ABSTRACT
Dengue virus (DENV) infection can cause severe, life-threatening events, and no specific treatments of DENV infection are currently approved. Although thrombocytopenia is frequently observed in dengue patients, its pathogenesis is still not fully understood. Previous studies have suggested that DENV-induced thrombocytopenia occurs through viral-replication-mediated megakaryopoiesis inhibition in the bone marrow; however, the exact mechanism for megakaryopoiesis suppression remains elusive. In this study, a reductionist approach was applied, in which C57B/6J mice were inoculated with recombinant DENV-envelope protein domain III (DENV-EIII) instead of the full viral particle. Our results demonstrated that DENV-EIII-suppressed megakaryopoiesis is similar to those observed with DENV infection. Furthermore, in agreement with our in vivo analyses, DENV-EIII sufficiently suppressed the megakaryopoiesis of progenitor cells from murine bone marrow and human cord blood in vitro. Additional analyses suggested that autophagy impairment and apoptosis are involved in DENV-EIII-mediated suppression of megakaryopoiesis. These data suggest that, even without viral replication, the binding of DENV-EIII to the cell surface is sufficient to suppress megakaryopoiesis.
Introduction
Dengue virus (DENV) is transmitted by mosquitos, and infection causes dengue and severe dengue (also known as dengue hemorrhagic fever), with nearly 400 million reported cases annually.Citation1 Although thrombocytopenia is a frequently observed manifestation of DENV infection significantly associated with severe dengue patientsCitation2 and is one of the warning signs in the World Health Organization 2009 Guidelines,Citation3 its mechanism remains elusive.Citation4,5 The increased destruction of platelets in the peripheral blood and the decreased production of platelets in the bone marrow are 2 major causes of thrombocytopenia.Citation4,6
A previous report demonstrated that the monoclonal anti-nonstructural protein 1 (NS1) of DENV could cross-react with human fibrinogen, thrombocytes, and endothelial cells.Citation7 Anti-DENV NS1 antibodies could recognize protein on platelets and inhibit platelet aggregation, thus causing platelet lysis in the presence of complements.Citation8-10 DENV NS1 protein elicited platelet-bound immunoglobulins (Igs) accelerated clearance by phagocytes, complement-mediated lysis, and that activation of platelets, leading to the development of thrombocytopenia.Citation11 Studies have revealed that platelets were infected by DENV and produced dengue viral-like particles in patients with dengue.Citation12,13 All of these studies have demonstrated that thrombocytopenia can be induced by anti-DENV NS1 antibody elicited-platelet destruction or directly through DENV infected platelet induced-platelet dysfunction in the peripheral blood.
Megakaryocytes (MKs) are platelet precursor cells present in the bone marrow. DENV was observed to inhibit megakaryopoiesis by infecting and inducing the apoptosis of early megakaryocytic progenitors in vitro.Citation14 Consistently, thrombocytopenia resulting from the suppression of the bone marrow and platelet synthesis has been observed in both human patientsCitation15 and DENV-infected humanized mice,Citation16 respectively. Furthermore, direct infection of the bone marrow MKs by DENV has been demonstrated in a nonhuman primate model and in dengue patients,Citation17-19 and the production of viral particles in MK–erythrocyte progenitor cellsCitation20 has also been demonstrated. All of these evidences collectively suggest that DENV-infection-elicited thrombocytopenia is partly induced by megakaryopoiesis inhibition in the bone marrow, but the mechanism remains elusive.
The DENV virion is a spherical, enveloped virus with a diameter of approximately 50 nm. The virion contains 3 structural proteins [capsid (C), membrane (M), and envelop (E)] and the RNA genome. The E protein is the major protein on the virion surface. The E glycoprotein can be divided into 3 functional domains: the central domain (domain I; EI), the dimerization and fusion domain (domain II; EII), and the receptor-binding domain (domain III; EIII).Citation21 In the absence of direct viral infection, human hepatitis C virus envelope glycoprotein 2 can induce an inflammatory response and apoptosis in human umbilical vein endothelial cells.Citation22,23 However, whether the binding of DENV-EIII to the cell-surface receptors can similarly induce cellular signaling and toxicity remains unclear. The differentiation of haematopoietic progenitor cells, including MKs, is extremely sensitive to the perturbation of cellular signaling.Citation24-26 Accordingly, in this study, we hypothesized that DENV-EIII could suppress megakaryopoiesis. To test this hypothesis, the in vivo effect of DENV-EIII on megakaryopoiesis suppression was evaluated in a mouse model. A colony-forming unit (CFU) assay of MKs (CFU-MK assay) and megakaryocytic differentiation were performed in vitro using human cord blood-derived CD34+ cells and mouse bone marrow cells.
Materials and methods
Ethics statement
Cord blood cells were collected from full-term pregnancy cases at the Department of Obstetrics and Gynecology, Mennonite Christian Hospital, Hualien, Taiwan. Written, informed consent was provided by participants, and this study followed the protocols approved by the Research Ethics Committee of Hualien Tzu Chi Hospital, Buddhist Tzu Chi Medical Foundation (approval ID: IRB101–76). All experimental protocols related to animal studies were conducted in accordance with the national guidelines of the Animal Protection Act (Taiwan) and were approved by the Institutional Animal Care and Use Committee, Tzu Chi University (approval ID: 98026).
