. scabrum water extracts. They include two Gram-negative strains, Escherichia coli ATCC 25922 and Salmonella enterica subsp. enterica ATCC 13076, and two Gram-positive strains, Listeria monocytogenes ATCC 19114 and Staphylococcus aureus subsp. aureus ATCC 33862. These strains belong to the culture collection of the Agricultural Microbiology Unit of the Department of Agricultural, Food and Forest Sciences (University of Palermo, Italy), where they were stored at –80 °C. All bacteria were reactivated using test tubes containing Mueller Hinton Broth (Oxoid, Milan, Italy) and incubated at 37 °C for 24 h.
2.5.2. In vitro antibacterial activity
The antibacterial activity of L. scabrum extracts was tested by applying the paper disk diffusion method reported by Ren et al. (Citation2014) with some modifications. Briefly, the MH plates were inoculated spreading 10 µL of 107 CFU/mL of each pathogen. Subsequently, sterilised paper disks (6 mm in diameter) were placed onto the inoculated plates and soaked with 10 µL of each L. scabrum extract (100 mg/mL). Paper disks soaked with 10 µL of sterile distilled water were used as the negative control, while paper disks soaked with 10 µL of streptomycin (10% w/v) were used as the positive control. The plates were incubated for 24 h at 37 °C, and the inhibitory activity was considered positive if a clear halo surrounded the disks soaked with fungal extracts. Data were expressed by measuring the diameter (mm) of the halos, and all tests were performed in triplicate.
2.5.3. Minimum inhibitory concentration (MIC)
The extracts exhibiting antibacterial activity were also analysed to determine their MIC values by using the microdilution method (Ren et al. Citation2014). The microdilution test was performed in a sterile round-bottomed 96-well microplate (). Eight two-fold serial dilutions of each extract were prepared at concentrations ranging from 100 to 0.78125 mg/mL. In detail, 100 µL of MH broth was added to all the wells of the plate. In the first well of columns 1 to 4, 100 µL of MAE extract (100 mg/mL) was added, while in columns 5 to 8, 100 µL of UAE extract (100 mg/mL) was added. Then, serial dilutions were performed by passing 100 µL of wells 1 to 8 of line A to the wells of line B and so forth with the following proportions: 1:1(A), 1:2 (B), 1:4(C), 1:8(D), 1:16(E), 1:32(F), 1:64(G), and 1:128(H). Subsequently in each well (columns 1 to 8) were added 10 µL of each bacterial pathogen inoculum. For the negative controls (columns 9 to 12, lines A-B-C) 10 µL of inoculum was added, while for the positive control (columns 9 to 12, lines D-E-F) 10 µL of streptomycin (10% w/v) was added. The test was performed in triplicate.
Figure 1. Microplates assay with MAE and UAE extracts of Leccinum scabrum (MAE = Microwave-assisted extraction; UAE = Ultrasound-assisted extraction).
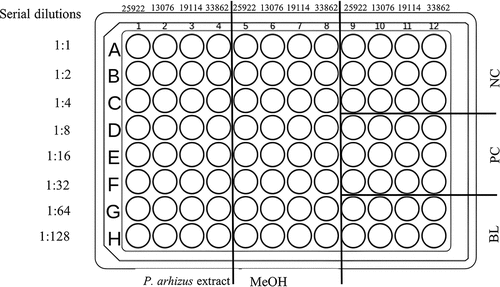
Wells in columns 9 to 12, lines G-H were used as blanks. Before incubation, the plate was put in a spectrophotometer (ScanReady, Life Real Biotechnology CO., Ltd., Hangzhou, China) to read the absorbance (OD 540 nm) at T0. Subsequently, the plate was incubated at 37 °C for 24 h. After incubation, the antibacterial activity was measured by comparing the absorbance of wells containing dilutions of fungal extracts and negative controls. The percentage of inhibition of bacterial growth was calculated using the following formula: Inhibition (%) = [(AB–AC)/AB] × 100 where AB was the difference between the absorbance of the negative control at T1 and T0; AC was the difference between the absorbance of the mixture containing the bacterial inoculum and fungal extract at T1 and T0. The obtained percentages of inhibition were expressed as reported by Ren et al. (Citation2014), i.e. low activity (+, ≤35%); moderate activity (++, >35% and ≤70%); high activity (+++, >70%); no antibacterial activity (-).
