Abstract
Paracoccidioides species are dimorphic fungi that initially infect the lungs but can also spread throughout the body. The spreading infection is most likely due to the formation of a biofilm that makes it difficult for the host to eliminate the infection. Biofilm formation is crucial for the development of infections and confines the pathogen to an extracellular matrix. Its presence is associated with antimicrobial resistance and avoidance of host defenses. This current study provides the first description of biofilm formation by Paracoccidioides brasiliensis (Pb18) and an analysis of gene expression, using real-time PCR, associated with 3 adhesins and 2 hydrolytic enzymes that could be associated with the virulence profile. Biofilm formation was analyzed using fluorescence microscopy, scanning electron microscopy (SEM) and confocal laser scanning microscopy (CLSM). Metabolic activity was determined using the XTT reduction assay. P. brasiliensis was able to form mature biofilm in 144 h with a thickness of 100 μm. The presence of a biofilm was found to be associated with an increase in the expression of adhesins and enzymes. GP43, enolase, GAPDH and aspartyl proteinase genes were over-expressed, whereas phospholipase was down-regulated in biofilm. The characterization of biofilm formed by P. brasiliensis may contribute to a better understanding of the pathogenesis of paracoccidioidomycosis as well as the search for new therapeutic alternatives; while improving the effectiveness of treatment.
Introduction
The incidence of systemic fungal diseases has been growing worldwide in recent years, highlighting fungal diseases as an important topic in the field of medicine. Paracoccidioidomycosis (PCM) is a systemic mycosis with higher incidence in Latin America. The majority of cases occur in Brazil, which has the highest concentration of endemic areas, with more than 80% of the reported cases occurring in Brazil. The etiological agent of PCM is Paracoccidioides spp.Citation1,2 This genus is composed of 2 species, Paracoccidioides lutzii and P. brasiliensis; the latter is sub-classified into 3 different phylogenetic groups.Citation1,2
An occupational predisposing factor for acquiring PCM is a work environment that exposes the population to soil, e.g., work on plantations in rural areas.Citation3 In such an environment, propagules from the mycelial form have access to the lungs.
The ability of microorganisms to adapt to environmental changes is of fundamental importance, as this ability enables pathogens to survive and cause disease. Tissue necrosis generates hypoxic conditions, and both host and pathogens adapt to survive. The adaptations of fungi to hostile environments with low oxygen levels are underexplored, especially because granulomas appear to have a hypoxic environment.Citation4
Pathogenic fungi, such as Paracoccidioides spp, have multiple virulence factors that can damage the host. A necessary step in colonization and the development of disease involves the ability of microorganisms to adhere to host surfaces. Adherence is a widely distributed biological phenomenon, which enables microorganisms to colonize their particular habitats. Many fungi, especially pathogenic fungi, are able to adhere to host tissue, the first step in the process of biofilm formation.Citation5,6 One important event during infection by Paracoccidioides spp. is adherence to pulmonary epithelial cells. Several proteins of this fungus (adhesins) have been shown to be ligands of the extracellular matrix in studies performed primarily with lung epithelial cells.Citation7–10 Various molecules have been described as adhesins in Paracoccidioides spp The 43-kDa glycoprotein is involved in Paracoccidioides adhesion,Citation11 as are the 30-, 32- and 54-kDa forms.Citation8,12 The glycoprotein gp43 and the 30-kDa protein are able to bind laminin.Citation11,13 Enolase is a fibronectin-binding protein in Paracoccidioides spp. that is also present in the cytoplasm and the cell wall, but at a higher levels in the cell wall, suggesting that it performs additional functions related to the glycolytic pathway.Citation8,12 In vitro studies have shown that the glyceraldehyde-3-phosphate-dehydrogenase (GAPDH) adhesin may be involved in the pathogenesis of the fungus because this molecule is capable of mediating the ability of the fungus to enter the cell.Citation14,15
Additionally, certain hydrolytic enzymes, such as phospholipase, are used by various microorganisms to invade host tissues.Citation16 These molecules have been described in P. brasiliensis and may play an important role in invasion by this fungus.Citation16–18 P. brasiliensis is also known to produce a variety of proteinases.Citation19,20 The aspartyl proteinases, also known as acid proteases, constitute one of the 4 superfamilies of proteolytic enzymes. They are generally similar to pepsin and show specificity for preferential cleavage at peptide bonds between hydrophobic amino acid residues.Citation19
Biofilms are a form of natural microbial growth that is important in the development of infections. They serve as niches for pathogens and are associated with high levels of resistance to antimicrobial agents.Citation21 The growth and antibiotic resistance of microorganisms differ based on whether they are located in biofilms or in planktonic form.
Fungi of many types have demonstrated the ability to colonize surfaces and form biofilms (Cryptococcus neoformans, Rhodotorula espécies, Aspergillus fumigatus, Malassezia pachydermatis, Histoplasma capsulatum, Pneumocystis species, Coccidioides immitis, Fusarium species, Saccharomyces cerevisiae, Trichosporon asahii, Zygomycetes, Blastoschizomyces and more recently Trichophyton rubrum and Trichophyton mentagrophytes). Most of the previous studies have focused on biofilms of Candida albicans, but other species of Candida, other yeasts and filamentous fungi are known to form biofilms.Citation22–25 Most importantly, fungal biofilms are of clinical importance, particularly in the context of chronic diseases.Citation26 For biofilms to successfully form in a host, microorganisms must first adhere to target tissues and concurrently obtain essential nutrients for growth and development. Recently, an in vitro study demonstrated the efficiency with which Histoplasma capsulatum var. capsulatum forms biofilms on abiotic surfaces.Citation23 Biofilm formation has been associated with the expression of various adhesins.Citation27 Therefore, the current study aimed to verify the ability of P. brasiliensis to form a biofilm and to investigate the gene expression of several adhesins that could be associated with the biofilm formation of this pathogen.
