ABSTRACT
Most of what is known about fungi in the human vagina has come from culture-based studies and phenotypic characterization of single organisms. Though valuable, these approaches have masked the complexity of fungal communities within the vagina. The vaginal mycobiome has become an emerging field of study as genomics tools are increasingly employed and we begin to appreciate the role these fungal communities play in human health and disease. Though vastly outnumbered by its bacterial counterparts, fungi are important constituents of the vaginal ecosystem in many healthy women. Candida albicans, an opportunistic fungal pathogen, colonizes 20% of women without causing any overt symptoms, yet it is one of the leading causes of infectious vaginitis. Understanding its mechanisms of commensalism and patho-genesis are both essential to developing more effective therapies. Describing the interactions between Candida, bacteria (such as Lactobacillus spp.) and other fungi in the vagina is funda-mental to our characterization of the vaginal mycobiome.
Introduction
The community of fungal organisms residing within the lower female reproductive tract is referred to as the vaginal mycobiota. Among those is one of the leading causes of vaginal infection, Candida albicans, a well-studied fungal pathogen. Because vulvovaginal candidiasis (VVC) is the second most commonly reported form of infectious vaginitis, a great deal of effort has been invested in studying the mechanisms of C. albicans pathogenesis. However, the totality of fungal organisms present within the vagina has been grossly underappreciated. While we have extensive knowledge of the types of bacteria present in the vaginal milieu, very little is known about their fungal counterparts. Recent studies that explored the composition of fungi within the vagina have pointed to an exciting new frontier of research. Exploring the types, functional and compositional dynamics of fungal species in the context of the vaginal environment is an important objective, with potential implications for treating and preventing VVC, improving obstetric outcomes, and reproductive health in general. Recent advances in next-generation sequencing tools have enabled the high-throughput identification of fungi and provide burgeoning insights into fungal ecology.
Mycology of the vagina
The sum of the genomes and genes carried by fungal species that exist within a particular environmental or biological niche is termed the “mycobiome.”Citation1 In humans, the mycobiome is poorly understood compared to the microbiome, which was extensively described by the Human Microbiome Project — the largest and most comprehensive survey of bacterial taxa in healthy adults.Citation2-5 In the midst of an explosive new era of genomics, rapidly developing sequencing technologies and cutting-edge bioinformatics tools, the mycobiome has developed into its own “sub-specialty” within the field of microbial genomics, but one that lags far behind its bacterial counterpart.Citation6
The earliest studies of vaginal microbiology underestimated the complexity of this ecosystem, in part, due to the limitations of the culture-dependent techniques used.Citation7,8 While bacteria have long been known to dominate the vaginal milieu, leading to a number of studies on the bacterial community, early investigators of vaginal mycology have attempted to draw attention to the importance of fungal members of this community.Citation9
Using classical, culture-dependent methods, investigators have measured point prevalence of vaginal fungi in healthy volunteers and diabetics, as well as adolescents and pregnant women.Citation10-12 Fungi were recovered by culture in 20–60% of the samples. Without exception, the predominant member of the fungal community is identified as C. albicans (often >70%), though the rank prevalence for non-albicans species (including C. krusei, C. parapsilosis, C. tropicalis, C. glabrata, C. guilliermondii, C. pseudotropicalis, C. stellatoidea, and others) varies by population studied, geography, and cultivation methods. More recent epidemiological studies of Candida carriage report even greater representation by C. albicans, making up 85–95% of isolates recovered.Citation13-15
A study comparing Candida isolates from the United States and the United Kingdom concluded that physiological biotyping could not significantly distinguish the isolates examined.Citation16 According to the authors, the evidence does not support strain tropism — a selection for “vaginotropic” vs. “vaginopathic” strains — since strains isolated during infection are statistically identical to those collected in asymptomatic colonization.Citation16,17 Using these same biotyping methods Odds et alCitation17,18 reported consistency in isolates from different sites (and tissue types) in the same patient and isolates collected months apart. Using different methods, a separate group also reported that no significant differences exist among Candida strains isolated from different body sites; but added that a single host can be colonized by multiple species, and even multiple genotypes of the same species.Citation19
Using a cultivation-independent 18S rRNA gene clone library, Guo et alCitation20 identified 3 fungal phyla in vaginal samples: Ascomycota (22/28), of which Candida was the predominant genera, Basidiomycota (5/28), and Oomycota (1/28). They reported larger proportions of C. albicans and lower proportions of S. cerevisiae and uncultured (unidentified) fungi in women with allergic rhinitis and recurrent vaginal candidiasis, as compared to healthy controls. Fungal diversity was also increased in these patient populations compared to controls. The authors suggested that fungal dysbiosis might be correlated with the pathophysiology of recurrent vaginal candidiasis, highlighting a role for fungal community in disease.
Though next-generation sequencing technology has profoundly expanded our appreciation of the bacterial microbiota within the human vaginaCitation21, these sophisticated methods have been applied to the study of the vaginal mycobiota to a far lesser degree. In fact, the very first next-generation sequencing-based survey of fungal communities within the vagina was published in 2013 by a group whose aim was to describe the bacterial and fungal communities of women in Estonia.Citation22 This cross-sectional study sequenced the Internal Transcribed Spacer 1 (ITS1) in 251 vaginal samples from 294 healthy women to characterize fungal taxonomic composition. Fifty-eight percent of sequences belonged to the Ascomycota Division, though Basidiomycota was also represented in a small number of sequences (3%). Within Ascomycota, hits to Saccharomycetales (dominated by the genus Candida), Capnodiales, Eurotiales, Pleosporales, and Helotiales were observed. The resulting mean taxonomic richness for each sample was about 8 fungal Operational Taxonomic Units (OTUs). Candida OTUs could be detected in 70% of samples. Within the group of sequences mapping to Candida, C. albicans was not surprisingly predominant (68%). Strikingly, 161 unique hits at the species-level were obtained from this data set, yet 38% of OTUs were unspecified — no taxonomic assignment lower than kingdom was available. These results speak to a very serious problem in molecular-based fungal taxonomy. The quality and relatively low representation of fungal species in reference databases has direct consequences on quality and accuracy of taxonomic assignments returned. Current databases not only lack the rich volume of sequences that exist for bacterial 16S rRNA gene, but taxonomic synonyms and misclassifications are widespread.Citation23-25 To protect the integrity of these investigations, careful sequence annotation and database curation is absolutely essential. The results published by Drell et al,Citation22 nonetheless, reveal the underappreciated diversity of fungal members within the vaginal ecosystem and warrant follow-up on these findings. In light of our limited understanding of vaginal mycology, most of what we know derives from studies focused on the key player C. albicans.
