ABSTRACT
Clostridium botulinum, a polyphyletic Gram-positive taxon of bacteria, is classified purely by their ability to produce botulinum neurotoxin (BoNT). BoNT is the primary virulence factor and the causative agent of botulism. A potentially fatal disease, botulism is classically characterized by a symmetrical descending flaccid paralysis, which is left untreated can lead to respiratory failure and death. Botulism cases are classified into three main forms dependent on the nature of intoxication; foodborne, wound and infant. The BoNT, regarded as the most potent biological substance known, is a zinc metalloprotease that specifically cleaves SNARE proteins at neuromuscular junctions, preventing exocytosis of neurotransmitters, leading to muscle paralysis. The BoNT is now used to treat numerous medical conditions caused by overactive or spastic muscles and is extensively used in the cosmetic industry due to its high specificity and the exceedingly small doses needed to exert long-lasting pharmacological effects. Additionally, the ability to form endospores is critical to the pathogenicity of the bacteria. Disease transmission is often facilitated via the metabolically dormant spores that are highly resistant to environment stresses, allowing persistence in the environment in unfavourable conditions. Infant and wound botulism infections are initiated upon germination of the spores into neurotoxin producing vegetative cells, whereas foodborne botulism is attributed to ingestion of preformed BoNT. C. botulinum is a saprophytic bacterium, thought to have evolved its potent neurotoxin to establish a source of nutrients by killing its host.
Introduction – the organism
Clostridium botulinum is a Gram-positive, anaerobic, endospore-forming bacillus responsible for botulism, a severe neuroparalytic disease that affects humans and vertebrate animals [Citation1–3]. The causative agent is botulinum neurotoxin (BoNT), renowned as the most potent biological substance known to humankind [Citation4,Citation5]. A metalloprotease, BoNT specifically cleaves the Soluble N-Ethylmaleimide-Sensitive Factor Attachment Protein Receptor (SNARE) proteins in postsynaptic nerve terminals, preventing the release of neurotransmitter and blocking neural transmission to effector muscles [Citation6].
Ubiquitous in nature, humans and animals routinely encounter this bacterium in the form of endospores [Citation7]. Formed under stressful conditions to allow survival, spores are a characteristic trait of the genus Clostridium, members of which includes other notable pathogens, such as Clostridium difficile (now reclassified as Clostridioides difficile), Clostridium tetani and Clostridium perfringens. These pathogens are the causative agents of chronic diarrhoea, tetanus, and gas gangrene, respectively, [Citation8,Citation9]. The spores of C. botulinum usually present no threat to people unless they germinate and form neurotoxin-producing, vegetative cells. As the healthy human digestive system is not conducive to spore germination, these routinely pass through our bodies and are excreted without causing any harm, except for infant and adult gut dysbiosis botulism cases [Citation10].
Clostridium botulinum has become one of the most notorious organisms in medical history, not only for outbreaks of fatal botulism cases but also for the development of pharmaceutical applications of the botulinum neurotoxin [Citation11]. Commonly used for treatment of numerous medical conditions in fields of dermatology, ophthalmology and neurology, the botulinum neurotoxins are now perhaps most famous for cosmetic applications [Citation12–15]. Consequently, the toxicology and pharmacology of the BoNTs is well studied, but less is known of the physiology of the bacteria responsible for their production.
C. botulinum - A historical perspective
Since humans have stored foods, C. botulinum and its neurotoxin have been problematic for causing cases of foodborne botulism [Citation16]. Following the poverty caused by the Napoleonic war in Europe in the late 1700s a mysterious illness attributed to poor food production led to many deaths [Citation17]. It wasn’t until in the early 18th century that the source was postulated. Across Southwest Germany, an increasing number of fatal food poisoning cases were reported after consumption of a traditional uncooked blood sausage meal. This led a young local medical officer and poet, Justinus Kerner, now known as the godfather of botulinum research, to connect the blood sausage to the paralytic illness. He published the first complete descriptions of botulism in 1820 [Citation18]. He termed the ailment “sausage poisoning” making it a reportable disease, and was the first to suggest the cause as a biological poison, performing experiments with the poison upon himself. Kerner even postulated the use of the “poison” to treat a variety of diseases in 1822, which was realised over 150 years after it was first envisaged [Citation18,Citation19].
In December 1895, a group of musicians, having just played at a funeral in Belgium, shared a customary meal of smoked and pickled ham. They all subsequently developed paralytic symptoms and three out of the 34 band members died [Citation20]. Physician, Emile van Ermengem, investigated the outbreak and isolated the same anaerobic bacteria from both samples of infected ham and the victim’s corpses. Ermengem named the bacteria Bacillus botulinus (botulinus translating to sausage in Latin) and demonstrated its production of an unknown toxin [Citation21]. In 1917, the anaerobic bacilli were reclassified into the genus Clostridium (from the Greek word “Kloster,” meaning spindle shape) and was subsequently renamed to Clostridium botulinum [Citation22].
Georgina Burke was responsible for the letter designation of different serotypes in 1919 (BoNT/A and B) [Citation23]. It took until the 1920s for the toxin to be purified when Herman Sommer managed to form an acid precipitate of BoNT/A, and in 1946, the microbiologist Carl Lamanna produced a crystallized form [Citation24,Citation25]. Following the crystallization Burgen’s group was the first to discover that BoNT blocked the release of the neurotransmitter acetylcholine at the neuromuscular junction [Citation26]. Some 43 years later, Schiavo identified BoNT and the tetanus neurotoxin toxin (TeNT) as metalloproteases, which cleave SNARE proteins within presynaptic nerve terminals [Citation27].
Although the potential of the toxin for medical applications was acknowledged by Kerner over 200 years ago, the use of BoNT as a therapeutic only began to be realised in the 1980s [Citation28,Citation29]. Ophthalmologist Alan Scott was the first to successfully demonstrate its therapeutic use as a treatment for strabismus (eye misalignment) through a collaboration with Edward Schantz at the University of Wisconsin, who supplied the purified the BoNT/A needed for the early clinical studies [Citation30–32]. In 1989, the FDA (Food and Drug Administration) gave the approval of the BoNT/A orphan drug “Oculinum” for the treatment of strabismus, blepharospasm and hemifacial spasms [Citation28]. The pharmaceutical company Allergan (Irvine, CA) acquired the rights to Oculinum soon after and later changed its name to the well-known Botox® (onabotulinumtoxinA) [Citation33,Citation34].
In parallel to Scott’s developments in the US, a partnership between biotechnology company Porton International (Ipsen Biopharm Ltd purchased the successor company) and the Centre for Applied Microbiology and Research (CAMR) at Porton Down were developing Dysport (abobotulinumtoxinA) in the United Kingdom. Shortly after the Oculinum approval, Dysport® (Dystonia/Porton Down) was approved in Europe in 1990 for the treatment of dystonia and was later the FDA approved in 2009 [Citation34]. Four other BoNT products are currently FDA approved; incobotulinumtoxinA (Xeomin®; Merz Pharmaceuticals, Frankfurt, Germany), prabotulinumtoxinA (Jeuveau®; Evolus Inc, CA, USA), daxibotulinumtoxinA (DAXXIFY™; Revance Therapeutics Inc, TN, USA) and BoNT/B preparation rimabotulinumtoxinB (Myobloc® in the USA; Supernus Pharmaceuticals Inc, MD, USA/Neurobloc® in Europe; Sloan Pharma, Switzerland) [Citation35]. Other BoNT/A formulations are available but are marketed chiefly in Asia [Citation36]. Further developments have allowed the list of approved therapeutic applications of BoNT to constantly expand beyond strabismus and dystonia, with over 12 different medical conditions now approved in 2022, and a growing cosmetic market for BoNT treatments [Citation13,Citation14,Citation37].
Classification of C. botulinum strains and neurotoxins – is a species a species?
The species of C. botulinum historically encompassed all bacteria that produced a BoNT, this single criterion for inclusion has led it to be a highly diverse species of bacteria [Citation38,Citation39]. This taxonomy was adopted in 1950s to prevent confusion among clinicians and scientists when handling or diagnosing the dangerous bacterium. Within the species, four phylogenetically distinct lineages are apparent, known as C. botulinum Groups I-IV, collated based on genetic heterogeneity and physiological factors [Citation40,Citation41]. Greater similarity can be seen to other clostridial species than between the groups of C. botulinum, the only common denominator in the groups being the production of a BoNT [Citation42]. This diversity can be seen in .
Figure 1. Classification of C. botulinum and the other BoNT producing strains. (a) A phylogenetic dendrogram of a representative selection of strains producing the seven different serotypes of BoNT and other related clostridia (NT – nontoxigenic strain). Constructed by average nucleotide identify from published whole genome assembly sequences and neighbour-joining tree method (b) Dendrogram comparing the protein sequences of all BoNT toxin types including the tetanus toxin (TeNT). Amino Acid sequences analysed by ClustalW alignment and tree created using the Maximum Likelihood method. (c) Table summarising the Clostridium spp. known to produce the BoNT; depicting the six phylogenetically distinct BoNT neurotoxic clostridia. Colours throughout refer to the group the strain or toxin type belongs to. All evolutionary analyses were conducted in MEGA11, with prior multiple sequence alignment of whole genomes by CLC Genomics Workbench 21.0.3 (CLC Bio, Aarhus, Denmark).
Group I and II strains are most commonly associated to human botulism cases, whereas Group III strains are responsible for avian and some mammalian animal cases. Group IV strains, renamed to Clostridium argentinense, are rarely associated to illness and produce BoNT/G [Citation43]. Neurotoxigenic strains of Clostridium sporogenes (classified in Group I), Clostridium baratii (often referred to as Group V) and Clostridium butyricum (often referred to as Group VI) are also known to produce BoNT/B, BoNT/F and BoNT/E neurotoxins, respectively [Citation40].
The BoNTs have traditionally been divided into seven toxin serotypes from A to G, with subtype variants designated within each toxin type, labelled by a sequential number in order of discovery [Citation41]. In 2016, a novel serotype was discovered in an existing C. botulinum strain, strain 111 which also produces BoNT/B2 neurotoxin, by whole genome and bioinformatic approaches. This serotype was named BoNT/X and although the toxin was proved to cleave a range of SNARE targets it is unknown if the BoNT/X gene cluster is expressed natively [Citation44]. A chimeric toxin, BoNT/FA, was briefly thought to be a new BoNT/H serotype until further analysis uncovered it to be a chimeric toxin [Citation45–47]. Toxin serotypes are categorized based on the toxin’s ability to be neutralized by monoclonal antibodies, while subtypes tend to be classified through differences in amino acid sequences, although subtypes can vary by 2.6–31.6% at the amino acid sequence level [Citation42,Citation48,Citation49]. By this criterion, only four serotypes have designated sub-serotypes; BoNT/A, BoNT/B, BoNT/E and BoNT/F.
Strains from different groups can produce the same toxin serotype (e.g. BoNT/F is produced by Groups I, II and V) and subtypes of the same serotype can vary in biological activity [Citation50]. Most characterized strains produce only a single serotype of neurotoxin, but some have been observed to produce two different serotype toxins, termed bivalent strains. A nomenclature for such strains has been devised where; (i) AB expresses equivalent amounts of each encoded toxin (ii) Ab, Af, Ba, and Bf predominantly express the capitalized major toxin and less of the lower case minor toxin (iii) A(B) called “A silent B” where a truncated BoNT/B produces no active toxin [Citation51]. Additionally, mosaic toxins sometimes known as chimeric toxins, naturally exist which are a single BoNT composed of domains derived from different serotypes, such as BoNT/CD, BoNT/DC, and BoNT/FA. For instance, the BoNT/DC toxin has a C-terminal heavy chain similar to that of BoNT/C toxins but a light chain and an N-terminal heavy chain almost identical to BoNT/D [Citation52–54]. Group III strains can additionally produce a non-neurotoxic binary toxin known as C2, a cytotoxic actin ADP-ribosylating toxin, and an C3 exoenzyme which has led to BoNT/C confusingly sometimes referred to as the C1 toxin [Citation55,Citation56].
The harbouring of the toxin gene clusters on plasmids and bacteriophages, as well as the presence of mobile genetic elements immediately flanking the toxin genes in several species, indicates horizontal gene transfer has played a role in acquisition of the cluster and explains the large diversity of BoNT-producing isolates identified [Citation42]. Through the use of whole-genome sequencing and bioinformatics methods, in 2015 the first non-clostridial BoNT homologue was discovered in Weissella oryzae SG25 (BoNT/Wo) able to cleave VAMP2, and later in 2018 a second was found in Enterococcus sp. 3G1_DIV0629, a close relative of E. faecium, and termed eBoNT/J, with the ability to cleave VAMP 2 and SNAP 25 [Citation57–59]. A more distantly related BoNT homolog in the genome of Chryseobacterium piperi (BoNT/Cp1) was discovered, but was designated a BoNT-like toxin, clustering outside the BoNT family of toxins with an approximately 15% overall sequence identity [Citation60]. These discoveries challenge the established dogma that BoNTs are exclusively clostridial toxins. Of particular importance are the BoNT/A1 strains since they induce the most severe, longer-lasting forms of human botulism, and for that reason this specific toxin is predominantly selected for therapeutics and aesthetics [Citation61,Citation62].
With each C. botulinum Group generally considered to be its own distinct species, it has been proposed, although currently not widely adopted by the field, that the Group names I-IV be replaced using the old Latin binomial nomenclature. To differentiate the proteolytic from the nonproteolytic neurotoxin-producing clostridia, the proteolytic C. botulinum Group I species could be known as Clostridium parabotulinum, whilst the nonproteolytic Group II strains remain as Clostridium botulinum [Citation63]. The Group III BoNT/C and BoNT/D producing bacteria, due to the similarities to Clostridium novyi, are proposed to be called “C. novyi sensu lato” [Citation64]. Whilst Group IV, C. argentinense, preserves its current species name, as should the other BoNT-producing clostridia [Citation65,Citation66].
Botulinum and bioterrorism
The use of biological agents for bioterrorism or bio-crime purposes continues to be of global concern [Citation67,Citation68]. BoNT is classified as among the highest risk threats, being listed as a Category A agent by the CDC in the US and a Schedule 5 biological agent in the United Kingdom [Citation69,Citation70]. Due to its potential to be utilized as a biological weapon, the storage and handling of BoNT neurotoxic strains requires enhanced security arrangements and regular audits. BoNT is one of the most potent biological agents having the lowest lethal dose of any natural substance known [Citation71,Citation72]. The human lethal dose (LD50) is dependent on the route of entry into the body, but assuming an average body weight of 70 kg, 70 µg of ingested, 0.7–0.9 µg of inhaled and 0.09–0.15 µg of intravenously administered concentrated BoNT/A toxin would be lethal [Citation73].
Historically, there have been several examples of state-sponsored BoNT production for use as a biological weapon [Citation74]. Under the leadership of Saddam Hussein, an extensive biological weapons program was overseen in Iraq between 1985 and 1991, where 19,000 L of concentrated BoNT was stockpiled for weaponization [Citation75]. This would have been enough to kill the entire human population three times over. An aerosolized release of purified toxin is a scenario of concern to counter-terrorism organizations, however, BoNT is rather unstable in the environment, and would degrade within a couple of hours of release [Citation76]. The most notorious report of BoNT bioterrorism is by the Japanese religious cult, Aum Shinrikyo, who failed to successfully release aerosolized BoNT preparations on three separated occasions in Tokyo prior to the group releasing Sarin nerve gas on the Tokyo subway in March 1995 [Citation73].
Another scenario of concern is a deliberate contamination of beverages with BoNT. In liquid preparations, BoNT has been described as being comparatively stable, with 50% residual toxicity after 70 days [Citation77]. An intentional release of BoNT into the water supply in the developed world would be prevented by routine chloride treatment destroying the toxin [Citation78]. The tasteless, colorless and odorless nature of BoNT in solution, alongside the short incubation times that leads to a potentially fatal condition, makes a BoNT-based attack through injection or lacing of foods a worrying scenario [Citation79]. Close monitoring of botulism cases, development of suitable diagnostics and improvement of treatments remains crucial.
Botulism – a neuroparalytic disease
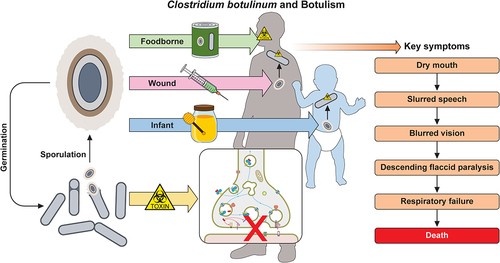
Botulism is a serious and potentially fatal neuroparalytic disease of humans and animals, in which the sole causative agent is the BoNT. Human botulism is characterized as a bilateral descending muscle weakness, symptoms generally begin in the cranial nerves presenting as blurred or double vision, dry mouth, and difficulty speaking [Citation3]. The classical early physical presentation of botulism can be remembered using the “four D’s” mnemonic: dysarthria, diplopia and dysphonia, dysphagia. As the disease progresses to the extremities of the body with the dissemination of the neurotoxin, weakness to the muscles of the torso and upper limbs occurs. If left untreated, the respiratory muscle groups are affected, and death is caused by respiratory failure [Citation80].
