Abstract
A review of fermentation practised in Ayurveda, together with the literature produced on various aspects of Ayurvedic fermentation, is presented. The analyses may be viewed in the following categories: classical prescriptions for fermentative production of Ayurvedic drugs, physicochemical parameters of fermented Ayurvedic drugs, changes observed in medicinal tinctures due to fermentation, significance of changes due to fermentation, clinical evaluation of fermented drug products of Ayurveda, prospects for research on fermented Ayurvedic drugs and solid-state fermentation in Ayurveda. The strength of fermentation as a unique method of preparing herbal drugs as described in classical texts of Ayurveda, as well as deficiencies in the analyses of the Ayurvedic fermentative process as evidenced in research publications, is also assessed. The review of the process also highlights the significance of solid-state fermentation, employed in the preparation of certain Ayurvedic pills as a tradition in Kerala, India, as an improvement on the classical text, Ashtangahrudayam. Emphasis is also given to the need for critical studies to understand the differences between tinctures and fermented liquors and their therapeutic applications, to improve and find new applications of the fermented Ayurvedic drugs. Rational drug design-protocol based modification and synthesis of analogues, supported and guided by the biotransformation evidenced in fermented polyherbal formulae, as prescribed effectively in Ayurvedic classics, would be a novel working principle for achieving better therapeutics for other systems of medicine as well.
Fermentation and drug preparation
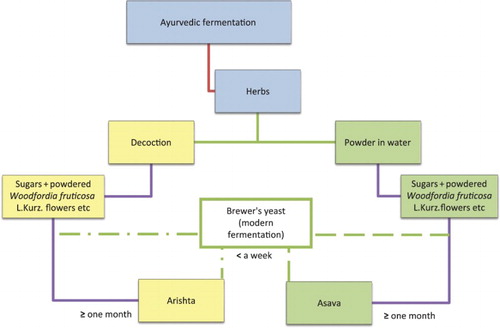
Fermentation is prescribed as a method for drug preparation in Ayurveda (Shrivastava Citation1998; Valiathan Citation2003, Citation2007, Citation2009). No other system of medicine uses fermentative technology as in Ayurveda. Rudimentary fermentation may take place in certain medicinal preparations of the Siddha and Unani systems of medicines. However, since no analytical reports using modern instrumentation are available in the public domain, systems other than Ayurveda are not considered in this review. No theoretical basis is provided in any of the Ayurvedic classics for adopting fermentative technology (Valiathan Citation2003, Citation2007, Citation2009). Neither it is explained explicitly why it is adopted, apart from the very strong tradition (Shrivastava Citation1998; Valiathan Citation2003, Citation2007, Citation2009). The texts provide only recipes to make the medicines (). Valiathan (Citation2009, pp. xvi–xviii) has also reviewed the transformation of Ayurvedic practices from faith to objective science, while considering the contributions of Vagbhata (the latest of the Great Trinity of Ayurveda). Asavas (alcoholic medicaments prepared from powdered herbal drugs) and arishtas (alcoholic medicaments prepared from decoctions of herbal drugs) are the types of fermented Ayurvedic herbal drug preparations (API Citation2008). In general, the use of asavas or arishtas is prescribed by the physician, depending on the patient's condition. Asavas and arishtas are considered generally superior to tinctures for absorption in the gut, since they are partly ‘digested’. This ‘digestion’ of the herbal extracts (arishta) or powders (asava) used in the respective preparations is scarcely described in any of the Ayurvedic classics by Caraka, Susruta or Vagbhata (Valiathan Citation2003, Citation2007, Citation2009).
However, there is a mention of arishta by Susruta (Susruta, 44th Chapter) which reads, ‘arishta has better properties and functions than any other mode of drug preparation because of the combination of different types of medical materials and their transformation’. It may also be inferred that the materials on which fermentative procedures are used for Ayurvedic preparations such as asavas and arishtas undergo changes described in the classical texts of Ayurveda (Chandra et al. Citation2015). This clearly suggests that the making of arishta (and also asava) may impart better or greater therapeutic potential to the medical decoction from which arishta is prepared. So far, no direct interpretation has been given to the above connections, expressing the biotransformation of the constituent compounds present in the herbs used in the preparation of arishta and asava. However, Thatte and Dahanukar (Citation1986) and Valiathan and Thatte (Citation2010) might have encouraged many investigators to the search for certain missing links in Ayurvedic literature and to offer explanations for some unexplained texts and practices. It seems that the insight of Susruta, as expressed in the translation given above, has not propelled many into research in that area, although a lot of research related to the fermentation of Ayurvedic preparations has been published from the last couple of decades of the twentieth century onwards (Valiathan Citation2007; Katiyar Citation2008; Sekar & Mariappan Citation2008; Mulay & Khale Citation2011; Sayyad et al. Citation2012; Kadam et al. Citation2012). This is evident from an overview of the available literature on arishta and asava fermentation. Hardly anyone seems to be interested in the structural and functional changes in the compounds present in the starting materials, brought about by fermentation. The basic character of Ayurveda, always to see the sum total of all components, may have inculcated such a deficiency when seen from the viewpoint of modern science.