Fungal commensalism in the vagina
Witkin and LedgerCitation26 described the characteristics and qualities of the human vaginal environment that make it both unique and complex. In contrast to oft-used animal models, the vagina of reproductive-age women is distinctly acidic (pH of 4.5 or less), owing to the presence of lactic-acid producing bacteria that thrive in this anaerobic nicheCitation27 not otherwise described in the Class Mammalia.Citation28 Further intricacies of the vaginal environment in humans include cycles of growth, production of glycogen and its breakdown products by human α-amylasesCitation29, and shedding of the epithelium in response to reproductive hormones, and primarily innate (as opposed to adaptive) mucosal immune protection.Citation30-32 These physiologic features are certainly key determinants of microbial colonization of the vaginal mucosa.Citation7,33
In 1976, Goplerud et al isolated Lactobacillus spp and C. albicans at consistently increased rates over all 3 trimesters in healthy pregnant women.Citation34 These early observations provided preliminary evidence for a positive correlation between estrogen levels and vaginal colonization of microbes. The data were further substantiated by Larsen and GalaskCitation35 who demonstrated obligate estrogenization to achieve yeast colonization in a rat model of vaginal Candidiasis. At present, all laboratory animal models of vaginal candidiasis require estrogen-treatment to establish colonization and subsequent infection.Citation36, 37 Some Candida species possess a cytosolic estrogen receptor that could mediate direct transcriptional responses to host hormones.Citation38 Furthermore, estrogen has been shown to disrupt neutrophil chemotaxis to the vaginal epitheliumCitation39 and inhibit Th17 cell differentiation,Citation40 resulting in heightened host vulnerability to pathogens, such as Candida. Clinical and anecdotal reports frequently link symptomatic Candida vaginitis to the luteal phase, just prior to menses, which is marked by both a high estrogen state and increased vaginal pH.Citation7 Researchers have documented enhanced C. albicans adherence to vaginal epithelial cells through estrogen signaling.Citation41 Further, while Candida is known for its broad pH-range tolerability, adherence to vaginal epithelial cells is significantly enhanced at pH 6 compared to pH 3–4.Citation7
The hormone-dependent production and accumulation of glycogen (and its breakdown productsCitation29) by human vaginal epithelial cells should not be understated in its contribution to fungal colonization,Citation42 however, Candida can utilize other nutrients (including lactate), making Candida highly adaptable to shifts in the nutritional microenvironment in the vagina.Citation7 The food source used by Candida in a particular niche is not a trivial detail, as studies have clearly indicated the effects of environmental cues, such as nutrient availability, on cell wall architectureCitation43-45 which impacts interactions between Candida and immune cells. Recent in vitro work has shown that in the presence of lactic acid (as the sole carbon source) C. albicans is taken up by macrophages less efficiently and can alter immune cell cytokine profiles, specifically by increasing IL-10 and decreasing IL-17 production.Citation46, 47 Interestingly, cells grown in a mixed lactate-glucose media behave more like lactate-grown cells. This has particular relevance in the vaginal context because of the abundance of both glycogen (and its breakdown sugar products) and lactic acid, which could effectively promote anti-inflammatory responses to Candida. Other studies have corroborated these findings and suggest that Candida may have evolved to curb immune responses to promote its own persistence and commensalism.Citation48 It has also been shown that Lactobacillus indoleamine 2,3-dioxygenase 1 (IDO1) in the gut leads to the production of tryptophan catabolites that act on regulatory T cells, resulting in increased local expression of IL-22Citation49 and immunoprotection to VVC.Citation50 This suggests that the bacterial microbiome could be mediating tolerance to C. albicans on the mucosa.
Attachment to the mucosal epithelium is mediated by binding to specific host receptors, of which the ALS (agglutinin-like sequence) adhesion family is best studied.Citation51-53 Hyphal formation is an important attachment factor as well.Citation54,55 In response to quorum sensing mechanisms, yeast growth is favored by high cell densities (>10 7 cells mL−1) whereas hyphal formation is stimulated by lower cell densities (<10 7 cells mL−1).Citation53 C. albicans adheres to vaginal and oral epithelial cells with greater levels than other speciesCitation56 — an important, but most likely partial, explanation for the predominance of C. albicans-associated infections. Interestingly, Sobel et alCitation57 reported marked person-to-person variability in Candida adherence to exfoliated vaginal epithelial cells, but enhanced attachment of C. albicans to epithelial cells from women with recurrent VVC.Citation58
Pathogenesis of Candida and vulvovaginal candidiasis
Despite its prominence as the second most common vaginal infection among US women of reproductive-age,Citation13,59 epidemiologic data on the incidence of VVC remains incomplete — primarily because this is not a reportable infection by public health authority standards.Citation60 And while exceptionally common—3 in 4 women will be affected at least once over their lifetimeCitation61—asymptomatic carriage rates for C. albicans in healthy women are estimated at around 20%.Citation12,62 Mechanisms of immunoprotection are still debated, and the factors that trigger the transition from commensal to pathogenic yeast are still obscure. However, it is generally accepted that predispositions for Candida growth/invasion are niche specificCitation63,64, though immune defects, breaches in epithelial integrity and microbial dysbiosis are common themes.Citation55 While many similarities exist between the mucosal environments of the mouth and vagina, the immunology of Candida vaginitis is undoubtedly distinct.Citation65,66 Excellent reviews have been written on the immunology of VVC, thus will not be discussed here.Citation67-69
A statistically significant increase was noted in number of C. albicans colonies cultured from swabs of women with VVC, representing an increase in fungal concentration, as compared to controls (healthy, no VVC), though no difference was measured with non-albicans Candida species.Citation70 Peeters et alCitation70 noted a positive correlation between the number of C. albicans colonies grown and amount/severity of vaginal discharge, as well as reported pruritus (itching). These findings support the theory of a fungal burden threshold above which inflammatory cells are recruited, resulting in the vaginal symptoms often reported, including itching, irritation, burning, and discharge.Citation68
Candida are polymorphic fungi, whose morphogenic transitions are essential mechanisms of pathogenesis in the human host.Citation71,72 The yeast form (blastoconidia) is typically associated with asymptomatic colonization, transmission or spread (particularly in the bloodstream)Citation60,73, while the hyphal (mycelial) form contributes mostly to adherence and mucosal invasion, characteristic of symptomatic disease.Citation57,60,74,75 Peters et alCitation76 recently noted that the genes which control C. albicans morphogenesis are required for the immunopathology associated with VVC. A variety of environmental stimuli affect a cell's morphology, including nutrient availability, pH, and temperature.Citation72 Using sophisticated quorum sensing mechanisms, C. albicans regulates morphogenesis in response to these external cues.Citation77