Despite the extraordinary potency, of BoNT cases of human botulism are now relatively rare with only an average of 160 reported cases of botulism annually in the United States according to CDC data [Citation81]. Deaths due to botulism are now also rare, over the last century the fatality rate of human botulism cases dramatically decreased from 70% to currently less than 5% in the developed world [Citation82–84]. This drop is primarily due to improved clinical recognition, treatment, and rigorous food processing measures. In animals, however, botulism is still prevalent in both wild and domesticated communities, in which deadly outbreaks occur through natural cycles of environmental transmission [Citation85].
In humans, the disease is classified into three main clinical types dependent on the route of entry into the body; foodborne, wound, and infant botulism. The prevalence of each can be seen in . Symptoms across all forms of botulism are broadly uniform, with the exception that foodborne cases may include additional gastrointestinal symptoms of diarrhoea, nausea, vomiting, and abdominal cramps prior to neurological symptoms [Citation86].
Figure 2. Reported botulism cases in the U.S. from 2001–2018. (a) Graph depicts the number of cases of each type of botulism, demonstrating the prevalence of infant botulism compared to other clinical types. (b) Shows the causative toxin type of reported cases if specified. All data obtained from the CDC national botulism surveillance annual summaries [Citation110].
![Figure 2. Reported botulism cases in the U.S. from 2001–2018. (a) Graph depicts the number of cases of each type of botulism, demonstrating the prevalence of infant botulism compared to other clinical types. (b) Shows the causative toxin type of reported cases if specified. All data obtained from the CDC national botulism surveillance annual summaries [Citation110].](/cms/asset/7cfe6647-edbb-4693-900c-13da55a0a423/kvir_a_2205251_f0002_oc.jpg)
Foodborne botulism
Foodborne botulism is caused by the ingestion of food contaminated with preformed BoNT, with as little as a few milligrams of contaminated food enough to cause symptoms and perhaps death if left untreated [Citation10]. Symptoms typically present 12–72-h post-consumption of the contaminated food, dependent on the quantity ingested [Citation73]. Foodborne botulism was historically the most prevalent form of human botulism and is usually associated with uncooked foods or the inadequate processing of food stuffs both commercially and when home prepared. Preservation techniques such as fermentation or pickling, followed by canning or bottling of foods without prior heat treatments pose the greatest threat as they create the anaerobic conditions required for spore germination and bacterial growth [Citation3]. The introduction of certain food control measures; the “botulinum cook” (121 °C heat treatment for 3 min), freezing, and refrigeration (below 4 °C), when applied correctly, have greatly decreased foodborne outbreaks this century [Citation87–90].
Vegetative bacterial growth and the generation of toxin occurs only under anaerobic, low salt (salt concentration >5% throughout food), high water activity, and non-acidic environments (pH < 4.6) [Citation91–93]. Bearing this in mind, the product formulation can help prevent incidences. BoNT is temperature sensitive, and any preformed toxin in the food stuffs can be inactivated by heating at 85 °C for at least 5 min [Citation78]. The spores can only be inactivated by the “botulinum cook,” although temperature can be reduced in the presence of increased pressure [Citation10,Citation87,Citation94].
Group I and II C. botulinum are associated with outbreaks of human foodborne botulism. BoNT/A and BoNT/B toxin serotypes are the most prevalent, with cases of BoNT/E and BoNT/F being rarely reported (see ) [Citation91]. Proteolytic, Group I C. botulinum strains are usually associated with outbreaks linked to shelf-stable commercially processed canned foods due to their high spore heat resistance [Citation91,Citation92]. Group I strains are mesophilic and as such have a minimum growth temperature of 12 °C [Citation10]. However, non-proteolytic Group II strains are able to grow at temperatures as low as 3 °C are more commonly associated with minimally heated or chilled food cases, such as fish or marine products [Citation92,Citation95]. When an outbreak does occur, it can have significant commercial implications for the food and drink companies involved. In a recent outbreak in the US, it was found that inadequate refrigeration and/or heat treatment of cans of chili sauce for hot dogs resulted in the product being compromised, necessitating the recall of 111 million cans and ultimately leading to the closure of the canning company [Citation96].
Food-borne botulism remains a prevalent disease in domesticated and wild animals, where outbreaks among farm livestock and wetland birds tend to spread rapidly, which can lead to devastating epidemics due to the favorable cycles of transmission in the environment [Citation97]. C. botulinum bacterial growth in anaerobic niches of decaying organic matter leads to production BoNT, which is ingested by animals, birds, or fish leading to death. Death can either be directly from a lethal dose of neurotoxin or consumption of a non-lethal quantity that decreases the animal’s fitness, causing it to become prey or outcompeted for resources. This contaminated carcass can then propagate further infections to other animals that feed on it [Citation7,Citation98]. This infection cycle can be further enhanced through neurotoxin ingestion by BoNT-insensitive insects and other invertebrates, such as maggots, worms or shellfish and subsequent consumption by a toxin sensitive animal [Citation99]. Animal outbreaks tend to be associated with BoNT/C and BoNT/D toxin serotypes, although interestingly equine botulism is more commonly related to BoNT/B [Citation100].
Infant botulism
Environmental C. botulinum spores routinely pass through the human digestive system with no ill effect as the healthy, mature human gut is not conducive to clostridial spore germination [Citation3]. However, an infant’s immature gastrointestinal tract (typically between 1 and 6 months old but reported in babies up to 12 months of age) is more amenable for C. botulinum colonization following the ingestion and germination of spores [Citation101]. This is due to immature gut physiology and/or underdeveloped gut microbiota. This results in the endogenous production of the neurotoxin by the bacteria, absorption from the bowel lumen, and so-called “floppy babies” with an inability to swallow or suck, with symptoms typically beginning 18 to 36-h post-ingestion [Citation102]. The majority of cases arise from consumption of untreated natural food stuffs, honey, and corn syrup, for example, are frequently found to contain a high concentration of spores [Citation103–105]. Other sources of spores have originated from contaminated powdered baby formula and household dust [Citation106,Citation107]. Infant botulism was first reported in 1976 and this century it has been the predominant form of human botulism cases in the US, as can be seen in [Citation108,Citation109].
Wound botulism
Wound botulism was first formally reported in 1951 and is caused by the absorption of BoNT into the blood stream following contamination of a wound with C. botulinum spores from the environment [Citation111]. The anaerobic conditions within a wound abscess provide the environment needed for spore germination and colonization. Due to the nature of infection, the presentation of clinical symptoms typically takes longer than those of other clinical forms of botulism, typically presenting between 4 and 14 days [Citation112]. Historically, cases were comparatively rare and usually implicated with trauma events, however the increasing prevalence of injecting drug-users led to a dramatic increase in the number of wound botulism cases [Citation113–115]. In particular, an epidemic of cases in the state of California in the United States arose due to increased usage of black tar heroin through the late 1980s into 1990s [Citation116,Citation117].
Less prevalent forms of botulism
Rarely cases of botulism arise that do not fit into the three main forms and are caused by much rarer manifestations of the condition. Adult intestinal toxemia botulism is caused when adults, like infants, suffer from C. botulinum colonization of the gastrointestinal tract due to depletion of the natural gut microbiota. This can arise following heavy antimicrobial use, gastrointestinal surgery, or inflammatory bowel disease [Citation118–120]. Rare botulism cases also occur due to the incorrect administration of concentrated BoNT for therapeutic and cosmetic purposes, called latrogenic botulism [Citation121–123]. An extremely rare non-natural form of intoxication following the absorption of BoNT across the respiratory mucosal membranes has been termed inhalational botulism [Citation124,Citation125]. This mechanism of transmission is generally discussed when hypothesizing the potential for a bioterrorism event utilizing aerosolised BoNT [Citation126]. No person-to-person transmission of botulism has ever been reported.
Treatment of botulism
The present treatment for botulism relies on symptomatic control, mechanical respiratory support, and the passive intravenous administration of botulinum antitoxin (BAT). It is possible to survive botulinum intoxication without antitoxin therapy through modern patient supportive care, such as mechanical ventilators, but recovery and the duration of hospitalization will be prolonged and costly [Citation127,Citation128]. The current antitoxin is a heptavalent polyclonal antibody formulation (HBAT®) which contains neutralizing antibodies against seven toxin serotypes; A, B, C, D, E, F, and G and has superseded the older trivalent antitoxin in US and UK, which is only able to neutralize serotypes A, B, and E [Citation129,Citation130]. Some trivalent antitoxins are still used in several European countries [Citation131]. BAT is derived from equine sources, by blending sterile sera obtained from horses hyperimmunized with C. botulinum toxoid strains for each serotype. It has been recently proposed that safer recombinant subunit fragments of the toxins can effectively trigger the equine immune response and lead to production of BAT with a similar high efficacy against the neurotoxins [Citation132]. HBAT® was approved by the FDA in the US in March 2013 but is used unlicensed in the UK [Citation133].
Serum-based antitoxin treatment has numerous limitations, not least its inability to neutralize toxin already in the intracellular environment. The antitoxin can only bind and neutralize toxin in the bloodstream, through blocking binding of the heavy-chain receptor to the presynaptic receptors, resulting in the removal of the toxin-antitoxin complex from circulation [Citation134]. Consequently, the antitoxin should ideally be administered as quickly as possible after the onset of symptoms, to minimize the amount of toxin internalized [Citation135]. Data suggests that patients treated with HBAT® within 48 h of the onset of symptoms require fewer days in hospital and require less intense care [Citation127,Citation129]. Hence, clinicians are advised that all cases of suspected botulism should be treated without delay and not to await laboratory diagnosis due to the fast-irreversible progression of intoxication [Citation128].
As BAT is an animal-derived from antibody preparation, and as such is foreign to the human body there is a risk of serious hypersensitivity side effects, such as serum sickness and anaphylaxis [Citation136,Citation137]. Anaphylaxis occurs in less than 2% of the treated patients but incidence of serum sickness and other milder allergic reactions is less well documented [Citation138]. Considering these complications sometimes seen with equine antitoxin treatment in adults, a bivalent human-derived immunoglobulin preparation (BIG-IV) has been used to treat infant botulism since the FDA in the US approved the use of BabyBiG® in 2003 and outside of the US since 2005 [Citation139–141]. BIG-IV is a purified immunoglobulin preparation against serotypes A and B only, derived from adults immunized with recombinant C. botulinum vaccine strains, and as such has a larger half-life with respect to equine antitoxin [Citation139]. BabyBIG is effective and despite its high cost per treatment, $45,300 per vial as of 2017, it was predicted to have saved >$85 million in hospital charges in the US in its first 12 years post-licensure in 2003. It also reduced the average length of hospital stay by 3.6 weeks per infant treated compared to the placebo-treated patients in the 1992–1997 BIG-IV pivotal clinical trial [Citation142]. Despite these benefits, availability of BIG-IV is limited in some countries due to high upfront costs when compared to traditional equine antitoxin (Vanella de Cuetos et al. 2011).
For the treatment of wound botulism cases, the antitoxin is administered followed by reduction of the bacterial load by antibiotic treatment (penicillin and metronidazole) and surgical debridement of the infected wound abscess to prevent further toxin production [Citation143]. This is in sharp contrast to infant and adult intestinal botulism cases where antibiotic treatment is not recommended due to possible worsening of neurological symptoms following further gut dysbiosis and potential lysis of vegetative C. botulinum cells resulting in the release of intracellular neurotoxin [Citation141,Citation144,Citation145]. In cases of foodborne botulism gastrointestinal decontamination may be considered, such as gastric lavage or induced emesis, only in cases were the ingestion of contaminated meal was recent [Citation131].
Supply of antitoxin is limited due to the long immunization course required before adequate levels of antibodies are developed in the sera of the equine production host and the short half life of the administered immunoglobins in circulation requires large quantities of the antitoxin per dose [Citation146–148]. In view of the shortfall of serum-based antitoxins, alternative therapeutics for botulism poisonings have been explored. Several human monoclonal antibody therapeutics to BoNTs are currently undergoing clinical trials, following demonstration of prior efficacy and safety [Citation149–153] and single-domain antibodies (VHH) have also shown promise [Citation154,Citation155]. Antibodies by nature are short lived however, and therefore do not persist in the human body. Recently, a recombinant adeno-associated virus expressing a BoNT/A antibody has been reported to give long-term protection in animals to an otherwise lethal dose of toxin [Citation156].
The threat of BoNT being utilized as a biological weapon has led to the exploration into several vaccines to provide protection against the effects of BoNTs, allowing clearance of the toxin from the circulation before it can bind to nerve endings and cause symptoms [Citation157,Citation158]. The current rarity of the disease, combined with the fact that vaccination precludes the individual from the potential therapeutic benefits of BoNT, means that population-wide immunization programs have not been established. A pentavalent (ABCDE) formalin-inactivated toxoid vaccine against botulism was used for nearly 50 years in a program to vaccinate workers at high occupational risk of exposure to botulinum toxins, such as researchers and manufacturers in the US [Citation159,Citation160]. This program ended in November 2011 due to the expiry of the initial batch of vaccines and reduced efficacy of the toxoid vaccine [Citation161]. Since then sera, protein-based, recombinant viral vectors and DNA vaccines are currently being explored as replacements [Citation147,Citation162].
Dependent on the quantity and serotype of the causative BoNT, recovery from the condition can range from a few weeks to several months. With prompt treatment and administration of the antitoxin, a full recovery can occur in as little as two weeks with no lasting symptoms [Citation128,Citation163]. Recovery from intoxication and restoration of neurotransmitter release is a consequence of the natural protein turnover causing degradation of the BoNT in the neuronal termini and the regeneration of nerve endings from the intoxicated neuron [Citation164–167]. The alterative toxicity and persistence of toxin serotypes is thought to be associated with the resistance of the proteins to ubiquitin-proteasome system (UPS) mediated protein turnover [Citation168,Citation169]. Treatments to accelerate botulism recovery times by increasing internalized BoNT turnover are being explored [Citation170].
Diagnostics for botulism and BoNT
For the successful treatment of botulism, a rapid diagnosis of the condition is essential to dictate treatment and prevent fatal consequences. When diagnosing botulism, in addition to the observation of clinical symptoms and patient history, physicians should seek a positive laboratory diagnosis. Symptoms of other neurological conditions, such as stroke, Guillain-Barré syndrome, myasthenia gravis, tick paralysis, and Eaton-Lambert syndrome, may appear similar to those of botulism, especially in the early stages of intoxication [Citation86,Citation171–173]. However, treatment should not be excessively delayed in anticipation of laboratory test confirmation, due to the increased morbidity associated with delay in positive cases [Citation128]. Differential diagnosis is more commonly associated with sporadic or unconnected cases, whereas the observation of several linked cases often helps make a botulism diagnosis more apparent.
A confirmed diagnosis can be made if biologically active BoNT is found in consumed food, debrided wound tissue, fecal matter, or serum of the patient. The mouse lethality bioassay is the current gold standard for BoNT detection, which involves injection of the patient sample into laboratory mice [Citation128,Citation174]. If BoNT is present, the mice will develop classical symptoms of botulism typically within 24–48-h post-injection dependent of concentration of neurotoxin present [Citation175]. The toxin type of the causative BoNT can be identified through administration of specific antitoxins to the infected mice. Although in vivo mouse bioassays are extremely sensitive (lower detection limits in the range of 5–10 pg/mL of toxin) and versatile in sample types that can be tested, they are relatively expensive, can take several days requiring specialized facilities and trained personnel, and due to the use of animals are ethically unfavourable [Citation176]. In 2019, it was estimated that approximately 400,000 animals are used for industrial BoNT batch testing per year in Europe alone [Citation177].
Cases can also be confirmed following detection of BoNT-producing bacterial species in the stool or wound tissue of suspected individuals. This can be confirmed by polymerase-chain reaction (PCR) tests to detect the presence of the toxin genes DNA (bont/A-G) in samples [Citation178–181]. Results can be provided within hours and are generally satisfactory for diagnosis but cannot definitively confirm the presence of the causative proteinaceous toxin. A laboratory diagnosis provides evidence to identify and isolate the suspected food source [Citation176,Citation182].