Review
The literature considered in this review should be considered representative, rather than exhaustive, since many articles were published outside the mainstream journals and are unavailable. However, the study of such literature may be effective to show the total output from those efforts. The above-mentioned literature may be categorized as follows:
classical prescriptions for fermentative production of Ayurvedic drugs
physicochemical parameters of fermented Ayurvedic drugs
changes observed in medicinal tinctures due to fermentation
significance of changes due to fermentation
clinical evaluation of fermented drug products of Ayurveda
prospects for research on fermented Ayurvedic drugs
solid-state fermentation in Ayurveda.
Classical prescriptions for fermentative production of Ayurvedic drugs
The protocols for fermentative production of Ayurvedic drugs have been increasingly enriched by adding to the prescriptions available in the oldest of the classics (Valiathan Citation2003). Thus, the other texts (Valiathan Citation2007, Citation2009; Shrivastava Citation1998) enriched and enlarged the medicinal repertoire of asava and arishta. These prescriptions prevailed for millennia, up to the present. The framework of the protocols for the production of such medicines was sufficiently flexible to allow local practitioners to invent new versions or modify the medicines according to local demands by introducing effective substitutions. However, the basic tenets were followed rigorously so that certain components were invariably constant. One such component was the use of Woodfordia fruticosa flowers. Another basic characteristic was the difference between the preparations of asava and arishta, from powdered herbs in the fermenting medium and the tincture of herbs in the fermenting medium, respectively. The final product was always a medicated wine. Many research groups have studied the protocols for fermentative production of asava and arishta prescribed in the classics, by reviewing the literature only, conducting experiments or analysing the final products made as per classical prescriptions, to establish common factors and parameters of asava and arishta preparations and their composition.
Joshi and Jha (Citation1990) reviewed various fermentative protocols provided in the three cardinal classics of Ayurveda, to identify the different types of constituents required for their preparation together with their ratios, method of preparation, time required to complete the process, fermentation pots, fermenting materials, place and time (season) of fermentation, etc. The aim was to develop certain standards for their preparation. However, they could find only the flexibility of the system and a few common norms. Kroes et al. (Citation1993) analysed the significance of W. fruticosa flowers on immunomodulatory activity, and the alcohol and sugar contents of the Ayurvedic drug Nimba arishta. They found that the yeasts from the W. fruticosa flowers were responsible for the ethanol production in Ayurvedic fermentation. Alam et al. (Citation1982, Citation1988) studied fermentation experimentally, as prescribed in Ayurvedic system, to record the differences in physicochemical parameters before and after fermentation. However, they did not correlate the fermentation with its functions or uses.
Sekar and Mariappan (Citation2008) reviewed the fundamental concepts in designing arishta and asava production, focusing on the art of preparation, storage and use of the products. Arishtas and asavas had improved shelf-life. The enhancement of therapeutic properties of the active constituents owing to microbial transformation was also speculated on. Mishra et al. (Citation2010) gave a detailed account on the preparation of asavas and arishtas in Ayurveda, and described the advantages of fermentation. Fermentation helped in rupturing the cells of herbs and exposing their contents to microorganisms for transformation. They observed that fermentation removed undesirable sugars from plant materials and made the product more bioavailable; however, the undesirable sugars were not specified. They speculated that arishta and asava may have enhanced therapeutic properties owing to microbial transformation of the initial ingredients, but no attempt was made in this study to observe the changes in the ingredients as a result of biotransformation. However, they concluded that the aqueous alcoholic milieu improved the drug delivery in the body. Bhondave et al. (Citation2013) found that the yeast consortium present in W. fruticosa flowers was responsible for the fermentation of Ayurvedic drugs. They also found that the yeast isolates that were individually less efficient in producing alcohol were more efficient in producing and mobilizing targeted active phytoconstituents only as a consortium. In addition, the preparations with yeast consortia were equivalent to the W. fruticosa flowers in showing anti-inflammatory activity based on the inhibition of complete Freund's adjuvant-induced paw oedema. They concluded that that production and/or mobilization of active phytoconstituents was independent of alcohol generation, and perhaps microbial transformation played an important role. Thatte and Dahanukar (Citation1986) reported from their analysis of Arjunarishta that the origin of Ayurvedic fermentation was indeed difficult to pinpoint in time exactly. It had been placed by scholars of Ayurveda and ancient Indian literature at somewhere around 6000 BC. However, they did not throw any more light on the basic issues regarding Ayurvedic fermentation. Bouldin et al. (Citation1999) observed that Ayurvedic remedies were considered cheap, inherently safe and with few side-effects. Chaudhury et al. (Citation2011) reviewed the literature related to asava and arishta from the Vedic period to recent publications of the Government of India, i.e. the Ayurvedic Formulary of India. They discussed factors such as the nature and amount of carbohydrate, type of containers, optimum temperature, variety and relevance of initiators of fermentation, and manufacture of asava and arishta fermentation.