In vitro proteolytic activity of C. albicans isolates from women with symptomatic VVC was greater than isolates from asymptomatic carriers.Citation78 Proteolytic enzymes, namely the secreted aspartyl protease (SAP) family, are well-studied virulence factors employed by C. albicans, in particular, to invade the mucosal layer during VVC and induce immunopathology.Citation79-83 In 1940, it was proposed that the immunopathology of VVC is caused by a Candida toxin.Citation84 Decades later, researchers negated this hypothesis by suggesting Candida cell wall glycoproteins resemble bacterial endotoxins in their structural location, pyrogenicity and immunogenicity, though much less potent.Citation85 Earlier this year, however, Moyes et alCitation86 identified a secreted cytolytic peptide toxin produced by C. albicans that is essential for mucosal pathogenesis in a murine model of oropharyngeal candidiasis. The C. albicans extent of cell elongation 1 (ECE1) gene, which encodes the toxin they named Candidalysin,Citation86 is also among the most highly expressed genes during murine VVC.Citation87
Like many pathogenic bacteria, Candida species in general and C. albicans in particular, are efficient at biofilm formation within the human host.Citation88,89 C. albicans biofilms have been identified on dentures, catheters, as well as mucosal epithelia.Citation90 Contact sensing (contact with abiotic or host substrates) has been described as an important trigger for C. albicans biofilm formation.Citation91 Furthermore, yeast cells are stimulated to form hyphae upon contact with a surface,Citation91 which in some cases may facilitate active penetration of host tissuesCitation92,93 and in others may lead to mature biofilm formation.Citation94 Transcriptional regulation of biofilm formation has been mainly attributed to Bcr1, Tec1, and Efg1,Citation90 however, recent studies revealed novel transcription factors associated with this process: Ndt80, Rob1, Brg1.Citation95 Harriott et alCitation96 were the first to show using in vivo and ex vivo models of murine Candida vaginitis that C. albicans does, indeed, form Bcr1- and Efg1-dependent biofilms. Similar to other body sites, biofilms in the vagina are of major concern, as they have been implicated in immunopathology of VVC, anti-fungal treatment failures and recurrent infections.Citation97,98 Genomic microvariations in C. albicans, which include rearrangements, loss of heterozygosity, polymorphisms, and copy number variations, have also been associated with fungal persistence in a host and antifungal resistance.Citation99,100
Candida-Bacteria interactions in the vagina
As early as 1930, Doderlein's bacillus,Citation101 which is now widely known as Lactobacillus, was positively attributed to protection of the vaginal mucosa and a “healthy” microenvironment.Citation102 Because the lack of Lactobacillus spp. within the vaginal microbiota has been associated with susceptibility to urogenital infections, such as bacterial vaginosis,Citation103,104 HIV,Citation105 and urinary tract infection,Citation106 it stands to reason that Lactobacillus spp may, similarly, protect women from vulvovaginal candidiasis (VVC). Among others, Odds proposed in 1979 a model where symbiosis between some fungi and bacteria is established,Citation107,108 though these relationships have not been clearly elucidated within the vagina. Early bacterial-yeast co-culture experiments led to the hypothesis that the role of vaginal bacteria is not likely to prevent colonization of Candida but rather prevent their uninhibited proliferation.Citation35 In support of this hypothesis, short chain fatty acids and lactate produced by Lactobacillus spp. and other lactic-acid producing bacteria were shown to inhibit the yeast-to-hyphae switch in C. albicans.Citation109 Further, investigators have reported lower numbers of Lactobacillus spp in vaginal cultures from women with symptomatic VVC.Citation70,102 Supporting evidence for this hypothesis includes increased susceptibility to Candida vaginitis following antibiotic therapy, a well-documented risk factor for VVC.Citation110-112 But not all studies have substantiated the link between VVC and antibiotic usage,Citation113 and even anecdotal reports are inconsistent. This is consistent with the different types of vaginal microbiota which could be differentially affected by antibiotics.Citation114,115 Not all women who take antibiotics develop VVC and most women who report VVC have not recently taken antibacterial therapy. Colonization with Candida appears to be a prerequisite risk factor for developing VVC following antibiotic therapy.Citation116 Recently, however, one group has posited that their findings are more consistent with Lactobacillus being associated with greater risk for vulvovaginal candidiasis.Citation117 By elucidating the mechanism by which lactic acid suppresses immune responses to C. albicans, Ene et alCitation46 have supplied evidence for this claim.
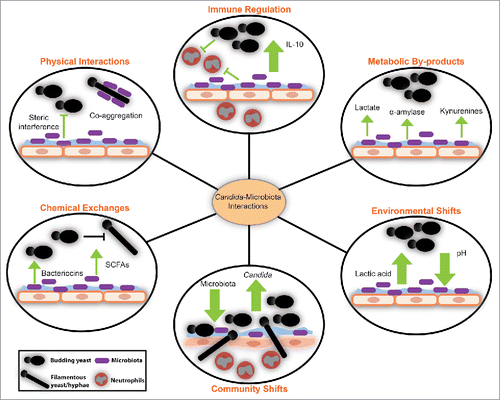
Candida-bacteria interactions within the vagina likely take place within the context of a polymicrobial biofilm on the epithelial surface.Citation118,119 An in vitro model of various C. albicans-bacterial biofilms concluded that bacteria negatively impact C. albicans biofilm formation by inhibiting fungal growth and suppressing genes responsible for hyphae formation.Citation120 Peleg et alCitation121 described 5 types of bacterial-fungal interactions and many speculate these also take place within the vagina: physical interactions,Citation122,123 chemical exchanges,Citation124 use of metabolic by-products,Citation109,118 changes in the environment,Citation124 and alteration of the host immune response.Citation125 Further studies are required to better understand this important relationship between vaginal bacteria, Candida spp. and other fungi in health and diseases.
Importance of the fungal mycobiome
It is becoming increasingly clear that fungal communities play a more significant role in human health and disease than once assumed.Citation126,127 Concerted effort, such as that given to surveying bacterial composition and abundance, is required to carry this field into the translational and clinical arenas. Characterization of the human mycobiome has the potential to produce widespread clinical advances in diagnosis, treatment and prevention of fungal infectionsCitation23,128 and vulvovaginal candidiasis, in particular, but also potentially bacterial and viral infections. Made possible by next-generation, culture-independent sequencing technologies, new developments in fungal contribution to human health and disease have proven to be very promising. Though far less abundant than bacterial members of the environment, fungi (but primarily Candida albicans) have a pronounced effect on vaginal health, and thus require more in depth studies of the interaction between the mycobiome and the microbiome. While pathogenic mechanisms attributed to single fungal species have consumed much of mycology, it is believed that mycobiome studies will establish correlation between composition and function of the entire fungal community, and cross-kingdom interactions to disease processes. Notably, fungi-fungi interactions have been implicated in the pathogenic process; C. glabrata binds to the hyphae of C. albicans to establish oropharyngeal candidiasis,Citation129 thus supporting the need for mycobiome studies that consider the full context in which infection takes place. Culture-based isolation and characterization of pathogens remains of great necessity, however, development of novel in vitro (and even in vivo) models of polymicrobial communities would be ideal to test hypotheses into the role of the vaginal mycobiome in health and disease. And while we invest scientific capital to understand pathogenic mechanisms, we must not neglect to appreciate mechanisms of commensalism, as this will likely lead to preventative strategies that prohibit the commensal-to-pathogen transition.Citation130
Figure 1. Interactions between Candida and microbiota at the mucosal interface have profound effects on the vaginal ecosystem.Citation109,120-125 Metabolites and small molecules made by the microbiota affect the metabolism and morphology of Candida species. Changes in microbiota relative abundance also impact the abundance of Candida and its ability to access the mucosal surface, where invasion occurs. In healthy states, when microbiota-derived lactic acid is produced, Candida can alter host cytokine production and promote anti-inflammatory signaling. The contribution of bacterial-fungal interactions to the ecology of the vaginal microbiota remains to be described.