Virus preparation
The DENV-2 PL046 strain was amplified in a mosquito C6/36 cell line (ATCC CRL-1660), and the virus titer was determined through a plaque assay using BHK-21 cells (ATCC CCL-10) as described previously.Citation10,26,27
Construction and protein purification
The plasmid Den2E3/pET21b containing DENV-EIII (amino acids 578–674) was kindly provided by Prof. Yi-Ling Lin (Institute of Biomedical Sciences, Academia Sinica, Taiwan). To achieve a sufficient quantity and easy purification of the induction protein, a DNA fragment encoding DENV-EIII was subcloned into a pET28a expression vector (Novagen, Madison, WI) through EcoRI cutting. The BamHI cutting site was filled in to enable the in-frame construct to translate recombinant DENV-EIII protein fused to a 6-His-Tag. The plasmid Den2E3/pET28a was transformed into the Escherichia coli strain BL21 (DE3), which was induced to express recombinant protein at 37°C for 4 h by adding isopropyl β-D-1-thiogalactopyranoside at a 1 mM final concentration when the culture medium reached the mid-log phase (OD600 between 0.6–0.8). Recombinant DENV-EIII protein was purified using nickel-nitrilotriacetic acid (Ni-NTA) metal-affinity chromatography matrices (Qiagen, TAIGEN Bioscience, Taipei, Taiwan) under denaturing conditions, according to the manufacturer's instructions with some modifications. Briefly, the inclusion body containing DENV-EIII obtained after sonication in a lysis buffer (50 mM Tris-HCl, 50 mM NaCl, and 1 mM EDTA) was denatured by 8 M urea. The cell lysate and Ni-NTA resin were incubated in a binding buffer (8 M urea, 100 mM NaH2PO4, and 10 mM Tris-HCl; pH = 8) at room temperature for 1 h and were then loaded into a column and washed with a washing buffer (8 M urea, 100 mM NaH2PO4, and 10 mM Tris-HCl; pH = 6.3) containing 1% Triton X-114 (Sigma–Aldrich) to remove endotoxins.Citation27 The recombinant protein was eluted by an elution buffer (8 M urea, 100 mM NaH2PO4, and 10 mM Tris-HCl; pH = 4.5) and refolded using a linear 4–0 M urea gradient in a dialysis buffer (2 mM reduced glutathione, 0.2 mM oxidized glutathione, 80 mM glycine, 1 mM EDTA, 50 mM Tris-HCl, 50 mM NaCl, and 0.1 mM phenylmethylsulfonyl fluoride) at 4°C for 2–3 h. Glutathione-S transferase (GST) recombinant protein was used as the control protein. The expression plasmid pGEX-2KSCitation28,29 was transformed into the E. coli strain BL21 (DE3) and induced to express the protein, which was purified according to the protocol of our previous study.Citation30 Endotoxin contamination was verified through the Limulus Amoebocyte Lysate assay (Lonza), according to the manufacturer's instructions as described previously.Citation28,31
Antibodies generation and purification
Antibodies against DENV-EIII were generated and purified following the procedure detailed in a previous study with some modifications.Citation30 Protein G Sepharose 4 Fast Flow (GE Healthcare) was used to purify immunoglobulin G (IgG) from the serum of DENV-EIII immunized rabbits according to the manufactory's instructions. Eluted IgG was dialyzed using normal saline (0.9% NaCl) and concentrated using polyethylene glycol 20000. The IgG from preimmunized rabbit serum was purified using the same protocol.
In vivo assay
An in vivo assay was performed as described in our previous study with some modifications.Citation24 To test whether DENV-EIII has the ability to suppress megakaryopoiesis in vivo, DENV (0.6–1.2 × 105 PFU/mouse, 3–6.8 × 104 PFU/mL) in a range approximately equivalent to the average viral load in dengue patients (3.265 × 104 PFU/mL) was used.Citation32 C57BL/6J mice (males, 9–12 weeks) with a body weight of 25 g, who had approximately 1750–2000 μL of blood in circulation,Citation33 were treated with DENV (0.6–1.2 × 105 PFU/mouse) through a retro-orbital injection twice every 24 h or treated with recombinant GST or DENV-EIII protein (3 mg/kg, equal to 37.5–42.9 μg/ml in the circulation and functionally equivalent to 5 × 104 PFU DENV based on the competition analysis as described previously;Citation34 Fig. S1) through retro-orbital injection every 12 h for 3 cycles. Peripheral blood was collected at 24 and 48 h, and MKs were isolated from the bone marrow and analyzed as described previouslyCitation24 at 48 h after the first DENV, GST, or DENV-EIII injection. Mice treated with saline at the same volume for the same time course were used as controls.
MK differentiation from mouse bone marrow cells
Mouse bone marrow cell isolation and in vitro MK differentiation were performed as described previouslyCitation24 except that 100 ng/mL recombinant mouse thrombopoietin (rmTPO, PeproTech) was added to the differentiation medium. On Day 0 and Day 3, mouse bone marrow cells were treated with recombinant DENV-EIII (25 μg/mL; functionally equivalent to 3 × 104 PFU, approximately the dose that can trigger megakaryopoiesis suppression in vivo and block approximately 30% of the infection and replication of DENV in BHK21 cells; Fig. S1) or GST protein (25 μg/mL), and the cell surface marker CD41 and DNA content were analyzed on Day 6 as described previously.Citation24
Binding assay of DENV-EIII protein and MKs
MKs were isolated from the mouse bone marrow as described previously.Citation24 A total of 1 × 106 MKs were incubated with 25 μg of purified recombinant DENV-EIII protein at 4°C for 1 h in 250 μL of MK buffer with 3% bovine serum albumin (BSA) [Ca2+/Mg2+-free phosphate buffered saline (PBS) containing 3% BSA; 2.8 μM prostaglandin E1; 5.5 mM D-glucose; 10.2 mM tridodium citrate, pH 7.3]. After washing with PBS, cells were stained with anti-CD61 antibody conjugated with allophycocyanin, anti-DENV-EIII antibody, and the IgG-purified from preimmunized rabbit serum at 4°C for 30 min in 300 μL of MK buffer with 3% BSA. Cells were washed with PBS again, and then they were incubated with goat anti-rabbit IgG (H+L) F(ab’)2 fragment conjugated with fluorescein isothiocyanate at 4°C for 30 min in 300 μL of MK buffer with 3% BSA. After washing with PBS, cells were dissolved in 500 μL of PBS and analyzed using flow cytometry as described previously.Citation24
CFU-MK assay
The experimental protocol was performed according to the manufacturer's instructions (MegaCult-C, StemCell Technologies, Vancouver, Canada) and the procedures described in our previous publication.Citation24 Briefly, 1 × 105 mononuclear cells (MNCs) derived from human umbilical cord blood were seeded in a double-chamber slide containing cytokines for MK differentiation. MNCs were treated with DENV-EIII (25 μg/mL) and GST protein (25 μg/mL) and incubated at 37°C for 12 d. MK colonies were identified through anti-GPIIb/IIIa staining and counted through counterstaining with 1% Evans Blue.