It can be seen that the above studies did not present new findings which were not implied in the ancient classics. However, they made fresh attempts to gain new insights into an ancient method of drug preparation.
Physicochemical parameters of fermented Ayurvedic drugs
The physicochemical parameters considered for studying the fermented Ayurvedic drugs were those generally assessed in the case of any fermented product for human consumption. A review of such studies on fermented Ayurvedic drugs gave the picture described below. Prabhu et al. (Citation2011a) attempted physicochemical standardization of Viburnum coriaceum bark arishta as a model of prescriptions in Ayurvedic classics. The extract of this plant is known to contain phenolic compounds. The arishta was prepared by traditional methods using W. fruticosa flowers. A large number of physicochemical parameters was determined. They conducted a primary organic analysis on the species and revealed the presence of bioactive molecules such as tannins, saponins, phenolic compounds (flavonoids) and other phenolic glycosides as the principal phytoconstituents. The authors claimed that such parameters could serve to standardize and characterize Ayurvedic drugs. Alam et al. (Citation1984,Citation1988) studied the effect of period of fermentation and storage of Ayurvedic drugs. The usual fermenting period according to the texts is 1 month or more. However, their study did not conclusively show any specific merit of a prolonged period of fermentation and storage based on the selected criteria. Also, the methodology adopted was not sufficient to demonstrate their objective.
Alam et al. (Citation1986) studied the size and shape of the fermenting pot and the quantity of the drug taken in a pot for fermentation, since this was not mentioned in the literature. This study showed that better results were obtained when the drug filled up to three-quarters of the volume of the earthen pot than when the pots contained various other volumes of fermenting liquor. This study showed only how better alcohol production could be achieved by limiting the fermenting liquor in the pot.
Prabhu and Ponnudurai (Citation2011), Prabhu et al. (Citation2011b, Citation2011c) and Sayyad et al. (Citation2012) studied the formulation and physicochemical standardization of V. coriaceum arishta. The crude drug (plants) was formulated into an asava using a conventional anaerobic fermentation process for about 90 days. They studied physicochemical parameters such as the boiling and congealing range of temperatures, freezing point, ethanol content, loss on drying, loss on ignition, pH, refractive index, viscosity, density, total organic content and total free sugar. Their conclusion was that every formulation of the Ayurvedic system of medicine has its own parameters, implying that generalization is far from conclusive.
Mulay and Khale (Citation2011) showed that a shorter incubation period was desirable to produce a greater yield of alcohol in fermented Ayurvedic drugs, which was thought to be good for no reason that was explained. Bhondave et al. (Citation2013) described the role of W. flowers in biomedical fermentation. Six yeast cultures were isolated from the flowers and identified as Saccharomyces species and Rhoduntonula muciliginosa. These isolates were used to ferment curcuminoids, and fermentation proceeded more quickly with isolated microbes than with Woodfordia flowers. The authors speculated that R. muciliginosa, a non-fermenting yeast, also had a role in the biotransformation of the active constituents. Kadam et al. (Citation2012) compared the constituents of mustharishta prepared traditionally in earthen pots with that in wooden and stainless steel vessels. They observed a higher percentage of alcohol in steel containers. Wadher et al. (Citation2007) described a gas–liquid chromatography method for the determination of ethanol in abhayarishtam. Parihar et al. (Citation2010) described a thin-layer chromatography method for the standardization of asokarishtam by quantitative estimation of major compounds such as catechin, catechol and epicatechin Rasheed and Roja (Citation2012) characterized, fingerprinted, and attempted to standardize and evaluate a polyherbal fermented Ayurvedic drug. They found that the parameters were within the limits prescribed by the World Health Organization for herbal medicines. Gharate et al. (Citation2011) evaluated the quantitative and certain physicochemical parameters of the fermented Ayurvedic medicine Kanakasava. Weerasooriya et al. (Citation2006) compared the physicochemical parameters of different fermented Ayurvedic medicines and found that they were all within a narrow range.