Funding
This review was supported by the National Institute for Allergy and Infectious Diseases and the National Institute of Nursing Research of the National Institutes of Health under awards number U19AI084044, R01AI116799 and R01NR015495. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
References
- Ghannoum MA, Jurevic RJ, Mukherjee PK, Cui F, Sikaroodi M, Naqvi A, Gillevet PM. Characterization of the oral fungal microbiome (mycobiome) in healthy individuals. PLoS Pathogens 2010; 6:e1000713; PMID:20072605; http://dx.doi.org/10.1371/journal.ppat.1000713
- Gevers D, Knight R, Petrosino JF, Huang K, McGuire AL, Birren BW, Nelson KE, White O, Methe BA, Huttenhower C. The Human Microbiome Project: a community resource for the healthy human microbiome. PLoS Biol 2012; 10:e1001377; PMID:22904687; http://dx.doi.org/10.1371/journal.pbio.1001377
- Human Microbiome Project C. Structure, function and diversity of the healthy human microbiome. Nature 2012; 486:207-14; PMID:22699609; http://dx.doi.org/10.1038/nature11234
- Markowitz VM, Chen IM, Chu K, Szeto E, Palaniappan K, Jacob B, Ratner A, Liolios K, Pagani I, Huntemann M, et al. IMG/M-HMP: a metagenome comparative analysis system for the Human Microbiome Project. PloS One 2012; 7:e40151; PMID:22792232; http://dx.doi.org/10.1371/journal.pone.0040151
- Gevers D, Pop M, Schloss PD, Huttenhower C. Bioinformatics for the human microbiome project. PLoS Comput Biol 2012; 8:e1002779; PMID:23209389; http://dx.doi.org/10.1371/journal.pcbi.1002779
- Huffnagle GB, Noverr MC. The emerging world of the fungal microbiome. Trends Microbiol 2013; 21:334-41; PMID:23685069; http://dx.doi.org/10.1016/j.tim.2013.04.002
- Galask RP. Vaginal colonization by bacteria and yeast. Am J Obstet Gynecol 1988; 158:993-5; PMID:3284368; http://dx.doi.org/10.1016/0002-9378(88)90111-1
- Carter BJC. A study of the vaginal flora in the normal female. South Med J 1937; 30:298-304; http://dx.doi.org/10.1097/00007611-193703000-00012
- Carter B, Jones CP, Creadick RN, Parker RT, Turner V. The vaginal fungi. Ann N Y Acad Sci 1959; 83:265-79; PMID:13808012; http://dx.doi.org/10.1111/j.1749-6632.1960.tb40900.x
- Barousse MM, Van Der Pol BJ, Fortenberry D, Orr D, Fidel PL, Jr. Vaginal yeast colonisation, prevalence of vaginitis, and associated local immunity in adolescents. Sex Transm Infect 2004; 80:48-53; PMID:14755036; http://dx.doi.org/10.1136/sti.2002.003855
- Nowakowska D, Kurnatowska A, Stray-Pedersen B, Wilczynski J. Prevalence of fungi in the vagina, rectum and oral cavity in pregnant diabetic women: relation to gestational age and symptoms. Acta Obstet Gynecol Scand 2004; 83:251-6; PMID:14995920; http://dx.doi.org/10.1111/j.0001-6349.2004.0361.x
- Goldacre MJ, Milne LJ, Watt B, Loudon N, Vessey MP. Prevalence of Yeast and fungi other than Candida albicans in the vagina of normal young women. Br J Obstet Gynaecol 1981; 88:596-600; PMID:7248216; http://dx.doi.org/10.1111/j.1471-0528.1981.tb01214.x
- Sobel JD. Epidemiology and pathogenesis of recurrent vulvovaginal candidiasis. YMOB 1985; 152:924-35
- Sobel JD. Recurrent vulvovaginal candidiasis. A prospective study of the efficacy of maintenance ketoconazole therapy. N Engl J Med 1986; 315:1455-8; PMID:3537785; http://dx.doi.org/10.1056/NEJM19861204315-2305
- Landers DV, Wiesenfeld HC, Heine RP, Krohn MA, Hillier SL. Predictive value of the clinical diagnosis of lower genital tract infection in women. Am J Obstet Gynecol 2004; 190:1004-10; PMID:15118630; http://dx.doi.org/10.1016/j.ajog.2004.02.015
- Odds FC. Genital candidosis. Clin Exp Dermatol 1982; 7:345-54; PMID:7127883; http://dx.doi.org/10.1111/j.1365-2230.1982.tb02441.x
- Odds FC. Ecology and epidemiology of Candida species. Zentralbl Bakteriol Mikrobiol Hyg A 1984; 257:207-12; PMID:6385559
- Odds FC, Webster CE, Fisk PG, Riley VC, Mayuranathan P, Simmons PD. Candida species and C. albicans biotypes in women attending clinics in genitourinary medicine. J Med Microbiol 1989; 29:51-4; PMID:2657069; http://dx.doi.org/10.1099/00222615-29-1-51
- Xu J, Boyd CM, Livingston E, Meyer W, Madden JF, Mitchell TG. Species and genotypic diversities and similarities of pathogenic yeasts colonizing women. J Clin Microbiol 1999; 37:3835-43; PMID:10565893
- Guo R, Zheng N, Lu H, Yin H, Yao J, Chen Y. Increased diversity of fungal flora in the vagina of patients with recurrent vaginal candidiasis and allergic rhinitis. Microb Ecol 2012; 64:918-27; PMID:22767123; http://dx.doi.org/10.1007/s00248-012-0084-0
- Ma B, Forney LJ, Ravel J. Vaginal microbiome: rethinking health and disease. Annu Rev Microbiol 2012; 66:371-89; PMID:22746335; http://dx.doi.org/10.1146/annurev-micro-092611-150157
- Drell T, Lillsaar T, Tummeleht L, Simm J, Aaspollu A, Vain E, Saarma I, Salumets A, Donders GG, Metsis M. Characterization of the vaginal micro- and mycobiome in asymptomatic reproductive-age Estonian women. PloS One 2013; 8:e54379; PMID:23372716; http://dx.doi.org/10.1371/journal.pone.0054379
- Cui L, Morris A, Ghedin E. The human mycobiome in health and disease. Genome Med 2013; 5:63; PMID:23899327; http://dx.doi.org/10.1186/gm467
- Dollive S, Peterfreund GL, Sherrill-Mix S, Bittinger K, Sinha R, Hoffmann C, Nabel CS, Hill DA, Artis D, Bachman MA, et al. A tool kit for quantifying eukaryotic rRNA gene sequences from human microbiome samples. Genome Biol 2012; 13:R60; PMID:22759449
- Nilsson RH, Ryberg M, Kristiansson E, Abarenkov K, Larsson KH, Koljalg U. Taxonomic reliability of DNA sequences in public sequence databases: a fungal perspective. PloS One 2006; 1:e59; PMID:17183689; http://dx.doi.org/10.1371/journal.pone.0000059
- Witkin SS, Ledger WJ. Complexities of the Uniquely Human Vagina. Sci Transl Med 2012; 4:132fs11-fs11; PMID:22553249; http://dx.doi.org/10.1126/scitranslmed.3003944