For successful laboratory confirmation specimens must be collected as soon as a botulism case is suspected, as toxin levels swiftly decrease in serum and stool samples over time as it is internalized into neurons. A delay in sample collection, or storage above refrigerator temperature (2 °C − 8 °C), can lead to false-negative results. Serum samples must also be collected prior to BAT administration, but stool samples may be collected post-treatment as Clostridium species are not affected by the antitoxin, but prior to antibiotic therapy [Citation128].
For some years, an in vitro replacement for the mouse bioassay has been pursued that would provide; (i) accelerated results within hours, (ii) an equivalent or higher level of sensitivity, (iii) a more cost-effective solution per test, (iv) better accessibility both in terms of ease of use and in the equipment and facilities required and, (v) effective detection in various matrices. Additionally, a candidate would ideally be able to accurately detect multiple serotypes of active toxin simultaneously. An in vitro assay would be beneficial not only for patient diagnosis, but for industrial food safety and environmental surveillance. The advancement of alternative BoNT detection methods has been an active area of research over recent years [Citation183–187].
Pharmaceutical companies producing BoNT preparations for therapeutic or cosmetic applications are currently leading the way in the development of alternatives to the mouse lethality assay. Several have developed the FDA and EU approved cell-based assays (CBAs) to dramatically reduce their use of animal testing to detect and test BoNT potency [Citation177]. Allergan was the first to develop a BoNT CBA, which was approved by the FDA in 2011 (and in the EU in 2012) and was outlined in a publication in 2012 [Citation188]. Merz and Ipsen later developed their own CBAs, with EU approval granted in 2015 and 2018, respectively [Citation189,Citation190]. A limitation of these assays is that they were developed for potency testing of purified single serotypes and are not suitable for clinical diagnostics or food testing with complex sample types [Citation191].
In a detailed review on the advances in BoNT diagnostic methods by Hobbs et al. in 2019, numerous exciting technologies were discussed, highlighting a centrifugal microfluidic assay, a colorimetric assay, an SPR-based biosensor, and the EIS (Electrochemical Impedance Spectroscopy) platform as particularly promising based on sensitivity, speed, and cost [Citation192–196]. Each require further validation and optimizations before they could be considered as an industry-wide replacement for the standard mouse bioassay.
The causative agent – the botulinum neurotoxin
BoNTs are encoded by bont genes, which are located either on chromosomal, plasmid, or phage elements [Citation197]. Common to all serotypes, the toxin is initially translated as a 150 kDa single-chain peptide [Citation198–200]. This precursor then undergoes a post-translational cleavage losing 11 amino acids in a process termed nicking, which occurs 50 kDa from the N-terminal end of the polypeptide [Citation201]. The mature and active toxin (known as the di-chain holoenzyme) is formed of two chains, a 100 kDa heavy chain and 50 kDa light chain, linked by non-covalent interactions and a single interchain disulphide bridge () [Citation203,Citation204]. The light chain incorporates the catalytic zinc-dependent metalloprotease activity, a zinc atom bound to a HEXXH motif, sheltered within the core of the structure [Citation205,Citation206]. The heavy chain is composed of two domains; the N-terminal translocation domain (HN) and the C-terminal receptor-binding domain (HC). The HC is further broken down into two subunits (HC-N and HC-C) with the interface between the subunits providing the space required for the glycosylated receptors (gSV2C) of the synaptic terminal to bind [Citation207]. The HC-N is responsible for interacting with membrane lipids, while the HC-C subunit is involved in nerve cell binding and is crucial regarding the internalization into, and trafficking within the nerve cell [Citation208–210]. The HN translocation domain includes a peptide belt that wraps around the light chain and facilitates translocation of this catalytic domain from the vesicles into the synaptic cytosol. It is also thought that this belt functions to partially occlude the active site, preventing any catalytic activity of the light chain prior to separation from the heavy chain [Citation211–213].
Figure 3. The classical structure of the BoNTs. Depicted is the BoNT/A1 from c. botulinum ATCC 3502. The bontA1 gene is translated into a single 1295 amino acid polypeptide (c.), which is cleaved into a light chain (L – coloured red) and a heavy chain joined by a disulphide bridge between Cys430 and Cys454 residues (shown in black). The heavy chain contains an N-terminal translocation domain (HN – coloured light green) and a C-terminal receptor binding domain, which is formed of two subunits (HC-N and HC-C coloured dark and light blue respectively). The light chain is the catalytic domain and consists of a zinc metalloprotease (the Zn2+ atom shown in yellow). The light chain is encircled by a hydrophobic peptide belt domain of the HN (coloured in dark green in c.). Crystal structures were prepared using UCSF ChimeraX (PBD ID: 3BTA) with a. displayed in ribbon format and b. a spacefill representation [Citation198,Citation202].
![Figure 3. The classical structure of the BoNTs. Depicted is the BoNT/A1 from c. botulinum ATCC 3502. The bontA1 gene is translated into a single 1295 amino acid polypeptide (c.), which is cleaved into a light chain (L – coloured red) and a heavy chain joined by a disulphide bridge between Cys430 and Cys454 residues (shown in black). The heavy chain contains an N-terminal translocation domain (HN – coloured light green) and a C-terminal receptor binding domain, which is formed of two subunits (HC-N and HC-C coloured dark and light blue respectively). The light chain is the catalytic domain and consists of a zinc metalloprotease (the Zn2+ atom shown in yellow). The light chain is encircled by a hydrophobic peptide belt domain of the HN (coloured in dark green in c.). Crystal structures were prepared using UCSF ChimeraX (PBD ID: 3BTA) with a. displayed in ribbon format and b. a spacefill representation [Citation198,Citation202].](/cms/asset/bd97d302-939a-464a-9119-c442d032711a/kvir_a_2205251_f0003_oc.jpg)
The mature BoNT forms a progenitor complex (PC) with one or more neurotoxin associated proteins (NAPs) through non-covalent interactions [Citation214]. In all serotypes, the BoNT forms an interlocking heterodimer with the non-toxic non-hemagglutinin (NTNH) protein, which is structurally similar to the toxin but lacking the zinc-binding motif characteristic of the clostridial neurotoxin metalloproteases [Citation215,Citation216] (). The BoNT-NTNH dimer complex associates with a number of other NAPs. In some strains, the NAPs are hemagglutinin proteins (HA) and in others OrfX proteins (OrfX1, OrfX2, OrfX3, and P47) [Citation42]. The OrfX proteins are found in BoNT/E, BoNT/F and some BoNT/A serotypes such as BoNT/A2. The HAs are found in BoNT/B, BoNT/C, BoNT/D, BoNT/G, and all BoNT/A1 serotypes, although one HA− OrfX+ A1 strain has been described [Citation42,Citation217].
Figure 4. Structure of the progenitor complex (PC) and BoNT-NTNH heterodimer. The NTNH and BoNT interlock to form a heterodimer with the catalytic light chain positioned on the exterior, held in place by the peptide belt of the heavy chain translocation domain (HN). The HA proteins assemble to form a symmetric tripod structure (B.). The NTNH interacts with the three HA70 proteins of each “arm” to complete the PC. Crystal structure of BoNT/A1- NTNH/A1 is shown in the bottom right and was prepared with UCSF ChimeraX with the BoNT heavy chain coloured dark red (PDB ID:3VOA) [Citation202,Citation216].
![Figure 4. Structure of the progenitor complex (PC) and BoNT-NTNH heterodimer. The NTNH and BoNT interlock to form a heterodimer with the catalytic light chain positioned on the exterior, held in place by the peptide belt of the heavy chain translocation domain (HN). The HA proteins assemble to form a symmetric tripod structure (B.). The NTNH interacts with the three HA70 proteins of each “arm” to complete the PC. Crystal structure of BoNT/A1- NTNH/A1 is shown in the bottom right and was prepared with UCSF ChimeraX with the BoNT heavy chain coloured dark red (PDB ID:3VOA) [Citation202,Citation216].](/cms/asset/821959ef-c5d6-4b3f-ac82-db1e5b1e8ab7/kvir_a_2205251_f0004_oc.jpg)
In most BoNT/A1 strains, including the model strain ATCC 3502 which was the first C. botulinum to have its genome sequenced in 2007, the progenitor complex is formed of three HA proteins named; HA33, HA17, and HA70, the number referring to the protein size in kilodaltons (alternatively named HA1, HA2, and HA3, respectively) [Citation217,Citation218]. The PC is thought to be approximately 760 kDa in a 1:1:3:3:6 stoichiometric ratio (BoNT:NTNH:HA17:HA70:HA33). The HAs form a trigonal three-armed structure on which the BoNT-A1/NTNH-A1 complex sits, to make a “phage-like” structure () [Citation219]. This structure suggests that the NTNH protects the toxin from chemical or enzymatic attack, such as low pH conditions and proteases associated with oral ingestion or present within decaying biological matter [Citation15,Citation216]. Conversely, the HA complex is believed to form a docking station on the intestinal lumen, as they contain multiple-carbohydrate binding sites, to allow the efficient transport of the BoNT across the intestinal epithelium into the bloodstream [Citation220–223]. Additionally, the HA proteins are known to disrupt the intestinal epithelial barrier through binding of E-cadherin between epithelial cells, compromising the integrity of the epithelium and facilitating absorption of the toxin increasing oral toxicity [Citation224–227]. The PC structures formed by the Orf proteins remain unknown but are thought to perform a similar function to the HA proteins, having been shown to be involved in lipid binding [Citation228,Citation229]. In culture, BoNT/A1 PCs have been found to exist in three different forms; a 12S M-complex (~300 kDa; BoNT and NTNH only), 16S L-complex (~760 kDa; BoNT, NTNH, HA17, HA33, and HA70) and 19S LL-complex (~900 kDa; a dimer of 16S L-PCs). The 19S complexes are thought to only occur in high concentration [Citation219,Citation230].
Mechanism of action
With the aid of the PC, the BoNT enters the blood circulation, via the lymphatic system if ingested, and disperses throughout the host tissues, localizing at peripheral nerve endings [Citation231]. A dual receptor-binding approach to a polysialoganglioside (PSG) and a synaptic vesicle receptor (Syt or gSV2C) allows the BoNT to selectively target the presynaptic membrane of these nerves [Citation232,Citation233]. The receptor-bound toxin then enters the presynaptic cell via receptor mediated endocytosis (RME) [Citation234]. The neurotransmitter refilling of the synaptic vesicles creates an acidic pH, causing a structural change of the heavy-chain translocation domain to form a pore-like structure, which allows translocation of the unfolded light chain from the vesicle to the neuronal cytosol [Citation235–239]. In the cytosol, the interchain di-sulphide bond is reduced, releasing the catalytic light chain of the toxin, which subsequently folds to allow specific cleavage of their SNARE targets [Citation240,Citation241]. The SNARE proteins are part of the neurotransmitter exocytosis machinery, which allows fusion of the neurotransmitter vesicle and the neural membrane [Citation242–244]. Cleavage of these SNARE proteins blocks neurotransmitter release into the neuromuscular junction, preventing postsynaptic excitation [Citation245]. This results in the classical flaccid paralysis or muscle weakness observed in botulism cases. The overal mechanism of action is summarised in .
Figure 5. The mechanism of BoNTs at nerve endings. The signalling between the pre and postsynaptic cell is mediated by small molecules called neurotransmitters, at neuromuscular junctions (NMJ) this is acetylcholine. The neurotransmitter is stored in membrane bound synaptic vesicles inside the neuronal cytosol. Endocytosis of the empty vesicles triggers a V-ATPase proton pump to generate a pH gradient across the vesicular membrane, which drives the newly synthesised neurotransmitter molecules to enter the vesicles. The loaded vesicles bind to the interleaflet of the presynaptic membrane by the VAMP and synaptotagmin proteins in a process called docking. On docking, SNARE complexes form around the vesicle, and facilitate the fusing of the vesicle and the membrane in mechanism known as priming. As a result of a Ca2+ influx, caused by the depolarisation of the presynaptic nerve, SNARE proteins undergo a conformational change to allow the primed vesicle to release its neurotransmitter into the synaptic cleft. Through diffusion the neurotransmitter binds to receptors on the postsynaptic cell membrane, causing its excitation. This cycle is then repeated in readiness for future depolarisations. However, BoNT blocks neurotransmitter release from the presynaptic nerve terminal. The toxin is endocytosed along with empty vesicles and the light chain is translocated into the neuronal cytosol. This catalytic protein then cleavages SNARE proteins on the vesicular membrane or inner leaflet of the presynaptic membrane, dependent on the toxin serotype. This prevents vesicle docking and fusion to the presynaptic membrane and subsequently the release of neurotransmitter.
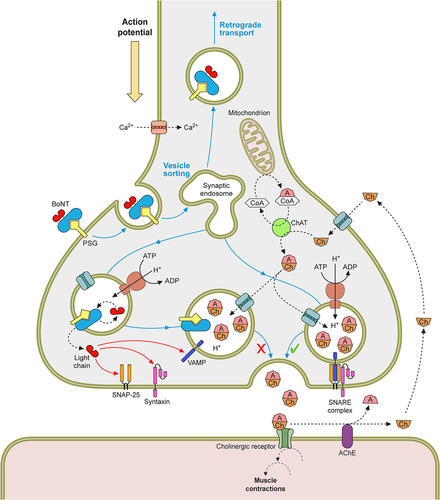
The BoNT SNARE protein targets include the vesicle associated membrane protein (VAMP – overwise known as synaptobrevin), syntaxin, and the synaptosomal-associated protein of 25 kDa (SNAP-25) [Citation246]. As the name suggests, VAMP is anchored on the outer membrane of the synaptic vesicle, whereas syntaxin and SNAP-25 are located on the inner leaflet of the neuronal membrane. Dependant on the serotype of the toxin, the light-chain metalloprotease cleaves the SNARE proteins at alternative peptide bonds, removing a segment of the cytosolic proteins and preventing the formation of the SNARE complex essential for neuronal vesicle docking and priming to the inner presynaptic membrane [Citation245]. These alternative SNARE targets and cleavage sites are summarized in .
Figure 6. Alternative SNARE cleavage sites of BoNT serotypes. Specific residues cleaved in each of the SNARE targets and their respective BoNT serotypes are shown in the table.
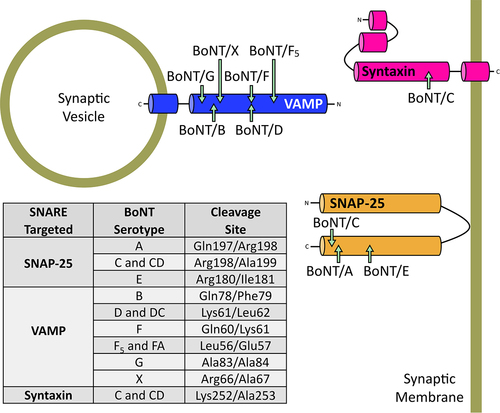
Evidence strongly supports that the long duration of BoNT/A intoxication results from the retention of the toxin within the nerve termini [Citation247]. However, some data suggests that short nature of the BoNT/A SNAP-25 truncation may play a contributing factor to its prolonged action [Citation248]. The cleavage of SNAP-25 by BoNT/E, known to cause the shortest exocytosis blockade of only lasts 1–4 weeks in humans, removes a 26 residue segment from its C-terminus and does not allow for SNARE-complex formation [Citation61,Citation249]. Whereas in the case of BoNT/A serotypes, the cleavage of SNAP-25 removes only a small segment (9 residues) of the protein. This truncated SNAP-25 is still able to form a SNARE complex, yet this SNARE complex is non-functional, and is thought to give the cleaved protein a dominant negative effect, which enhances the persistence of BoNT/A function in neurons [Citation247,Citation250].
Historically, it was thought that BoNT exclusively acted at peripheral nerve terminals. Substantial evidence now suggests that some degree of retrograde transport occurs, similar to that of TeNT, which can give the toxin long-distance effects [Citation251]. Axonal transport of BoNTs has been demonstrated in several studies, predominantly through the use of animal models, for instance where cleaved SNARE proteins were detected in neurons at least two synapses from the site of injection [Citation252]. Evidence has also been presented in therapeutic studies in humans where BoNT activity has been observed distal to the site of peripheral muscular injection, e.g. through reduction in tibial nerve reflex following injection in the ankle plantar flexor [Citation253,Citation254]. Effects on the central nervous system (CNS) which have been observed following administration of BoNT can broadly be separated into two categories, indirect and direct. Many studies have demonstrated that BoNTs may indirectly affect the functional organization of the CNS through altered peripheral inputs, i.e. that the blockade of the neuromuscular junction (NMJ) has a knock-on effect, which may bring about plastic adaptive reorganization of the neuron.