2.5.4. Statistical analysis
The effect of the two extracts against each bacterial strain was analysed by one-way ANOVA using STATGRAPHICS Plus 5.1. Mean values were compared using the Tukey HSD test. Statistical significance was established as P ≤ 0.01.
3. Results
3.1. Identification and collection site of L. scabrum
L. scabrum collected in Białowieża Forest () presented the following features. Cap up to 15 cm wide (), off-white to light grey with yellowish hues, with margin wavy. The cuticle is velvety or smooth and becomes viscous with moisture. Hymenium with tubules and pores. Tubes small, circular, broadly adnexed to the stem, 1–2 cm long, off-white, gradually turning slightly browner when bruised. Stem, white or buff, sometimes turns slightly pink when it is cut or broken, very long relative to the width of the cap, about 15 cm × 2 cm, firm, cylindrical, slightly tapered near the cap; off-white or grey, covered with many small dark grey scales. The flesh is off-white in colour, tender in cap, and leathery in stem, and turns black when handled or after cooking. Smell: pleasant, light, and aromatic. Taste grateful, sweet. Basidiospores (), 13–21 × 4–6 µm, yellow in mass with cinnamon shades, narrowly ellipsoid to subfusiform, smooth, guttulate, thin-walled, with vacuole inclusions. To confirm morphological identification, a phylogenetic analysis based on ITS sequences was performed. The phylogenetic tree showed that our isolate grouped with other L. scabrum isolates with high bootstrap values ().
Figure 2. The habitat of Leccinum scabrum in Białowieża Forest (Tilio – Carpinetum subcontinental oak-hornbeam forest).

Figure 3. (a) Leccinum scabrum basidiomata collected in situ; (b) Leccinum scabrum basidiospores at light microscope 100× magnification (scale bar = 10 µm).

Figure 4. Neighbour-joining tree based on phylogenetic analysis of the ITS1–5.8S rDNA-ITS2 sequences of Leccinum spp. Bootstrap percentages calculated from 1,000 re-samplings are indicated at nodes. The GenBank number with a triangle represents the sequence obtained in this study and deposited in the GenBank database.
3.2. Mushroom proximate composition and content in elements and vitamins
The results obtained from the analysis performed on a dry sample obtained from fresh basidiomata of L. scabrum, pulverised, show significant content of vitamins and minerals, including vitamin D3, vitamin B2, and among minerals, sodium, potassium, iron, and calcium (). On the other hand, the carbohydrate, protein, and dietary fibre content are noteworthy, giving the mushroom an energy value of 1,361 kJ (). Regarding total sugars and total free amino acids, they were found to be 2.964 and 5.31 g/100 g, respectively (). Numerous acids also result from the analysis, among which polyunsaturated fatty acids represent the most pronounced value (1.98 g/100 g), while saturated fatty acids represent the lowest value (0.523 g/100 g).
Table 1. Protein, carbohydrate, and dietary fibre content of Leccinum scabrum.
Table 2. Composition of sugars in Leccinum scabrum.
Table 3. Vitamins and minerals content of Leccinum scabrum.
Table 4. Acids composition by weight of Leccinum scabrum.
Table 5. Free amino acid composition of Leccinum scabrum.
3.3. Antibacterial activity
3.3.1. Paper disk diffusion assay
The antibacterial activity of L. scabrum aqueous extracts (MAE and UAE) evaluated in this survey by the paper disk diffusion assay is reported in . Both extracts showed inhibitory activity against all tested bacteria, showing a diameter of inhibition zones ranging from 10.5 ± 0.2 to 13.5 ± 0.2 mm for MAE extract and from 10 ± 0.2 to 12.5 ± 0.2 mm for UAE extract. In general, MAE extract exhibits a higher inhibition activity against L. monocytogenes ATCC 19114, which was the most sensitive, with a 13.5 ± 0.2 mm halo. Both extracts showed the same effect, i.e. S. aureus ATCC 33862 with a halo of 12.5 ± 0.1 mm, and, in general, the weaker effect was expressed on Gram-negative strains.
