Abstract
Objective
Pivotal clinical trials revealed good clinical efficiency of ocrelizumab while having a good safety profile in the management of multiple sclerosis (MS). However, real-world data of ocrelizumab in daily clinical practice remain scarce. The aim of this study was to evaluate the preliminary safety profile and effectiveness of ocrelizumab treatment for MS in an Arab population in a real-world clinical setting.
Methods
In this retrospective single-center observational study in Qatar, we reviewed the medical records and analyzed the clinical and MRI data of all patients with relapsing-remitting MS (RRMS) and active secondary progressive MS (aSPMS)—between October 2017 through December 2020—who had received at least one infusion of ocrelizumab (Q-OCRE).
Results
A total of 60 MS patients were included (57 with RRMS, three SPMS). The Median follow-up period was 19 months (range, 1–32). The most common reason for switching to ocrelizumab was increased disease activity and three-quarters of the patients were on a previous disease-modifying drug (DMD). No evidence of disease activity (NEDA) status at year 1 was achieved in 73% of the cohort. Mild infusion-related reactions (IRR) and infections were reported (mainly upper respiratory tract infections followed by urinary tract infection) with a declining percentage over the follow-up applications. No severe side effects were observed.
Conclusion
Our real-world experience confirms good efficacy, tolerability, and safety of ocrelizumab in our Arab population.
Introduction
Multiple sclerosis (MS) is a chronic autoimmune disease that targets the central nervous system and leads to progressive neuro-axonal demyelination eventually resulting in neurological disability and cognitive impairment.
MS presents in phenotypes according to the patterns and course of the disease [Citation1] clinically isolated syndrome (CIS), relapsing-remitting MS (RRMS) which usually evolves to secondary progressive MS (SPMS) and primary progressive MS (PPMS). Between 5 and 10% of MS adult patients have PPMS [Citation2]. Over the last three decades, numerous randomized clinical trials [Citation3–15] have demonstrated the efficacy of several so-called disease-modifying therapies (DMT) that have now become the cornerstone in long-term MS management.
In the past decade, the role of B cells in the pathogenesis of MS has come to the forefront. Depletion of B cells by anti-CD20 monoclonal antibodies (mAbs) has proved to decrease the activity of the RRMS and the progression of PPMS [Citation16–20]. The treatment efficacy and safety of ocrelizumab in patients with RRMS was demonstrated in two identical, double-blind, randomized phase III trials in patients with RRMS: In the OPERA I and II trials [Citation21] ocrelizumab was able to reduce the annualized relapse rate by 46–47% compared with interferon β-1a.
Both OPERA studies didn’t report major safety concerns as serious AEs were reported in 6.9 and 7% of the active group compared with the 7.8 and 9.6% of patients treated with interferon-β-1a. The most common AEs were infusion reactions, nasopharyngitis, upper respiratory tract infection, headache, and urinary tract infection in patients treated with ocrelizumab.
In the PPMS trial, ORATORIO [Citation22], 32.9% of patients in the ocrelizumab and 39.3% in the placebo group had disease progression confirmed after 12 weeks.
As of 31 July 2020, an estimated 174,508 people with MS worldwide have been treated with ocrelizumab, including ∼167,684 in the commercial post-marketing setting, and 6824 in clinical trials resulting in exposure of 249,971 patient-years [Citation23].
Although, as previously mentioned, the clinical trials have established the efficacy of Ocrelizumab, real-world data is limited. In addition, none of the 141 sites that participated in the clinical development program of ocrelizumab were in the Middle East, therefore, efficacy and safety are lacking in the Arab population. This study was designed to evaluate the generalizability of data from ocrelizumab phase 3 clinical trials in RRMS and its effectiveness in a real-world setting of an Arab population within a rapidly developing country, such as Qatar.
Methods
Study design and patients
This is a single-center, retrospective observational cohort study analyzing data collected from patients in clinical routine practice presented to Hamad General Hospital (HGH). HGH belongs to Hamad Medical Corporation, the public healthcare network in the country of Qatar.
All patients had received at least one infusion of ocrelizumab and were included from October 2017 through December 2020.
All patients were diagnosed according to the 2017 McDonald criteria, but only RRMS and active SPMS were included.
Ethics
The institutional ethics committee of the Hamad General Hospital approved the study (reference number: MRC-01-20-440) and all research was completed in accordance with the Declaration of Helsinki guidelines for research practice.