Although the neurotoxin action at the CNS is not conclusive, there is increasing evidence of a direct effect of BoNTs on the CNS through retrograde transport, whereby following intramuscular injection, a small proportion of the BoNT travels into the axon rather than remain at the NMJ [Citation255]. In one such study, the presence of cleaved SNAP25 in a mouse model has been observed through immunostaining in sections of hind paw, to sciatic nerve, dorsal root ganglia, and spinal cord, a strong indication for axonal transport of the BoNT along peripheral nerve to the spinal cord [Citation256,Citation257]. Although the application of these new findings to BoNT-based therapeutics targeting specific regions of the CNS is still in its infancy, it is an exciting and developing area, which may open up promising treatments for challenging neurodegenerative disorders.
Neurotoxin gene regulation
The genes encoding the botulinum neurotoxin and its non-toxic accessory proteins are always located proximal to each other, and this locus is termed the neurotoxin gene cluster. The location and overall composition of genes that comprise the cluster varies between strains, although the ntnh is always located upstream of the bont toxin gene and is co-expressed alongside the toxin in the same operon [Citation258] (). The conservation of the ntnh alongside the bont gene suggests they arose from a common ancestor, possibly through a gene duplication event [Citation259,Citation260]. The other accessory protein genes are generally located in a divergent polycistronic operon and consist of three genes grouped into two categories; the orfX genes or the ha genes. In addition to orfX1, orfX2,orfX3, an additional gene, p47 (encoding a 47kDa protein), is always found in the cluster upstream of the ntnh [Citation42]. There is no structural homology between the two sets of accessory proteins, and the function of the OrfX and P47 proteins is poorly understood. The role of OrfX-P47 may be similar to that of HA proteins in that they facilitate binding and transport of the PC into circulation through the intestinal barrier [Citation223]. However, the fact that orfX-p47 clusters have been found in numerous other unrelated bacteria and are frequently associated with other bacterial toxins suggests that their function is not exclusive to BoNT pathogenicity [Citation261].
Figure 7. Botulinum neurotoxin gene cluster variation in a representative selection of neurotoxic strains. the diversity in the gene clusters and flanking regions, with ha clusters at the top and orfX at the bottom. The location of the gene cluster is indicated, if not chromosomal, on the left. The Group I strains either have the classical ha70-ha17-ha33-botR-ntnh-bont genes or the orfX3-orfX2-orfX1-botR-p47-ntnh-bont cluster. Group III are contained in ha clusters, although the alternative sigma factor gene, botR, is upstream of ha70. Group IV C. argentinese strains also have ha accessory proteins but the ha70 and ha33 genes are inverted relative to the conventional structure. Group II strains (with the exception of bont/B4 containing a ha70-ha17-ha33-botR-ntnh-bont cluster) have orfX3-orfX2-orfX1-p47-ntnh-bont clusters, which lack the botR gene completely; which is also observed in C. baratii F7 and the neurotoxic C. butyricum strains [Citation267,Citation268]. This diversity is likely attributable to recombination events and horizontal gene transfer between strains, which have also led to neurotoxin gene clusters present in non-Clostridium sp. such as Enterococcus sp. 3G1_DIV0629 and Weissella oryzae SG25 [Citation59]. The Weissella neurotoxin gene is present without accessory genes, the ntnh-like gene downstream of bont/Wo lacks significant domains compared to the well conserved ntnh [Citation269]. The insertion elements (IS) and flagellin genes flanking and internal to the gene clusters indicate the probable method of transfer into the strain as it evolved.
![Figure 7. Botulinum neurotoxin gene cluster variation in a representative selection of neurotoxic strains. the diversity in the gene clusters and flanking regions, with ha clusters at the top and orfX at the bottom. The location of the gene cluster is indicated, if not chromosomal, on the left. The Group I strains either have the classical ha70-ha17-ha33-botR-ntnh-bont genes or the orfX3-orfX2-orfX1-botR-p47-ntnh-bont cluster. Group III are contained in ha clusters, although the alternative sigma factor gene, botR, is upstream of ha70. Group IV C. argentinese strains also have ha accessory proteins but the ha70 and ha33 genes are inverted relative to the conventional structure. Group II strains (with the exception of bont/B4 containing a ha70-ha17-ha33-botR-ntnh-bont cluster) have orfX3-orfX2-orfX1-p47-ntnh-bont clusters, which lack the botR gene completely; which is also observed in C. baratii F7 and the neurotoxic C. butyricum strains [Citation267,Citation268]. This diversity is likely attributable to recombination events and horizontal gene transfer between strains, which have also led to neurotoxin gene clusters present in non-Clostridium sp. such as Enterococcus sp. 3G1_DIV0629 and Weissella oryzae SG25 [Citation59]. The Weissella neurotoxin gene is present without accessory genes, the ntnh-like gene downstream of bont/Wo lacks significant domains compared to the well conserved ntnh [Citation269]. The insertion elements (IS) and flagellin genes flanking and internal to the gene clusters indicate the probable method of transfer into the strain as it evolved.](/cms/asset/29918186-d3f5-4b59-9072-a8a0cd957d43/kvir_a_2205251_f0007_oc.jpg)
Located between these two divergent operons is the botR gene, previously termed orf21, which encodes a 21 kDa alternative sigma factor 70 (σ [Citation70] that is responsible for the positive regulation of the entire toxin cluster, equivalent to tetR in C. tetani [Citation262]. Binding sites for the BotR sigma factor are found in the promoters of cluster operons, binding directly to the core RNA polymerase enzyme, controlling the expression of the entire cluster. A BotR binding site is also found in the upstream promoter region of the botR gene itself, suggesting a positive regulatory loop of the entire gene cluster [Citation263–265]. However, botR is not present in toxin clusters of BoNT/E producing strains, neurotoxic C. baratii, BoNT/X, and eBoNT/J strains, suggesting another form of regulation present in these cases [Citation44,Citation59,Citation266].
In the C. baratii BoNT/F7 strain lacking botR, the promoters of the neurotoxin cluster were surprisingly similar to that of BotR regulated promoters. Immediately upstream of the neurotoxin gene cluster, a UviA-like regulatory protein was discovered with a similar DNA-binding-domain to that of σ [Citation70] family, suggesting this UviA-like protein may act as an alternative sigma factor for the toxin cluster expression [Citation270]. Interestingly, the toxin cluster promotor PorfX1 of non-proteolytic BoNT/E and BoNT/F6 strains in which botR is also absent, does not share the highly conserved−35 promoter elements of σ [Citation70] alternative sigma factors. Rather, the promoters share the primary sigma factor recognition sequence (σA), inferring the neurotoxin cluster in these strains may be regulated by σA rather than an alternate σ [Citation70] family sigma factor [Citation270].
The neurotoxin cluster can be found on different genetic elements; chromosomal, bacteriophage or plasmid borne. C. botulinum strains producing C and D or C/D, D/C mosaic toxin types are encoded on bacteriophages, which tend not to integrate into the genome but exist as a plasmid prophage. The prophages exhibit unstable lysogeny, known as pseudo-lysogeny, and can be easily lost through passage resulting in a non-toxigenic strain [Citation271,Citation272]. Alternatively, bont genes, including those of C. botulinum Group I, II, and IV strains, are frequently found on plasmids ranging in size from ~ 48kb for pCLL of Eklund 17B to ~ 270 kb for pCLJ of strain 657 [Citation273–275]. The presence of the toxin gene on a plasmid is widespread with serotypes BoNT A, B, E, F, and G and in strains carrying multiple BoNT types [Citation273,Citation276–279]. The bontB gene in particular is found to be often encoded on a plasmid, in one study 32 out of 60 strains BoNT/B producing strains carried the bontB as such [Citation275]. It has been demonstrated that these bont-bearing plasmids can be transferred via conjugation across the different physiological Groups of C. botulinum and even into nontoxigenic clostridial strains [Citation280,Citation281]. A rare integration event has recently been identified in which an entire bont-harbouring plasmid was inserted into the chromosome in three distinct species by homologous recombination [Citation282].
When chromosomally encoded, the cluster appears to be located at specific positions in the genome with unifying characteristics [Citation283,Citation284]. Noticeably, regions in the cluster neighborhood frequently contain insertion sequence (IS) elements or genes associated with transposition or recombination. IS elements usually encode transposases, which mediate their mobility. The IS elements flanking Group I neurotoxin clusters are, however, no longer intact suggesting the insertion of the cluster occurred early in the strain’s evolution [Citation273]. The presence of flagellin genes in close proximity to the neurotoxin cluster in some strains is in line with the observation that they appear to be recombination hotspots in other bacterial species [Citation285,Citation286]. Through sequence analysis of multiple C. botulinum strains, it has been shown that the BoNT gene clusters and the rest of the respective genomes evolved independently of one another [Citation91,Citation287]. This evidence has compounded the role of horizontal gene transfer in the acquisition and evolution of BoNT producing strains, giving reason for the diversity seen within and between the different groups of bacteria able to produce the BoNT.
The expression and production of BoNT is growth phase dependent, peaking at late exponential-early stationary phase and rapidly declining through stationary phase [Citation288,Citation289]. In 2006, environmental temperature was shown to control BotR regulation, and since then several positive and negative regulatory systems have been described responsible for the tight control of bont expression [Citation290–292]. Evidence suggests that the agr quorum sensing system has a regulatory effect on BoNT production, and numerous two component regulatory systems (TCSs) have been found to both positively and negatively regulate toxin expression [Citation293–295]. The production of BoNT is also influenced by nutritional availability, with increased levels of arginine repressing BoNT expression and conversely a decrease in glucose concentration also reducing BoNT expression [Citation296–299]. The approximate 1000-fold reduction in BoNT/A production in the presence of high concentrations of arginine was found to be a due to a pH shift to more alkaline conditions as an indirect consequence of the catabolism of arginine. It was hypothesized that the production of neurotoxin is regulated on a post-translational level by an uncharacterized pH-dependent protease produced by the bacteria that only functions under alkaline conditions [Citation300]. Regulation of metabolism and BoNT expression have been shown to be linked by the global regulator, CodY, which binds to the ntnh-bontA promoter region to positively regulate its expression [Citation301]. The regulation of toxin production by nutrient and quorum sensing signals was integrated into a computational model of toxin production by Ihekwaba and colleagues in 2016 [Citation302].
Sporulation and germination of C. botulinum
In addition to the neurotoxin, a key virulence factor in the pathogenesis of C. botulinum and prevalence of botulism, is the sporulation and germination survival strategy. This capability enables the bacterium to protect itself from adverse environmental conditions as a dormant and metabolically inert endospore, returning to vegetative cell growth once conditions become favorable, through the process of germination. It is precisely these processes that enable the ubiquitous environmental persistence of C. botulinum and its infiltration and neurotoxin production in the specific environmental niches associated with the different forms of botulism [Citation10].
Owing to the genetic and metabolic diversity between C. botulinum Groups I-IV, there exists an array of sporulation and germination mechanisms and kinetics within the species botulinum.
Sporulation
A paradigm for bacterial sporulation has been established from an extensive study of the model spore former, Bacillus subtilis, aiding in the prediction and elucidation of the sporulation genes and pathways of Gram-positive spore forming bacteria [Citation303,Citation304].
The factors, which initiate the morphological changes required for the process of sporulation remain ambiguous, but are likely to be stimuli representative of hostile microenvironments, such as extremes of temperature, oxygen, ultraviolet radiation, and desiccation; threatening the survival of cells unable to maintain vegetative growth [Citation305]. The cell to cell communication agrBD quorum sensing system may also play a role in the initiation of sporulation, triggered through assessment of cell population density [Citation293].
The sporulation process is initiated when the DNA-binding response regulator Stage 0 sporulation protein, Spo0A, becomes phosphorylated. In C. botulinum, the phosphorylation of Spo0A occurs directly by a yet to be determined orphan sensor histidine kinase [Citation306]. This mechanism is thought to have evolved from an ancestral phosphorelay system, lost through convergent and reductive evolution [Citation307]. Phosphorylated Spo0A activates the transcription of stage I and II sporulation genes as well as regulatory sigma factors. These early-stage genes halt binary cell division, inducing the formation of an asymmetric septum and the morphogenesis of the forespore within the mother cell [Citation308]. Spo0A has been demonstrated to be actively expressed in the exponential growth phase in the Group I C. botulinum ATCC 3502, and subsequently the expression of the sigma factors essential for sporulation increases in the stationary phase as expression levels of Spo0A decrease [Citation309,Citation310]. As well as having a role in sporulation initiation, Spo0A is also hypothesized as a regulator of toxin production, having been observed to bind directly to the promoter upstream of the botE gene in the Group II type E C. botulinum Beluga strain [Citation311].
Late-stage sporulation genes are temporally and spatially separated between the mother cell and forespore, with co-ordinated activities between the distinct compartments. These later stages of sporulation involve the sequential addition of multiple surrounding layers and forespore engulfment by the mother cell. The outer layers of the spore; the cortex, outer membrane, and spore coat contribute to the resistance properties of the spore [Citation312,Citation313].
Finally, prior to the release of the mature spore, the exosporium envelopes the spore, a structure, which is thought to enhance resilience and pathogenicity through hydrophobic interactions facilitating adhesion. Spores of C. botulinum exhibit varying levels of tolerance to environmental stressors, with Group I strains producing highly heat-resistant spores, Group III spores exhibiting moderate heat resistance, and Group II spores the least resistant to heat, hypothesized to be a consequence of diverse exosporia morphotypes () [Citation314].
Figure 8. Sporulation and germination of the C. botulinum Groups. The vegetative cell enters the sporulation program when environmental stressors are encountered. Following lysis of the mother cell, the mature spore is released into the extracellular environment, with diverse spore morphotypes. The elucidated spore morphotypes are presented for isolates of Groups I-III. All spore sub-types possess the fundamental spore core (Co), cortex (Cx) and coat (Ct). Exosporium (Exo) present in Group I (thick and loose fitting) and Group III (thin and tight fitting). Appendages (App) present in Group II spore isolates. The diverse germination mechanisms of Groups I-IV: germinant receptors (GR) and core lytic enzymes (CLE).
Germination
Germination is the process of spore recrudescence to neurotoxin producing vegetative cells, crucial to the pathogenesis of C. botulinum and botulism [Citation315]. Improper preservation and storage of food enabling the survival and subsequent germination of C. botulinum spores is a potentially lethal series of events in food-borne botulism, where the neurotoxin is ingested. Toxicoinfection may result from the germination of C. botulinum spores in environmental favorable conditions, such as the anaerobic environments of a deep wound, or in the underdeveloped infant gut following ingestion of spores, as well as in adults with a depleted gut microbiota.
The germination of spores is triggered by the interaction of small nutrient molecules, termed germinants, with receptors embedded in the inner membrane of the spore [Citation316]. The spores of Groups I and II initiate germination in response to the stereospecific interaction of certain amino acids with germinant receptors [Citation317,Citation318]. Following the binding of a germinant to its cognate germinant receptor, monovalent cations, and dipicolinic acid are released, prior to hydrolysis of the cortex peptidoglycan by core lytic enzymes. This results in core rehydration and vegetative cell outgrowth [Citation319].
As with sporulation, the germination mechanisms of the groups making up the C. botulinum species are diverse and not fully understood. Two separate pathways have been hypothesized for Groups I and III and Groups II and IV involving subtly distinct germinant receptors and core lytic enzymes () [Citation317]. Furthermore, the functional germinant receptor essential for spore germination of Group II C. botulinum has been identified [Citation320].
Concluding remarks
Although cases of botulism have decreased over the last decade, C. botulinum remains a notable pathogen of importance to public health, not least due to the potential bioterrorism risk and the extensive pharmaceutical applications of the BoNT. The relevance of BoNT in the medical and cosmetic fields has stimulated research interest in pathogens. Due to dangers associated with handling the highly toxic neurotoxin and its listing as a select biological agent, research into this pathogen is restricted to only a few authorized laboratories across the world. This has been the main barrier to elucidating a greater understanding of the pathogen. An established set of genetic tools to produce mutants in the bacteria is now available [Citation321–324] which can allow the development of safe, non-toxigenic strains of C. botulinum, which may offer the possibility for further work outside of these select facilities in the future [Citation325].