Results
Infection of pneumocytes and alveolar macrophages by P. brasiliensis
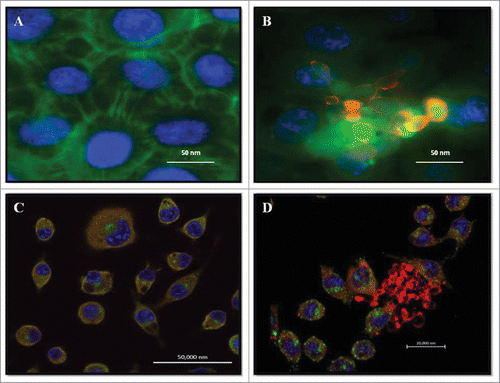
The notion that P. brasiliensis can form biofilms is based on experiments showing that the fungus can appear in clumps that strongly resemble the formation of a fungal mass, which is highly characteristic of biofilms. Infections of P. brasiliensis (Pb18) yeast cells in pneumocytes and macrophages were evaluated by confocal laser microscopy and In Cell Analyzer 2000. Pb18 was able to adhere to pneumocytes, as fungal masses were attached to several areas of these cells (). The same pattern of interaction occurred with phagocytic cells, in which an agglomeration of fungal cells was observed around the alveolar macrophage (). show uninfected macrophages and A549 cells, respectively.
Figure 1. Double Immunofluorescence for epithelial cell line A549 and alveolar macrophages murine AMJ2-C11. (A) Uninfected A549 cells. (B) A549 cells infected with P. brasiliensis. A549 cells were labeled with FITC-phalloidin (green), P. brasiliensis stained with anti-cell free antibody and Alexa Fluor® 594 conjugate and nucleus was labeled with DAPI (blue). (C) Uninfected macrophages. (D) Macrophages infected with P. brasiliensis. Macrophages were immunolabeled with the primary antibodies anti- cytoplasmic protein and secondary conjugated Alexa Fluor R 488 (green), and P. brasiliensis immunolabeled with anti-cell-free antibody (red) and secondary conjugated Alexa Fluor ® 594, and nucleus was labeled with DAPI (blue) (Zeiss LSM 510 Meta Confocal Microscope).
Kinetics of biofilm formation by P. brasiliensis
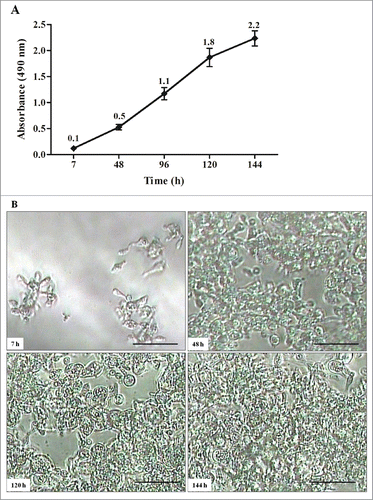
All biofilm standardization was performed with P. brasiliensis (Pb18), the strain used in virulence studies. The kinetics of biofilm formation by P. brasiliensis was measured on polystyrene microtiter plates using the XTT reduction assay to determine the amount of metabolic activity. The formation of a biofilm was consistent after 144 h of incubation at 37°C in a CO2 incubator. The initial formation of a biofilm was observed after 7 h. This initial period included pre-adhesion, during which P. brasiliensis (Pb18) became attached to the plastic surface in a monolayer arrangement. Over a period of 48 – 120 h, an increase in the biofilm was observed, and the metabolic activity of the biofilm, measured using the XTT reduction assay, increased over time as the cellular mass increased. During the maturation stage (48 – 144 h), the architecture of the P. brasiliensis biofilm became more complex ().
Figure 2. Kinetics of biofilm formation by P. brasiliensis in microdilution plates. (A) Measurements determined by the XTT reduction assay. Each point represents the mean of 3 measurements of absorbance at 490nm on a microtiter reader (iMarkTM Microplate Reader; BIO-RAD). (B) Kinetics monitored by Microphotograph taken using a camera attached to an inverted microscope. Bars = 30 nm for all panels.
Biofilm morphology of P. brasiliensis
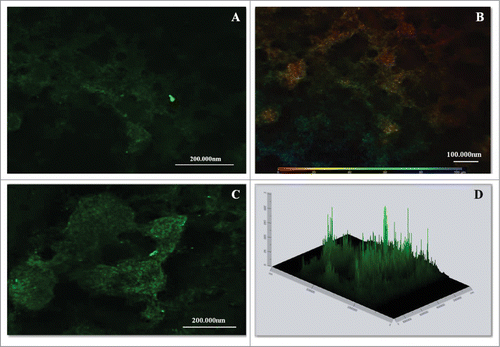
The morphology of the P. brasiliensis biofilm was evaluated using calcofluor white fluorescence microscopy, SEM and CLMS. The P. brasiliensis biofilm showed an intense blue coloration, derived from the binding of fluorochrome to the fungal cell wall (). SEM revealed a highly organized network of fungal cells in the form of a biofilm and an extracellular matrix (). The Pb18 biofilm consisted of a dense network of yeast; an orthogonal image was analyzed to determine the thickness and architecture of the biofilm. The sections of the 3-dimensional image () showed that the P. brasiliensis biofilm had a thickness of approximately 100 μm, as observed by CLMS.
Figure 3. Images of mature biofilms of P. brasiliensis (Pb 18), formed after incubation for 144 h at 37°C 40× magnification. Images were acquired by Fluorescence microscopy and the fungi biofilms were stained by Calcofluor White Stain reagent (Fluka ®). All scale bars are 125 μm.