Patients
The main inclusion criteria for retrospective data analysis was a history of initiation of ocrelizumab in RRMS patients after approval in 2017 by FDA. Besides, there were no exclusion criteria for our retrospective data analysis, hence any type of immunomodulatory or immunosuppressive MS pretreatment was allowed before ocrelizumab initiation.
In our center, the standard patient follow-up protocol after ocrelizumab initiation included visits at 3-month intervals. During the COVID-19 pandemic, however, every three months, follow-up visits were conducted via teleconference, through which variables were recorded. So if the patient did not report any clinical relapse or worsening symptoms, the EDSS recorded for that encounter was the same as the previous one in person.
Variables and outcomes assessed during follow-up included demographics (gender, age at diagnosis, and disease duration), clinical (comorbidities, number of ocrelizumab infusions, number of relapses the two years before and after treatment initiation, previous treatments, and reason for discontinuation, EDSS score at baseline and the last in-person visit, radiological parameters (number of gadolinium-enhancing lesions (GELs) or new/enlarged T2 lesions on the first MRI scan before ocrelizumab initiation (before 3 months at maximum) and after one year of ocrelizumab.
Adverse events in each patient as documented during the follow-up were examined and depending on their severity grouped according to Common Terminology Criteria for Adverse Events (CTCAE) version 5.0 [Citation24], in mild (i.e. self-limiting, short-lasting, without need for any intervention), moderate (i.e. manageable outpatient, in need for short-term standard medication), or severe (i.e. extended or new hospitalization for any reason, application of rescue medication, death).
Clinical and MRI outcomes
A relapse was defined as new or recurrent symptoms and objective typical findings of MS with a duration of at least 24 h, in the absence of any other causes (such as fever or infections) [Citation25].
Disability progression was defined as a sustained (≥3 months) EDSS score increase of ≥1 point in patients with a baseline EDSS score of ≤5 or EDSS score increase of ≥0.5 point in patients with a baseline EDSS score of >5.5.
Disability improvement was defined as a sustained (≥3 months) EDSS score reduction of 0 reduction from the baseline EDSS score of at least 1.0 point for baseline EDSS score <5.5 or a reduction of 0.5 points if the baseline EDSS score was >5 [Citation26].
A patient was considered NEDA if they showed a combination of lack of clinical activity (relapses and progression of the disability) and no evidence of disease activity on MRI. Only patients who showed no signs of disease activity (NEDA) after a year of follow-up were considered [Citation27].
MRI activity was defined as the presence of T1 GELs in the year of the follow-up scan.
Patients underwent brain and spinal cord 3 T MRI with gadolinium before ocrelizumab initiation (baseline), after 12 months of the treatment initiation, and every year thereafter.
Scans were assessed by experienced neuro-radiologists and by the attending neurologist.
Description of the standard ocrelizumab application scheme in our center
Ocrelizumab was administered according to the standard protocol guidelines in our center [Citation26]. Vital signs were controlled before initiation, every 30 min during application and, at the end of each infusion as well as in case of infusion-related reaction (IRR) arising.
Pretreatment was routinely prescribed comprising intravenous 125 mg methylprednisolone, 50 mg of diphenhydramine, and paracetamol 1000 mg.
Routinely, ocrelizumab infusion rates were gradually accelerated every 30 min and adapted respecting patients’ tolerance. After application, clinical observation was continued for 60 min with reporting of possible adverse events.
Patients remained at the hospital during the ocrelizumab infusion and 1 h after for monitoring IRRs, which included all symptoms and events occurring during or within 24 h of the infusion and were graded as mild, moderate, severe, or life-threatening.
Statistics
SPSS 26.0 statistical package was used for the analysis. Quantitative variables were described using mean and standard deviations (for normally distributed variables) or using median and interquartile range (IQR) for non-normally distributed variables. We tested the normal distribution of each of the quantitative variables using Shapiro–Wilk’s test.
For categorical variables, we determined absolute and relative frequencies.
To compare proportions between Q-OCRE and OPERA I/II, we used the chi-square test. To compare the means between Q-OCRE and OPERA I/II, we used the t-test for independent samples.
The annualized relapse rate (ARR) was calculated by dividing the number of relapses on ocrelizumab by the number of patient-years. The adjusted ARR was estimated using a negative binomial regression adjusted for the number of relapses in the two years before ocrelizumab was initiated.