- Linhares IM, Summers PR, Larsen B, Giraldo PC, Witkin SS. Contemporary perspectives on vaginal pH and lactobacilli. Am J Obstet Gynecol 2011; 204:120-e1-5; PMID:20832044; http://dx.doi.org/10.1016/j.ajog.2010.07.010
- Mirmonsef P, Gilbert D, Veazey RS, Wang J, Kendrick SR, Spear GT. A comparison of lower genital tract glycogen and lactic acid levels in women and macaques: implications for HIV and SIV susceptibility. AIDS Res Hum Retroviruses 2012; 28:76-81; PMID:21595610; http://dx.doi.org/10.1089/aid.2011.0071
- Spear GT, French AL, Gilbert D, Zariffard MR, Mirmonsef P, Sullivan TH, Spear WW, Landay A, Micci S, Lee BH, et al. Human α-amylase present in lower-genital-tract mucosal fluid processes glycogen to support vaginal colonization by Lactobacillus. J Infect Dis 2014; 210:1019-28; PMID:24737800; http://dx.doi.org/10.1093/infdis/jiu231
- Larsen B, Markovetz AJ, Galask RP. Spatial relationship of the vaginal microflora to the vaginal epithelium of female rats: scanning electron microscopy. App Environ Microbiol 1977; 34:80-7; PMID:889330
- Mirmonsef P, Hotton AL, Gilbert D, Burgad D, Landay A, Weber KM, Cohen M, Ravel J, Spear GT. Free glycogen in vaginal fluids is associated with Lactobacillus colonization and low vaginal pH. PloS One 2014; 9:e102467; PMID:25033265; http://dx.doi.org/10.1371/journal.pone.0102467
- Amjadi F, Salehi E, Mehdizadeh M, Aflatoonian R. Role of the innate immunity in female reproductive tract. Adv Biomed Res 2014; 3:1; PMID:24592358; http://dx.doi.org/10.4103/2277-9175.124626
- Brock M. Fungal metabolism in host niches. Curr Opin Microbiol 2009; 12:371-6; PMID:19535285; http://dx.doi.org/10.1016/j.mib.2009.05.004
- Goplerud CP, Ohm MJ, Galask RP. Aerobic and anaerobic flora of the cervix during pregnancy and the puerperium. Am J Obstet Gynecol 1976; 126:858-68; PMID:793391; http://dx.doi.org/10.1016/0002-9378(76)90674-8
- Larsen B, Galask RP. Influence of estrogen and normal flora on vaginal candidiasis in the rat. J Reprod Med 1984; 29:863-8; PMID:6394756
- Cassone A, Sobel JD. Experimental Models of Vaginal Candidiasis and Their Relevance to Human Candidiasis. Infect Immun 2016; 84:1255-61; PMID:26883592; http://dx.doi.org/10.1128/IAI.01544-15
- Fidel PL, Jr., Cutright J, Steele C. Effects of reproductive hormones on experimental vaginal candidiasis. Infect Immun 2000; 68:651-7; PMID:10639429; http://dx.doi.org/10.1128/IAI.68.2.651-657.2000
- Skowronski R, Feldman D. Characterization of an estrogen-binding protein in the yeast Candida albicans. Endocrinology 1989; 124:1965-72; PMID:2647470; http://dx.doi.org/10.1210/endo-124-4-1965
- Lasarte S, Samaniego R, Salinas-Munoz L, Guia-Gonzalez MA, Weiss LA, Mercader E, Ceballos-Garcia E, Navarro-Gonzalez T, Moreno-Ochoa L, Perez-Millan F, et al. Sex Hormones Coordinate Neutrophil Immunity in the Vagina by Controlling Chemokine Gradients. J Infect Dis 2016; 213:476-84; PMID:26238687; http://dx.doi.org/10.1093/infdis/jiv402
- Chen RY, Fan YM, Zhang Q, Liu S, Li Q, Ke GL, Li C, You Z. Estradiol inhibits Th17 cell differentiation through inhibition of RORgammaT transcription by recruiting the ERalpha/REA complex to estrogen response elements of the RORgammaT promoter. J Immunol 2015; 194:4019-28; http://dx.doi.org/10.4049/jimmunol.1400806
- Tarry W, Fisher M, Shen S, Mawhinney M. Candida albicans: the estrogen target for vaginal colonization. J Surg Res 2005; 129:278-82; PMID:16111702; http://dx.doi.org/10.1016/j.jss.2005.05.019
- McCourtie J, Douglas LJ. Relationship between cell surface composition of Candida albicans and adherence to acrylic after growth on different carbon sources. Infect Immun 1981; 32:1234-41; PMID:7019091
- Ene IV, Heilmann CJ, Sorgo AG, Walker LA, de Koster CG, Munro CA, Klis FM, Brown AJ. Carbon source-induced reprogramming of the cell wall proteome and secretome modulates the adherence and drug resistance of the fungal pathogen Candida albicans. Proteomics 2012; 12:3164-79; PMID:22997008; http://dx.doi.org/10.1002/pmic.201200228
- Ene IV, Adya AK, Wehmeier S, Brand AC, MacCallum DM, Gow NAR, Brown AJP. Host carbon sources modulate cell wall architecture, drug resistance and virulence in a fungal pathogen. Cell Microbiol 2012; 14:1319-35; PMID:22587014; http://dx.doi.org/10.1111/j.1462-5822.2012.01813.x
- Sosinska GJ, de Groot PW, Teixeira de Mattos MJ, Dekker HL, de Koster CG, Hellingwerf KJ, Klis FM. Hypoxic conditions and iron restriction affect the cell-wall proteome of Candida albicans grown under vagina-simulative conditions. Microbiology 2008; 154:510-20; PMID:18227255; http://dx.doi.org/10.1099/mic.0.2007/012617-0
- Ene IV, Cheng SC, Netea MG, Brown AJP. Growth of Candida albicans Cells on the Physiologically Relevant Carbon Source Lactate Affects Their Recognition and Phagocytosis by Immune Cells. Infect Immun 2012; 81:238-48; PMID:23115042; http://dx.doi.org/10.1128/IAI.01092-12
- Masson L, Salkinder AL, Olivier AJ, McKinnon LR, Gamieldien H, Mlisana K, Scriba TJ, Lewis DA, Little F, Jaspan HB, et al. Relationship between female genital tract infections, mucosal interleukin-17 production and local T helper type 17 cells. Immunology 2015; 146:557-67; PMID:26302175; http://dx.doi.org/10.1111/imm.12527
- Noverr MC, Phare SM, Toews GB, Coffey MJ, Huffnagle GB. Pathogenic yeasts Cryptococcus neoformans and Candida albicans produce immunomodulatory prostaglandins. Infect Immun 2001; 69:2957-63; PMID:11292712; http://dx.doi.org/10.1128/IAI.69.5.2957-2963.2001
- Zelante T, Iannitti RG, Cunha C, De Luca A, Giovannini G, Pieraccini G, Zecchi R, D'Angelo C, Massi-Benedetti C, Fallarino F, et al. Tryptophan catabolites from microbiota engage aryl hydrocarbon receptor and balance mucosal reactivity via interleukin-22. Immunity 2013; 39:372-85; PMID:23973224; http://dx.doi.org/10.1016/j.immuni.2013.08.003