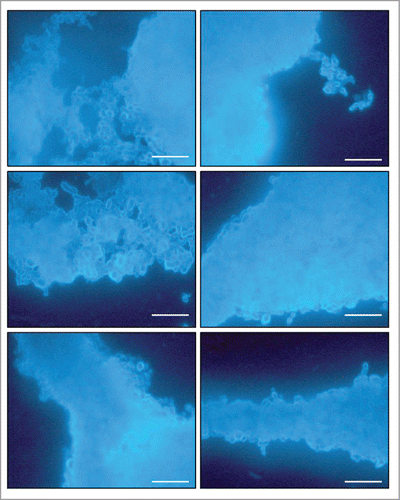
Figure 4. SEM images of mature biofilms of P. brasiliensis (Pb 18), in different dimensions, formed after incubation for 144 h at 37°C (A–D). In the arrows in showing the presence of extracellular matrix.
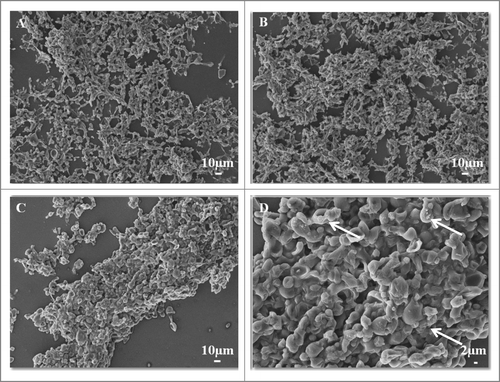
Figure 5. CLSM images of mature biofilms of P. brasiliensis (144 h). Fluorescence labeling of P. brasiliensis biofilms. (A and C) Biofilm was immunolabeled with primary antibodies anti-cell-free and secondary conjugated Alexa Fluor ® 488. (B) Scale depth image A showing the thickness of the biofilm. (D) Projection of biofilm formation of P. brasiliensis 2.5D (Zeiss LSM 510 Meta Confocal Microscope).
Gene expression analysis using real-time PCR (qRT-PCR)
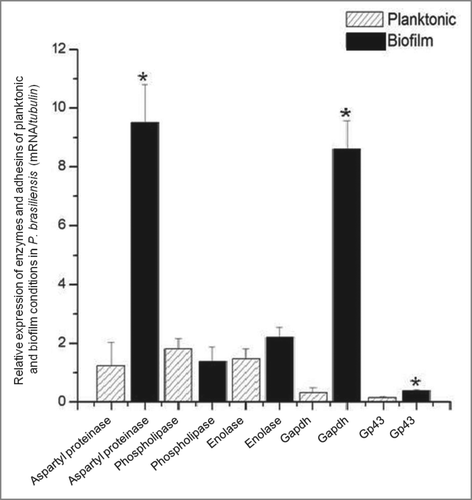
The gene expression of certain adhesins and enzymes of P. brasiliensis was compared between the biofilm and planktonic conditions. The expression of GP43, GAPDH and aspartyl proteinase was significantly higher in the biofilm when compared to the planktonic condition (). The expressions of aspartyl proteinase, GAPDH, GP43 and enolase were 10, 9, 2.5 and 1.5 times greater in the biofilm, respectively, than in the planktonic form. The expression of phospholipase was less in the biofilm when compared to the planktonic form.
Discussion
Biofilm formation has been described for various fungi, including Candida species, C. neoformans, C. immitis, A. fumigatus, Fusarium solani and, more recently, H. capsulatum.Citation21,Citation23–25 Adhesion is a biological process that allows various organisms to colonize their habitats. The organisms that form biofilms may grow differently in the planktonic and biofilm forms. The aim of the present research was to investigate the ability of P. brasiliensis to form a biofilm and to analyze the expression of genes encoding adhesins and hydrolytic enzymes that could be associated with biofilm formation.
In our study, P. brasiliensis was able to form a biofilm in vitro under hypoxic conditions. Studies by Bonhomme et al.Citation40 and Stichternoth & Ernst,Citation41 have indicated that hypoxia is required for C. albicans to form biofilms.
The kinetics of biofilm formation by the Pb18 strain showed that the biofilm initially formed before 7 h had elapsed, which included the adhesion period. Mature biofilms were produced from 120 to 144 h. P. brasiliensis is a slowly growing fungus. In terms of the time required for formation, Pb18 shows a longer delay and slower growth when compared with other fungi. In contrast, growth is more rapid in the dimorphic fungus, H. capsulatum (72 h), and in C. albicans (24 h).Citation23,42 The thickness of the mature biofilm was approximately 100 μm, which is greater than that found for other fungi, such as C. neoformans, which exhibited a biofilm thickness of 76 μm, as demonstrated by Martinez et al.Citation43 The metabolic activity of the biofilm increased over time as the cellular mass increased.
The Pb18 fungal cells may also form an agglomeration resembling a biofilm. However, this structure occurs in ex vivo cells, shown in . Pb18 formed fungal masses in epithelial cells () and in phagocytic cells (). It was thought that this fungus has the ability to form biofilm, since histopathological findings found fungal mass granulomas. This aspect may be similar to that observed in histopathological observations of organized granulomas, with compact aggregates of macrophages, epithelial cells and masses of yeasts.Citation44 The same pattern was observed for H. capsulatum, which formed a ring of fungal cells in phagocytic cells. This structure may result from a particular type of interaction of this fungus with the tissue. Biofilm formation may contribute to a chronic state of this disease.Citation23
The up-regulation of selected genes was demonstrated using qRT-PCR. The results allowed the identification of a potential adhesin of P. brasiliensis. Adhesins have an important role in biofilm formation, as demonstrated by C. albicans, in which ALS genes exhibited increased expression during the formation of biofilms.Citation45 The real-time PCR performed in the current study detected the expression of GP43, GAPDH, aspartyl proteinase and phospholipase in both planktonic form and biofilm growth, but at different levels. The GP43, GAPDH and aspartyl proteinase genes were significantly (P ≤ 0.05) overexpressed in the biofilm, whereas phospholipase was downregulated. These results are consistent with data demonstrating an increased expression of adhesins in Candida biofilms,Citation46 the most studied fungal biofilm, as well as in bacterial studies.Citation47
Various studies have indicated that the 43-kDa glycoprotein is involved in Paracoccidioides adhesion.Citation11,29 In the present study, GP43 was up-regulated in biofilm formation, demonstrating that this glycoprotein could be involved in adhesion and biofilm formation (P < 0.05).