We estimated the median follow-up time with NEDA on Ocrelizumab using Kaplan Meier survival analysis. We constructed a Cox proportional hazard model to determine the factors associated with the occurrence of disease activity in patients on Ocrelizumab among the following covariates: age, gender, duration of illness, and the number of relapses during the two years before Ocrelizumab initiation. We calculated the hazard ratios (HRs) with their 95% confidence intervals.
Results
Baseline characteristics and pretreatments
A total of 60 patients (57 RRMS and three SPMS) fulfilling our inclusion criteria were included. Their demographics and clinical baseline characteristics are summarized in . For comparison, the data of the pivotal clinical trials have been added ().
Table 1. Baseline characteristics of Q-OCRE cohort compared with OPERA I/II.
As documented in the medical charts, we found that 24 of the patients (38%) were treatment-naive.
From the group coming from a previous disease-modifying treatment (n = 36) the most commonly used were the oral group including dimethyl fumarate (n = 12), fingolimod (n = 7), and teriflunomide (n = 5), whereas four and eight patients were switched from interferon-beta and natalizumab, respectively.
The most common reason for switching or initiating ocrelizumab was increased disease activity, measured either clinically, by MRI, or both (61%, n = 37) or clinical progression (11%, n = 7).
Six patients switched to ocrelizumab due to adverse events on previous DMT: (a) severe lymphopenia (two fingolimod, one DMF), (b) high liver function test (one fingolimod), and (c) intolerance to the drug (one IFN, one DMF).
Two patients on natalizumab were switched due to safety concerns (JC virus seroconversion). The other reasons included lack of compliance (2 DMF), and unknown as there was no reason documented in the medical charts for change in eight patients (in most of them their treatment was started abroad), and only one patient requested for change due to convenience.
From the clinical perspective, 31 patients (55%) had at least one relapse in the previous 2 years before treatment initiation, while 34 had GEL in the MRI scan done prior (at a maximum of 3 months) starting the ocrelizumab treatment.
Relevant comorbidities () were present in 36 patients (60%) which included cardiovascular risk factors as the most frequent one (33%) followed by other autoimmune and neurological diseases (5% for each group).
Table 2. Comorbidities in our patients treated with ocrelizumab (n = 37/60).
Interestingly our cohort exhibits a high number of patients with vitamin D deficiency (66%, n = 40).
During the treatment, six patients underwent surgical procedures, mainly bariatric surgery without any adverse events in terms of disease activity.
Clinical and MRI outcome after ocrelizumab initiation
The clinical and radiological evaluation () was performed with a global median-follow up of 19 months (range from 6 to 39 months). The median number of ocrelizumab infusions was 3.4 (range 1–7).
Table 3. Clinical and MRI outcomes in our Q-OCRE cohort.
After starting treatment with ocrelizumab, 11 patients (18%), all from the RRMS group experimented with a relapse. However, in one case the relapse happened just a month after treatment initiation.
The mean annualized relapse rate in the cohort fell from 0.61 (95%CI: 0.44–0.77) before the ocrelizumab initiation to 0.2 (95%CI: 0.10–0.45) after the treatment initiation (p < .001). Three patients (7%) had MRI activity at a 12-month scan after ocrelizumab treatment.
The mean EDSS post-treatment was 2.2, with four patients showing improvement in EDSS score during the follow-up, whereas disability progression occurred in three patients.
Finally, NEDA status was achieved in 73% of the RRMS patients with more than 12 months year of follow-up.
Adverse events
The most frequent adverse events were infusion-related reactions and infections (). IRRs (n = 10) were mostly mild or moderate, and responded well to standard management (reduction of infusion rate and administration of symptomatic treatment when needed). The rate of infusion reactions decreased over the period of follow-up. All patients received the standard premedication protocol (including antihistaminic, steroids, and antipyretic). No patient discontinued the treatment due to IRRs.
Table 4. Safety data in Q-OCRE cohort vs. pooled OPERA I/II.