Table 6. Inhibitory activity of Leccinum scabrum extracts on Gram + and Gram – bacteria strains.
3.3.2. Minimum inhibitory concentration (MIC)
Since both MAE and UAE extracts had an inhibitory effect against all bacterial pathogens in the paper disk diffusion assay, we decided to determine the Minimum Inhibitory Concentration (MIC) values for both of them by the microdilution test.
Results are shown in . In general, an inhibition effect was detected for all the bacterial pathogens, with both extracts, at all tested concentrations, except for the lowest concentration of MAE on E. coli ATCC 25922.
Table 7. Growth inhibition percentages obtained with microdilution assay used to test antimicrobial activity of Leccinum scabrum extracts on pathogenic bacterial strains.
According to the classes used to evaluate the inhibitory activity of L. scabrum extracts, based on inhibition percentages, high antibacterial activities of MAE extract were detected at MIC value of 25 mg/mL for L. monocytogenes ATCC 19114 and E. coli ATCC 25922, at MIC value of 50 mg/mL for S. enterica ATCC 13076 and at MIC value of 100 mg/mL for S. aureus ATCC 33862; moderate activity was registered at MIC value of 3.125 mg/mL, 6.25 mg/mL, 12.5 mg/mL, and 25 mg/mL for S. enterica ATCC 13076, E. coli ATCC 25922, L. monocytogenes ATCC 19114, and S. aureus ATCC 33862, respectively; low antibacterial activity was detected at MIC value of 0.78125 mg/mL (the lowest concentration) for all the bacteria strains except for E. coli ATCC 25922.
Regarding UAE, high antibacterial activity was detected at MIC values of 50 mg/mL for S. enterica ATCC 13076 and L. monocytogenes ATCC 19114 and at MIC value of 100 mg/mL for S. aureus ATCC 33862 and E. coli ATCC 25922; moderate activity was detected at 6.25 mg/mL for S. enterica ATCC 13076 and at 25 mg/mL for L. monocytogenes ATCC 19114, S. aureus ATCC 33862, and E. coli ATCC 25922; low antibacterial activity was registered at MIC value of 0.78125 mg/mL for all the pathogens.
Results show that MAE had a higher inhibitory effect at lower concentrations than UAE both on L. monocytogenes ATCC 19114 and E. coli ATCC 25922 with MIC values of 25 mg/mL for MAE and 50 and 100 mg/mL, respectively, for UAE.
4. Discussion
Based on the data reported in this study, L. scabrum can be included among the foods useful to humans for a varied and balanced diet. The mineral content, which is essential for growth and health, bone formation, regulation of body fluids, and participation in cellular life processes, is higher than that detectable in eggs, grana cheese (except calcium), and veal as also when compared with the contents of the most common edible mushrooms namely Agaricus bisporus (J.E. Lange) Imbach and Boletus edulis Bull (Venturella et al. Citation2021). The energy value is similar to that of grana cheese, the carbohydrate content is higher than that of potatoes, porcini, and champignon, and the protein content and dietary fibre are higher than that found in eggs, veal, potatoes, porcini, and champignon. In addition, the glucose content is almost double that of champignon and the fructose values exceed those found in potatoes and champignon (Fidanza Citation1984). Glutamic acid has higher values than estimated on potatoes, porcini mushrooms, and champignons (Fidanza Citation1984). Although it is a non-essential amino acid, it is an important excitatory neurotransmitter and plays an important role in cellular energy production and protein synthesis (Wang et al. Citation2007). Alanine, which is essential for improving athletic performance by reducing fatigue and increasing endurance in high-intensity training, has higher values than those found in grana cheese, eggs, potatoes, porcini, and champignon (Fidanza Citation1984; Artioli et al. Citation2012). The arginine, glycine, histidine, serine, tyrosine, threonine, and total free amino acid values of L. scabrum are higher than those found in potatoes, porcini, and champignon (Fidanza Citation1984). These are very important elements for human health being directly involved in the functioning of the circulatory system, protein synthesis, immune response, digestion, regulating sexual function and sleep-wake cycles, creating barriers against viruses and bacteria, and the synthesis of important neurotransmitters (Goodman Citation2010). Also of interest is the octadecenoic acid content (18:1 total), which is higher than that of eggs, grana cheese, potatoes, porcini, and champignon (Fidanza Citation1984). It is known that consumption of monounsaturated fats is associated with a reduction in low-density lipoprotein (LDL) cholesterol. Eicosenoic acid (20:1 total), a prominent component of some fish oils including cod liver oil, is present in L. scabrum in higher amounts than in eggs while, overall, the amount of saturated and polyunsaturated fatty acids is higher than in champignons (Fidanza Citation1984).