A remarkable level of expression of the GAPDH adhesin was observed; almost 10-fold greater in the biofilm form when compared to the planktonic form (P < 0.05). Studies performed by Bailão and collaborators,Citation14 demonstrated that this adhesin was also upregulated in the yeast form based on recovery of the compound from infected mice.
Verstrepen and Klis,Citation48 also emphasized the remarkable ability of fungi to express adhesins and form biofilms. This characteristic is of great medical importance, because the presence of a mature biofilm hinders the action of antifungal agents, and biofilms can become a reservoir of cells that show resistance to certain drugs. Therefore, there is a considerable interest in the persistence of infection due to biofilms in infectious diseases.
Aspartyl proteinase and phospholipase were up-regulated and down-regulated, respectively, in the P. brasiliensis biofilm when compared with the planktonic form. The results of the present study are consistent with previous research by Ramage et al.Citation27 and Nailis et al.,Citation46 in which an increased expression of aspartyl proteinase and a decreased expression of phospholipase were demonstrated in a C. albicans biofilm. Ramage et al.Citation27 reported that the production of proteinase by C. albicans aided and established adhesion, invasion and tissue destruction and may be related to the severity of disease because the expression of this enzyme was significantly higher in mature biofilms.
Proteinases produced by P. brasiliensis, A. fumigatus, T. rubrum, and C. neoformans have been described in previous studies and are of great importance because they may split the major components of the basement membrane in vitro and are, therefore, potentially relevant for the spread of this fungus.Citation19,49,50 Additionally, these proteins are hydrolytic enzymes capable of either hydrolyzing large substrates into small units for transportation into the cell to serve as a nutrient or for degrading tissue and facilitating colonization or invasion, serving as a virulence factor for the pathogen.
The current paper is the first study of P. brasiliensis biofilms. Additional studies are required to determine the role of these biofilms in vivo in association with pathogenesis.
Materials and Methods
Microorganism
Experiments were performed using a clinical isolate of P. brasiliensis (Pb18). The strain was cultivated in Fava-Netto at 37°C for 5 d in an atmosphere with a reduced level of oxygen (5% CO2). This strain was isolated from a case of paracoccidioidomycosis (PMC) and then maintained at the Faculty of Medicine, University of São Paulo, Brazil. This strain is considered a highly virulent isolate (Pb18 isolate), as described in the intraperitoneal infection of susceptible, genetically homogeneous B10A mice.Citation28 Pb18 also presents a high level of adhesion.Citation29
Preparation of inoculum
A suspension of P. brasiliensis (Pb18) was prepared in PBS and was adjusted to 10Citation8 cells/ml for the infection assay based on observations performed with a Neubauer counting chamber.
Antisera and reagents
A cell-free antigen was obtained using Pb18 and was prepared as described elsewhere.Citation30 Approximately 300 mg of the fungus was added to 1 ml of sterile PBS. This mixture was vortexed for 30 seconds and centrifuged at 400 g for 1 minute. The supernatant (cell-free antigen) was collected, aliquoted and stored at −20°C. The Bradford method (BioRad, São Paulo,SP, Brazil) was used to quantify the protein concentration and the samples were analyzed using the SDS-PAGE method. A polyclonal antibody produced against P. brasiliensis cell-free antigen was prepared as described in Mendes-Giannini et al.Citation5 Rabbits were inoculated with injections of 1.0 ml of antigen mixed with 1.0 ml of complete Freund´s adjuvant. After 3 months of injections of antigen, the rabbits were bled, and the fractions of antisera were separated by precipitation with ammonium sulfate and stored at – 70°C.
The following antibodies were used in this current study: Alexa Fluor 488 goat anti-rabbit IgG (Molecular Probes, USA), Alexa Fluor® 594 goat anti-rabbit IgG (Molecular Probes, Invitrogen, USA) and Alexa Fluor® 488 goat anti-mouse IgG (Molecular Probes, USA). Fluorescein isothiocyanate (FITC)-labeled phalloidin and all other reagents were purchased from Sigma-Aldrich, USA.
Macrophages and epithelial cell line A549 culture
Macrophages, alveolar line AMJ2-C11 (ATCC CRL-2456), were cultivated overnight in Dulbecco's modified Eagle's medium (DMEM) (Sigma-Aldrich, Brazil), supplemented with 10% fetal calf serum (Cultilab, Brazil). Cultures of a human lung adenocarcinoma, cell line A549, were obtained from the American Type Culture Collection (ATCC-Rockville, MD). These cells were seeded in Ham's F-12 medium supplemented with 10% fetal calf serum.
Infection assay
Macrophages and continuous epithelial cell line A549 cells were cultured at 36.5°C in 24-well plates with the well bottoms covered with coverslips. The cultures were adjusted to 1 × 10Citation5 cells per well. A total of 1 × 10Citation5 Pb18 yeast-phase cells/ml were added to the cells to obtain a yeast/macrophage ratio of 1:1. After infection, these cover slips were fixed with 4% paraformaldehyde and submitted to immunofluorescence. The coverslips were permeabilized with a solution of Triton X-100, 0.5% in PBS, for 20 minutes. The coverslips were then washed, the "cell-free" antiserum was added, and the samples were incubated at room temperature for 1 hour, washed again, and conjugated anti-rabbit labeled with Alexa 594 (INVITROGEN) at a ratio of 1:300 was added. Phalloidin-FITC conjugate (SIGMA) at a ratio of 1:100 at 4°C was added and the specimens sat overnight. The specimens were again washed, 100 μl DAPI (4 '- 6 – diamidino – 2 – phenylindole) was added for 10 minutes to mark the cores, washed with PBS, mounted on slides with buffered glycerol, and examined using a confocal microscope (Zeiss LSM 510 Meta Confocal Microscope).