Central to its pathogenicity and virulence is the BoNT, whose pathological mechanism of action is reliant on inhibition of the release of neurotransmitters at nerve terminals. Although the structural architecture is generally well defined for many serotypes, the molecular mechanisms of the neurotoxins are far from fully elucidated, including the molecular dynamics of the neurotoxin within the cell. The function of the neurotoxin accessory proteins, particularly the OrfX proteins, and their role in virulence remains unknown. Some evidence suggests that the toxin may also have action at the CNS, via retrograde transport of the toxin following entry via the presynaptic nerve membrane. Further research is required to fully understand the mechanism behind this, which may lead to targeted CNS-based therapies in the future. A greater understanding at the molecular level could open the possibilities for further structure-based engineering of the toxin, producing recombinant toxin forms with enhanced clinical efficacy, safer production methods of pharmaceutical BoNTs, and targeted cellular delivery due to BoNTs binding specificity [Citation326–330]. Numerous regulators of neurotoxin expression in the bacterium have been identified, but a full picture of the regulatory network underpinning neurotoxin production would remain useful for public safety measures and pharmaceutical synthesis.
Second only to the neurotoxin in C. botulinum virulence factors is the bacteria’s ability to sporulate, allowing persistence through challenging and diverse environments. Gaining a greater understanding of the mechanisms responsible for this crucial part of the pathogen’s life cycle could lead to more preventatives and therapies. Specific germination agents, although well defined for many other clostridia, remain largely unknown in the case of C. botulinum; exclusion of these germinant molecules from high-risk foods and preservation methods could be a valuable preventative strategy. Further elucidation of germinant mechanisms and receptors of C. botulinum spores could also yield novel preventatives. The development of new botulism treatments and diagnostics has remained an active area and will hopefully provide greater safety to populations in the event of a bioterror attack, as well as allow improved ethical surveillance of imminent risks.
The decreasing cost of next-generation sequencing and resultant availability of high-quality genome sequences has highlighted the genetic diversity within the species. The heterogeneity of neurotoxin gene clusters has given clues regarding the horizontal transfer of the neurotoxin locus between distantly related strains. The single-species taxonomy of 1950s has led to a convoluted classification of C. botulinum strains, and there is now a compelling argument for the strict implementation of a more rigorous taxonomic system of nomenclature, as the field continues to discover more strains of this important pathogen.
Disclosure statement
No potential conflict of interest was reported by the authors.
Data availability statement
Data sharing is not applicable to this article as no new data were created or analysed in this study.
Additional information
Funding
References
- Hauschild AHW. Clostridium botulinum. In: Doyle M, editor. Foodborne bacterial pathogens. New York: Marcel Dekker Inc; 1989. pp. 111–28.
- Hatheway CL. Botulism. In: Barlows A, WJ Hausler Jr., M Ohashi, A Turano, Lennete EH, editors. Laboratory diagnosis of infectious diseases. New York: Springer; 1988. p. 111–133.
- Sobel J. Botulism. Clin Infect Dis. 2005;41(8):1167–1173.
- Johnson EA. Clostridium botulinum. In: Doyle M, Buchanan R, editors. Food Microbiology. Washington DC: ASM Press; 2019. pp. 487–512.
- Johnson EA. Clostridium botulinum and the Most Poisonous Poison. In: Gurtler J, Doyle M, Kornacki J, editors. Foodborne Pathogens. Cam, Switzerland: Springer; 2017. pp.553–601.
- Rossetto O, Pirazzini M, Fabris F, et al. Botulinum Neurotoxins: Mechanism of Action . Handbook of Experimental Pharmacology. 2021;263 :35–47.
- Hauschild AHW. Clostridium botulinum. In: editors, Hauschild A, and K Dodds. Ecology and Control in Foods. 1st ed. Boca Raton: CRC Press; 1993.
- Minton NP, Clarke DJ. Clostridia. New York: Plenum; 1989.
- Hatheway CL. Toxigenic clostridia. Clinical Microbiology Reviews. 1990;3(1):66–98.
- Peck MW. Biology and Genomic Analysis of Clostridium botulinum. Adv Microb Physiol. 2009;55:183–320.
- Shukla HD, Sharma SK. Clostridium botulinum: a bug with beauty and weapon. Crit Rev Microbiol. 2005;31(1):11–18.
- Nigam P, Nigam A. Botulinum toxin. Indian J Dermatol. 2010;55(1):8–14.
- Dorizas A, Krueger N, Sadick NS. Aesthetic uses of the botulinum toxin. Dermatol Clin. 2014;32(1):23–36.
- Chen S. Clinical uses of botulinum neurotoxins: current indications, limitations and future developments. Toxins (Basel). 2012;4:913–939.
- Rossetto O, Pirazzini M, Montecucco C. Botulinum neurotoxins: genetic, structural and mechanistic insights. Nature Rev Microbiol. 2014;12(8):535–549.
- Lund BM, Peck M. Clostridium botulinum. In: Lund BM, Baird-Parker AC, GW Gould, editors. The Microbiological Safety and Quality of Food. Maryland, MD, USA: Aspen; 2000. pp. 1057–1109.
- Erbguth FJ. From poison to remedy: the chequered history of botulinum toxin. J Neural Transm. 2008;115(4):559–565.
- Kerner J Neue beobachtungen über die in württemberg so häufig vorfallenden tödlichen vergiftungen durch den genuss geräucherter würste. Tübingen, Oisander 1820.
- Erbguth FJ, Naumann M. Historical aspects of botulinum toxin: justinus Kerner (1786-1862) and the sausage poison. Neurology. 1999;53(8):1850.
- Erbguth FJ. Historical notes on botulism, Clostridium botulinum, botulinum toxin, and the idea of the therapeutic use of the toxin. Mov Disord. 2004;19(S8):S2–6.
- van Ermengem E. A New Anaerobic Bacillus and Its Relation to Botulism. Clin Infect Dis. 1979;1(4):701–719.
- Winslow CE, Broadhurst J, Buchanan RE, et al. THE families and genera of the bacteria preliminary report of the committee of the society of American bacteriologists on characterization and classification of bacterial types. J Bacteriol. 1917;2(5):505.
- Burke GS. The Occurrence of Bacillus botulinus in Nature. J Bacteriol. 1919;4(5):541.
- Sommer H, Nealon PJ, Snipe PT. Studies on Botulinum Toxin: 4. Dialysis Experiments. J Infect Dis. 1928;43(2):161–166.
- Lamanna C, Eklund HW, McElroy OE. Botulinum Toxin (Type A); Including a Study of Shaking with Chloroform as a Step in the Isolation Procedure. J Bacteriol. 1946;52(1):1.
- Burgen ASV, Dickens F, Zatman LJ. The action of botulinum toxin on the neuromuscular junction. Journal of Physiology. 1949;109(1–2):10–24.
- Schiavo GG, Benfenati F, Poulain B, et al. Tetanus and botulinum-B neurotoxins block neurotransmitter release by proteolytic cleavage of synaptobrevin. Nature. 1992;359(6398):832–835.
- Schantz EJ, Johnson EA. Botulinum toxin: the story of its development for the treatment of human disease. Perspect Biol Med. 1997;40(3):317–327.
- Pellett S. Learning from the past: historical aspects of bacterial toxins as pharmaceuticals. Curr Opin Microbiol. 2012;15(3):292–299.
- Scott AB. Botulinum toxin injection to correct strabism. Trans Am Ophthalmol Soc. 1979;79:924–927.
- Scott AB. Botulinum Toxin Injection into Extraocular Muscles as an Alternative to Strabismus Surgery. Ophthalmol. 1980;87(10):1044–1049.
- Schantz EJ, Scott AB. Use of crystalline type a botulinum toxin in medical research. In: Lewis E, editor. Biomedical Aspects of Botulism. San Diego, CA: Academic Press; 1981. pp. 143–150.
- Ting PT, Freiman A. The story of Clostridium botulinum: from food poisoning to Botox. Clin Med. 2004;4:258–261.
- Monheit GD, Pickett A. AbobotulinumtoxinA: a 25-year history. Aesthet Surg J. 2017;37(suppl_1):S4–11.
- Choudhury S, Baker MR, Chatterjee S, et al. Botulinum toxin: an update on pharmacology and newer products in development. Toxins (Basel). 2021;13(1):58.
- Frevert J, Ahn KY, Park MY, et al. Comparison of botulinum neurotoxin type a formulations in Asia. 2018;11:327.
- Duplantier J, A DK, Bavari C, et al. Searching for therapeutics against botulinum neurotoxins: a true challenge for drug discovery. Curr Top Med Chem. 2016;16(21):2330–2349.
- Hill KK, Smith TJ, Helma CH, et al. Genetic diversity among botulinum neurotoxin-producing clostridial strains. J Bacteriol. 2007;189(3):818–832.
- Holdeman LV, Brooks JB. Variation among strains of Clostridium botulinum and related clostridia. In: Herzberg M, editor. Proceedings of the 1st US-Japan Conference on Toxic Microorganisms. Washington, DC.: U.S. Government Printing Office; 1970. p. 278–286.
- Collins C, East E. Phylogeny and taxonomy of the food-borne pathogen Clostridium botulinum and its neurotoxins. J Appl Microbiol. 1998;84:5–17.
- Peck MW, Smith TJ, Anniballi F, et al. Historical perspectives and guidelines for botulinum neurotoxin subtype nomenclature. Toxins (Basel). 2017;9(1):38.
- Hill KK, Smith TJ. Genetic Diversity Within Clostridium botulinum Serotypes, Botulinum Neurotoxin Gene Clusters and Toxin Subtypes. Curr Top Microbiol Immunol. 2012;364:1–20.
- Suen JC, Hatheway CL, Steigerwalt AG, et al. Clostridium argentinense sp. nov.: a genetically homogeneous group composed of all strains of Clostridium botulinum toxin type G and some nontoxigenic strains previously identified as Clostridium subterminale or Clostridium hastiforme. Int J Bacteriol. 1988;38(4):375–381.
- Zhang S, Masuyer G, Zhang J, et al. Identification and characterization of a novel botulinum neurotoxin. Nat Commun. 2017;8(1):1–10.
- Dover N, Barash JR, Hill KK, et al. Molecular characterization of a novel botulinum neurotoxin type H gene. J Infect Dis. 2014;209(2):192–202.
- Kalb SR, Baudys J, Raphael BH, et al. Functional Characterization of Botulinum Neurotoxin Serotype H as a Hybrid of Known Serotypes F and a (BoNT F/A). Anal Chem. 2015;87(7):3911–3917.
- Maslanka SE, Lúquez C, Dykes JK, et al. A novel botulinum neurotoxin, previously reported as Serotype H, has a hybrid-like structure with regions of similarity to the structures of serotypes a and F and is neutralized with Serotype a antitoxin. J Infect Dis. 2016;213(3):379–385.
- Leuchs J. Beiträge zur Kenntnis des Toxins und Antitoxins des Bacillus botulinus. Zeitschrift für Hygiene und Infektionskrankheiten. 1910;76(1):55–84.
- Smith TJ, Lou J, Geren IN, et al. Sequence variation within botulinum neurotoxin serotypes impacts antibody binding and neutralization. Infect Immun. 2005;73(9):5450–5457.
- Rossetto O, Montecucco C. Tables of Toxicity of Botulinum and Tetanus Neurotoxins. Toxins (Basel). 2019;11(12):686.
- Franciosa G, Floridi F, Maugliani A, et al. Differentiation of the gene clusters encoding botulinum neurotoxin type a complexes in Clostridium botulinum type A, Ab, and A(B) strains. Appl Environ Microbiol. 2004;70(12):7192–7199.
- Moriishi K, Koura M, Fujii N, et al. Molecular cloning of the gene encoding the mosaic neurotoxin, composed of parts of botulinum neurotoxin types C1 and D, and PCR detection of this gene from Clostridium botulinum type C organisms. Appl Environ Microbiol. 1996;62(2):662.
- Moriishi K, Koura M, Abe N, et al. Mosaic structures of neurotoxins produced from Clostridium botulinum types C and D organisms 1. Biochimica Et Biophysica Acta (BBA) - Gene Structure and Expression. 1996;1307(2):123–126.
- Nakamura K, Kohda T, Umeda K, et al. Characterization of the D/C mosaic neurotoxin produced by Clostridium botulinum associated with bovine botulism in Japan. Vet Microbiol. 2010;140:147–154.
- Schleberger C, Hochmann H, Barth H, et al. Structure and action of the binary C2 toxin from Clostridium botulinum. J Mol Biol. 2006;364:705–715.
- Sakaguchi Y, Suzuki T, Yamamoto Y, et al. Genomics of Clostridium botulinum group III strains. Res Microbiol. 2015;166:318–325.
- Zornetta I, Azarnia Tehran D, Arrigoni G, et al. The first non Clostridial botulinum-like toxin cleaves VAMP within the juxtamembrane domain. Sci Rep. 2016;6.
- Zhang S, Lebreton F, Mansfield MJ, et al. Identification of a Botulinum Neurotoxin-like Toxin in a Commensal Strain of Enterococcus faecium. Cell Host & Microbe. 2018;23(2):169–176.e6.
- Brunt J, Carter AT, Stringer SC, et al. Identification of a novel botulinum neurotoxin gene cluster in Enterococcus. FEBS Lett. 2018;592(3):310–317.
- Wentz TG, Muruvanda T, Lomonaco S, et al. Closed genome sequence of Chryseobacterium piperi strain CTMT/ATCC BAA- 1782, a Gram-negative bacterium with clostridial neurotoxin-like coding sequences. Genome Announc. 2017; 5(48).
- Eleopra R, Tugnoli V, Rossetto O, et al. Different time courses of recovery after poisoning with botulinum neurotoxin serotypes a and E in humans. Neurosci Lett. 1998;256:135–138.
- Keller JE. Recovery from botulinum neurotoxin poisoning in vivo. Neuroscience. 2006;139(2):629–637.
- Seddon HR. Bulbar Paralysis in Cattle Due to the Action of a Toxicogenic Bacillus, with a Discussion on the Relationship of the Condition to Forage Poisoning (Botulism). J Comp Pathol Ther. 1922;35:147–190.
- Skarin H, Håfström T, Westerberg J, et al. Clostridium botulinum group III: a group with dual identity shaped by plasmids, phages and mobile elements. BMC Genomics. 2011;12(1):185.
- Smith T, Williamson CHD, Hill K, et al. Botulinum neurotoxin-producing bacteria. Isn’t it time that we called a species a species? MBio. 2018;9(5).
- Tamura K, Stecher G, Kumar S. MEGA11: molecular Evolutionary Genetics Analysis Version 11. Mol Biol Evol. 2021;38(7):3022–3027.
- Janik E, Ceremuga M, Bijak JS, et al. Biological toxins as the potential tools for bioterrorism. Int J Mol Sci. 2019;20.
- Carus WS. Bioterrorism and Biocrimes: the Illicit Use of Biological Agents Since 1900. . Washington DC, USA: Center for Counterproliferation Research, National Defense University; 2001. p. 209.
- U.K. Government. Schedule 5 Pathogens and toxins [Internet]. 2001 [cited 20 October 2022]. Available from: http://www.legislation.gov.uk/ukpga/2001/24/contents.
- Centers for Disease Control and Prevention (CDC). Bioterrorism Agents/Diseases [Internet]. 2018 [cited 20 October 2022]; Available from: https://emergency.cdc.gov/agent/agentlist-category.asp.
- Josko D. Botulin toxin: a weapon in terrorism. Clin. Lab Sci. 2004;17:30–34.
- Patocka J, Splino M, Merka V. Botulism and bioterrorism: how serious is this problem? Acta medica (Hradec Králové). Acta Medica (Hradec Kralove). 2005;48(1):23–28.
- Arnon SS, Schechter R, V IT, et al. Botulinum toxin as a biological weapon. JAMA. 2001;285:1059–1070.
- Smith TJ, Roxas-Duncan V, Smith L. Botulinum neurotoxins as biothreat agents. J Bioterror Biodef. 2012;2:S2:003.
- Zilinskas RA. Iraq’s Biological Weapons. JAMA. 1997;278:418–424.
- Villar RG, Elliott SP, Davenport KM. Botulism: the Many Faces of Botulinum Toxin and its Potential for Bioterrorism. Infect Dis Clin North Am. 2006;20:313–327.
- Kazdobina IS. Stability of botulin toxins in solutions and beverages. Gig Sanit. 1995;1:9–12.
- Siegel LS. Destruction of Botulinum Toxins in Food and Water. In: Hauschild A, Dodds K, editors. Ecology and Control in Foods. 1st ed. Boca Raton: CRC Press; 1993. pp. 323–341.
- Cenciarelli O, Riley PW, Baka A. Biosecurity Threat Posed by Botulinum Toxin. Toxins (Basel). 2019;11(12):681.
- Johnson EA, Montecucco C. Botulism. In: Andrew GE, editor. Handbook of Clinical Neurology. Vol. 91. Amsterdam, NL: Elsevier; 2008. p. 333–368. DOI:10.1016/S0072-9752(07)01511-4
- Centers for Disease Control and Prevention (CDC). National Botulism Surveillance Summary, 2017 [Internet]. [cited 20 Occtober 2022]. Available from: https://www.cdc.gov/botulism/surv/2017/index.html
- Gangarosa EJ, Donadio JA, Armstrong RW, et al. Botulism in the United States, 1899–1969. Am J Epidemiol. 1971;93(2):93–101.