- De Luca A, Carvalho A, Cunha C, Iannitti RG, Pitzurra L, Giovannini G, Mencacci A, Bartolommei L, Moretti S, Massi-Benedetti C, et al. IL-22 and IDO1 affect immunity and tolerance to murine and human vaginal candidiasis. PLoS Pathog 2013; 9:e1003486
- Chaffin WL. Candida albicans cell wall proteins. Microbiol Mol Biol Rev 2008; 72:495-544; PMID:18772287; http://dx.doi.org/10.1128/MMBR.00032-07
- Moyes DL, Richardson JP, Naglik JR. Candida albicans-epithelial interactions and pathogenicity mechanisms: scratching the surface. Virulence 2015; 6:338-46; PMID:25714110; http://dx.doi.org/10.1080/21505594.2015.1012981
- Mayer FL, Wilson D, Hube B. Candida albicans pathogenicity mechanisms. Virulence 2013; 4:119-28; PMID:23302789; http://dx.doi.org/10.4161/viru.22913
- Sobel JD, Muller G, Buckley HR. Critical role of germ tube formation in the pathogenesis of candidal vaginitis. Infect Immun 1984; 44:576-80; PMID:6327527
- Iliev ID, Underhill DM. Striking a balance: fungal commensalism versus pathogenesis. Curr Opin Microbiol 2013; 16:366-73; PMID:23756050; http://dx.doi.org/10.1016/j.mib.2013.05.004
- King RD, Lee JC, Morris AL. Adherence of Candida albicans and other Candida species to mucosal epithelial cells. Infect Immun 1980; 27:667-74; PMID:6991423
- Sobel JD, Myers PG, Kaye D, Levison ME. Adherence of Candida albicans to human vaginal and buccal epithelial cells. J Infect Dis 1981; 143:76-82; PMID:7012245; http://dx.doi.org/10.1093/infdis/143.1.76
- Trumbore DJ, Sobel JD. Recurrent vulvovaginal candidiasis: vaginal epithelial cell susceptibility to Candida albicans adherence. Obstet Gynecol 1986; 67:810-2; PMID:3517722; http://dx.doi.org/10.1097/00006250-198606000-00012
- Fleury FJ. Adult vaginitis. Clin Obstet Gynecol 1981; 24:407-38; PMID:7307366; http://dx.doi.org/10.1097/00003081-198106000-00008
- Sobel JD. Pathogenesis of Candida vulvovaginitis. Curr Topics Med Mycol 1989; 3:86-108; PMID:2688924; http://dx.doi.org/10.1007/978-1-4612-3624-5_5
- Hurley R, De Louvois J. Candida vaginitis. Postgrad Med J 1979; 55:645-7; PMID:523355; http://dx.doi.org/10.1136/pgmj.55.647.645
- Drake TE, Maibach HI. Candida and candidiasis. One. Cultural conditions, epidemiology and pathogenesis. Postgrad Med 1973; 53:83-7; PMID:4569505; http://dx.doi.org/10.1080/00325481.1973.11713368
- Rane HS, Hardison S, Botelho C, Bernardo SM, Wormley F, Jr., Lee SA. Candida albicans VPS4 contributes differentially to epithelial and mucosal pathogenesis. Virulence 2014; 5:810-8; PMID:25483774; http://dx.doi.org/10.4161/21505594.2014.956648
- Luna-Tapia A, Peters BM, Eberle KE, Kerns ME, Foster TP, Marrero L, Noverr MC, Fidel PL, Jr., Palmer GE. ERG2 and ERG24 Are Required for Normal Vacuolar Physiology as Well as Candida albicans Pathogenicity in a Murine Model of Disseminated but Not Vaginal Candidiasis. Eukaryot Cell 2015; 14:1006-16; PMID:26231054; http://dx.doi.org/10.1128/EC.00116-15
- Fidel PL, Jr. Distinct protective host defenses against oral and vaginal candidiasis. Medical Mycol 2002; 40:359-75; PMID:12230215; http://dx.doi.org/10.1080/714031126
- Yano J, Noverr MC, Fidel PL, Jr. Cytokines in the host response to Candida vaginitis: Identifying a role for non-classical immune mediators, S100 alarmins. Cytokine 2012; 58:118-28; PMID:22182685; http://dx.doi.org/10.1016/j.cyto.2011.11.021
- Fidel PL, Jr., Sobel JD. Immunopathogenesis of recurrent vulvovaginal candidiasis. Clin Microbiol Rev 1996; 9:335-48; PMID:8809464
- Fidel PL, Jr. Immunity in vaginal candidiasis. Curr Opin Infect Dis 2005; 18:107-11; PMID:15735412; http://dx.doi.org/10.1097/01.qco.0000160897.74492.a3
- Fidel PL, Jr. History and update on host defense against vaginal candidiasis. Am J Reprod Immunol 2007; 57:2-12; PMID:17156186; http://dx.doi.org/10.1111/j.1600-0897.2006.00450.x
- Peeters F, Snauwaert R, Segers J, Amery W, van Cutsem J. Observations on candidal vaginitis. Vaginal pH, microbiology, and cytology. Am J Obstet Gynecol 1972; 112:80-6; PMID:4621407; http://dx.doi.org/10.1016/0002-9378(72)90533-9
- Jacobsen ID, Wilson D, Wachtler B, Brunke S, Naglik JR, Hube B. Candida albicans dimorphism as a therapeutic target. Expert Rev Anti Infect Ther 2012; 10:85-93; PMID:22149617; http://dx.doi.org/10.1586/eri.11.152
- Biswas S, Van Dijck P, Datta A. Environmental sensing and signal transduction pathways regulating morphopathogenic determinants of Candida albicans. Microbiol Mol Biol Rev 2007; 71:348-76; PMID:17554048; http://dx.doi.org/10.1128/MMBR.00009-06
- Saville SP, Lazzell AL, Monteagudo C, Lopez-Ribot JL. Engineered control of cell morphology in vivo reveals distinct roles for yeast and filamentous forms of Candida albicans during infection. Eukaryot Cell 2003; 2:1053-60; PMID:14555488; http://dx.doi.org/10.1128/EC.2.5.1053-1060.2003
- Kimura LH, Pearsall NN. Relationship between germination of Candida albicans and increased adherence to human buccal epithelial cells. Infect Immun 1980; 28:464-8; PMID:6995309
- Sobel JD, Muller G. Ketoconazole in the prevention of experimental candidal vaginitis. Antimicrob Agents Chemother 1984; 25:281-2; PMID:6324671; http://dx.doi.org/10.1128/AAC.25.2.281
- Peters BM, Palmer GE, Nash AK, Lilly EA, Fidel PL, Jr., Noverr MC. Fungal morphogenetic pathways are required for the hallmark inflammatory response during Candida albicans vaginitis. Infect Immun 2014; 82:532-43; PMID:24478069; http://dx.doi.org/10.1128/IAI.01417-13
- Albuquerque P, Casadevall A. Quorum sensing in fungi–a review. Med Mycol 2012; 50:337-45; PMID:22268493; http://dx.doi.org/10.3109/13693786.2011.652201