MK differentiation from human cord blood CD34+ cells
The purification of human cord blood CD34+ cells and in vitro MK differentiation were performed according to our previous studyCitation24 with some modification. To obtain a large number of CD34+ cells for performing the 16-day MK differentiation, methylcellulose (3.75 g/L) was added for 4–5 d to slow the cell movement and increase the cell number.Citation35 CD34+ cells were treated with DENV-EIII (25 μg/mL) or GST protein (25 μg/mL) on Days 0, 4, 8, and 12 and were incubated with an MK differentiation medium. Flow cytometry was used to monitor MK differentiation and apoptosis on Day 16, as described previously.Citation24 Autophagic cells were stained using the Cyto-ID Autophagy Detection Kit (Enzo Life Science, NY, USA) on Day 16 and were analyzed using a flow cytometer (FACSCalibur, Becton–Dickinson, USA).
Statistics
The mean, standard deviation (SD), and statistics for the quantifiable data were calculated using Microsoft Office Excel 2003. Comparisons between groups were made using the 2-tailed Student t test. P values less than 0.05 were considered significant.
Results
DENV-EIII suppressed megakaryopoiesis in vivo
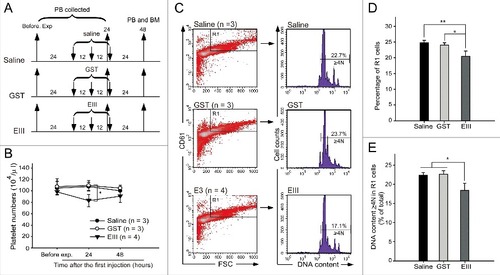
After treatment with DENV (0.6–1.2 × 105 PFU/mouse, a range approximately equivalent to the average viral load in dengue patientsCitation32), our analysis revealed that DENV could reduce the platelet number in the peripheral blood of mice ( and ), as previously reported.Citation36 The expression of specific surface markers (CD41, CD61, and CD42b) and polyploid were used to identify MKs.Citation37-39 Flow cytometry and propidium iodide (PI) staining revealed that the percentages of differentiated MK (large, CD61+ cells in the R1-gated region) and polyploid cell (DNA content ≥ 4N) populations in the R1 region were significantly suppressed in DENV-treated mice compared with saline-treated mice (, , and ), suggesting that DENV suppressed megakaryopoiesis in mice. To investigate whether DENV-EIII is sufficient to suppress megakaryopoiesis in mice, experimental mice were treated with DENV-EIII (3 mg/kg) every 12 h for 3 cycles (). Our data revealed that compared with saline and GST-treated groups, DENV-EIII-treated mice displayed significantly lower counts of R1 cells and a lower percentage of polyploid cells in the R1 region (, , and ); a similar response was observed in DENV-treated mice (, , and ). These results suggest that DENV-EIII-mediated suppression of megakaryopoiesis plays a crucial role in the DENV-induced negative impact on MKs in mice.
Figure 1. DENV suppressed megakaryopoiesis in vivo. The experimental outlines are indicated (A). C57BL/6J mice were retro-orbitally injected with DENV (0.6–1.2 × 105 PFU/mouse). The peripheral blood cells were collected, and the platelet counts were monitored at 24 h before the experiment and at 24 and 48 h after the first injection (B). Saline-treated groups were used as controls. Flow cytometry analyses were performed on bone marrow cells at 48 h (C) after the first injection. High-FSC (cell size) and CD61+ cells were gated as the R1 region. PI staining revealed the cellular DNA contents. The percentage of cells in the R1 region (D) and the percentages of polyploid cells (DNA content ≥ 4 N) in the R1 region are illustrated (E). Data are reported as the mean ± standard deviation (SD) and represent 3–5 independent experiments. ## P < 0.01 compared with the saline-treated groups
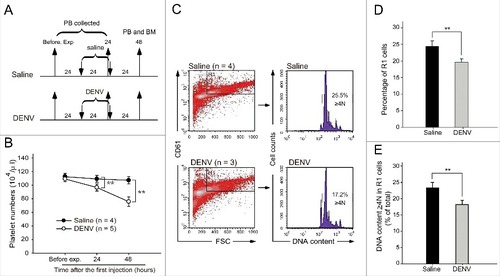
Figure 2. DENV-EIII suppressed megakaryopoiesis in vivo. The experimental outlines are indicated (A). C57BL/6J mice were retro-orbitally injected with DENV-EIII. Saline and recombinant GST protein were used as controls. The peripheral blood cells were collected and the platelet counts were monitored at 24 h before the experiment and at 24 and 48 h after the first injection (B). Flow cytometry analyses were performed on BM cells at 48 h (C) after the first injection. The percentage of cells in the R1 region (D) and the percentages of polyploid cells (DNA content ≥ 4 N) in R1 region are illustrated (E). Data are reported as the mean ± SD and represent 3–4 independent experiments. #P < 0.05, ##P < 0.01 compared with the indicated groups
DENV-EIII suppressed megakaryopoiesis from mouse bone marrow cells
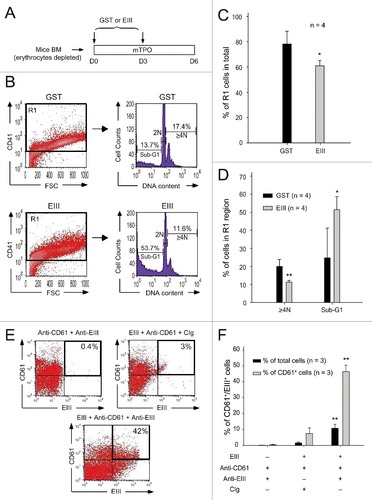
To investigate whether DENV-EIII exerted a suppressive effect on murine MKs, we performed in vitro megakaryocytic differentiation by using erythrocyte-depleted mouse bone marrow cells, according to a described previously method.Citation24 Treatment with DENV-EIII (25 μg/mL) reduced the percentage of the CD41+ cell population ( and ; R1-gated region). DNA content analysis using PI staining revealed that compared with GST, DENV-EIII reduced the percentage of polyploid cells and increased the percentage of sub-G1 cells significantly ( and ). This suggests that DENV-EIII suppresses megakaryopoiesis in primary cultured mouse haematopoietic progenitor cells present in the bone marrow. To investigate whether DENV-EIII can directly bind MKs, murine MKs purified from the bone marrow were incubated with DENV-EIII at 4°C for 1 h. Then, they were stained with anti-CD61 antibody to identify MKs and stained with anti-DENV-EIII antibody (Western blot analysis revealed that anti-DENV-EIII antibody specifically bound to DENV-EIII, Fig. S2). After flow cytometry analysis, the data revealed that the percentages of CD61 and DENV-EIII double positive cells are significantly increased when compared with controls without adding DENV-EIII or anti-DENV-EIII antibodies ( and ). This indicated that DENV-EIII could bind directly on the surface of MKs.