Sixteen patients (26%) developed infections. Upper respiratory infections were the most common followed by urinary tract infections. During the follow-up period, two serious adverse events were observed, severe COVID pneumonia and a severe urinary tract infection that required hospitalization. The patient who contracted COVID is a 34-years-old gentleman, with no previous comorbidities and an EDSS score of 1. He required admission to the hospital due to the requirement of oxygen. Fortunately, the patient recovered fully with standard anti-COVID medication without sequelae and returned back to his previous neurological and physical baseline. The second patient was a 59-years-old gentleman, active smoker, known case of HTN, epilepsy, and with a baseline EDSS score of 7.5. He required ICU admission due to urinary sepsis and multiorgan failure however after appropriate antibiotic and supportive management the patient recovered fully without an increase in his EDSS score.
Herpes zoster infection and prolonged event of gastroenteritis were observed in one patient, respectively.
No opportunistic infections were detected and except for these two patients, none required hospitalization.
One patient discontinued the medication during the follow-up due to unplanned pregnancy. Pregnancy happened just one month after receiving the second dose of ocrelizumab infusion, however, the outcome of the mother and newborn were uneventful without teratogenic effect or hematological abnormalities. She didn't experiment with any medical relapse or medical complications during the pregnancy. The delivery was through natural vaginal birth and the newborn had a normal weight.
Survival analysis and Cox regression ( and ).
Figure 1. Kaplan–Meier survival curves showing the proportions of patients with multiple sclerosis started on Ocrelizumab remaining with no evidence of disease activity (NEDA) over follow-up time. NEDA: no evidence of disease activity.
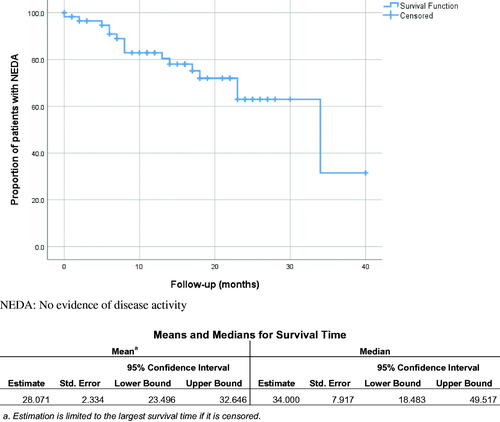
Table 5. Cox regression model with the estimated hazard ratios of having evidence of disease activity on Ocrelizumab for different covariates.
The median time to show evidence of disease activity for patients started on ocrelizumab was 34 months with a 95% confidence interval of [18.5; 49.5].
The Cox regression model showed that the number of relapses during the two years before starting ocrelizumab (but not gender, age, or duration of illness) was associated with higher hazards of exhibiting evidence of disease activity on the ocrelizumab (p = .046, hazard ratio = 2.45 [1.02; 5.87]).
Discussion
Ocrelizumab has been approved for the treatment of patients with MS more than three years ago but data on its real-world use is limited [Citation28–30]. We present the largest and longest real-world study to date in an Arab population, the population that was not included in the initial pivotal studies.
Our data on ocrelizumab administration in a cohort of 60 patients (RRMS and SPMS) with various immunomodulatory pretreatments demonstrated generally excellent efficacy and safety of this antibody in clinical routine application.
Compared with the cohort of the OPERA trial, our RMS cohort was slightly younger and had a longer duration of disease. Another important difference is the male preponderance in our cohort. The reason behind that is probably the childbearing age of most of the female patients in our MS clinics, therefore female patients and their physicians are more prompt to choose other disease-modifying treatments with a better profile in family planning than ocrelizumab.
About three-quarters of the RRMS patients included in the OPERA trials were treatment naïve, and the most common previous therapies were interferon and glatiramer acetate. In contrast, in our cohort three-quarters of the MS patients have been previously treated as they exhibit either clinical, radiological (or both) activity with most patients receiving highly effective DMT. Moreover, none of our patients was on glatiramer acetate as it is not available in Qatar.
The only variable that increased the hazards of exhibiting evidence of disease activity on ocrelizumab in our cohort was the number of relapses during the two years before starting ocrelizumab (but not gender, age, or duration of illness) data that has not been established in the clinical trial.
Nevertheless, ocrelizumab still exhibited high effectiveness not only after switching from first-line injectable treatments, such as interferons but also after switching from moderate or highly effective agents, such as dimethyl fumarate, fingolimod, and natalizumab and NEDA status at year 1 were achieved in 73% of the patients.