The studies presented above may not have any specific relevance to the therapeutic value of the fermented Ayurvedic products. Such parameters are only considered for generalizing fermented Ayurvedic medicinal products as fermented products for human consumption. Studies of such parameters have little therapeutic significance and may be redundant in the case of asava and arishta. They may be useful, to some extent, for determining certain values important in industrial products for human consumption.
Changes observed in medicinal tinctures due to fermentation
There are many reports showing the changes in the constituents of herbal tinctures. Most of them are studies related to asava or arishta preparation. Only a few studies have evaluated such changes brought about by preparation of asava or arishta. Mishra et al. (Citation2010) critically overviewed and analysed the fermented Ayurvedic products. They discussed the transformation of chemical compounds during fermentation, leading to a lowering of toxicity and better extraction of herbal constituents into the water/alcohol medium of the fermented drug. Mulay and Khale (Citation2011) reviewed the production of asava and arishta by improved fermentation technology. They found that fermentation could also reduce the toxicity of some of the toxic compounds present in plants. They observed that fermentation actively ruptured the cells of herbs, exposing them more to the solvent, and bacterial enzymes broke the cell walls of herbal materials to further the leaching process. The yeast Saccharomyces cerevisiae was commonly used. Okutsu et al. (Citation2007) studied the concentration of phenyl ethanol, the breakdown product of phenylalanine by yeast, and found that it was higher in ginger asava than in the tincture of ginger. In ginger asava, only traces of aldehydes, such as geranial and neral, were found. The percentages of geraniol and nerol were higher in asavas than in tincture. Thus, it was concluded that the yeast reduced geranial and neral to geraniol and nerol, respectively. Also, geraniol was released during fermentation by the hydrolysis of geraniol disaccharides.
Horsten et al. (Citation1990) studied hydrolysis of gallotannins by two Aspergillus niger strains isolated from a model preparation of nimba arishta. Although this investigation was not into the fermentation of any true Ayurvedic medicinal preparations, it mimicked partly the preparation of an arishta described in Ayurvedic classics. It was conducted to compare the composition of a tincture and its arishta. Chen et al. (Citation2003) reported that ginger asava showed only trace of aldehydes such as geranial and neral, which were present in ginger tincture. In contrast, the relative contents of geraniol and nerol in the asava were higher than in tincture. Baker's yeast reduced ketone to alcohol. Thus, it was thought that yeasts reduced geranial or neral to geraniol or nerol. Another possibility was that yeast hydrolysed the geraniol glycosides. This was also a model study, performed as the preparation of an asava. Ress et al. (Citation2003) studied the detoxifying action by asava preparation. Geranial and neral, which are added to foods as flavouring agents, cause forestomach and kidney lesions. Asavas which contain monoterpenols in place of such aldehydes could be milder drugs than tinctures.
The above reports reveal two aspects: the changes in constituents found in tinctures and the asavas or arishtas prepared from the tinctures; and the changes in constituents found in tinctures and their asava- or arishta-like compounds. However, both types of analyses show the significance of the changes to the constituents of the herbal raw materials of asavas and arishtas.
Significance of changes due to fermentation
There are indirect statements in Ayurvedic classics (Valiathan Citation2007) that fermentation will enhance the therapeutic properties of herbal drugs. So far, little direct evidence has been shown for such oblique statements. However, Chandra et al. (Citation2011, Citation2012) provided experimental proof for the enhancement of enzyme inhibition relevant in therapeutic action by fermentative transformation of the constituents of a herbal tincture. Biotransformation of berberine by fermentation produced its hydroxyl derivatives, monohydroxy- or dihydroxy-berberine. Surface plasmon resonance and enzyme kinetic studies showed that these derivatives had a higher inhibitory potential than berberine towards phospholipase A2 (PLA2). The X-ray crystal structures showed that the biotransformed derivative of berberine was bound to PLA2 in an inverted orientation with respect to the binding of berberine. This study revealed the significance of biotransformation in the generation of better enzyme-inhibitory compounds.
Fermentation processes help in rupturing the cells of the herbs and exposing their contents to biotransformation. Fermentation also creates an active transport system with dissolved constituents from the herbal material (Sharma & Dash Citation2001). There are claims that yeast cell walls naturally bind heavy metals and pesticide residues and act as natural cleaning system, making fermentation of herbal products safer than powder or tinctures. Fermentation also creates an active transport system that moves the dissolved constituents from the herbal material to the solvent (Katiyar Citation2008). The above studies provide validation of the speculated benefit of fermentation of herbal tinctures as described in Ayurvedic classics.