The cultures were then incubated for 7 h at 36.5°C to observe adhesion. The assay was analyzed using conventional fluorescence microscopy and scanning confocal laser microscopy (CLMS) (Zeiss LSM 510 Meta Confocal Microscope, Carl Zeiss, Jena, Göttingen).
Biofilm assay
The assay was performed as described by Silva et al.Citation31 with slight modifications. Initially, 500 μl of a culture of 1 × 10Citation8 cells/ml in saline was added to the wells of a 24-well plate (TPP, Trasadingen, Switzerland) and covered with coverslips. The plates were incubated at 37°C for 7 h in 5% CO2 for biofilm pre-adhesion. After pre-adhesion for 7 h, the supernatant was removed from each well. Subsequently, 1000 μL of mFUM (Modified Fluid Universal Medium – Guggenheim et al.Citation32) was added to each well and the plates were further incubated for 144 h. The culture medium was renewed every 48 hours. After biofilm formation for 144 h, the supernatant was again removed, and the wells were rinsed using 400 μl of PBS. Six wells were filled with a sterile medium as a control. All assays were repeated at least 3 times.. To characterize the biofilm, measurements of biofilm metabolic activity were made using XTT. At the structural level, P. brasiliensis biofilm formation was characterized using Calcofluor White Stain (Fluka®, São Paulo, SP., Brazil) and scanning electron microscopy (SEM), and biofilm measurements were performed using confocal laser scanning microscopy (CLSM).
Measurement of biofilm metabolic activity
A quantitative measurement of P. brasiliensis biofilm formation was obtained from the XTT reduction assay. For this measurement, 50 μL of XTT salt solution (1 mg ml−Citation1 in PBS) and 4 μl of menadione solution (1 mM in ethanol; Sigma-Aldrich, São Paulo, SP., Brazil) were added to each well of the microtiter plates and incubated at 37°C for 3 h, resulting in a colorimetric reaction that is correlated with cell viability. This reaction was measured using a microtiter reader (iMarkTM Microplate Reader; BIORAD, Brazil) at 490 nm. In all assays, culture media were included as negative controls.Citation31–33
Fluorescence microscopy
For fluorescence microscopy, coverslips with biofilm were stained with Calcofluor White Stain reagent (1g/L – Fluka®). To prepare the coverslips, 50 μl of dye were added. The coverslips were carefully removed from each well and transferred to a slide for observation under a fluorescence microscope. Calcofluor is a non-specific fluorochrome that binds to cellulose and chitin in the fungal cell wall. This staining procedure provides a rapid method for the detection of yeasts and pathogenic fungi.Citation34 A range of wavelengths, from 300 to 440 nm, can be used for excitation. Emission occurred at a wavelength of 355 nm.
Scanning electron microscopy
Biofilms formed on coverslips were processed as described by Morris et al.Citation35 and modified by Ells and Truelstrup Hansen.Citation36 Briefly, the specimens were washed 3 times with PBS to remove planktonic cells and then fixed with 1% glutaraldehyde in 0.2 M sodium cacodylate buffer for 18 h at 4°C. After three PBS washes of 10 min each, the biofilms that still adhered to the coverslips were fixed with 1% osmium tetroxide for 2 h. The specimens were washed with PBS and dehydrated with an increasing gradient of ethanol, from 50% to 100% ethanol, at room temperature. The samples were dried using the critical point method in a Samdri 780A desiccator (Rockville, MD, USA) using CO2. Topographic features of the biofilms were analyzed with a Zeiss-Leica/440 SEM at the Institute of Chemistry of Sao Carlos, University of Sao Paulo, using a voltage of 25 kV and a 10 mm working distance.
Indirect immunofluorescence
After biofilm formation, the biofilm was fixed with 4% paraformaldehyde, washed in PBS, and permeabilized in 0.5% Triton X-100 for 30 minutes. After permeabilization, anti-P. brasiliensis (cell-free) serum was added for 1 h, unbound antibodies were removed by washing with PBS, and Alexa Fluor®488 goat anti-rabbit IgG was then added for 1 h. The biofilm was then washed 3 times with PBS and analyzed under confocal laser scanning microscopy (LSM 510 – META, Zeiss). For the adhesion assay, the same procedure was used, but the secondary antibody was Alexa Fluor®594 goat anti-rabbit IgG, added for 1 h. FITC-labeled phalloidin was then added for 1 h and observed using confocal microscopy.