Besides, protein contents, in agreement with data reported by La Guardia et al. (Citation2005) are significantly higher than those found in other edible mushrooms in which values range from 1.2 to 6 g/100 g [i.e. Armillaria mellea (Vahl) P. Kumm. (1.6 g/100 g), Boletus edulis Bull. (2.8 g/100 g), Tuber melanosporum Vittad. (5.5 g/100 g), Cantharellus cibarius Fr. (1.5 g/100 g), and Amanita caesarea (Scop.) Pers. (2.0 g/100 g)]. Such differences are also noticeable in the case of fats in commonly used edible fungi such as A. mellea, Lactarius deliciosus (L.) Gray, Leccinum aurantiacum (Bull.) Gray, and Pleurotus eryngii (DC.) Quél. Never exceed 0.8 g/100 g and also of carbohydrates whose highest value corresponds to 3.4 g/100 g in P. eryngii. All mineral elements in L. scabrum are higher than those of A. mellea, B. edulis, C. cibarius, L. deliciosus, A. caesarea, and P. eryngii. On the contrary, the amino acid and vitamin content of L. scabrum is lower than that detected in other fungi, such as C. cibarius, B. edulis, P. eryngii, and L. aurantiacum.
The antibacterial activity of L. scabrum aqueous extracts (MAE and UAE) represents another important potential aspect for the enhancement of L. scabrum as a medicinal mushroom. Both extracts showed inhibitory activity against L. monocytogenes, S. aureus subsp. aureus, S. enterica subsp. enterica, and E. coli, although MAE had a higher inhibitory effect at lower concentrations (lower MIC values) than UAE both on L. monocytogenes ATCC 19114 and E. coli ATCC 25922. In this regard, similar MIC values were reported by Alves et al. (Citation2012) who tested extracts of different basidiomycetes against E. coli and L. monocytogenes. Moreover, previous research supports the Gram-positive bacteria’s susceptibility to mushroom extracts (Barros et al. Citation2007; Alves et al. Citation2012; Cateni et al. Citation2021; Venturella et al. Citation2021; Cardoso et al. Citation2022). This occurrence can be explained by the fact that, in contrast to Gram-positive bacteria, Gram-negative bacteria have a periplasmic gap and an outer membrane enclosing the cell wall. The presence of these peculiar structures makes them more resistant to the action of extracts (Ren et al. Citation2014).
Several studies reported the antimicrobial properties of fungal methanolic, ethanolic, and acetone extracts against Gram-positive and Gram-negative bacteria, but to our knowledge, this is the first study that tests the aqueous extract of L. scabrum. Our results are in line with those reported by Nowacka et al. (Citation2014) who tested the antimicrobial potential of ethanolic extracts of several edible mushrooms, including L. scabrum, against some human pathogenic bacteria (i.e. E. coli and S. aureus). On the other hand, Vukajlović et al. (Citation2021) tested the acetone extract of L. scabrum against E. coli, S. aureus, and other bacterial strains obtaining lower MIC values (between 0.078 and 0.039 mg/mL). This could be explained by the fact that acetone can extract from L. scabrum more effective antibiotic compounds than other solvents, including water. To our knowledge, no literature data are reported about the antimicrobial activity of L. scabrum against L. monocytogenes and S. enterica. Regarding the antimicrobial activity of other Boletaceae against bacterial human pathogens, Kosanić et al. (Citation2012) reported MIC values of acetone and methanolic extracts of Boletus aestivalis, B. edulis, and L. carpini ranging from 5 to 10 mg/mL for E. coli and S. aureus, comparable to those obtained in our study.