Clinical evaluation of fermented drug products of Ayurveda
There are several reports demonstrating the clinical significance of fermentation of herbal tinctures as described in Ayurvedic texts. Many such prescriptions are available for fermented herbal preparations for specific situations. The following are some of the plants often used in fermented drug preparations. Viburnum Linn. species have been reported to contain sesquiterpenes (Khosa et al. Citation1979), triterpenes and phytosterols; phenolic compounds and their derivatives such as tannins, flavonoids and anthocyanins and iridoid glycosides in their stems, roots and leaves, and have been shown to possess uterine sedative, diuretic cardiovascular stimulant, antimicrobial, anti-inflammatory, antinociceptive, antispasmodic, antiasthmatic and astringent activities (Altun et al. Citation2009; NISCAIR Citation2003; Nadkarni Citation2002). In the late 1960s to early 1980s, scientific studies on the genus Viburnum Linn. were voluminous (Hoerhammer et al. Citation1965; Wahi et al. Citation1981). However, the number of species studied and the area of investigation were remarkably small. After a couple of decades, a few Viburnum species re-emerged to undergo extensive phytochemical and pharmacological investigation. Typical examples are: iridoid aldehydes and their glycosides in Viburnum luzonicum (Tomassini et al. Citation2006), and their cytotoxic effect; vibsane-type diterpenes from Viburnum awabuki (Fukuyama et al. Citation2005); iridoid glycosides from Viburnum tinos; antinociceptive and anti-inflammatory activities of Viburnum lanata (Sever et al. Citation2007) and Viburnum opulus (Fukuyama et al. Citation2005); and iridoid glycoside from Viburnum rhytidophyllum (Tomassini et al. Citation1997). Fermented products containing some of the above-mentioned plants showed significant therapeutic properties, as described below.
Prabhu and Ponnudurai (Citation2011) determined the anticonvulsant activity of V. coriaceum arishta in mice by the maximal electroshock seizures (MES) test, considered to be a predictor of likely therapeutic efficacy against generalized tonic–clonic seizures. Their study revealed that the Viburnum arishta blocked tonic seizures induced by MES significantly (p < 0.001) at doses of 200, 400 and 600 mg/kg body weight. The study showed that testing increasing doses of the drug may result in an increase in therapeutic efficacy. It showed that V. coriaceum arishta to be an anticonvulsant formulation. Bharadwaj et al. (Citation2005) studied the antibacterial activity of Takrarishta and suggested that it would be useful in gastrointestinal infections and food poisoning. Belge and Belge (Citation2012) showed that Gomuthrasava prepared according to ancient texts was clinically significant in treating vitiligo, resulting from the destruction of melanocytes. They showed that treatment with Gomuthrasava resulted in significant repigmentation of the affected regions. Prabhu et al. (Citation2011) demonstrated that the antihelminthic activity of asava of Viburrnum stem was comparable to piperazine citrate. It was concluded that phenolic compounds such as tannins and flavonoids bind with intestinal enzymes and cause the death of the worms.
Prospects for research on fermented Ayurvedic drugs
The literature (Khosa et al. Citation1979; NISCAIR Citation2003; Nadkarni Citation2002) shows that Viburnum species produce several therapeutic effects by their chemical components. Studies (Prabhu & Ponnudurai Citation2011; Prabhu et al. Citation2011c) have shown that asava and arishta prepared with Viburnum species are effective therapeutic agents. However, it remains to be demonstrated whether fermentation of Viburnum species' tincture into asava or arishta imparts greater therapeutic potential to the tincture. Similar studies may be considered with other medicinal herbs having therapeutic potential to identify effective compounds and/or polyherbal preparations with enhanced therapeutic potential.
Solid-state fermentation in Ayurveda
Solid-state fermentation (SSF) is generally defined as the growth of microorganisms on moist solid material in the absence or near absence of free water. The solid material is either a natural substrate or an inert support used as a solid base. Based on the type of microorganism involved, SSF processes can be classified into two main groups: natural (indigenous) SSF and pure culture SSF using individual strains or a mixed culture. There are indications on the application of SSF in the preparation of traditional Ayurvedic preparations in Kerala. However, it is never referred to by the exact term ‘solid-state fermentation’. For example, in the preparation of Bilwadi Gutika (pills), used in Ayurvedic treatment for bites from scorpions, rodents, insects and spiders, gastroenteritis, dyspepsia, fever, toxic and psychological conditions, etc., a crude form of SSF is employed. The fine powder of the ingredients is triturated with goat's urine and tablets are prepared (Valiathan Citation2009). In the past, Bilwadi Gutika was made by grinding the mix for 1 or 2 hours daily for about 8–9 months continuously (grinding used to be done in goat's urine), and rolling into small spheres. When processed this way, the pills never dried up. The daily grinding helps the substrate (here the medicinal raw materials) to acquire an adequate particle size with recharging of the microbial inocula, which may include yeasts and lactic acid bacteria. This may lead to SSF. Although this practice is not as per the prescription in the classical text, Ashtangahrudayam, Uttarasthana 36/84-85 (Valiathan Citation2009), this was followed widely in Kerala as an improvement on the classical text. There is a report on the changes in the microflora of Bilwadi Gutika mix under preparation for a period of 1 month, showing characteristic changes seen in SSF (Thankamani Citation2005). However, that report (Thankamani Citation2005) is silent on the changes in the Bilwadi Gutika mix under a long duration of preparation. It may be assumed that the biotransformation of berberine, observed elsewhere (Chandra et al. Citation2012), also happens in Bilwadi Gutika mix under a long duration of preparation, since it is rich in herbs containing berberine, the biotransforming microflora, and has conditions suitable for biotransformation.