RNA isolation and cDNA synthesis
RNA extraction was performed from mature biofilms (144h) and planktonic cells (144h). Planktonic cells were cultured in mFUM in bottles. Every 48 hours, the cells in suspension were withdrawn and centrifuged, then placed in fresh medium in a new flask for a period of 144 hours. After 144 hours, the biofilm and planktonic cells were washed and centrifuged. The resulting pellet was stored in Ultra freezer for subsequent RNA extraction. Total RNA was obtained by the addition of TRIZOL® (Invitrogen, Carlsbad, CA, USA) after growing the Pb18 strain in both the biofilm and planktonic forms under 5% CO2 conditions. Total RNA was treated with DNase I (Invitrogen, Carlsbad, CA, USA). RNA quality (i.e., the presence of discrete 18S and 28S rRNA peaks) was determined in 1.5% agarose gels in 1× TBE buffer for 2 h at 100 V. The gels were stained with GelRed and observed under UV light. Two independent RNA samples were prepared for use in the experiments. First-strand cDNA synthesis was performed using 1 μg/μl RNA and the enzyme Superscript III reverse transcriptase (Invitrogen, Carlsbad, CA, USA).Citation37
Gene expression analysis for real-time PCR (qRT-PCR)
The expression levels of 5 genes involved in various cellular functions of P. brasiliensis were measured and described during growth in biofilm and planktonic forms, both under low oxygen tension. Quantitative real-time PCR (qRT-PCR) was performed for genes using the primer constants shown in . The concentrations of primers used were adjusted to 0.5 μM for improved amplification efficiency. The quantity of cDNA used was 1 μl, and 12.5 μl of Maxima®SYBR Green (Fermentas, São Paulo, SP., Brazil) was added. The final volume was adjusted to 25 μl. PCR was performed with a starting temperature of 50°C for 2 minutes, followed by 10 minutes at 95°C, then 40 cycles at 95°C for 15 seconds, followed by annealing and synthesis at 60°C for 1 minute. The reactions were performed in triplicate using an Applied Biosystems 7500 thermal cycler. Variations in mRNA expression were calculated using the 2−ΔCT formula, where ΔCT is the difference between the targets and the housekeeping gene β-tubulin in accordance with previous studies performed by several authors. The data were analyzed using the 2−ΔΔCT method.Citation38 The specific primers for qRT-PCR were designed using the Primer 3 software.Citation39
Table 1 Primers utilized in this study
Statistical analysis
The data were analyzed using the Origin 6.0 software (Origin Lab Corporation, Northampton, MA). A P value ≤0.05 was considered statistically significant.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Funding
The authors thank CNPq (National Council for Scientific and Technological Development) and PADC (Support Program for Scientific, Faculty of Pharmaceutical Sciences, UNESP) for their financial support.
References
- Marques-da-Silva SH, Rodrigues AM, de Hoog GS, Silveira-Gomes F, Camargo ZP. Occurrence of Paracoccidioides lutzii in the Amazon region: description of two cases. Am J Trop Med Hyg 2012; 87:710-4; PMID:22927496; http://dx.doi.org/10.4269/ajtmh.2012.12-0340
- Arantes TD, Theodoro RC, Da Graça Macoris SA, Bagagli E. Detection of Paracoccidioides spp. in environmental aerosol samples. Med Mycol 2013; 51:83-92; PMID:22762209; http://dx.doi.org/10.3109/13693786.2012.698444
- Franco M, Montenegro MR, Mendes RP, Marques SA, Dillon NL, Mota NG. Paracoccidioidomycosis: a recently proposed classification of its clinical forms. Rev Soc Bras Med Trop 1987; 20:129-32; PMID:3507739; http://dx.doi.org/10.1590/S0037-86821987000200012
- Grahl N, Shepardson KM, Chung D, Cramer RA. Hypoxia and fungal pathogenesis: to air or not to air? Eukaryot Cell 2012; 11:560-70; PMID:22447924; http://dx.doi.org/10.1128/EC.00031-12
- Mendes-Giannini MJ, Taylor ML, Bouchara JB, Burger E, Calich VL, Escalante ED, Hanna SA, Lenzi HL, Machado MP, Miyaji M, et al. Pathogenesis II: fungal responses to host responses: interaction of host cells with fungi. Med Mycol 2000; 38 (Suppl 1):113-23; PMID:11204137; http://dx.doi.org/10.1080/mmy.38.s1.113.123
- Mendes-Giannini MJ, Soares CP, da Silva JL, Andreotti PF. Interaction of pathogenic fungi with host cells: Molecular and cellular approaches. FEMS Immunol Med Microbiol 2005; 45:383-94; PMID:16087326; http://dx.doi.org/10.1016/j.femsim.2005.05.014
- Hernández O, Almeida AJ, Tamayo D, Torres I, Garcia AM, López A, Restrepo A, McEwen JG. The hydrolase PbHAD32 participates in the adherence of Paracoccidioides brasiliensis conidia to epithelial lung cells. Med Mycol 2012; 50:533-7; http://dx.doi.org/10.3109/13693786.2011.619583
- Donofrio FC, Calil AC, Miranda ET, Almeida AM, Benard G, Soares CP, Veloso SN, Soares CM, Mendes Giannini MJ. Enolase from Paracoccidioides brasiliensis: isolation and identification as a fibronectin-binding protein. J Med Microbiol 2009; 58:706-13; PMID:19429745; http://dx.doi.org/10.1099/jmm.0.003830-0
- Caro E, Gonzalez A, Muñoz C, Urán ME, Restrepo A, John Hamilton A, Elena Cano L. Recognition of laminin by Paracoccidioides brasiliensis conidia: a possible mechanism of adherence to human type II alveolar cells. Med Mycol 2008; 46:795-804; PMID:18608937; http://dx.doi.org/10.1080/13693780802073108