Comorbidities affect the quality of life (QoL) and long-term disability in patients with MS (PwMS). Depression, anxiety, cardiovascular disease, epilepsy, metabolic disease, and autoimmune diseases are some of the most common conditions found in patients. Real-world studies offer crucial information about patients with different comorbidities and prior treatments, who are rarely included in clinical trials [Citation30]. In our cohort, the most frequent comorbidities included cardiovascular risk factors, diabetes, and mental health conditions. All of them presented with a similar profile as described in previous studies [Citation30,Citation31] but with a lower frequency for all of them. Depression and anxiety are reported to be prevalent in patients with MS, with prevalence rates ranging from 20% to 50%. Although previously published data suggested that Middle Eastern [Citation32] PwMS may have lower rates of mood disorders, our cohort exhibited mood disorders even lower than reported. This is probably related to the underestimation of patient self-reporting (persistent social stigma around mental health problems). It's well known that not only MS but also several other autoimmune disorders are associated with vitamin D deficiency [Citation33]. Our cohort shows a very high rate of vitamin D deficiency (related to lack of sun exposure given the high temperatures during some seasons of the year, heat sensitivity in MS patients, and social lifestyle with regards to dressing code).
In terms of efficacy, our cohort matches with the findings in the OPERA I/II (see ). Most of the patients had no disease progression whereas disability progression occurred in four patients. It’s worth mentioning that our study might have been limited as the last EDSS score was estimated from the last face-to-face visit if no new relapses were reported by phone, given the measures imposed after the COIVD-19 outbreak, which lead to virtual clinics during most of the last year.
Matching the phase 3 clinical trial, our study showed that mild-to-moderate IRRs and infections were the most common adverse events.
The percentage of IRRs in our study (26%) was lower than those in the pivotal clinical trials (ORATORIO: 39.9%, OPERA: 34.3%) [Citation21,Citation22]. Probably this is related to the modified premedication protocol used in our center, in concordance with previously reported data [Citation34].
The most common infections observed in the OPERA trials of ocrelizumab were upper respiratory tract and urinary tract infections. Most of the infections reported in two recent observational studies were classified as minor in 8 and 5% of the patients [Citation28,Citation29]. However, in our cohort, this proportion was higher (16%), even slightly higher than the one reported in the latest published study [Citation30]. This might be explained by the longer follow-up interval in our series. Furthermore, upper respiratory tract infections were likely underreported due to two main causes, firstly; most patients do not consult their physicians for symptoms of mild upper respiratory (underestimation) and secondly; the adoption of non-pharmacological measures during the COVID-19 outbreak (such as social lockdown, mask adoption, and handwashing) had provoked a decrease in the total number of infections worldwide [Citation35,Citation36].
On the contrary, in our cohort, the rate of serious infections requiring hospitalization, which is rarely affected by reporting bias, was actually lower.
The rate of COVID infections was low in our group, (1.6%) lower than reported in the literature [Citation23]. The ongoing coronavirus pandemic impacted initially the management of the PwMS as there were some concerns that patients receiving immunosuppressive therapy could increase the risk of severe COVID infections. Even more than one year after the outbreak, the available data is scarce, and initially published studies suggested that after adjusting for region, age, sex, progressive MS course, and recent methylprednisolone use, the therapy with an anti-CD20 agent (ocrelizumab or rituximab) was significantly associated with an increased risk of severe Covid-19 course [Citation37]. According to a variety of data sources including ongoing clinical trials of ocrelizumab, post-marketing safety reports, as well as the OPTUMR database, most SARS-CoV-2 infections were mild-to-moderate, so no treatment was needed. No association was found between ocrelizumab exposure duration and the incidence of COVID-19. Furthermore, as in the general population, people with higher risk factors (older age, disability status, male, and comorbidities) seem to experience the condition more often. In the post-marketing safety database, case-fatality rates appear to be in-line with those of PwMS and the general population, ranging from 1.6 to 4.9%. In the Optum real-world COVID-19 dataset, similar rates of hospitalization, ventilation, and death were observed in the ocrelizumab and general MS groups [Citation23].
Our study is subject to limitations; firstly, due to the retrospective design, underestimation of adverse events due to reporting or documentation bias cannot be excluded; secondly, phone interviews and last face-to-face EDSS could mislead the potential diseases activity and progression in some patients. Nevertheless, our study contained a long follow-up that could elucidate long-term side effects and tolerance problems to ocrelizumab which is crucial to estimate the risk for delayed side effects.