- Cassone A, De Bernardis F, Mondello F, Ceddia T, Agatensi L. Evidence for a correlation between proteinase secretion and vulvovaginal candidosis. J Infect Dis 1987; 156:777-83; PMID:3309073; http://dx.doi.org/10.1093/infdis/156.5.777
- De Bernardis F, Agatensi L, Ross IK, Emerson GW, Lorenzini R, Sullivan PA, Cassone A. Evidence for a role for secreted aspartate proteinase of Candida albicans in vulvovaginal candidiasis. J Infect Dis 1990; 161:1276-83; PMID:2189009; http://dx.doi.org/10.1093/infdis/161.6.1276
- Al- Hedaithy SS. Spectrum and proteinase production of yeasts causing vaginitis in Saudi Arabian women. Med Sci Monitor 2002; 8:CR498-501
- Taylor BN, Staib P, Binder A, Biesemeier A, Sehnal M, Rollinghoff M, Morschhauser J, Schroppel K. Profile of Candida albicans-secreted aspartic proteinase elicited during vaginal infection. Infect Immun 2005; 73:1828-35; PMID:15731084; http://dx.doi.org/10.1128/IAI.73.3.1828-1835.2005
- Naglik JR, Rodgers CA, Shirlaw PJ, Dobbie JL, Fernandes-Naglik LL, Greenspan D, Agabian N, Challacombe SJ. Differential expression of Candida albicans secreted aspartyl proteinase and phospholipase B genes in humans correlates with active oral and vaginal infections. J Infect Dis 2003; 188:469-79; PMID:12870130; http://dx.doi.org/10.1086/376536
- Schaller M, Bein M, Korting HC, Baur S, Hamm G, Monod M, Beinhauer S, Hube B. The secreted aspartyl proteinases Sap1 and Sap2 cause tissue damage in an in vitro model of vaginal candidiasis based on reconstituted human vaginal epithelium. Infect Immun 2003; 71:3227-34; PMID:12761103; http://dx.doi.org/10.1128/IAI.71.6.3227-3234.2003
- Robertson WH. Mycology of vulvovaginitis. Am J Obstet Gynecol 1988; 158:989-91; PMID:3284367; http://dx.doi.org/10.1016/0002-9378(88)90109-3
- Cutler JE, Friedman L, Milner KC. Biological and chemical characterization of toxic substances from Candida albicans. Infect Immun 1972; 6:616-27; PMID:4564290
- Moyes DL, Wilson D, Richardson JP, Mogavero S, Tang SX, Wernecke J, Hofs S, Gratacap RL, Robbins J, Runglall M, et al. Candidalysin is a fungal peptide toxin critical for mucosal infection. Nature 2016; 532:64-8; PMID:27027296; http://dx.doi.org/10.1038/nature17625
- Bruno VM, Shetty AC, Yano J, Fidel PL, Jr., Noverr MC, Peters BM. Transcriptomic analysis of vulvovaginal candidiasis identifies a role for the NLRP3 inflammasome. mBio 2015; 6; PMID:25900651; http://dx.doi.org/10.1128/mBio.00182-15
- Silva S, Henriques M, Martins A, Oliveira R, Williams D, Azeredo J. Biofilms of non-Candida albicans Candida species: quantification, structure and matrix composition. Med Mycol 2009; 47:681-9; PMID:19888800; http://dx.doi.org/10.3109/13693780802549594
- Paiva LC, Vidigal PG, Donatti L, Svidzinski TI, Consolaro ME. Assessment of in vitro biofilm formation by Candida species isolates from vulvovaginal candidiasis and ultrastructural characteristics. Micron 2012; 43:497-502; PMID:22001373; http://dx.doi.org/10.1016/j.micron.2011.09.013
- Fanning S, Mitchell AP. Fungal biofilms. PLoS Pathogens 2012; 8:e1002585; PMID:22496639; http://dx.doi.org/10.1371/journal.ppat.1002585
- Kumamoto CA. Molecular mechanisms of mechanosensing and their roles in fungal contact sensing. Nat Rev Microbiol 2008; 6:667-73; PMID:18679170; http://dx.doi.org/10.1038/nrmicro1960
- Wachtler B, Wilson D, Haedicke K, Dalle F, Hube B. From attachment to damage: defined genes of Candida albicans mediate adhesion, invasion and damage during interaction with oral epithelial cells. PloS One 2011; 6:e17046; PMID:21407800; http://dx.doi.org/10.1371/journal.pone.0017046
- Dalle F, Wachtler B, L'Ollivier C, Holland G, Bannert N, Wilson D, Labruere C, Bonnin A, Hube B. Cellular interactions of Candida albicans with human oral epithelial cells and enterocytes. Cell Microbiol 2010; 12:248-71; PMID:19863559; http://dx.doi.org/10.1111/j.1462-5822.2009.01394.x
- Finkel JS, Mitchell AP. Genetic control of Candida albicans biofilm development. Nat Rev Microbiol 2011; 9:109-18; PMID:21189476; http://dx.doi.org/10.1038/nrmicro2475
- Nobile CJ, Fox EP, Nett JE, Sorrells TR, Mitrovich QM, Hernday AD, Tuch BB, Andes DR, Johnson AD. A recently evolved transcriptional network controls biofilm development in Candida albicans. Cell 2012; 148:126-38; PMID:22265407; http://dx.doi.org/10.1016/j.cell.2011.10.048
- Harriott MM, Lilly EA, Rodriguez TE, Fidel PL, Jr., Noverr MC. Candida albicans forms biofilms on the vaginal mucosa. Microbiology 2010; 156:3635-44; PMID:207056-67; http://dx.doi.org/10.1099/mic.0.039354-0
- Muzny CA, Schwebke JR. Biofilms: An Underappreciated Mechanism of Treatment Failure and Recurrence in Vaginal Infections. Clin Infect Dis 2015; 61:601-6; PMID:25935553; http://dx.doi.org/10.1093/cid/civ353
- Ramage G, Rajendran R, Sherry L, Williams C. Fungal biofilm resistance. Int J Microbiol 2012; 2012:528521; PMID:22518145; http://dx.doi.org/10.1155/2012/528-521
- Diogo D, Bouchier C, d'Enfert C, Bougnoux ME. Loss of heterozygosity in commensal isolates of the asexual diploid yeast Candida albicans. Fungal Genet Biol 2009; 46:159-68; http://dx.doi.org/10.1016/j.fgb.2008.11.005
- Coste A, Selmecki A, Forche A, Diogo D, Bougnoux ME, d'Enfert C, Berman J, Sanglard D. Genotypic evolution of azole resistance mechanisms in sequential Candida albicans isolates. Eukaryot Cell 2007; 6:1889-904; PMID:17693596; http://dx.doi.org/10.1128/EC.00151-07
- Cruickshank R. Doderlein's Vaginal Bacillus: A Contribution to the Study of the Lacto-Bacilli. J Hygiene 1931; 31:375-81; PMID:20475100; http://dx.doi.org/10.1017/S0022172400010901
- Auger P, Joly J. Microbial flora associated with Candida albicans vulvovaginitis. Obstet Gynecol 1980; 55:397-401; PMID:7360441
- Donders GG, Bosmans E, Dekeersmaecker A, Vereecken A, Van Bulck B, Spitz B. Pathogenesis of abnormal vaginal bacterial flora. Am J Obstet Gynecol 2000; 182:872-8; PMID:10764465; http://dx.doi.org/10.1016/S0002-9378(00)70338-3