- Shapiro RL. Botulism in the United States: a clinical and epidemiologic review. Ann internal med. 1998;129(3):221–228.
- Sobel J, Tucker N, Sulka A, et al. Foodborne Botulism in the United States, 1990–2000. Emerg Infect Dis. 2004;10(9):1606–1611.
- Critchley EM. A comparison of human and animal botulism: a review. J R Soc Med. 1991;84(5):295.
- Hughes JM, Blumenthal JR, Merson MH, et al. Clinical features of types a and B food-borne botulism. Ann Intern Med. 1981;95:442–445.
- Lund BM, Peck MW. Heat resistance and recovery of spores of non-proteolytic Clostridium botulinum in relation to refrigerated, processed foods with an extended shelf-life. J Appl Bacteriol. 1994;76:115S–128S.
- Peck MW. Clostridium botulinum and the safety of refrigerated processed foods of extended durability. Trends Food Sci Technol. 1997;8:186–192.
- McLauchlin J, Grant KA, Little CL. Food-borne botulism in the United Kingdom. J Public Health. 2006;28(4):337–342.
- International Commission on Microbiological Specifications for Foods (ICMSF). Clostridium botulinum. In: Roberts TA, Baird-Parker AC, Tompkin RB, editors. Microorganisms in Foods 5: characteristics of microbial pathogens. London, UK: Blackie Academic & Professional; 1996. p. 68–111.
- Carter AT, Peck MW. Genomes, neurotoxins and biology of Clostridium botulinum Group I and Group II. Res Microbiol. 2015;166(4):303–317.
- Peck MW. Clostridium botulinum and the safety of minimally heated, chilled foods: an emerging issue? J Appl Microbiol. 2006;101(3):556–570.
- Peck MW, Stringer SC. The safety of pasteurised in-pack chilled meat products with respect to the foodborne botulism hazard. Meat Sci. 2005;70(3):461–475.
- Stumbo CR, Purohit KS, Ramakrishnan TV. Thermal process lethality guide for low‐acid foods in metal containers. J Food Sci. 1975;40(6):1316–1323.
- Lindström M, Kiviniemi K, Korkeala H. Hazard and control of group II (non-proteolytic) Clostridium botulinum in modern food processing. Int J Food Microbiol. 2006;108:92–104.
- Juliao PC, Maslanka S, Dykes J, et al. National outbreak of type a foodborne botulism associated with a widely distributed commercially canned hot dog chili sauce. Clin Infect Dis. 2013;56(3):376–382.
- Rocke TE, Samuel MD. Water and Sediment Characteristics Associated with Avian Botulism Outbreaks in Wetlands. J Wildl Manage. 1999;63(4):1249.
- Espelund M, Klaveness D. Botulism outbreaks in natural environments – an update. Front Microbiol. 2014;5:287.
- Duncan RM, Jensen WI. A relationship between avian carcasses and living invertebrates in the epizootiology of avian botulism. J Wildl Dis. 1976;12:116–126.
- Johnson AL, McAdams-Gallagher SC, Aceto H. Accuracy of a Mouse Bioassay for the Diagnosis of Botulism in Horses. J Vet Intern Med. 2016;30:1293–1299.
- Cagan E, Peker E, Dogan M, et al. Infant Botulism. Eurasian J Med. 2010;42(2):92.
- Fox CK, Keet CA, Strober JB. Recent advances in infant botulism. Pediatr Neurol. 2005;32(3):149–154.
- Tanzi MG, Gabay MP. Association between honey consumption and infant botulism. Pharmacotherapy. 2002;22(11):1479–1483.
- Arnon SS, Midura TF, Damus K, et al. Honey and other environmental risk factors for infant botulism. J Paediatr. 1979;94(2):331–336.
- Aureli P, Franciosa G, Fenicia L. Infant botulism and honey in Europe: a commentary. Pediatr Infect Dis J. 2002;21(9):866–868.
- Brett MM, McLauchlin J, Harris A, et al. A case of infant botulism with a possible link to infant formula milk powder: evidence for the presence of more than one strain of Clostridium botulinum in clinical specimens and food. J Med Microbiol. 2005;54:769–776.
- Nevas M, Lindstrom M, Virtanen A, et al. Infant botulism acquired from household dust presenting as sudden infant death syndrome. J Clin Microbiol. 2005;43(1):511–513.
- Midura TF, Arnon SS. Infant botulism. Identification of Clostridium botulinum and its toxins in faeces. Lancet. 1976;308(7992):934–936.
- Koepke R, Sobel J, Arnon SS. Global occurrence of infant botulism, 1976-2006. Pediatrics. 2008;122(1):122.
- Centers for Disease Control and Prevention (CDC). National Botulism Surveillance [Internet]. 2018 [cited 20th October 2022]. Available from: https://www.cdc.gov/botulism/surveillance.html.
- Davis JB, Mattman LH, Wiley M. Clostridium botulinum in a Fatal Wound Infection. J Am Med Assoc. 1951;146:646–648.
- Merson MH, Dowell VR. Epidemiologic, Clinical and Laboratory Aspects of Wound Botulism. N Engl J Med. 1973;289:1005–1010.
- Elston HR, Wang M, Loo LK. Arm abscesses caused by Clostridium botulinum. J Clin Microbiol. 1991;29:2678.
- Brett MM, Hood J, Brazier JS, et al. Soft tissue infections caused by spore-forming bacteria in injecting drug users in the United Kingdom. Epidemiol Infect. 2005;133:575–582.
- Kuehn B. Wound Botulism Outbreak. JAMA. 2019;321(6):538.
- Werner SB, Passaro D, McGee J, et al. Wound Botulism in California, 1951–1998: recent Epidemic in Heroin Injectors. Clin Infect Dis. 2000;31:1018–1024.
- Passaro DJ, Werner SB, McGee J, et al. Wound botulism associated with black tar heroin among injecting drug users. J Am Med Assoc. 1998;279:859–863.
- McCroskey LM, Hatheway CL. Laboratory findings in four cases of adult botulism suggest colonization of the intestinal tract. J Clin Microbiol. 1988;26(5):1052.
- Fenicia L, Anniballi F, Aureli P. Intestinal toxemia botulism in Italy, 1984-2005. Eur J Clin Microbiol Infect Dis. 2007;26:385–394.
- Harris RA, Anniballi F, Austin JW. Adult Intestinal Toxemia Botulism. Toxins (Basel). 2020;12(2):81./81.
- Bakheit AMO, Ward CD, McLellan DL. Generalised botulism-like syndrome after intramuscular injections of botulinum toxin type A: a report of two cases [3]. J Neurol Neurosurg Psychiatry. 1997;62(2):198.
- Chertow DS, Tan ET, Maslanka SE, et al. Botulism in 4 adults following cosmetic injections with an unlicensed, highly concentrated botulinum preparation. JAMA. 2006;296(20):2476–2479.
- Crowner BE, Brunstrom JE, Racette BA. Iatrogenic botulism due to therapeutic botulinum toxin a injection in a pediatric patient. Clin Neuropharmacol. 2007;30(5):310–313.
- Holzer E. Botulism caused by inhalation. Medizinische Klin. 1962;57:1735–1738.
- Park JB, Simpson LL. Inhalational poisoning by botulinum toxin and inhalation vaccination with its heavy-chain component. Infect Immun. 2003;71(3):1147–1154.
- Pitt MLM, LeClaire RD. Pathogenesis by Aerosol. In: Lebeda F , Korch G, Lindler L, editors. Biological Weapons Defense. Totowa, NJ: Humana Press; 2005. pp. 65–78.
- O’Horo JC, Harper EP, El Rafei A, et al. Efficacy of Antitoxin Therapy in Treating Patients with Foodborne Botulism: a Systematic Review and Meta-analysis of Cases, 1923-2016. Clin Infect Dis. 2017;66:S43–56.
- Rao AK, Sobel J, Chatham-Stephens K, et al. Clinical Guidelines for Diagnosis and Treatment of Botulism, 2021. MMWR Recommendations Rep. 2021;70(2):1.
- Yu PA, Lin NH, Mahon BE, et al. Safety and Improved Clinical Outcomes in Patients Treated with New Equine-Derived Heptavalent Botulinum Antitoxin. Clin Infect Dis. 2018;66(suppl_1):S57–64.
- Barker D, Gillum KT, Niemuth NA, et al. Therapeutic efficacy of equine botulism heptavalent antitoxin against all seven botulinum neurotoxins in symptomatic guinea pigs. PLoS ONE. 2019;14:e0222670. DOI:10.1371/journal.pone.0222670
- Lonati D, Schicchi A, Crevani M, et al. Foodborne Botulism: clinical diagnosis and medical treatment. Toxins (Basel). 2020;12(8):509.
- Ben David A, Barnea A, Torgeman A, et al. Immunologic and Protective Properties of Subunit- vs. Whole Toxoid-Derived Anti-Botulinum Equine Antitoxin. Vaccines. 2022;10(9):1522.
- US Food and Drug Administration (FDA). Approval History, Letters, Reviews, and Related Documents - BAT (Botulism Antitoxin Heptavalent (A B, C D, E F, G)) (Equine) [Internet]. 2013 [cited 20th October 2022]; Available from: https://www.fda.gov/vaccines-blood-biologics/approved-blood-products/bat-botulism-antitoxin-heptavalent-b-c-d-e-f-g-equine
- Atassi MZ. Immune recognition of BoNTs a and B: how anti-toxin antibodies that bind to the heavy chain obstruct toxin action. Toxicon. 2009;54(5):600–613.
- Tacket CO, Shandera WX, Mann JM, et al. Equine antitoxin use and other factors that predict outcome in type a foodborne botulism. Am j med. 1984;76(5):794–798.
- Black RE, Gunn RA. Hypersensitivity reactions associated with botulinal antitoxin. Am j med. 1980;69(4):567–570.
- Pirazzini M, Rossetto O. Challenges in searching for therapeutics against Botulinum Neurotoxins. Expert Opin Drug Discov. 2017;12(5):497–510.
- Schussler E, Sobel J, Hsu J, et al. Workgroup Report by the Joint Task Force Involving American Academy of Allergy, Asthma & Immunology (AAAAI); Food Allergy, Anaphylaxis, Dermatology and Drug Allergy (FADDA) (Adverse Reactions to Foods Committee and Adverse Reactions to Drugs, Biologicals, and Latex Committee); and the Centers for Disease Control and Prevention Botulism Clinical Treatment Guidelines Workgroup—Allergic Reactions to Botulinum Antitoxin: a Systematic Review. Clin Infect Dis an off Publ Infect Dis Soc Am. 2018;66:S65.
- Arnon SS, Schechter R, Maslanka SE, et al. Human Botulism Immune Globulin for the Treatment of Infant Botulism. N Engl J Med. 2006;354(5):462–471.
- US Food and Drug Administration (FDA). BabyBIG [Internet]. 2003 [cited 20th October 2022]; Available from: https://www.fda.gov/vaccines-blood-biologics/approved-blood-products/babybig
- Fenicia L, Anniballi F. Infant botulism. Ann Ist Super Sanita. Annali dell’Istituto superiore di sanita. 2009;45(2):134–146.
- Payne JR, Khouri JM, Jewell NP, et al. Efficacy of Human Botulism Immune Globulin for the Treatment of Infant Botulism: the First 12 Years Post Licensure. J Paediatr. 2018;193:172–177.
- Schulte M, Hamsen U, Schildhauer TA, et al. Effective and rapid treatment of wound botulism, a case report. BMC Surg. 2017;17(1):103.
- Brook I. Infant botulism. Journal of Perinatology. 2007;27(3):175–180.
- Arnon SS. Botulism as an intestinal toxemia. In: S BM, RJ PD GH GR, editors. Infections of the gastrointestinal tract. New York: Raven Press; 1995. pp. 257–271.
- Fagan RP, Neil KP, Sasich R, et al. Initial Recovery and Rebound of Type F Intestinal Colonization Botulism After Administration of Investigational Heptavalent Botulinum Antitoxin. Clin Infect Dis. 2011;53(9):e125–8.
- Rasetti-Escargueil C, Popoff MR. Antibodies and vaccines against botulinum toxins: available measures and novel approaches. Toxins (Basel). 2019;11:528. DOI:10.3390/toxins11090528
- Thanongsaksrikul J, Chaicumpa W. Botulinum neurotoxins and botulism: a novel therapeutic approach. Toxins (Basel). 2011;3(5):469–488.
- Nowakowski A, Wang C, Powers DB, et al. Potent neutralization of botulinum neurotoxin by recombinant oligoclonal antibody. Proc Natl Acad Sci, USA. 2002;99(17):11346–11350.
- Fan Y, Garcia-Rodriguez C, Lou J, et al. A three monoclonal antibody combination potently neutralizes multiple botulinum neurotoxin serotype F subtypes. PLoS ONE. 2017;12:e0174187. DOI:10.1371/journal.pone.0174187
- Snow DM, Riling K, Kimbler A, et al. Safety and Pharmacokinetics of a Four Monoclonal Antibody Combination Against Botulinum C and D Neurotoxins. Antimicrob Agents Chemother. 2019;63:e01270–19. DOI:10.1128/AAC.01270-19
- Matsumura T, Amatsu S, Misaki R, et al. Fully Human Monoclonal Antibodies Effectively Neutralizing Botulinum Neurotoxin Serotype B. Toxins (Basel). 2020;12(5):12.
- Garcia-Rodriguez C, Razai A, Geren IN, et al. A Three Monoclonal Antibody Combination Potently Neutralizes Multiple Botulinum Neurotoxin Serotype E Subtypes. Toxins (Basel). 2018;10(3):10.
- Godakova SA, Noskov AN, Vinogradova ID, et al. Camelid VHHs Fused to Human Fc Fragments Provide Long Term Protection Against Botulinum Neurotoxin a in Mice. Toxins (Basel). 2019;11:464. DOI:10.3390/toxins11080464
- Mukherjee J, Ondeck CA, Tremblay JM, et al. Intramuscular delivery of formulated RNA encoding six linked nanobodies is highly protective for exposures to three Botulinum neurotoxin serotypes. Sci Rep. 2022;12(1):12.
- Derkaev AA, Ryabova EI, Esmagambetov IB, et al. rAAV expressing recombinant neutralizing antibody for the botulinum neurotoxin type a prophylaxis. Front Microbiol. 2022;13.
- Rusnak JM, Smith LA. Botulinum neurotoxin vaccines: past history and recent developments. Hum Vaccines. 2009;5(12):794–805.
- Smith LA. Botulism and vaccines for its prevention. Vaccine. 2009;27 Suppl 4:D33–9. DOI:10.1016/j.vaccine.2009.08.059
- Graham R, Thorp F. The Effect of Formalin on Botulinum Toxins A, B and C. J Immunol. 1929;16(4):391–401.
- Keller JE. Characterization of new formalin-detoxified botulinum neurotoxin toxoids. Clin Vaccine Immunol. 2008;15(9):1374–1379.
- Centers for Disease Control and Prevention (CDC). Notice of CDC’s discontinuation of investigational pentavalent (ABCDE) botulinum toxoid vaccine for workers at risk for occupational exposure to botulinum toxins. Morb Mortal Wkly Rep. 2011;60:1454–1455.
- Sundeen G, Barbieri JT. Vaccines against botulism. Toxins (Basel). 2017;9(9):268.
- Hatami F, Shokouhi S, Mardani M, et al. Early recovery of botulism: one decade of experience. Clin Toxicol. 2021;59(7):628–632.
- De Paiva A, Meunier FA, Molgó J, et al. Functional repair of motor endplates after botulinum neurotoxin type a poisoning: biphasic switch of synaptic activity between nerve sprouts and their parent terminals. Proc Nat Acad Sci. 1999;96(6):3200–3205.
- Holland RL, Brown MC. Nerve growth in botulinum toxin poisoned muscles. Neuroscience. 1981;6(6):1167–1179.
- Adler M, Franz DR. Toxicity of botulinum neurotoxin by inhalation: implications in bioterrorism. In: Salem H, Katz S, editors. Aerobiology: the Toxicology of Airborne Pathogens and Toxins. Cambridge, U.K: Royal Society of Chemistry; 2016. pp. 167–185.
- Meunier FA, Schiavo G, Molgó J. Botulinum neurotoxins: from paralysis to recovery of functional neuromuscular transmission. J Physiol Paris. 2002;96(1–2):105–113.