In conclusion, our real-world experience confirms tolerability and safety of ocrelizumab in a particular area of the world that was not included in the pivotal trials supporting their findings. Further studies and post-marketing surveillance are needed to confirm these beneficial findings and to reveal safety concerns in the treatment with ocrelizumab in the longer-term follow-up.
Transparency
Declaration of funding
No funding was received to produce this article.
Declaration of financial/other relationships
B.G.-C. and D.D. have received funds as consultant/advisor from Roche, Merck, Sanofi, and Novartis. B.G.-C. and D.D. have received funds as speakers from Roche, Merck, Sanofi, and Novartis. Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Acknowledgements
Open Access funding is provided by the Qatar National Library.
References
- Lublin FD, Reingold SC, Cohen JA, et al. Defining the clinical course of multiple sclerosis: the 2013 revisions. Neurology. 2014;83(3):278–286.
- Rice CM, Cottrell D, Wilkins A, et al. Primary progressive multiple sclerosis: progress and challenges. J Neurol Neurosurg Psychiatry. 2013;84(10):1100–1106.
- The IFNB Multiple Sclerosis Study Group. Interferon beta-1b is effective in relapsing-remitting multiple sclerosis. I. Clinical results of a multicenter, randomized, double-blind, placebo-controlled trial. Neurology. 1993;43(4):655–661.
- Jacobs LD, Cookfair DL, Rudick RA, et al. Intramuscular interferon beta-1a for disease progression in relapsing multiple sclerosis. The multiple sclerosis collaborative research group (MSCRG). Ann Neurol. 1996;39(3):285–294.
- Johnson KP, Brooks BR, Cohen JA, et al. Copolymer 1 reduces relapse rate and improves disability in relapsing-remitting multiple sclerosis: results of a phase III multicenter, double-blind placebo-controlled trial. The copolymer 1 multiple sclerosis study group. Neurology. 1995;45(7):1268–1276.
- PRISMS (Prevention of Relapses and Disability by Interferon beta-1a Subcutaneously in Multiple Sclerosis) Study Group. Randomised double-blind placebo-controlled study of interferon beta-1a in relapsing/remitting multiple sclerosis. Lancet. 1998;352(9139):1498–1504.
- Hartung HP, Gonsette R, König N, et al. Mitoxantrone in progressive multiple sclerosis: a placebo-controlled, double-blind, randomised, multicentre trial. Lancet. 2002;360(9350):2018–2025.
- Polman CH, O'Connor PW, Havrdova E, et al. A randomized, placebo-controlled trial of natalizumab for relapsing multiple sclerosis. N Engl J Med. 2006;354(9):899–910.
- Kappos L, Radue E-W, O'Connor P, et al. A placebo-controlled trial of oral fingolimod in relapsing multiple sclerosis. N Engl J Med. 2010;362(5):387–401.
- O'Connor P, Wolinsky JS, Confavreux C, et al. Randomized trial of oral teriflunomide for relapsing multiple sclerosis. N Engl J Med. 2011;365(14):1293–1303.
- Fox RJ, Miller DH, Phillips JT, et al. Placebo-controlled phase 3 study of oral BG-12 or glatiramer in multiple sclerosis. N Engl J Med. 2012;367(12):1087–1097.
- Coles AJ, Twyman CL, Arnold DL, et al. Alemtuzumab for patients with relapsing multiple sclerosis after disease-modifying therapy: a randomised controlled phase 3 trial. Lancet. 2012;380(9856):1829–1839.
- Calabresi PA, Kieseier BC, Arnold DL, et al. Pegylated interferon-1a for relapsing-remitting multiple sclerosis (ADVANCE): a randomised, phase 3, doubleblind study. Lancet Neurol. 2014;13(7):657–665.
- Secondary Progressive Efficacy Clinical Trial of Recombinant Interferon-Beta-1a in MS (SPECTRIMS) Study Group. Randomized controlled trial of interferon-beta-1a in secondary progressive MS: clinical results. Neurology. 2001;56(11):1496–1504.
- La Mantia L, Vacchi L, Rovaris M, et al. Interferon β for secondary progressive multiple sclerosis: a systematic review. J Neurol Neurosurg Psychiatry. 2013;84(4):420–426.
- Salzer J, Svenningsson R, Alping P, et al. Rituximab in multiple sclerosis: a retrospective observational study on safety and efficacy. Neurology. 2016;87(20):2074–2081.