- Mendes-Giannini MJ, Hanna SA, da Silva JL, Andreotti PF, Vincenzi LR, Benard G, Lenzi HL, Soares CP. Invasion of epithelial mammalian cells by Paracoccidioides brasiliensis leads to cytoskeletal rearrangement and apoptosis of the host cell. Microbes Infect 2004; 6:882-91; PMID:15310464; http://dx.doi.org/10.1016/j.micinf.2004.05.005
- Vicentini AP, Gesztesi JL, Franco MF, de Souza W, de Moraes JZ, Travassos LR, Lopes JD. Binding of Paracoccidioides brasiliensis to laminin through surface glycoprotein gp43 leads to enhancement of fungal pathogenesis. Infect Immun 1994; 62:1465-9; PMID:8132354
- Marcos CM, de Fátima da Silva J, de Oliveira HC, Moraes da Silva RA, Mendes-Giannini MJ, Fusco-Almeida AM. Surface-expressed enolase contributes to the adhesion of Paracoccidioides brasiliensis to host cells. FEMS Yeast Res 2012; 12:557-70; PMID:22443156; http://dx.doi.org/10.1111/j.1567-1364.2012.00806.x
- Andreotti PF, Monteiro da Silva JL, Bailão AM, Soares CM, Benard G, Soares CP, Mendes-Giannini MJ. Isolation and partial characterization of a 30 kDa adhesin from Paracoccidioides brasiliensis. Microbes Infect 2005; 7:875-81; PMID:15862780; http://dx.doi.org/10.1016/j.micinf.2005.02.005
- Bailão AM, Schrank A, Borges CL, Dutra V, Walquíria Inês Molinari-Madlum EE, Soares Felipe MS, Soares Mendes-Giannini MJ, Martins WS, Pereira M, Maria de Almeida Soares C. Differential gene expression by Paracoccidioides brasiliensis in host interaction conditions: representational difference analysis identifies candidate genes associated with fungal pathogenesis. Microbes Infect 2006; 8:2686-97; http://dx.doi.org/10.1016/j.micinf.2006.07.019
- Barbosa MS, Báo SN, Andreotti PF, de Faria FP, Felipe MS, dos Santos Feitosa L, Mendes-Giannini MJ, Soares CM. Glyceraldehyde-3-phosphate dehydrogenase of Paracoccidioides brasiliensis is a cell surface protein involved in fungal adhesion to extracellular matrix proteins and interaction with cells. Infect Immun 2006; 74:382-9; PMID:16368993; http://dx.doi.org/10.1128/IAI.74.1.382-389.2006
- Ghannoum MA. Potential role of phospholipases in virulence and fungal pathogenesis. Clin Microbiol Rev 2000; 13:122-43, table of contents; PMID:10627494; http://dx.doi.org/10.1128/CMR.13.1.122-143.2000
- Parente JA, Salem-Izacc SM, Santana JM, Pereira M, Borges CL, Bailão AM, Soares CM. A secreted serine protease of Paracoccidioides brasiliensis and its interactions with fungal proteins. BMC Microbiol 2010; 10:292; PMID:21080956; http://dx.doi.org/10.1186/1471-2180-10-292
- Maza PK, Oliveira P, Toledo MS, Paula DM, Takahashi HK, Straus AH, Suzuki E. Paracoccidioides brasiliensis induces secretion of IL-6 and IL-8 by lung epithelial cells. Modulation of host cytokine levels by fungal proteases. Microbes Infect 2012; 14:1077-85; PMID:22687715; http://dx.doi.org/10.1016/j.micinf.2012.05.016
- Tacco BA, Parente JA, Barbosa MS, Báo SN, Gsóes Tde S, Pereira M, Soares CM. Characterization of a secreted aspartyl protease of the fungal pathogen Paracoccidioides brasiliensis. Med Mycol 2009; 47:845-54; PMID:20028235; http://dx.doi.org/10.3109/13693780802695512
- Longo LV, Nakayasu ES, Matsuo AL, Peres da Silva R, Sobreira TJ, Vallejo MC, Ganiko L, Almeida IC, Puccia R. Identification of human plasma proteins associated with the cell wall of the pathogenic fungus Paracoccidioides brasiliensis. FEMS Microbiol Lett 2013; 341:87-95; PMID:23398536; http://dx.doi.org/10.1111/1574-6968.12097
- Pierce GE. Pseudomonas aeruginosa, Candida albicans, and device-related nosocomial infections: implications, trends, and potential approaches for control. J Ind Microbiol Biotechnol 2005; 32:309-18; PMID:15868157; http://dx.doi.org/10.1007/s10295-005-0225-2
- Sánchez-Vargas LO, Estrada-Barraza D, Pozos-Guillen AJ, Rivas-Caceres R. Biofilm formation by oral clinical isolates of Candida species. Arch Oral Biol 2013; 58:1318-26; http://dx.doi.org/10.1016/j.archoralbio.2013.06.006
- Pitangui NS, Sardi JC, Silva JF, Benaducci T, Moraes da Silva RA, Rodríguez-Arellanes G, Taylor ML, Mendes-Giannini MJ, Fusco-Almeida AM. Adhesion of Histoplasma capsulatum to pneumocytes and biofilm formation on an abiotic surface. Biofouling 2012; 28:711-8; PMID:22784100; http://dx.doi.org/10.1080/08927014.2012.703659
- Kaur S, Singh S. Biofilm formation by Aspergillus fumigatus. Med Mycol 2013; 52(1):2-9; PMID:23962172
- Sengupta J, Saha S, Khetan A, Sarkar SK, Mandal SM. Effects of lactoferricin B against keratitis-associated fungal biofilms. J Infect Chemother 2012; 18:698-703; PMID:22410856; http://dx.doi.org/10.1007/s10156-012-0398-3
- Bjarnsholt T, Alhede M, Alhede M, Eickhardt-Sørensen SR, Moser C, Kühl M, Jensen PØ, Høiby N. The in vivo biofilm. Trends Microbiol 2013; 21:466-74; PMID:23827084; http://dx.doi.org/10.1016/j.tim.2013.06.002
- Ramage G, Coco B, Sherry L, Bagg J, Lappin DF. In vitro Candida albicans biofilm induced proteinase activity and SAP8 expression correlates with in vivo denture stomatitis severity. Mycopathologia 2012; 174:11-9; PMID:22302440; http://dx.doi.org/10.1007/s11046-012-9522-2