Conclusions
Clardy and Walsh (Citation2004) observed the significance of natural products in drug development. Rational drug design-protocol based modification and synthesis of analogues, supported and guided by the biotransformation evidenced in fermented polyherbal formulae, as prescribed effectively in Ayurvedic classics, could be a novel working principle for achieving better therapeutics. Valiathan and Thatte (Citation2010) emphasized the need to experiment with traditional medicine to obtain better products. Polyherbal products, either tinctures or fermented products, may exert their therapeutic properties as the sum of the interactions of their multiple components with the human body. It may be seen that many such interactions are dependent on the amounts of such components (compounds). It is known that the phytoconstituents vary greatly depending on factors exerting an influence on the herbs and processes employed in drug preparation. By developing knowledge on such factors and their critical role in expressing the therapeutic effects of polyherbal products, their amount in such products could be optimized. Experimental evidence (Prabhu & Ponnudurai Citation2011) for the enhancement of enzyme inhibition relevant to therapeutic action by fermentative biotransformation of the constituents of herbal tinctures may build confidence in this new field of research.
Acknowledgement
The authors are grateful to Dr M. S. Valiathan for fruitful suggestions and discussion.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Alam M, Dasan KKS, Joy S, Purushothaman KK. 1988. Comparative and fermentation standardization studies on Dasamularishta. Ancient Sci Life. 8(1):68–70.
- Alam M, Dasan KKS, Ramar C, Ali US, Purushothaman KK. 1982. Experimental studies on the fermentation in asavas and aristas part III – Drakshasava. Ancient Sci Life. 2(4):216–219.
- Alam M, Rukmani B, Joy S, Anandan T, Veluchamy G. 1986. Effect of Time on the Fermentation and Storage of Candanasava. Anc Sci Life. VI(1):51–55.
- Alam M, Rukumini B, Shanmughadasan KK, Purushothaman KK. 1984. Effect of time on the fermentation and storage of candanasava. Ancient Sci Life. 4(1):51–55.
- Altun ML, Citoglu GS, Yilmaz BS, Ozbek H. 2009. Antinociceptive and anti-inflammatory activities of Viburnum opulus. Pharm Biol. 47(7):653–658. doi: 10.1080/13880200902918345
- Ayurvedic Pharmacopoeia of India, Monographs-API, Part II, Vol. II, 2008.
- Belge RS, Belge AR. 2012. Clinical evaluation of the efficacy of gomutra aasava in shvitra vis-a-vis vitiligo. IOSR J Pharm Biol Sci. 2(3):10–13.
- Bharadwaj S, Achaliya GS, Meghare VS, Wadodkar SG, Dorle AK. 2005. In vitro antibacterial activity of Tarakarista, an ayurvedic formulation. Indian J Tradit Knowl. 4(3):325–328.
- Bhondave P, Burase R, Takale S, Paradkar A, Patil S, Mahadik K, Harsulkar A. 2013. Yeast consortium isolated from Woodfordia fruticosa flowers proved to be instrumental for traditional Ayurvedic fermentation. Int J Pharm Biomed Res. 4(1):37–43.
- Bouldin SA, Smith MC, Garner DD, Szeinbach SL, Frate DA, Croom EM. 1999. Pharmacy and herbal medicine in the US. Soc Sci Med. 49:279–289. doi: 10.1016/S0277-9536(99)00118-5
- Chandra DN, Prashanth GK, Singh N, Kumar S, Jithesh O, Sadasivan C, Sharma S, Singh TP, Haridas M. 2011. Identification of a novel and potent inhibitor of phospholipase A2 in a medicinal plant: Crystal structure at 1.93 Å and surface plasmon resonance analysis of phospholipase A2 complexed with berberine. Biochim Biophys Acta. 1814(5):657–663. doi: 10.1016/j.bbapap.2011.03.002
- Chandra DN, Joseph A, Prasanth GK, Sabu A, Sadasivan C, Haridas M. 2012. Inverted binding due to a minor structural change in berberine enhances its phospholipase A2 inhibitory effect. Int J Biol Macromol. 50(3):578–85. doi: 10.1016/j.ijbiomac.2012.01.029
- Chandra DN, Preethidan DS, Sabu A, Haridas M. 2015. Traditional fermentation of Ayurvedic medicine yields higher pro-inflammatory enzyme inhibition compared to wine-model product. Front Life Sci. 10.1080/21553769.2015.1005245.