Figure 3. DENV-EIII killed differentiated MKs derived from mouse bone marrow. Murine bone marrow cells (erythrocyte-depleted fraction) were isolated and triggered to differentiate into MKs in the presence of mouse thrombopoietin. The GST protein was used as a control. DENV-EIII or GST protein was added on Day 0 and Day 3, and data were analyzed on Day 6 through flow cytometry (A). Cells were stained with CD41 antibodies, and CD41+ cells were gated to analyze DNA content through PI staining (B). The percentages of R1 cells among all analyzed cells (C), and the percentages of polyploid cells (DNA content ≥ 4N) and sub-G1 hypoploid cells in the R1 region are shown (D). Murine MKs were isolated from bone marrow, incubated with DENV-EIII proteins for 1 h, and stained with antibodies against DENV-EIII and CD61. IgG isolated from preimmunized rabbit serum served as control Ig (CIg). Cells were divided into 3 groups: anti-CD61 + anti-EIII, EIII + anti-CD61 + CIg, and EIII + anti-CD61 + anti-EIII. The percentages of CD61+/DENV-EIII+ double positive cells among the total cells and total CD61+ cells were analyzed (E) and quantified (F). Data are reported as the mean ± SD and represent 4 ((C)and D) and 3 (F) independent experiments. #P < 0.05, ##P < 0.01 compared with the GST-treated groups (C, D). ##P < 0.01 compared with the anti-CD61 + anti-EIII groups (F)
DENV-EIII suppressed megakaryopoiesis in a CFU-MK assay
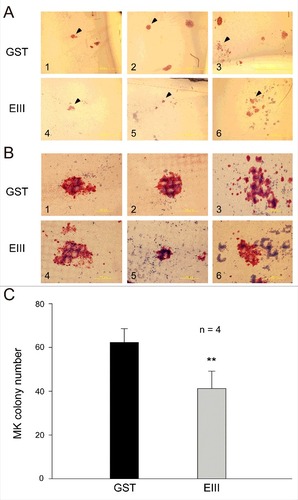
Compared with murine bone marrow, human umbilical cord blood is a relatively more accessible resource for haematopoietic progenitor cells. A CFU-MK assay was performed to investigate whether DENV-EIII has the ability to suppress megakaryopoiesis in human haematopoietic progenitor cells. Our data revealed that compared with GST-treated control groups, DENV-EIII-treated groups showed a significant reduction in the number of MK colonies (). These results suggest that DENV-EIII can suppress megakaryopoiesis in primary human haematopoietic progenitor cells.
Figure 4. DENV-EIII suppressed megakaryopoiesis in the CFU-MK assay. Mononuclear cells isolated from human umbilical cord blood were used in the CFU-MK assay. The morphology (A–B) and quantitative numbers (C) of MK colonies after GST (A1–A3 and B1–B3) and DENV-EIII (A4–A6 and B4–B6) treatments are shown. Arrowheads indicating specific colonies in the low magnification images (A1–A6) are highlighted in the high magnification photographs (B1–B6). Scale bar: 2 mm (A), 200 μm (B). Data are presented as the mean ± SD and represent 4 independent experiments. ## P < 0.01 compared with the GST-treated groups
DENV-EIII suppressed megakaryopoiesis in human cord blood CD34+ cells by autophagy impairment and apoptosis
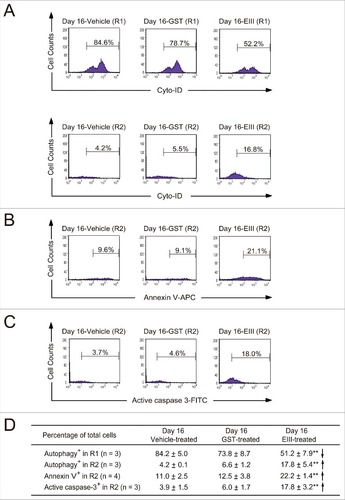
Next, we investigated the mechanism through which DENV-EIII treatments lead to megakaryopoiesis suppression. Megakaryocytic differentiation was performed using human umbilical cord blood-derived CD34+ cells in a 16-day experiment (). Consistent with a previous report,Citation24 the cell size (FSC) and cellular granularity (SSC) were increased after the induction of differentiation (; Day 0 vs. Day 16-Vehicle). Compared with the vehicle and GST treatments, DENV-EIII decreased the cell number in the R1 region and increased the cell number in the R2 region (, , and ). Flow cytometry and PI staining revealed that the percentages of CD61+ (premature MK), CD61+/CD42b+ (mature MK), and sub-G1 cells in the R2 region were increased significantly in the DENV-EIII-treated groups compared with the vehicle and GST-treated groups (, , and ). These results suggest that DENV-EIII suppresses megakaryopoiesis partly by initiating cell death in premature and mature MKs. Recent studies have revealed that autophagy plays an essential role in megakaryocytic differentiation, and the loss of autophagy leads to failure in megakaryopoiesis.Citation40,41 Our data revealed that the percentage of autophagic cells was decreased in the R1 region and increased in the R2 region in the DENV-EIII-treated groups compared with the vehicle-treated and GST-treated groups ( and ). In addition, the intensity of Cyto-ID in the R2 region was lower than that in the R1 region in the DENV-EIII treated groups [, Day 16-EIII (R1) vs. Day 16-EIII (R2)]. These results indicate that DENV-EIII triggers impaired autophagy. Furthermore, to verify whether the cell death of these CD61+ (premature MK), CD61+/CD42b+ (mature MK), and autophagy-impaired cells was associated with apoptosis, annexin V and activated caspase-3 staining was performed in flow cytometry. Our results revealed that compared with the vehicle and GST treatments, DENV-EIII produced significantly more annexin V+ and active caspase-3+ cells in the R2 region (, , and ). Taken together, our data indicate that DENV-EIII suppresses megakaryopoiesis in human cord blood CD34+ cell cultures through autophagy impairment and apoptosis.