The antimicrobial performance of our aqueous extracts of L. scabrum is similar to those reported for methanolic and ethanolic extracts of edible mushrooms belonging to other families (Venturella et al. Citation2021). Moreover, the MIC results obtained in this study are lower than those reported by Chowdhury et al. (Citation2015) and Fogarasi et al. (Citation2020) for methanolic extracts of edible mushrooms, such as B. edulis, A. bisporus, and P. ostreatus.
According to our results and literature data, it is important to highlight that the use of water as an extraction solvent has a lower environmental impact compared with ethanolic, methanolic, and acetone extracts, still maintaining high antimicrobial properties. Among the innovative extraction methods with low environmental impact and eco-friendly solvents, no substantial differences emerged between MAE and UAE. Moreover, the use of distilled water instead of other solvents, such as methanol and ethanol, not only reduces the costs of extraction but also reduces environmental impact and manipulation risks. This could be used as a measure for large-scale industrialised extraction, also minimising by-product disposal costs and increasing the added value of the products. Compared with the traditional methods, UAE and MAE also present the advantages of high yield, simple operation, high efficiency, and remarkable reduction of extraction time (few minutes vs. hours or days). Moreover, the novel extraction techniques destroy fungal cells promoting solute diffusion and increasing the mass transfer rate from the solid phase to the liquid phase, UAE by the application of ultrasound and MAE by increasing temperature and pressure. Both methods can therefore be considered for further studies also against other pathogenic microorganisms.
In conclusion, the results reported in the present study demonstrate that L. scabrum can be considered a valid source of nutrients that are important to balance the everyday diet. Its mineral, carbohydrate, and protein content, its energy value, and the high presence of monounsaturated fatty acid could exert important benefits on human health. Moreover, the antimicrobial activity of L. scabrum aqueous extracts, against four of the most common pathogenic bacteria for humans, makes this mushroom a potential source of natural antimicrobial compounds as an alternative to antibiotics, opening up important therapeutic perspectives.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Alves MJ, Ferreira ICFR, Martins A, Pintado M. 2012. Antimicrobial activity of wild mushroom extracts against clinical isolates resistant to different antibiotics. J Appl Microbiol. 113(2):466–475. doi: 10.1111/j.1365-2672.2012.05347.x.
- Artioli GG, Gualano B, Smith A, Stout J, Lancha AH Jr. 2012. Role of beta-alanine supplementation on muscle carnosine and exercise performance. Med Sci Sports Exerc. 42(6):1162–1173. doi: 10.1249/MSS.0b013e3181c74e38.
- Baldini M, Fabietti F, Giammarioli S, Onori R, Orefice L, Stacchin A. 1996. Metodi di analisi utilizzati per il controllo chimico degli alimenti. Istituto Superiore di Sanità, Rapporti ISTISAN 96/34, 265 pp.
- Barros L, Baptista P, Estevinho LM, ICFR F. 2007. Bioactive properties of the medicinal mushroom Leucopaxillus giganteus mycelium obtained in the presence of different nitrogen sources. Food Chem. 105(1):179–186. doi: 10.1016/j.foodchem.2007.03.063.
- Cardoso RV, Oludemi T, Â F, Ferreira IC, Barros L. 2022. Bioactive properties of mushrooms with potential health benefits. In: Stojković D, Barros L, editors. Edible fungi: chemical composition, nutrition and health effects. 1st ed. London, UK: Royal Society of Chemistry; p. 161–231.
- Cateni F, Gargano ML, Procida G, Venturella G, Cirlincione F, Ferraro V. 2021. Mycochemicals in wild and cultivated mushrooms: nutrition and health. Phytochem Rev. 21(2):339–383. doi: 10.1007/s11101-021-09748-2.
- Cavalluzzi MM, Lamonaca A, Rotondo NP, Miniero DV, Muraglia M, Gabriele P, Corbo F, De Palma A, Budriesi R, De Angelis E, et al. 2022. Microwave-assisted extraction of bioactive compounds from lentil wastes: Anti-oxidant activity evaluation and metabolomic characterization. Molecules. 27(21):7471. doi:10.3390/molecules27217471.