- Singer-Vermes LM, Burger E, Franco MF, Di-Bacchi MM, Mendes-Giannini MJ, Calich VL. Evaluation of the pathogenicity and immunogenicity of seven Paracoccidioides brasiliensis isolates in susceptible inbred mice. J Med Vet Mycol 1989; 27:71-82; PMID: 2746437; http://dx.doi.org/10.1080/02681218980000111
- Hanna SA, Monteiro da Silva JL, Giannini MJ. Adherence and intracellular parasitism of Paracoccidioides brasiliensis in Vero cells. Microbes Infect 2000; 2:877-84; PMID:10962270; http://dx.doi.org/10.1016/S1286-4579(00)00390-7
- Camargo ZP, Taborda CP, Rodrigues EG, Travassos LR. The use of cell-free antigens of Paracoccidioides brasiliensis in serological tests. J Med Vet Mycol 1991; 29:31-8; PMID:1905751; http://dx.doi.org/10.1080/02681219180000061
- Silva S, Henriques M, Oliveira R, Williams D, Azeredo J. In vitro biofilm activity of non-Candida albicans Candida species. Curr Microbiol 2010; 61:534-40; PMID:20401483; http://dx.doi.org/10.1007/s00284-010-9649-7
- Guggenheim B, Giertsen E, Schüpbach P, Shapiro S. Validation of an in vitro biofilm model of supragingival plaque. J Dent Res 2001; 80:363-70; PMID:11269730; http://dx.doi.org/10.1177/00220345010800011201
- Martinez LR, Casadevall A. Cryptococcus neoformans biofilm formation depends on surface support and carbon source and reduces fungal cell susceptibility to heat, cold, and UV light. Appl Environ Microbiol 2007; 73:4592-601; PMID:17513597; http://dx.doi.org/10.1128/AEM.02506-06
- Harrington BJ, Hageage GJ. Calcofluor white: a review of its uses and applications in clinical mycology and parasotology. Lab Med 2003; 34:361-7; http://dx.doi.org/10.1309/EPH2TDT8335GH0R3
- Morris CE, Monier J, Jacques M. Methods for observing microbial biofilms directly on leaf surfaces and recovering them for isolation of culturable microorganisms. Appl Environ Microbiol 1997; 63:1570-6; PMID:16535579
- Ells TC, Truelstrup Hansen L. Strain and growth temperature influence Listeria spp. attachment to intact and cut cabbage. Int J Food Microbiol 2006; 111:34-42; PMID:16824634; http://dx.doi.org/10.1016/j.ijfoodmicro.2006.04.033
- Voltan AR, Sardi JeC, Soares CP, Pelajo Machado M, Fusco Almeida AM, Mendes-Giannini MJ. Early Endosome Antigen 1 (EEA1) decreases in macrophages infected with Paracoccidioides brasiliensis. Med Mycol 2013; 51:759-64; PMID:23566224; http://dx.doi.org/10.3109/13693786.2013.777859
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001; 25:402-8; http://dx.doi.org/10.1006/meth.2001.1262
- Rozen S, Skaletsky H. Primer3 on the WWW for general users and for biologist programmers. Methods Mol Biol 2000; 132:365-86; PMID:10547847
- Bonhomme J, Chauvel M, Goyard S, Roux P, Rossignol T, d'Enfert C. Contribution of the glycolytic flux and hypoxia adaptation to efficient biofilm formation by Candida albicans. Mol Microbiol 2011; 80:995-1013; PMID:21414038; http://dx.doi.org/10.1111/j.1365-2958.2011.07626.x
- Stichternoth C, Ernst JF. Hypoxic adaptation by Efg1 regulates biofilm formation by Candida albicans. Appl Environ Microbiol 2009; 75:3663-72; PMID:19346360; http://dx.doi.org/10.1128/AEM.00098-09
- Pierce CG, Uppuluri P, Tummala S, Lopez-Ribot JL. A 96 well microtiter plate-based method for monitoring formation and antifungal susceptibility testing of Candida albicans biofilms. J Vis Exp 2010; 21: 2287; http://dx.doi.org/10.3791/2287
- Martinez LR, Casadevall A. Susceptibility of Cryptococcus neoformans biofilms to antifungal agents in vitro. Antimicrob Agents Chemother. 2006; 50:1021-1033; PMID:16495265; http://dx.doi.org/10.1128/AAC.50.3.1021-1033.2006
- Da Silva FC, Svidzinski TI, Patussi EV, Cardoso CP, De Oliveira Dalalio MM, Hernandes L. Morphologic organization of pulmonary granulomas in mice infected with Paracoccidioides brasiliensis. Am J Trop Med Hyg 2009; 80:798-804; PMID:19407127
- O'Connor L, Lahiff S, Casey F, Glennon M, Cormican M, Maher M. Quantification of ALS1 gene expression in Candida albicans biofilms by RT-PCR using hybridisation probes on the LightCycler. Mol Cell Probes 2005; 19:153-62; PMID:15797814; http://dx.doi.org/10.1016/j.mcp.2004.10.007
- Nailis H, Kucharíková S, Ricicová M, Van Dijck P, Deforce D, Nelis H, Coenye T. Real-time PCR expression profiling of genes encoding potential virulence factors in Candida albicans biofilms: identification of model-dependent and -independent gene expression. BMC Microbiol 2010; 10:114; PMID:20398368; http://dx.doi.org/10.1186/1471-2180-10-114
- Sanchez CJ, Shivshankar P, Stol K, Trakhtenbroit S, Sullam PM, Sauer K, Hermans PW, Orihuela CJ. The pneumococcal serine-rich repeat protein is an intra-species bacterial adhesin that promotes bacterial aggregation in vivo and in biofilms. PLoS Pathog 2010; 6:e1001044
- Verstrepen KJ, Klis FM. Flocculation, adhesion and biofilm formation in yeasts. Mol Microbiol 2006; 60:5-15; PMID:16556216; http://dx.doi.org/10.1111/j.1365-2958.2006.05072.x
- Leng WC, Wang LL, Wei CD, Yang J, Jin Q. Analysis of secreted proteases of Trichophyton rubrum. Wei Sheng Wu Xue Bao 2005; 45:601-5; PMID:16245880
- Pinti M, Orsi CF, Gibellini L, Esposito R, Cossarizza A, Blasi E, Peppoloni S, Mussini C. Identification and characterization of an aspartyl protease from Cryptococcus neoformans. FEBS Lett 2007; 581:3882-6; PMID:17651737; http://dx.doi.org/10.1016/j.febslet.2007.07.006