- Pybus V, Onderdonk AB. Microbial interactions in the vaginal ecosystem, with emphasis on the pathogenesis of bacterial vaginosis. Microbes Infect 1999; 1:285-92; PMID:10602662; http://dx.doi.org/10.1016/S1286-4579(99)80024-0
- Lai SK, Hida K, Shukair S, Wang YY, Figueiredo A, Cone R, Hope TJ, Hanes J. Human immunodeficiency virus type 1 is trapped by acidic but not by neutralized human cervicovaginal mucus. J Virol 2009; 83:11196-200; PMID:19692470; http://dx.doi.org/10.1128/JVI.01899-08
- Gupta K, Stapleton AE, Hooton TM, Roberts PL, Fennell CL, Stamm WE. Inverse association of H2O2-producing lactobacilli and vaginal Escherichia coli colonization in women with recurrent urinary tract infections. J Infect Dis 1998; 178:446-50; PMID:9697725; http://dx.doi.org/10.1086/515635
- Odds FC. Candida and candidosis. Baltimore: University Park Press, 1979
- Segal E, Savage DC. Adhesion of Candida albicans to mouse intestinal mucosa in vitro: development of the assay and test of inhibitors. J Med Veterinary Mycol 1986; 24:477-9; PMID:3553522; http://dx.doi.org/10.1080/02681218680000751
- Noverr MC, Huffnagle GB. Regulation of Candida albicans morphogenesis by fatty acid metabolites. Infect Immun 2004; 72:6206-10; PMID:15501745; http://dx.doi.org/10.1128/IAI.72.11.6206-6210.2004
- Pirotta MV, Gunn JM, Chondros P. “Not thrush again!” Women's experience of post-antibiotic vulvovaginitis. Med J Aust 2003; 179:43-6; PMID:12831384
- Bluestein D, Rutledge C, Lumsden L. Predicting the occurrence of antibiotic-induced candidal vaginitis (AICV). Fam Pract Res J 1991; 11:319-26; PMID:1755351
- Sobel JD. Vulvovaginal candidosis. Lancet 2007; 369:1961-71; PMID:17560449; http://dx.doi.org/10.1016/S0140-6736(07)60917-9
- Glover DD, Larsen B. Relationship of fungal vaginitis therapy to prior antibiotic exposure. Infect Dis Obstet Gynecol 2003; 11:157-60; PMID:15022876; http://dx.doi.org/10.1080/10647440300025514
- Gajer P, Brotman RM, Bai G, Sakamoto J, Schutte UM, Zhong X, Koenig SS, Fu L, Ma ZS, Zhou X, et al. Temporal dynamics of the human vaginal microbiota. Sci Transl Med 2012; 4:132ra52; PMID:22553250; http://dx.doi.org/10.1126/scitranslmed.3003605
- Ravel J, Gajer P, Abdo Z, Schneider GM, Koenig SS, McCulle SL, Karlebach S, Gorle R, Russell J, Tacket CO, et al. Vaginal microbiome of reproductive-age women. Proc Natl Acad Sci U S A 2011; 108 Suppl 1:4680-7
- Pirotta MV, Garland SM. Genital Candida species detected in samples from women in Melbourne, Australia, before and after treatment with antibiotics. J Clin Microbiol 2006; 44:3213-7; PMID:16954250; http://dx.doi.org/10.1128/JCM.00218-06
- McClelland RS, Richardson BA, Hassan WM, Graham SM, Kiarie J, Baeten JM, Mandaliya K, Jaoko W, Ndinya-Achola JO, Holmes KK. Prospective study of vaginal bacterial flora and other risk factors for vulvovaginal candidiasis. J Infect Dis 2009; 199:1883-90; PMID:19456235; http://dx.doi.org/10.1086/599213
- Shirtliff ME, Peters BM, Jabra-Rizk MA. Cross-kingdom interactions: Candida albicans and bacteria. FEMS Microbiol Lett 2009; 299:1-8; PMID:19552706; http://dx.doi.org/10.1111/j.1574-6968.2009.01668.x
- Domingue PA, Sadhu K, Costerton JW, Bartlett K, Chow AW. The human vagina: normal flora considered as an in situ tissue-associated, adherent biofilm. Genitourin Med 1991; 67:226-31; PMID:2071125
- Park SJ, Han KH, Park JY, Choi SJ, Lee KH. Influence of bacterial presence on biofilm formation of Candida albicans. Yonsei Med J 2014; 55:449-58; PMID:24532517; http://dx.doi.org/10.3349/ymj.2014.55.2.449
- Peleg AY, Hogan DA, Mylonakis E. Medically important bacterial-fungal interactions. Nat Rev Microbiol 2010; 8:340-9; PMID:20348933; http://dx.doi.org/10.1038/nrmicro2313
- Klotz SA, Gaur NK, De Armond R, Sheppard D, Khardori N, Edwards JE, Jr., Lipke PN, El-Azizi M. Candida albicans Als proteins mediate aggregation with bacteria and yeasts. Med Mycol 2007; 45:363-70; PMID:17510860; http://dx.doi.org/10.1080/1369378070129-9333
- Holmes AR, McNab R, Jenkinson HF. Candida albicans binding to the oral bacterium Streptococcus gordonii involves multiple adhesin-receptor interactions. Infect Immun 1996; 64:4680-5; PMID:8890225
- Boris S, Barbes C. Role played by lactobacilli in controlling the population of vaginal pathogens. Microbes Infect 2000; 2:543-6; PMID:10865199; http://dx.doi.org/10.1016/S1286-4579(00)00313-0
- Romani L, Zelante T, De Luca A, Iannitti RG, Moretti S, Bartoli A, Aversa F, Puccetti P. Microbiota control of a tryptophan-AhR pathway in disease tolerance to fungi. Euro J Immunol 2014; 44:3192-200; PMID:25256754; http://dx.doi.org/10.1002/eji.201344406
- Rizzetto L, De Filippo C, Cavalieri D. Richness and diversity of mammalian fungal communities shape innate and adaptive immunity in health and disease. Euro J Immunol 2014; 44:3166-81; PMID:25257052; http://dx.doi.org/10.1002/eji.201344403
- Underhill DM, Lliev LD. The mycobiota: interactions between commensal fungi and the host immune system. Nat Rev Immunol 2014; 14:405-16; PMID:24854590; http://dx.doi.org/10.1038/nri3684
- Seed PC. The human mycobiome. Cold Spring Harbor Perspectives Med 2015; 5:a019810; http://dx.doi.org/10.1101/cshperspect.a019810
- Tati S, Davidow P, McCall A, Hwang-Wong E, Rojas IG, Cormack B, Edgerton M. Candida glabrata Binding to Candida albicans Hyphae Enables Its Development in Oropharyngeal Candidiasis. PLoS Pathogens 2016; 12:e1005522; PMID:27029023; http://dx.doi.org/10.1371/journal.ppat.1005522
- d'Enfert C. Hidden killers: persistence of opportunistic fungal pathogens in the human host. Curr Opin Microbiol 2009; 12:358-64; PMID:19541532; http://dx.doi.org/10.1016/j.mib.2009.05.008