Figure 5. DENV-EIII suppressed cytokines-triggered megakaryopoiesis by killing differentiated cells in umbilical cord blood-derived CD34+ cells. A 16-day experiment on megakaryocytic differentiation was performed using expanded umbilical cord blood-derived CD34+ cells. DENV-EIII was added on Days 0, 4, 8, and 12, and flow cytometry data were analyzed on day 16 (A). The vehicle and GST protein were used as controls. The FSC and SSC are shown (B). The cell numbers in the R1 and R2 regions (C) and the percentages of CD61- and CD61/CD42b-expressing cells in the R2 region (D) are shown. PI staining revealed the cellular DNA contents, and the sub-G1 hypoploid cells were increased in the DENV-EIII-treated groups (E). Summarized events of (C), (D) and (E) are shown in (F) and (G), respectively. Data are reported as the mean ± SD and represent 3–5 independent experiments. # P < 0.05, ## P < 0.01 compared with the vehicle-treated groups
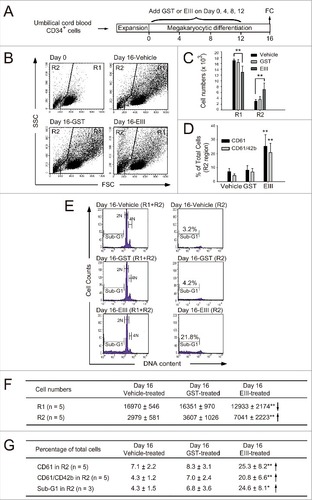
Figure 6. DENV-EIII killed differentiated MKs in umbilical cord blood–derived CD34+ cells through impaired autophagy and apoptosis. The 488-nm-excitable Cyto-ID green fluorescent reagents were used to monitor the autophagic cells in the R1 and R2 regions (A). Annexin V-allophycocyanin (B) and an antibody against activated caspase-3 conjugated with fluorescein isothiocyanate (C) were used to investigate the apoptotic changes in DENV-EIII-treated cells through flow cytometry. Summarized events are shown in (D). Data are reported as the mean ± SD and represent 3–4 independent experiments. ## P < 0.01 compared with the vehicle-treated groups
Discussion
Thrombocytopenia is an important manifestation of DENV infection and is associated with higher mortality;Citation2 however, the mechanism remains unclear.Citation4 Bone marrow suppression is one possible mechanism contributing to thrombocytopenia.Citation4,15 Studies have reported that DENV can directly infect MKs in the bone marrow of humans and monkeysCitation17-20 and interfere with megakaryopoiesis.Citation16 However, how DENV elicits thrombocytopenia and interferes with megakaryopoiesis and whether viral replication is required for the suppression remain elusive. Our data revealed that DENV-EIII suppresses megakaryopoiesis independent of viral infection, and DENV-EIII-suppressed megakaryopoiesis is associated with autophagy impairment and the apoptosis of differentiated MKs ( and ). These findings suggest that DENV-induced thrombocytopenia is partly mediated by the suppressive effect of DENV-EIII on megakaryopoiesis.
Theoretically, the presence of additional mechanisms, such as the infection and replication of DENV in MKs and/or the nearby supporting bone marrow stroma cells interfering with MK differentiation and cell survival, remains possible.Citation4 However, unlike humans, mice are not native hosts of DENV; therefore, DENV cannot normally replicate in wild type mice unless the mice are deficient in interferon (IFN) α/β and γ receptors or type I IFN receptors.Citation42,43 We demonstrated that DENV can't infect and replicate on mouse MKs (Fig. S3). Our data suggested that DENV-EIII can directly bind MKs ( and ) and that DENV-EIII alone is sufficient to elicit MK suppression and decrease platelet numbers, similarly to the in vivo effect of DENV ( and , 24 hr). The result is consistent with the in vitro data obtained from mouse () and human cells (). Our reductionist approach (use of mouse cells/mouse models) supports a simple way to focus on the effect of DENV-EIII on megakaryopoiesis irrespective of any viral replication; however, the approach can't totally mimic real DENV infections especially in vivo models. DENV cannot replicate in mice, DENV nonetheless induces elicitation of proinflammatory cytokines such as TNF-α,Citation36 which is well known to elicit thrombocytopenic responses.Citation44,45 This is probably the reason why DENV injections have a higher potency than DENV-EIII on the elicitation of thrombocytopenic response ( vs. , 48 hr).
Previous studies have demonstrated that impaired autophagy led to apoptotic death in various cell types, including MKs.Citation41,46 The deficiency of Atg7 resulted in mitochondrial damage, followed by apoptosis in mouse erythrocytes and mature T lymphocytes.Citation47 Similar to data from previous publications, our data showed that DENV-EIII-impaired autophagy is associated with apoptosis in differentiated MKs (). Further investigation is warranted to elucidate the detailed mechanism through which DENV-EIII induces impaired autophagy and apoptosis.
Currently, some live attenuated dengue vaccines are being assessed in clinical trials.Citation48-50 DENV-EIII is the protein subdomain responsible for DENV binding to target cells;Citation21 therefore, DENV-EIII is a feasible vaccine candidate to elicit protective antibodies.Citation51 A DENV-EIII-specific epitope-recognizing antibody that ameliorated the clinical symptoms of infection for all 4 serotypes of DENV was demonstrated in a humanized mouse model.Citation52 All 4 serotypes of DENV induce thrombocytopenia, and the sequences of DENV-EIII share high homology, with an estimated 68–87% similarity.Citation53 Therefore, use of the DENV-EIII subunit vaccine to produce neutralizing antibodies or treatment with the small-molecule DENV-EIII antagonist can theoretically not only block DENV infection but also ameliorate DENV-EIII-induced megakaryopoiesis suppression and could be a feasible approach to managing severe dengue.
In conclusion, this study provides a new prospect that DENV-EIII can be a virulence factor for suppressing megakaryopoiesis. DENV-EIII induced the cell death of differentiated MKs through autophagy impairment and apoptosis. DENV-EIII-induced megakaryopoiesis suppression is one of causes of the thrombocytopenia observed in DENV infection. Therefore, DENV-EIII could be considered a drug target to treat MK defects and thrombocytopenia in severe dengue.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Author contributions
GLL, TSL, PKC, HC, and MTS performed the experiments and analyzed the data. HHC and DSS conceived, designed the experiments and wrote the paper. CYL provided human umbilical cord bloods.