- Tsai YC, Maditz R, Kuo C-L, et al. Targeting botulinum neurotoxin persistence by the ubiquitin-proteasome system. Proc Nat Acad Sci. 2010;107(38):16554–16559.
- Tsai YC, Kotiy A, Kiris E, et al. Deubiquitinating enzyme VCIP135 dictates the duration of botulinum neurotoxin type a intoxication. Proc Nat Acad Sci. 2017;114(26):E5158–66.
- Sen E, Kota KP, Panchal RG, et al. Screening of a Focused Ubiquitin-Proteasome Pathway Inhibitor Library Identifies Small Molecules as Novel Modulators of Botulinum Neurotoxin Type a Toxicity. Front Pharmacol. 2021;12:2659.
- Rao AK, Lin NH, Jackson KA, et al. Clinical Characteristics and Ancillary Test Results Among Patients with Botulism—United States, 2002–2015. Clin Infect Dis. 2017;66(suppl_1):S4–10.
- Chatham-Stephens K, Fleck-Derderian S, Johnson SD, et al. Clinical Features of Foodborne and Wound Botulism: a Systematic Review of the Literature, 1932-2015. Clin Infect Dis. 2017;66:S11–6.
- Louis ME S, Peck SHS, Bowering D, et al. Botulism from chopped garlic: delayed recognition of a major outbreak. Ann Intern Med. 1988;108:363–368.
- Maslanka SE, Solomon HM, Sharma S, et al. Clostridium botulinum and its toxins. In: Tortorello M, F Downes, S Doores, K Ito, and Y Salfinger, editors. Compendium of methods for the microbiological examination of foods . Washington, DC: American Public Health Association; 2013. p. Chapter 32:1–11. DOI:10.2105/MBEF.0222.037
- Solomon HM, Lilly TJ. Chapter 17: Clostridium Botulinum. In: Jackson GJ , Merker RI, Bandler R, editors. Bacteriological Analytical Manual. 8th Ed. College Park, MD, USA: U.S. Food and Drug Administration (FDA), Centre for Food Safety and Applied Nutrition; 2001. https://www.fda.gov/food/laboratory-methods-food/bam-chapter-17-clostridium-botulinum
- Lindström M, Korkeala H. Laboratory diagnostics of botulism. Clin. Clinical Microbiology Reviews. 2006;19(2):298–314.
- Taylor K. Botulinum toxin testing on animals is still a Europe-wide issue. ALTEX. 2019;36(1):81–90.
- Kakinuma H, Maruyama H, Yamakawa K, et al. Application of nested polymerase chain reaction for the rapid diagnosis of infant botulism type B. Pediatr Int. 1997;39(3):346–348.
- De Medici D, Anniballi F, Wyatt GM, et al. Multiplex PCR for Detection of Botulinum Neurotoxin-Producing Clostridia in Clinical, Food, and Environmental Samples. Appl Environ Microbiol. 2009;75(20):6457.
- Szabo EA, Pemberton JM, Gibson AM, et al. Application of PCR to a clinical and environmental investigation of a case of equine botulism. J Clin Microbiol. 1994;32(8):1986–1991.
- Chellapandi P, Prisilla A. PCR-based molecular diagnosis of botulism (types C and D) outbreaks in aquatic birds. Ann Microbiol. 2018;68(12):835–849.
- Cai S, Singh BR, Sharma S. Botulism diagnostics: from clinical symptoms to in vitro assays. Crit Rev Microbiol. 2007;33(2):109–125.
- Thirunavukkarasu N, Johnson E, Pillai S, et al. Botulinum Neurotoxin Detection Methods for Public Health Response and Surveillance. Front Bioeng Biotechnol. 2018;6:80.
- Wictome M, Newton KA, Jameson K, et al. Development of in vitro assays for the detection of botulinum toxins in foods. FEMS Immunology & Medical Microbiology. 1999;24(3):319–323.
- Cheng LW, Land KM, Stanker LH. Chapter 1: Presence of Botulinum Neurotoxins in Food and Other Biological Samples. In: Morse SA, editor. Bioterrorism. Online Edition. London, UK: InTechOpen; 2012. p. 1–16. DOI:10.5772/33638
- Singh AK, Stanker LH, Sharma SK. Botulinum neurotoxin: where are we with detection technologies? Crit Rev Microbiol. 2013;39(1):43–56.
- Čapek P, Dickerson TJ. Sensing the deadliest toxin: technologies for botulinum neurotoxin detection. Toxins (Basel). 2010;2(1):24–53.
- Fernández-Salas E, Wang J, Molina Y, et al. Botulinum Neurotoxin Serotype a Specific Cell-Based Potency Assay to Replace the Mouse Bioassay. PLoS ONE. 2012;7(11):e49516.
- Pharma M. Press Release: alternative Test Method for Botulinum Neurotoxin Now Approved in Europe [Internet]. 2015 [cited 20th October 2022]; Available from: https://www.merz.com/blog/news/alternative-test-method-for-botulinum-neurotoxin-now-approved-in-europe/
- Ipsen Inc. Press Release: ipsen’s Cell-Based Assay Receives Approvals in the E.U. and Switzerland for its Botulinum Toxin [Internet]. 2018 [cited 20th October 2022]; Available from: https://www.ipsen.com/websites/IPSENCOM-PROD/wp-content/uploads/2018/08/28165732/00-IAW-ONLINE-POSITION-STATEMENT_Ipsens-CBA-implementation-2018-08-27.pdf
- Pellett S. Progress in Cell Based Assays for Botulinum Neurotoxin Detection. Curr Top Microbiol Immunol. 2013;364:257–285.
- Koh C-Y, Schaff UY, Piccini ME, et al. Centrifugal microfluidic platform for ultrasensitive detection of botulinum toxin. Anal Chem. 2015;87(2):922–928.
- Halliwell J, Gwenin C. A label free colorimetric assay for the detection of active botulinum neurotoxin type a by SNAP-25 conjugated colloidal gold. Toxins (Basel). 2013;5(8):1381–1391.
- Patel K, Halevi S, Melman P, et al. A novel surface plasmon resonance biosensor for the rapid detection of botulinum neurotoxins. Biosens (Basel). 2017;7(4):32.
- Savage AC, Buckley N, Halliwell J, et al. Botulinum neurotoxin serotypes detected by electrochemical impedance spectroscopy. Toxins (Basel). 2015;7(5):1544–1555.
- Hobbs RJ, Thomas CA, Halliwell J, et al. Rapid Detection of Botulinum Neurotoxins—A Review. Toxins (Basel). 2019;11(7):11.
- Smith TJ, Hill KK, Raphael BH. Historical and current perspectives on Clostridium botulinum diversity. Res Microbiol. 2015;166(4):290–302.
- Lacy DB, Tepp W, Cohen AC, et al. Crystal structure of botulinum neurotoxin type a and implications for toxicity. Nat Struct Biol. 1998;5(10):898–902.
- Swaminathan S, Eswaramoorthy S. Structural analysis of the catalytic and binding sites of Clostridium botulinum neurotoxin B. Nat Struct Biol. 2000;7(8):693–699.
- Kumaran D, Eswaramoorthy S, Furey W, et al. Domain organization in Clostridium botulinum neurotoxin type E is unique: its implication in faster translocation. J Mol Biol. 2009;386(1):233–245.
- Dekleva ML, Dasgupta BR. Purification and characterization of a protease from Clostridium botulinum type a that nicks single-chain type a botulinum neurotoxin into the di-chain form. J Bacteriol. 1990;172(5):2498–2503.
- Pettersen EF, Goddard TD, Huang CC, et al. UCSF ChimeraX: structure visualization for researchers, educators, and developers. Protein Sci. 2021;30:70–82.
- Bandyopadhyay S, Clark AW, DasGupta BR, et al. Role of the heavy and light chains of botulinum neurotoxin in neuromuscular paralysis. J Biol Chem. 1987;262(6):2660–2663.
- Kriegistein KG, DasGupta BR, Henschen AH. Covalent structure of botulinum neurotoxin type A: location of sulfhydryl groups, and disulfide bridges and identification of C-termini of light and heavy chains. Journal of Protein Chemistry. 1994;13(1):49–57.
- Montecucco C, Schiavo G. Structure and Function of Tetanus and Botulinum Neurotoxins. Q Rev Biophys. 1995;28(4):423–472.
- Pirazzini M, Montecucco C, Rossetto O. Toxicology and pharmacology of botulinum and tetanus neurotoxins: an update. Arch Toxicol. 2022;96(6):1521–1539.
- Yao G, Zhang S, Mahrhold S, et al. N-linked glycosylation of SV2 is required for binding and uptake of botulinum neurotoxin a. Nature Structural & Molecular Biology. 2016;23(7):656–662.
- Muraro L, Tosatto S, Motterlini L, et al. The N-terminal half of the receptor domain of botulinum neurotoxin a binds to microdomains of the plasma membrane. Biochem Biophys Res Commun. 2009;380(1):76–80.
- Montecucco C, Zanotti G. Botulinum neurotoxin A1 likes it double sweet. Nature Structural & Molecular Biology. 2016;23:619–621.
- Rummel A. Two feet on the membrane: uptake of clostridial neurotoxins. Curr Top Microbiol Immunol. 2017;406:1–37.
- Brunger AT, Breidenbach MA, Jin R, et al. Botulinum Neurotoxin Heavy Chain Belt as an Intramolecular Chaperone for the Light Chain. PLOS Pathog. 2007;3:e113.
- Galloux M, Vitrac H, Montagner C, et al. Membrane interaction of botulinum neurotoxin a translocation (T) domain: the belt region is a regulatory loop for membrane interaction. J Biol Chem. 2008;283:27668–27676.
- Fischer A, Montal M. Molecular dissection of botulinum neurotoxin reveals interdomain chaperone function. Toxicon. 2013;75:101–107.
- Gu S, Jin R. Assembly and Function of the Botulinum Neurotoxin Progenitor Complex. Curr Top Microbiol Immunol. 2013;364:21–44.
- Barrett AJ, Rawlings ND. Types and families of endopeptidases. Biochem Soc Trans. 1991;19:707–715.
- Gu S, Rumpel S, Zhou J, et al. Botulinum neurotoxin is shielded by NTNHA in an interlocked complex. Science. 2012;335:977–981.
- Jacobson MJ, Lin G, Raphael B, et al. Analysis of neurotoxin cluster genes in Clostridium botulinum strains producing botulinum neurotoxin serotype a subtypes. Appl Environ Microbiol. 2008;74:2778–2786.
- Sebaihia M, Peck MW, Minton NP, et al. Genome sequence of a proteolytic (Group I) Clostridium botulinum strain Hall a and comparative analysis of the clostridial genomes. Genome Res. 2007;17(7):1082–1092.
- Lee K, Gu S, Jin L, et al. Structure of a Bimodular Botulinum Neurotoxin Complex Provides Insights into Its Oral Toxicity. PLOS Pathogens. 2013;9(10):e1003690.
- Fujinaga Y, Inoue K, Watanabe S, et al. The haemagglutinin of Clostridium botulinum type C progenitor toxin plays an essential role in binding of toxin to the epithelial cells of guinea pig small intestine, leading to the efficient absorption of the toxin. Microbiology. 1997;143(12):3841–3847.
- Fujinaga Y, Inoue K, Nomura T, et al. Identification and characterization of functional subunits of Clostridium botulinum type a progenitor toxin involved in binding to intestinal microvilli and erythrocytes. FEBS Lett. 2000;467(2–3):179–183.
- Matsumura T, Sugawara Y, Yutani M, et al. Botulinum toxin a complex exploits intestinal M cells to enter the host and exert neurotoxicity. Nat Commun. 2015;6(1):6.
- Matsumura T, Fujinaga Y. Functional Analysis of Botulinum Hemagglutinin (HA). Methods Mol Biol. 2020;2132:191–200.
- Jin Y, Takegahara Y, Sugawara Y, et al. Disruption of the epithelial barrier by botulinum haemagglutinin (HA) proteins – differences in cell tropism and the mechanism of action between HA proteins of types a or B, and HA proteins of type C. Microbiology. 2009;155(1):35–45.
- Sugawara Y, Matsumura T, Takegahara Y, et al. Botulinum hemagglutinin disrupts the intercellular epithelial barrier by directly binding E-cadherin. J Cell Bio. 2010;189(4):691–700.
- Lee K, Zhong X, Gu S, et al. Molecular basis for disruption of E-cadherin adhesion by botulinum neurotoxin a complex. Science. 2014;344(6190):1405–1410.
- Fujinaga Y, Popoff MR. Translocation and dissemination of botulinum neurotoxin from the intestinal tract. Toxicon. 2018;147:13–18.
- Gustafsson R, Berntsson RP-A, Martínez-Carranza M, et al. Crystal structures of OrfX2 and P47 from a Botulinum neurotoxin OrfX-type gene cluster. FEBS Lett. 2017;591(22):3781–3792.
- Lam K-H, Qi R, Liu S, et al. The hypothetical protein P47 of Clostridium botulinum E1 strain Beluga has a structural topology similar to bactericidal/permeability-increasing protein. Toxicon. 2018;147:19–26.
- Inoue K, Fujinaga Y, Watanabe T, et al. Molecular composition of Clostridium botulinum type a progenitor toxins. Infect Immun. 1996;64(5):1589–1594.
- Simpson L. The life history of a botulinum toxin molecule. Toxicon. 2013;68:40–59.
- Montecucco C. How do tetanus and botulinum toxins bind to neuronal membranes? Trends Biochem Sci. 1986;11(8):314–317.
- Rummel A. Double Receptor Anchorage of Botulinum Neurotoxins Accounts for their Exquisite Neurospecificity. Curr Top Microbiol Immunol. 2013;364:61–90.
- Pirazzini M, Rossetto O, Bolognese P, et al. Double anchorage to the membrane and intact inter-chain disulfide bond are required for the low pH induced entry of tetanus and botulinum neurotoxins into neurons. Cell Microbiol. 2011;13(11):1731–1743.
- Cottone G, Chiodo L, Maragliano L, et al. In Silico Conformational Features of Botulinum Toxins A1 and E1 According to Intraluminal Acidification. Toxins (Basel). 2022;14(9):644. 2022. .
- Moriyama Y, Futai M. H±atpase, a primary pump for accumulation of neurotransmitters, is a major constituent of brain synaptic vesicles. Biochem Biophys Res Commun. 1990;173:443–448.
- Fischer A. Synchronized Chaperone Function of Botulinum Neurotoxin Domains Mediates Light Chain Translocation into Neurons. Curr Top Microbiol Immunol. 2013;364:115–137.
- Koriazova LK, Montal M. Translocation of botulinum neurotoxin light chain protease through the heavy chain channel. Nat Struct Biol. 2003;10(1):13–18.
- Hoch DH, Romero-Mira M, Ehrlich BE, et al. Channels formed by botulinum, tetanus, and diphtheria toxins in planar lipid bilayers: relevance to translocation of proteins across membranes. Proc Nat Acad Sci. 1985;82(6):1692–1696.
- Fischer A, Montal M. Crucial role of the disulfide bridge between botulinum neurotoxin light and heavy chains in protease translocation across membranes. J Biol Chem. 2007;282:29604–29611.
- Pirazzini M, Bordin F, Rossetto O, et al. The thioredoxin reductase-thioredoxin system is involved in the entry of tetanus and botulinum neurotoxins in the cytosol of nerve terminals. FEBS Lett. 2013;587(2):150–155.
- Sutton RB, Fasshauer D, Jahn R, et al. Crystal structure of a SNARE complex involved in synaptic exocytosis at 2.4 Å resolution. Nature. 1998;395(6700):347–353.
- Jahn R, Scheller RH. Snares — engines for membrane fusion. Nat Rev Mol Cell Biol. 2006;7(9):631–643.
- Südhof TC, Rizo J. Synaptic vesicle exocytosis. Cold Spring Harbor Perspect Biol. 2011;3(12):3.
- Binz T. Clostridial Neurotoxin Light Chains: devices for SNARE Cleavage Mediated Blockade of Neurotransmission. Curr Top Microbiol Immunol. 2013;364:139–157.
- Rossetto O, Schiavo G, Montecucco C, et al. SNARE motif and neurotoxins. Nature. 1994;372(6505):415–416.
- Shoemaker CB, Oyler GA. Persistence of Botulinum neurotoxin inactivation of nerve function. Curr Top Microbiol Immunol. 2013;364:179–196.
- Keller JE, Neale EA. The Role of the Synaptic Protein SNAP-25 in the Potency of Botulinum Neurotoxin Type a. J Biol Chem. 2001;276(16):13476–13482.
- Pantano S, Montecucco C. The blockade of the neurotransmitter release apparatus by botulinum neurotoxins. Cell Mol Life Sci. 2014;71(5):793–811.