- Chowdhury MMH, Kubra K, Ahmed AR. 2015. Screening of antimicrobial, antioxidant properties, and bioactive compounds of some edible mushrooms cultivated in Bangladesh. Ann Clin Microbiol Antimicrob. 14(1):8. doi: 10.1186/s12941-015-0067-3.
- den Bakker HC, Gravendeel B, Kuyper TW. 2004. An ITS phylogeny of Leccinum and analysis of the evolution of minisatellite-like sequences within ITS1. Mycologia. 96(1):102–118. doi: 10.1080/15572536.2005.11833001.
- den Bakker HC, Noordeloos M. 2005. A revision of European species of Leccinum Pers.: Gray and notes on extralimital species. Mol Phylogeny Evol Fungi. 18(4):511–574.
- Fidanza F. 1984. Alimenti e Tabelle di Composizione. In: Fidanza F, Liguori G, editors. Nutrizione Umana. Napoli, Italy: Idelson; p. 221–238.
- Fogarasi M, Diaconeasa ZM, Pop CR, Fogarasi S, Semeniuc CA, Fărcaş AC, Țibulcă D, Sălăgean CD, Tofană M, Socaci AA. 2020. Elemental composition, antioxidant and antibacterial properties of some wild edible mushrooms from Romania. Agronomy. 10(12):1972. doi: 10.3390/agronomy10121972.
- Gąsecka M, Siwulski M, Mleczek M. 2017. Evaluation of bioactive compounds content and antioxidant properties of soil-growing and wood-growing edible mushrooms. J Food Process Preserv. 42(1):1–10. doi: 10.1111/jfpp.13386.
- Goodman B. 2010. Insights into digestion and absorption of major nutrients in humans. Adv Physiol Educ. 34(2):44–53. doi: 10.1152/advan.00094.2009.
- Kosanić M, Ranković B, Dašić M. 2012. Mushrooms as possible antioxidant and antimicrobial agents. Iran J Pharm Res. 11(4):1095–1102.
- La Guardia M, Venturella G, Venturella F. 2005. On the chemical composition and nutritional value of Pleurotus taxa growing on umbelliferous plants (Apiaceae). J Agric Food Chem. 53(15):5997–6002. doi: 10.1021/jf0307696.
- Meng X, Wang G, Wu G, Wang P, Yang ZL, Li Y. 2021. The genus Leccinum (Boletaceae, Boletales) from China based on morphological and molecular data. J Fungi. 7(9):732. doi: 10.3390/jof7090732.
- Nowacka N, Nowak R, Drozd M, Olech M, Los R, Malm A. 2014. Analysis of phenolic constituents, antiradical and antimicrobial activity of edible mushrooms growing wild in Poland. LWT Food Sci Technol. 59(2):689–694. doi: 10.1016/j.lwt.2014.05.041.
- Ren L, Hemar Y, Perera CO, Lewis G, Krissansen GW, Buchanan PK. 2014. Antibacterial and antioxidant activities of aqueous extracts of eight edible mushrooms. Bioact Carbohydr Diet Fibre. 3(2):41–51. doi: 10.1016/j.bcdf.2014.01.003.
- Venturella G, Ferraro V, Cirlincione F, Gargano ML. 2021. Medicinal mushrooms: Bioactive compounds, use, and clinical trials. Int J Mol Sci. 22(2):634. doi: 10.3390/ijms22020634.
- Vukajlović JT, Kosanić M, Ranković B, Stanojković T, Marković A, Grujičić D, Djelić N, Radaković M, Milošević-Djordjević O. 2021. Evaluation of biological activities of acetone extract of the mushroom Leccinum scabrum. Farmacia. 69(5):974–979. doi: 10.31925/farmacia.2021.5.22.
- Wang L, Maher TJ, Wurtman RJ. 2007. Oral L-glutamine increases GABA levels in striatal tissue and extracellular fluid. Faseb J. 21(4):1227–1232. doi: 10.1096/fj.06-7495com.
- Zhang Z, Lu G, He W, Shi L, Pan H, Fan L. 2013. Effects of extraction methods on the antioxidant activities of polysaccharides obtained from Flammulina velutipes. Carbohydr Polym. 98(2):1524–1531. doi: 10.1016/j.carbpol.2013.07.052.