KVIR_S_1343769.docx
Download MS Word (402.5 KB)Acknowledgments
The authors are grateful to Prof. Nien-Tsung Lin (Department of Immunology, Tzu Chi University, Taiwan) for kindly providing E. coli stain BL21 (DE3) and Prof. Yi-Ling Lin (Institute of Biomedical Sciences, Academia Sinica, Taiwan) for generously providing the plasmid Den2E3/pET21b to reconstruct the Den2E3/pET28a plasmid for generation Den2E3 recombinant protein. We wish to appreciate Dr. Chi-Yuan Liao and the teams in Department of Obstetrics and Gynecology of Mennonite Christian Hospital for providing human umbilical cord bloods. We also thank Prof. Ming-Hseng Wang and his team (Experimental Animal Center, Tzu Chi University) for their help in maintaining experimental animals and pathogen-free environments. We thank Jhen-Cheng Wu, Tai-Hung Chen, and Chen-Ru Li for helping with the production of anti-DENV-EIII antibodies and the purification of CIg.
Funding
This work was supported by the grants of Tzu Chi Foundation (TCIRP 98001-05 and TCIRP 101001-03) and Ministry of Science and Technology (MOST 105-2311-B-320-001-MY3).
References
- Bhatt S, Gething PW, Brady OJ, Messina JP, Farlow AW, Moyes CL, Drake JM, Brownstein JS, Hoen AG, Sankoh O, et al. The global distribution and burden of dengue. Nature 2013; 496:504-7; PMID:23563266; https://doi.org/https://doi.org/10.1038/nature12060
- Huy NT, Van Giang T, Thuy DH, Kikuchi M, Hien TT, Zamora J, Hirayama K. Factors associated with dengue shock syndrome: a systematic review and meta-analysis. PLoS Negl Trop Dis 2013; 7:e2412; PMID:24086778; https://doi.org/https://doi.org/10.1371/journal.pntd.0002412
- WHO Guidelines Approved by the Guidelines Review Committee. Dengue: Guidelines for Diagnosis, Treatment, Prevention and Control: New Edition. Geneva, 2009
- de Azeredo EL, Monteiro RQ, de-Oliveira Pinto LM. Thrombocytopenia in Dengue: Interrelationship between Virus and the Imbalance between Coagulation and Fibrinolysis and Inflammatory Mediators. Mediators Inflamm 2015; 2015:313842; PMID:25999666; https://doi.org/https://doi.org/10.1155/2015/313842
- Martina BE, Koraka P, Osterhaus AD. Dengue virus pathogenesis: an integrated view. Clin Microbiol Rev 2009; 22:564-81; PMID:19822889; https://doi.org/https://doi.org/10.1128/CMR.00035-09
- Assinger A. Platelets and infection - an emerging role of platelets in viral infection. Frontiers Immunol 2014; 5:649; PMID:25566260; https://doi.org/https://doi.org/10.3389/fimmu.2014.00649
- Falconar A. The dengue virus nonstructural-1 protein (NS1) generates antibodies to common epitopes on human blood clotting, integrin/adhesin proteins and binds to human endothelial cells: potential implications in haemorrhagic fever pathogenesis. Arch Virol 1997; 142:897-916; PMID:9191856; https://doi.org/https://doi.org/10.1007/s007050050127
- Lin CF, Lei HY, Liu CC, Liu HS, Yeh TM, Wang ST, Yang TI, Sheu FC, Kuo CF, Lin YS. Generation of IgM anti-platelet autoantibody in dengue patients. J Med Virol 2001; 63:143-9; PMID:11170051; https://doi.org/https://doi.org/10.1002/1096-9071(20000201)63:2%3c143::AID-JMV1009%3e3.0.CO;2-L
- Lin CF, Lei HY, Liu CC, Liu HS, Yeh TM, Anderson R, et al. Patient and mouse antibodies against dengue virus nonstructural protein 1 cross-react with platelets and cause their dysfuction or depletion. Am J Infect Dis 2008; 4:69-75; https://doi.org/https://doi.org/10.3844/ajidsp.2008.69.75
- Cheng HJ, Lei HY, Lin CF, Luo YH, Wan SW, Liu HS, Yeh TM, Lin YS. Anti-dengue virus nonstructural protein 1 antibodies recognize protein disulfide isomerase on platelets and inhibit platelet aggregation. Mol Immunol 2009; 47:398-406; PMID:19822367; https://doi.org/https://doi.org/10.1016/j.molimm.2009.08.033
- Sun DS, King CC, Huang HS, Shih YL, Lee CC, Tsai WJ, Yu CC, Chang HH. Antiplatelet autoantibodies elicited by dengue virus non-structural protein 1 cause thrombocytopenia and mortality in mice. J Thromb Haemost 2007; 5:2291-9; PMID:17958746; https://doi.org/https://doi.org/10.1111/j.1538-7836.2007.02754.x
- Simon AY, Sutherland MR, Pryzdial EL. Dengue virus binding and replication by platelets. Blood 2015; 126:378-85; PMID:25943787; https://doi.org/https://doi.org/10.1182/blood-2014-09-598029
- Noisakran S, Gibbons RV, Songprakhon P, Jairungsri A, Ajariyakhajorn C, Nisalak A, Jarman RG, Malasit P, Chokephaibulkit K, Perng GC. Detection of dengue virus in platelets isolated from dengue patients. Southeast Asian J Trop Med Public Health 2009; 40:253-62; PMID:19323010
- Basu A, Jain P, Gangodkar SV, Shetty S, Ghosh K. Dengue 2 virus inhibits in vitro megakaryocytic colony formation and induces apoptosis in thrombopoietin-inducible megakaryocytic differentiation from cord blood CD34+ cells. FEMS Immunol Med Microbiol 2008; 53:46-51; PMID:18371071; https://doi.org/https://doi.org/10.1111/j.1574-695X.2008.00399.x
- La Russa VF, Innis BL. Mechanisms of dengue virus-induced bone marrow suppression. Baillieres Clin Haematol 1995; 8:249-70; PMID:7663049; https://doi.org/https://doi.org/10.1016/S0950-3536(05)80240-9