- Chaudhury A, Singh N, Dalvi M, Wele A. 2011. A progressive review of sandhana kalpana (biomedical fermentation): an advanced innovative dosage form of Ayurveda. Ayu. 2(3):408–417. doi: 10.4103/0974-8520.93925
- Chen Y, Inaba M, Abe N, Hirota A. 2003. Antimutagenic activity of 8-hydroxyisoflavones and 6-hydroxydiadzein from soybean miso. Biosci Biotechnol Biochem. 67(4):903–906. doi: 10.1271/bbb.67.903
- Clardy J, Walsh C. 2004. Lessons from natural molecules. Nature. 432(7019):829–837. doi: 10.1038/nature03194
- Fukuyama Y, Kubo M, Minami H, Yuasa H, Matsuo A, Fujii T, Morisaki M, Harada K. 2005. Rearranged vibsane-type diterpenes from Viburnum awabuki and photochemical reaction of vibsanin B. Chem Pharm Bull. 53(1):72–80. doi: 10.1248/cpb.53.72
- Gharate MK, Pawar R, Kasture V, Patil R. 2011. Evaluation of quantitative parameters of ayurvedic formulation: Kanakasava. Int J Pharm Pharm Sci. 3(1):43–45.
- Hoerhammer L, Wagner H, Reinhardt H. 1965. Isolation of flavonoids from the barks of Viburnum prunifolium. Dent Apothekerzer. 105(40):1371–1375.
- Horsten SFAJ, van den Berg AJJ, Kroes BH, Labadie RP. 1990. Hydrolysis of gallotannins by two Aspergillus niger strains isolated from a model preparation of Nimba arishta. Planta Med. 56:586–587. doi: 10.1055/s-2006-961193
- Joshi D, Jha CB. 1990. Critical study of the Asavarishta preparations of Brhatirayee. Ancient Sci Life. 9(3):125–133.
- Kadam PV, Yadav KN, Patel AN, Navsare VS, Narappanawar NS, Patil MJ. 2012. Comparative account of traditionally fermented biomedicine from Ayurveda: Mustakarishta. Int J Res. Ayurveda and Pharmacy. 3(3):429–432.
- Katiyar CK. 2008. Aqueous alcoholic extraction of medicinal and aromatic plants by fermentation. In: Handa SS, Khanuja SS, Longo G, Rakesh DD, editors. Extraction technologies for medicinal and aromatic plants. Trieste: International Centre for Science and High Technology; p. 107–112.
- Khosa RL, Wahi AK, Mohan Y. 1979. Isolation of bergenin from the roots of Viburnum nervosum. Indian J Pharm. 41(3):120–124.
- Kroes BH, van den Berg AJJ, Abeysekera AM, de Silva KT, Labadie RP. 1993. Fermentation in traditional medicine; the impact of Woodfordia fruticosa flowers on the immunomodulatory activity, and the alcohol and sugar contents of Nimba arishta. J Ethnopharmacol. 40:117–125. doi: 10.1016/0378-8741(93)90056-B
- Mishra AK, Gupta A, Gupta V, Sand R, Bansal P. 2010. Asava and arishta: an Ayrvedic medicine – an overview. Int J Pharm Biol Arch. 1(1):24–30.
- Mulay S, Khale A. 2011. Asavarishtas through improved fermentation technology. Int J Pharma Sci Res. 2(6):1421–1425.
- Nadkarni KM. 2002. Indian materia medica vol I; 2nd ed. Bombay, India: Popular Prakashan.
- National Institute of Science Communication and Information Resources (NISCAIR). 2003. The wealth of India. A dictionary of Indian raw materials and industrial products: raw material series. p. 437–446. New Delhi: Publication and Information Directorate, CSIR.
- Okutsu K, Yoshimitsu M, Kakiuchi N. 2007. Differences in volatile compounds between tincture and Ayurvedic herbal liquor asava made from ginger or jujube. J Tradit Med. 24: 93–199.
- Parihar SS, Mishra SK, Singh H, Rathore A. 2010. Standardization of Ashokarista formulation by TLC method. Int J Pharm Tech Res. 2(2):1427–1430.