- Alping P, Frisell T, Novakova L, et al. Rituximab versus fingolimod after natalizumab in multiple sclerosis patients. Ann Neurol. 2016;79(6):950–958.
- Granqvist M, Boremalm M, Poorghobad A, et al. Comparative effectiveness of rituximab and other initial treatment choices for multiple sclerosis. JAMA Neurol. 2018;75(3):320–327.
- Bar-Or A, Grove RA, Austin DJ, et al. Subcutaneous ofatumumab in patients with relapsing-remitting multiple sclerosis: the MIRROR study. Neurology. 2018;90(20):e1805–e1814.
- Sorensen PS, Lisby S, Grove R, et al. Safety and efficacy of ofatumumab in relapsing-remitting multiple sclerosis: a phase 2 study. Neurology. 2014;82(7):573–581.
- Hauser SL, Bar-Or A, Comi G, et al. Ocrelizumab versus interferon beta-1a in relapsing multiple sclerosis. N Engl J Med. 2017;376(3):221–234.
- Montalban X, Hauser SL, Kappos L, et al. Ocrelizumab versus placebo in primary progressive multiple sclerosis. N Engl J Med. 2017;376(3):209–220.
- Hughes R, Whitley L, Fitovski K, et al. COVID-19 in ocrelizumab-treated people with multiple sclerosis. Mult Scler Relat Disord. 2021;49:102725.
- National Cancer Institute. Common terminology criteria for adverse events v4.0 [cited 2020 Mar 8]. Available from: https://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03/CTCAE_4.03_2010-06-14_QuickReference_8.5x11.pdf
- Thompson AJ, Banwell BL, Barkhof F, et al. Diagnosis of multiple sclerosis: 2017 revisions of the McDonald criteria. Lancet Neurol. 2018;17(2):162–173.
- Ocrevus: EPAR-Product information [cited 2020 Oct 8]. Available from: https://www.ema.europa.eu/en/documents/product-information/ocrevus-epar-product-information_en.pdf
- Giovannoni G, Turner B, Gnanapavan S, et al. Is it time to target no evident disease activity (NEDA) in multiple sclerosis? Mult Scler Relat Disord. 2015;4(4):329–333.
- Ellwardt E, Rolfes L, Klein J, et al. Ocrelizumab initiation in patients with MS: a multicenter observational study. Neurol Neuroimmunol Neuroinflamm. 2020;7(4):e719.
- Prockl V, Nickel FT, Utz KS, et al. Real world application of ocrelizumab in multiple sclerosis: single-center experience of 128 patients. J Neurol Sci. 2020;415:116973.
- Fernandez‐Diaz E, Perez‐Vicente JA, Villaverde‐Gonzalez R, et al. Real-world experience of ocrelizumab in multiple sclerosis in a Spanish population. Ann Clin Transl Neurol. 2021;8(2):385–394.
- Hauer L, Perneczky J, Sellner J. A global view of comorbidity in multiple sclerosis: a systematic review with a focus on regional differences, methodology, and clinical implications. J Neurol. 2020. DOI:https://doi.org/10.1007/s00415-020-10107-y
- Alsaadi T, El Hammasi K, Shahrour TM, et al. Prevalence of depression and anxiety among patients with multiple sclerosis attending the MS Clinic at Sheikh Khalifa Medical City, UAE: cross-sectional study. Mult Scler Int. 2015;2015:487159.
- Miclea A, Bagnoud M, Chan A, et al. A brief review of the effects of vitamin D on multiple Sclerosis. Front Immunol. 2020;11:781.
- Conte WL, Arndt N, Cipriani VP, et al. Reduction in ocrelizumab-induced infusion reactions by a modified premedication protocol. Mult Scler Relat Disord. 2019;27:397–399.
- Huang QS, Wood T, Jelley L, et al. Impact of the COVID-19 nonpharmaceutical interventions on influenza and other respiratory viral infections in New Zealand. Nat Commun. 2021;12(1):1001.
- Cowling BJ, Ali ST, Ng TWY, et al. Impact assessment of non-pharmaceutical interventions against coronavirus disease 2019 and influenza in Hong Kong: an observational study. Lancet Public Health. 2020;5(5):e279–e288.
- Sormani MP, Italian Study Group on COVID-19 Infection in Multiple Sclerosis. An Italian programme for COVID-19 infection in multiple sclerosis. Lancet Neurol. 2020;19(6):481–482.