- Sridharan A, Chen Q, Tang KF, Ooi EE, Hibberd ML, Chen J. Inhibition of megakaryocyte development in the bone marrow underlies dengue virus-induced thrombocytopenia in humanized mice. J Virol 2013; 87:11648-58; PMID:23966397; https://doi.org/https://doi.org/10.1128/JVI.01156-13
- Clark KB, Noisakran S, Onlamoon N, Hsiao HM, Roback J, Villinger F, Ansari AA, Perng GC. Multiploid CD61+ cells are the pre-dominant cell lineage infected during acute dengue virus infection in bone marrow. PloS One 2012; 7:e52902; PMID:23300812; https://doi.org/https://doi.org/10.1371/journal.pone.0052902
- Noisakran S, Onlamoon N, Hsiao HM, Clark KB, Villinger F, Ansari AA, Perng GC. Infection of bone marrow cells by dengue virus in vivo. Exp Hematol 2012; 40:250-9 e4; https://doi.org/https://doi.org/10.1016/j.exphem.2011.11.011
- Noisakran S, Onlamoon N, Pattanapanyasat K, Hsiao HM, Songprakhon P, Angkasekwinai N, Chokephaibulkit K, Villinger F, Ansari AA, Perng GC. Role of CD61+ cells in thrombocytopenia of dengue patients. Int J Hematol 2012; 96:600-10; PMID:22987294; https://doi.org/https://doi.org/10.1007/s12185-012-1175-x
- Clark KB, Hsiao HM, Bassit L, Crowe JE, Jr, Schinazi RF, Perng GC, Villinger F. Characterization of dengue virus 2 growth in megakaryocyte-erythrocyte progenitor cells. Virology 2016; 493:162-72; PMID:27058763; https://doi.org/https://doi.org/10.1016/j.virol.2016.03.024
- Whitehead SS, Blaney JE, Durbin AP, Murphy BR. Prospects for a dengue virus vaccine. Nat Rev Microbiol 2007; 5:518-28; PMID:17558424; https://doi.org/https://doi.org/10.1038/nrmicro1690
- Balasubramanian A, Munshi N, Koziel MJ, Hu Z, Liang TJ, Groopman JE, Ganju RK. Structural proteins of Hepatitis C virus induce interleukin 8 production and apoptosis in human endothelial cells. J Gen Virol 2005; 86:3291-301; PMID:16298974; https://doi.org/https://doi.org/10.1099/vir.0.81056-0
- Urbaczek AC, Ribeiro LC, Ximenes VF, Afonso A, Nogueira CT, Generoso WC, Alberice JV, Rudnicki M, Ferrer R, Fonseca LM, et al. Inflammatory response of endothelial cells to hepatitis C virus recombinant envelope glycoprotein 2 protein exposure. Mem Inst Oswaldo Cruz 2014; 109(6):748-56; PMID:25317702; https://doi.org/https://doi.org/10.1590/0074-0276140090
- Chen PK, Chang HH, Lin GL, Wang TP, Lai YL, Lin TK, Hsieh MC, Kau JH, Huang HH, Hsu HL, et al. Suppressive effects of anthrax lethal toxin on megakaryopoiesis. PloS One 2013; 8:e59512; PMID:23555687; https://doi.org/https://doi.org/10.1371/journal.pone.0059512
- Chang HH, Wang TP, Chen PK, Lin YY, Liao CH, Lin TK, Chiang YW, Lin WB, Chiang CY, Kau JH, et al. Erythropoiesis suppression is associated with anthrax lethal toxin-mediated pathogenic progression. PloS One 2013; 8:e71718; PMID:23977125; https://doi.org/https://doi.org/10.1371/journal.pone.0071718
- Chang HH, Chiang YW, Lin TK, Lin GL, Lin YY, Kau JH, Huang HH, Hsu HL, Wang JH, Sun DS. Erythrocytic mobilization enhanced by the granulocyte colony-stimulating factor is associated with reduced anthrax-lethal-toxin-induced mortality in mice. PloS One 2014; 9:e111149; PMID:25384016; https://doi.org/https://doi.org/10.1371/journal.pone.0111149
- Aida Y, Pabst MJ. Removal of endotoxin from protein solutions by phase separation using Triton X-114. J Immunol Methods 1990; 132:191-5; PMID:2170533; https://doi.org/https://doi.org/10.1016/0022-1759(90)90029-U
- Chang HH, Shyu HF, Wang YM, Sun DS, Shyu RH, Tang SS, Huang YS. Facilitation of cell adhesion by immobilized dengue viral nonstructural protein 1 (NS1): arginine-glycine-aspartic acid structural mimicry within the dengue viral NS1 antigen. J Infect Dis 2002; 186:743-51; PMID:12198607; https://doi.org/https://doi.org/10.1086/342600
- Chang HH, Hu ST, Huang TF, Chen SH, Lee YH, Lo SJ. Rhodostomin, an RGD-containing peptide expressed from a synthetic gene in Escherichia coli, facilitates the attachment of human hepatoma cells. Biochem Biophys Res Commun 1993; 190:242-9; PMID:7916592; https://doi.org/https://doi.org/10.1006/bbrc.1993.1037
- Chang HH, Chen PK, Lin GL, Wang CJ, Liao CH, Hsiao YC, Dong JH, Sun DS. Cell adhesion as a novel approach to determining the cellular binding motif on the severe acute respiratory syndrome coronavirus spike protein. J Virol Methods 2014; 201:1-6; PMID:24530430; https://doi.org/https://doi.org/10.1016/j.jviromet.2014.01.022
- Sun DS, Lee PC, Kau JH, Shih YL, Huang HH, Li CR, Lee CC, Wu YP, Chen KC, Chang HH. Acquired coagulant factor VIII deficiency induced by Bacillus anthracis lethal toxin in mice. Virulence 2015; 6:466-75; PMID:25906166; https://doi.org/https://doi.org/10.1080/21505594.2015.1031454
- Thomas L, Verlaeten O, Cabie A, Kaidomar S, Moravie V, Martial J, Najioullah F, Plumelle Y, Fonteau C, Dussart P, et al. Influence of the dengue serotype, previous dengue infection, and plasma viral load on clinical presentation and outcome during a dengue-2 and dengue-4 co-epidemic. Am J Trop Med Hyg 2008; 78:990-8; PMID:18541782
- Harkness JE, Wagner JE. Clinical procedures: Biology and Medicine of Rabbits and Rodents. 1995. Chapter 2, Biology and Husbandry; p. 23-95.