- Prabhu K, Ponnudurai K. 2011. Formulation of Viburnum coriaceum arista and determination of its anticonvulsant activity in mice. Der Pharmacia Sinica. 2(4):159–163.
- Prabhu K, Karar PK, Hemalatha S, Ponnudurai K. 2011. Formulation and physicochemical standardization of Viburnum coriaceum bark arista. Int J Res Ayurved Pharma. 2(2):535–540.
- Prabhu K, Karar PK, Hemalatha S, Ponnudurai K. 2011a. Formulation and physicochemical standardization of Viburnum coriaceum bark arista. Int J Res Ayurved Pharma. 2(2):535–540.
- Prabhu K, Karar PK, Hemalatha S, Ponnudurai K, Ghatuary SK. 2011b. Preparation and physico chemical standardization of Viburnum punctatum arishta. Int J Res Ayurveda and Pharmacy. 2(4):1192–1197.
- Prabhu K, Karar PK, Hemalatha S, Ponnudurai K. 2011c. Formulation and phyto-chemical standardization of Viburnum coriaceum arishta. Der Pharmacia Sinica. 2(4):44–52.
- Rasheed A, Roja C. 2012. Formulation, standardization and pharmacological evaluation of a poly herbal traditional remedy – Aswagandharistam. Orient Pharm Exp Med. 12:51–58. doi: 10.1007/s13596-011-0050-2
- Ress NB, Hailey JR, Maronpot RR, Bucher JR, Travlos GS, Haseman JK, Orzech DP, Johnson JD, Hejtmancik MR. 2003. Toxicology and carcinogenesis studies of microencapsulated citral in rats and mice. Toxicol Sci. 71: 198–206. doi: 10.1093/toxsci/71.2.198
- Sayyad SF, Randive DS, Jagtap SM, Chaudhari SR, Panda BP. 2012. Preparation and evaluation of fermented Ayurvedic formulation: Arjunarishta. J Appl Pharma Sci. 5:122–124.
- Sekar S, Mariappan S. 2008. Traditionally fermented biomedicines, aristas and asavas from ayurveda. Indian J Tradit Knowl. 7(4):548–556.
- Sever YB, Saltan CG, Altun ML, Ozbek H. 2007. Antinociceptive and anti-inflammatory activities of Viburnum lantana. Pharm Biol. 45(3):241–245. doi: 10.1080/13880200701213187
- Sharma RK, Dash B. 2001. Charak Samhita, vol. I-VI. Varanasi, India: Chaukhambha Sanskrit Series.
- Shrivastava S. 1998. Sarangadhara Samhita. Varanasi, India: Chaukhamba Orientalia.
- Thankamani V. 2005. Antimicrobial activity of Vilwadi Gutika. Paper presented at Global Summit on Ayurveda and Expo; Kerala, India.
- Thatte UM, Dahanukar SA. 1986. Ayurveda and contemporary scientific thoughts. Trends Pharmacol Sci. 7:247–251. doi: 10.1016/0165-6147(86)90336-6
- Tomassini L, Dejan B, Foddai S, Nicoletti M. 1997. Iridoid glucosides from Viburnum rhytidophyllum. Phytochemistry. 44(4):751–753. doi: 10.1016/S0031-9422(96)00600-0
- Tomassini L, Gao J, Foddai S, Serafini M, Ventrone A, Nicoletti M. 2006. Iridoid glucosides from Viburnum chinshanense. Nat Prod Res. 20(8):697–700. doi: 10.1080/14786410500056678
- Valiathan MS. 2003. The legacy of Caraka. Hyderabad, India: Orient Longman Pvt. Ltd.
- Valiathan MS. 2007. The legacy of Susruta. Hyderabad, India: Orient Longman Pvt. Ltd.
- Valiathan MS. 2009. The legacy of Vagbhata. Hyderabad, India: Universities Press (India) Pvt. Ltd.
- Valiathan MS, Thatte U. 2010. Ayurveda: time to experiment. Int J Ayurveda Res. 1(1):3–3.
- Wadher SJ, Puranik M, Yeole PG, Lokhande CS. 2007. Determination of ethanol in abhayarista by gas chromatography. Indian J Pharm Sci. 69:152–154. doi: 10.4103/0250-474X.32136
- Wahi AK, Khosa RL, Mohan Y. 1981. Pharmacognostical studies on the roots of Viburunum nervosum Hook. Bulle Medico-Ethno Botanical Res. 3(2–4):205–211.
- Weerasooriya WMB, Liyanage JA, Pandya SS. 2006. Quantitative parameters of different brands of asava and arista used in ayurvedic medicine, an assessment. Indian J Pharmacol. 38(5):365–365. doi: 10.4103/0253-7613.27710