- Pirazzini M, Rossetto O, Eleopra R, et al. Botulinum neurotoxins: biology, pharmacology, and toxicology. Pharmacol Rev. 2017;69:200–235.
- Caleo M, Schiavo G. Central effects of tetanus and botulinum neurotoxins. Toxicon. 2009;54(5):593–599.
- Restani L, Novelli E, Bottari D, et al. Botulinum neurotoxin a impairs neurotransmission following retrograde transynaptic transport. Traffic. 2012;13(8):1083–1089.
- Marchand-Pauvert V, Aymard C, Giboin L-S, et al. Beyond muscular effects: depression of spinal recurrent inhibition after botulinum neurotoxin a. Journal of Physiology. 2013;591(4):1017.
- Aymard C, Giboin L-S, Lackmy-Vallée A, et al. Spinal plasticity in stroke patients after botulinum neurotoxin a injection in ankle plantar flexors. Physiological Reports. 2013;1(6):e00173.
- Mazzocchio R, Caleo M. More than at the neuromuscular synapse: actions of botulinum neurotoxin a in the central nervous system. Neuroscientist. 2015;21(1):44–61.
- Marinelli S, Vacca V, Ricordy R, et al. The Analgesic Effect on Neuropathic Pain of Retrogradely Transported botulinum Neurotoxin a Involves Schwann Cells and Astrocytes. PLoS ONE. 2012;7(10):e47977.
- Luvisetto S. Botulinum Neurotoxins in Central Nervous System: an Overview from Animal Models to Human Therapy. Toxins (Basel). 2021;13(11):751.
- Popoff MR, Marvaud J. Structural and genomic features of clostridial neurotoxins. In: Alouf JE J Freer, editors. The Comprehensive Sourcebook of Bacterial Protein Toxins. London, UK: Academic Press; 1999. pp. 174–201.
- Doxey AC, Lynch MDJ, Müller KM, et al. Insights into the evolutionary origins of clostridial neurotoxins from analysis of the Clostridium botulinum strain a neurotoxin gene cluster. BMC Evol Biol. 2008;8(1):14.
- Popoff MR, Bouvet P. Genetic characteristics of toxigenic Clostridia and toxin gene evolution. Toxicon. 2013;75:63–89.
- Nowakowska MB, Douillard FP, Lindström M. Looking for the X Factor in Bacterial Pathogenesis: association of orfX-p47 Gene Clusters with Toxin Genes in Clostridial and Non-Clostridial Bacterial Species. Toxins (Basel). 2019;12(1):19.
- Dupuy B, Matamouros S. Regulation of toxin and bacteriocin synthesis in Clostridium species by a new subgroup of RNA polymerase σ-factors. Res Microbiol. 2006;157(3):201–205.
- Marvaud JC, Gibert M, Inoue K, et al. botR/A is a positive regulator of botulinum neurotoxin and associated non-toxin protein genes in Clostridium botulinum a. Mol Microbiol. 1998;29(4):1009–1018.
- Raffestin S, Marvaud JC, Cerrato R, et al. Organization and regulation of the neurotoxin genes in Clostridium botulinum and Clostridium tetani. Anaerobe. 2004;10(2):93–100.
- Raffestin S, Dupuy B, Marvaud JC, et al. BotR/A and TetR are alternative RNA polymerase sigma factors controlling the expression of the neurotoxin and associated protein genes in Clostridium botulinum type a and Clostridium tetani. Mol Microbiol. 2004;55(1):235–249.
- Dover N, Barash JR, Hill KK, et al. Novel Structural Elements within the Nonproteolytic Clostridium botulinum Type F Toxin Gene Cluster. Appl Environ Microbiol. 2011;77(5):1904.
- Collins MD, East AK. Phylogeny and taxonomy of the food-borne pathogen Clostridium botulinum and its neurotoxins. J Appl Microbiol. 1998;84:5–17.
- Smith TJ, Williamson CHD, Hill KK, et al. The Distinctive Evolution of orfX Clostridium parabotulinum Strains and Their Botulinum Neurotoxin Type a and F Gene Clusters is Influenced by Environmental Factors and Gene Interactions via Mobile Genetic Elements. Front Microbiol. 2021;12:566908.
- Mansfield MJ, Adams JB, Doxey AC. Botulinum neurotoxin homologs in non-Clostridium species. FEBS Lett. 2015;589:342–348.
- Dover N, Barash JR, Burke JN, et al. Arrangement of the Clostridium baratii F7 Toxin Gene Cluster with Identification of a σ Factor That Recognizes the Botulinum Toxin Gene Cluster Promoters. PLoS ONE. 2014;9:e97983.
- Oguma K. The stability of toxigenicity in Clostridium botulinum types C and D. J Gen Microbiol. 1976;92(1):67–75.
- Hariharan H, Mitchell WR. Observations on bacteriophages of Clostridium botulinum type C isolates from different sources and the role of certain phages in toxigenicity. Appl Environ Microbiol. 1976;32:145–158.
- Smith TJ, Hill KK, Foley BT, et al. Analysis of the Neurotoxin Complex Genes in Clostridium botulinum A1-A4 and B1 Strains: BoNT/A3, /Ba4 and /B1 Clusters Are Located within Plasmids. PLoS ONE. 2007;2(12):e1271.
- Marshall KM, Bradshaw M, Pellett S, et al. Plasmid Encoded Neurotoxin Genes in Clostridium botulinum Serotype a Subtypes. Biochem Biophys Res Commun. 2007;361:49.
- Franciosa G, Maugliani A, Scalfaro C, et al. Evidence that plasmid-borne botulinum neurotoxin type B genes are widespread among Clostridium botulinum serotype B strains. PLoS ONE. 2009;4(3):4.
- Zhang Z, Hintsa H, Chen Y, et al. Plasmid-Borne Type E Neurotoxin Gene Clusters in Clostridium botulinum Strains. Appl Environ Microbiol. 2013;79:3856.
- Zhou Y, Sugiyama H, Nakano H, et al. The genes for the Clostridium botulinum type G toxin complex are on a plasmid. Infect Immun. 1995;63(5):2087.
- Raphael BH, Bradshaw M, Kalb SR, et al. Clostridium botulinum Strains Producing BoNT/F4 or BoNT/F5. Appl Environ Microbiol. 2014;80:3250.
- Dover N, Barash JR, Hill KK, et al. Clostridium botulinum Strain Af84 Contains Three Neurotoxin Gene Clusters: bont/A2, bont/F4 and bont/F5. PLoS ONE. 2013;8(4):e61205.
- Marshall KM, Bradshaw M, Johnson EA. Conjugative Botulinum Neurotoxin-Encoding Plasmids in Clostridium botulinum. PLoS ONE. 2010;5(6):e11087.
- Nawrocki EM, Bradshaw M, Johnson EA. Botulinum neurotoxin–encoding plasmids can be conjugatively transferred to diverse Clostridial strains. Sci Rep. 2018;8(1).
- Smith TJ, Tian R, Imanian B, et al. Integration of Complete Plasmids Containing Bont Genes into Chromosomes of Clostridium parabotulinum, Clostridium sporogenes, and Clostridium argentinense. Toxins (Basel). 2021;13(7):473.
- Dineen SS, Bradshaw M, Johnson EA. Neurotoxin gene clusters in Clostridium botulinum type a strains: sequence comparison and evolutionary implications. Curr Microbiol. 2003;46(5):345–352.
- Hill KK, Xie G, Foley BT, et al. Recombination and insertion events involving the botulinum neurotoxin complex genes in Clostridium botulinum types A, B, E and F and Clostridium butyricum type E strains. BMC Biol. 2009;7(1):66.
- Frankel G, Newton SMC, Schoolnik GK, et al. Intragenic recombination in a flagellin gene: characterization of the H1-j gene of Salmonella typhi. EMBO J. 1989;8(10):3149.
- Harrington CS, Thomson-Carter FM, Carter PE. Evidence for recombination in the flagellin locus of Campylobacter jejuni: implications for the flagellin gene typing scheme. J Clin Microbiol. 1997;35(9):2386–2392.
- Carter AT, Paul CJ, Mason DR, et al. Independent evolution of neurotoxin and flagellar genetic loci in proteolytic Clostridium botulinum. BMC Genomics. 2009;10(1):115.
- Bradshaw M, Dineen SS, Maks ND, et al. Regulation of neurotoxin complex expression in Clostridium botulinum strains 62A, Hall A-hyper, and NCTC 2916. Anaerobe. 2004;10(6):321–333.
- Bonventre PF, Kempe LL. Physiology of toxin production by Clostridium botulinum types a and B. I. Growth, autolysis, and toxin production. J Bacteriol. 1960;79:18–23.
- Couesnon A, Raffestin S, Popoff MR. Expression of botulinum neurotoxins a and E, and associated non-toxin genes, during the transition phase and stability at high temperature: analysis by quantitative reverse transcription-PCR. Microbiology. 2006;152(3):759–770.
- Ihekwaba AEC, Mura I, Malakar PK, et al. New elements to consider when modeling the hazards associated with botulinum neurotoxin in food. J Bacteriol. 2016;198(2):204–211.
- Connan C, Denève C, Mazuet C, et al. Regulation of toxin synthesis in Clostridium botulinum and Clostridium tetani. Toxicon. 2013;75:90–100.
- Cooksley CM, Davis IJ, Winzer K, et al. Regulation of neurotoxin production and sporulation by a putative agrBD signaling system in proteolytic Clostridium botulinum. Appl Environ Microbiol. 2010;76(13):4448–4460.
- Zhang Z, Korkeala H, Dahlsten E, et al. Two-Component Signal Transduction System CBO0787/CBO0786 Represses Transcription from Botulinum Neurotoxin Promoters in Clostridium botulinum ATCC 3502. PLOS Pathogens. 2013;9(3):e1003252.
- Connan C, Popoff MR. Two-component systems and toxinogenesis regulation in Clostridium botulinum. Res Microbiol. 2015;166(4):332–343.
- Bonventre PF, Kempe LL. Physiology of toxin production by Clostridium botulinum types a and B. III. Effect of pH and temperature during incubation on growth, autolysis. and toxin production. Appl Microbiol. 1959;7(6):374–377.
- Leyer GJ, Johnson EA. Repression of toxin production by tryptophan in Clostridium botulinum type E. Arch Microbiol. 1990;154(5):443–447.
- Fredrick CM, Lin G, Johnson EA. Regulation of botulinum neurotoxin synthesis and toxin complex formation by arginine and glucose in Clostridium botulinum ATCC 3502. Appl Environ Microbiol. 2017;83(13).
- Bowers LE, Williams OB. Effect of arginine on growth and lysis of Clostridium botulinum. J Bacteriol. 1963;85:1175.
- Inzalaco HN, Tepp WH, Fredrick C, et al. Posttranslational Regulation of Botulinum Neurotoxin Production in Clostridium botulinum Hall A- hyper. mSphere. 2021;6(4):6.
- Zhang Z, Dahlsten E, Korkeala H, et al. Positive regulation of botulinum neurotoxin gene expression by CodY in Clostridium botulinum ATCC 3502. Appl Environ Microbiol. 2014;80:7651–7658.
- Ihekwaba AEC, Mura I, Walshaw J, et al. An Integrative Approach to Computational Modelling of the Gene Regulatory Network Controlling Clostridium botulinum Type A1 Toxin Production. PLoS Comput Biol. 2016;12(11):e1005205.
- Piggot PJ, Hilbert DW. Sporulation of Bacillus subtilis. Curr Opin Microbiol. 2004;7:579–586.
- Paredes CJ, Alsaker KV, Papoutsakis ET. A comparative genomic view of clostridial sporulation and physiology. Nature Rev Microbiol. 2005;3(12):969–978.
- Tan IS, Ramamurthi KS. Spore formation in Bacillus subtilis. Environ Microbiol Rep. 2014;6:212–225.
- Wörner K, Szurmant H, Chiang C, et al. Phosphorylation and functional analysis of the sporulation initiation factor Spo0A from Clostridium botulinum. Mol Microbiol. 2006;59:1000–1012.
- Davidson P, Eutsey R, Redler B, et al. Flexibility and constraint: evolutionary remodeling of the sporulation initiation pathway in Firmicutes. PLoS Genet. 2018;14(9):e1007470.
- Hoch JA. Regulation of the phosphorelay and the initiation of sporulation in Bacillus subtilis. Annu Rev Microbiol. 1993;47(1):441–465.
- Kirk DG, Zhang Z, Korkeala H, et al. Alternative sigma factors SigF, SigE, and SigG are essential for sporulation in Clostridium botulinum ATCC 3502. Appl Environ Microbiol. 2014;80:5141–5150.
- Kirk DG, Palonen E, Korkeala H, et al. Evaluation of normalization reference genes for RT-Qpcr analysis of spo0A and four sporulation sigma factor genes in Clostridium botulinum Group I strain ATCC 3502. Anaerobe. 2014;26:14–19.
- Mascher G, Mertaoja A, Korkeala H, et al. Neurotoxin synthesis is positively regulated by the sporulation transcription factor Spo0A in Clostridium botulinum type E. Environ Microbiol. 2017;19(10):4287–4300.
- Imae Y, Strominger JL. Cortex content of asporogenous mutants of Bacillus subtilis. J Bacteriol. 1976;126(2):914–918.
- Setlow P. Spores of Bacillus subtilis: their resistance to and killing by radiation, heat and chemicals. J Appl Microbiol. 2006;101(3):514–525.
- Portinha IM, Douillard FP, Korkeala H, et al. Sporulation Strategies and Potential Role of the Exosporium in Survival and Persistence of Clostridium botulinum. Int J Mol Sci. 2022;23(2):23.
- Setlow P. Germination of spores of Bacillus species: what we know and do not know. J Bacteriol. 2014;196:1297–1305.
- Moir A. How do spores germinate? J Appl Microbiol. 2006;101:526–530.
- Brunt J, van Vliet AHM, van den Bos F, et al. Diversity of the germination apparatus in Clostridium botulinum groups I, II, III, and IV. Front Microbiol. 2016;7:1702. DOI:10.3389/fmicb.2016.01702.
- Clauwers C, Lood C, Van Noort V, et al. Canonical germinant receptor is dispensable for spore germination in Clostridium botulinum group II strain NCTC 11219. Sci Rep. 2017;7:1–10.
- Ross C, Abel-Santos E. The Ger Receptor Family from Sporulating Bacteria. Curr Issues Mol Biol. 2010;12:147.
- Brunt J, Carter AT, Pye HV, et al. The orphan germinant receptor protein GerXAO (but not GerX3b) is essential for L-alanine induced germination in Clostridium botulinum Group II. Sci Rep. 2018;8(1):1–10.
- Cañadas IC, Groothuis D, Zygouropoulou M, et al. RiboCas: a Universal CRISPR-Based Editing Tool for Clostridium. ACS Synth Biol. 2019;8(6):1379–1390.
- Mertaoja A, Nowakowska MB, Mascher G, et al. CRISPR-Cas9-Based Toolkit for Clostridium botulinum Group II Spore and Sporulation Research. Front Microbiol. 2021;12:32.
- Heap JT, Pennington OJ, Cartman ST, et al. The ClosTron: a universal gene knock-out system for the genus Clostridium. J Microbiol Methods. 2007;70:452–464.
- Heap JT, Ehsaan M, Cooksley CM, et al. Integration of DNA into bacterial chromosomes from plasmids without a counter-selection marker. Nucleic Acids Res. 2012;40(8):e59.
- Nowakowska MB, Selby K, Przykopanski A, et al. Construction and validation of safe Clostridium botulinum Group II surrogate strain producing inactive botulinum neurotoxin type E toxoid. Sci Rep. 2022;12(1):1790.
- Chen S, Barbieri JT. Engineering botulinum neurotoxin to extend therapeutic intervention. Proc Natl Acad Sci, USA. 2009;106:9180–9184.
- Vazquez-Cintron EJ, Vakulenko M, Band PA, et al. Atoxic derivative of botulinum neurotoxin a as a prototype molecular vehicle for targeted delivery to the neuronal cytoplasm. PLoS ONE. 2014;9(1):e85517.
- Vazquez-Cintron E, Tenezaca L, Angeles C, et al. Pre-clinical study of a novel recombinant botulinum neurotoxin derivative engineered for improved safety. Sci Rep. 2016;6:6.
- Tao L, Peng L, Berntsson RP-A, et al. Engineered botulinum neurotoxin B with improved efficacy for targeting human receptors. Nat Commun. 2017; 8(1).
- Guo J, Pan X, Zhao Y, et al. Engineering clostridia neurotoxins with elevated catalytic activity. Toxicon. 2013;74:158–166.