- Chiu MW, Yang YL. Blocking the dengue virus 2 infections on BHK-21 cells with purified recombinant dengue virus 2 E protein expressed in Escherichia coli. Biochem Biophys Res Commun 2003; 309:672-8; PMID:12963043; https://doi.org/https://doi.org/10.1016/j.bbrc.2003.08.053
- Leysi-Derilou Y, Robert A, Duchesne C, Garnier A, Boyer L, Pineault N. Polyploid megakaryocytes can complete cytokinesis. Cell Cycle 2010; 9:2589-99; PMID:20647776; https://doi.org/https://doi.org/10.4161/cc.9.13.12078
- Lien TS, Sun DS, Chang CM, Wu CY, Dai MS, Chan H, Wu WS, Su SH, Lin YY, Chang HH. Dengue virus and antiplatelet autoantibodies synergistically induce haemorrhage through Nlrp3-inflammasome and FcgammaRIII. Thrombosis Haemostasis 2015; 113:1060-70; PMID:25740324; https://doi.org/https://doi.org/10.1160/TH14-07-0637
- Pintado CO, Friend M, Llanes D. Characterisation of a membrane receptor on ruminants and equine platelets and peripheral blood leukocytes similar to the human integrin receptor glycoprotein IIb/IIIa (CD41/61). Vet Immunol Immunopathol 1995; 44:359-68; PMID:7538249; https://doi.org/https://doi.org/10.1016/0165-2427(94)05310-O
- Ravid K, Lu J, Zimmet JM, Jones MR. Roads to polyploidy: the megakaryocyte example. J Cell Physiol 2002; 190:7-20; PMID:11807806; https://doi.org/https://doi.org/10.1002/jcp.10035
- Yu M, Cantor AB. Megakaryopoiesis and thrombopoiesis: an update on cytokines and lineage surface markers. Methods Mol Biol 2012; 788:291-303; PMID:22130715
- You T, Wang Q, Zhu L. Role of autophagy in megakaryocyte differentiation and platelet formation. Int J Physiol Pathophysiol Pharmacol 2016; 8:28-34; PMID:27186320
- Cao Y, Cai J, Zhang S, Yuan N, Li X, Fang Y, Song L, Shang M, Liu S, Zhao W, et al. Loss of autophagy leads to failure in megakaryopoiesis, megakaryocyte differentiation, and thrombopoiesis in mice. Exp Hematol 2015; 43:488-94; PMID:25591498; https://doi.org/https://doi.org/10.1016/j.exphem.2015.01.001
- Plummer EM, Shresta S. Mouse models for dengue vaccines and antivirals. J Immunol Methods 2014; 410:34-8; PMID:24440090; https://doi.org/https://doi.org/10.1016/j.jim.2014.01.001
- Plummer E, Shresta S. Animal models in dengue. Methods Mol Biol 2014; 1138:377-90; PMID:24696349
- Wan SW, Yang YW, Chu YT, Lin CF, Chang CP, Yeh TM, Anderson R, Lin YS. Anti-dengue virus nonstructural protein 1 antibodies contribute to platelet phagocytosis by macrophages. Thrombosis Haemostasis 2016; 115:646-56; PMID:26632672; https://doi.org/https://doi.org/10.1160/TH15-06-0498
- Bozza FA, Cruz OG, Zagne SM, Azeredo EL, Nogueira RM, Assis EF, Bozza PT, Kubelka CF. Multiplex cytokine profile from dengue patients: MIP-1beta and IFN-gamma as predictive factors for severity. BMC Infect Dis 2008; 8:86; PMID:18578883; https://doi.org/https://doi.org/10.1186/1471-2334-8-86
- Huang SP, Chien JY, Tsai RK. Ethambutol induces impaired autophagic flux and apoptosis in the rat retina. Dis Model Mech 2015; 8:977-87; PMID:26092127; https://doi.org/https://doi.org/10.1242/dmm.019737
- Mortensen M, Ferguson DJ, Edelmann M, Kessler B, Morten KJ, Komatsu M, Simon AK. Loss of autophagy in erythroid cells leads to defective removal of mitochondria and severe anemia in vivo. Proc Natl Acad Sci U S A 2010; 107:832-7; PMID:20080761; https://doi.org/https://doi.org/10.1073/pnas.0913170107
- Saez-Llorens X, Tricou V, Yu D, Rivera L, Tuboi S, Garbes P, Borkowski A, Wallace D. Safety and immunogenicity of one versus two doses of Takeda's tetravalent dengue vaccine in children in Asia and Latin America: interim results from a phase 2, randomised, placebo-controlled study. Lancet Infect Dis 2017; 17:615-25; PMID:28365225; https://doi.org/https://doi.org/10.1016/S1473-3099(17)30166-4
- Whitehead SS. Development of TV003/TV005, a single dose, highly immunogenic live attenuated dengue vaccine; what makes this vaccine different from the Sanofi-Pasteur CYD vaccine? Expert Rev Vaccines 2016; 15:509-17; PMID:26559731; https://doi.org/https://doi.org/10.1586/14760584.2016.1115727
- Hadinegoro SR, Arredondo-Garcia JL, Capeding MR, Deseda C, Chotpitayasunondh T, Dietze R, Muhammad Ismail HI, Reynales H, Limkittikul K, Rivera-Medina DM, et al. Efficacy and long-term safety of a dengue vaccine in regions of endemic disease. N Engl J Med 2015; 373:1195-206; PMID:26214039; https://doi.org/https://doi.org/10.1056/NEJMoa1506223
- Guzman MG, Hermida L, Bernardo L, Ramirez R, Guillen G. Domain III of the envelope protein as a dengue vaccine target. Expert Rev Vaccines 2010; 9:137-47; PMID:20109025; https://doi.org/https://doi.org/10.1586/erv.09.139
- Robinson LN, Tharakaraman K, Rowley KJ, Costa VV, Chan KR, Wong YH, Ong LC, Tan HC, Koch T, Cain D, et al. Structure-Guided Design of an Anti-dengue Antibody Directed to a Non-immunodominant Epitope. Cell 2015; 162:493-504; PMID:26189681; https://doi.org/https://doi.org/10.1016/j.cell.2015.06.057
- Frei JC, Kielian M, Lai JR. Comprehensive mapping of functional epitopes on dengue virus glycoprotein E DIII for binding to broadly neutralizing antibodies 4E11 and 4E5A by phage display. Virology 2015; 485:371-82; PMID:26339794; https://doi.org/https://doi.org/10.1016/j.virol.2015.08.011

