Abstract
Objective
To understand current treatment patterns and health care resource utilization (HRU) of women with locally advanced or metastatic breast cancer (advanced breast cancer; ABC) in Korea overall and within patients who had progressed with prior endocrine therapy (as first-line treatment for metastatic disease) and patients with no prior systemic treatment (for advanced disease).
Methods
A chart review was conducted in 109 patients (women ≥ 18 years old with HR+/HER2- ABC diagnosed between 2015 and 2017) from 11 hospitals. Anonymized data on patient characteristics, treatment patterns and HRU was abstracted.
Results
Mean (range) age of all patients was 57.5 (40–81) years. Overall, the most common first-, second- and third-line systemic therapy after diagnosis of ABC were letrozole ± palbociclib (51%), endocrine therapy (ET)±everolimus (42%) or chemotherapy (ChT) (39%), and ChT (68%), respectively. In patients progressed with ET (n = 33) and those with no prior systemic treatment (n = 52), the most common first-line treatments were letrozole (82%) and letrozole + palbociclib (42%), respectively. The percentage of patients with at least one grade 3 or higher adverse event during first-line therapy was 93.1% vs 39.2% in patients on a ChT based regimen (N = 29) vs. ET (N = 74). Overall, oncologist visits, at an annual rate of 9.27 (95% CI: 8.87, 9.69) visits per month, and hospitalizations, with an annual rate of 0.44 (95% CI: 0.36, 0.54), and mean (SD) length of stay of 14.3 (10.32) days, were the key drivers of HRU.
Conclusions
These findings on real world HRU reflected clinical guidelines and severity of ABC. Results can inform future evaluations of new ABC treatments that estimate the health economic impact of their adoption in Korea.
Introduction
Cancer is one of the primary public health concerns in South Korea and it is expected that the burden due to cancer will continue to grow with the aging population. Cancer accounts for one in four deaths in Korea (27.9% in 2015) and more than 200,000 new cases are diagnosed each year. The number of women in Korea living with breast cancer (prevalent cases) was estimated to be 178,395 at the end of 2015 with 19,219 new cases of breast cancer and 17,399 deaths during the year. The Korean age-standardized incidence of all newly diagnosed breast cancer in 2015 was estimated to be 49.2 per 100,000 for women (crude incidence 75.1) and their five-year relative survival is among the highest in the developed world at over 90% (92.3% for cases diagnosed in 2011–15Citation1. A small proportion of new breast cancer cases are diagnosed with distant extent of disease (4.6% in 2015), and five-year relative survival for this group remains relatively low (38.3%)Citation2.
Breast cancer is a heterogeneous disease. In the United States (US), hormone receptor-positive (HR+)/human epidermal growth factor receptor-2-negative (HER2-) subtype is the most common subtype at initial presentation (67.8%), followed by HR+/HER2-overexpressing (HER2+) (13.3%), triple-negative (13.1%), and HR-/HER2+ (6.8%)Citation3. In another study, the distribution of cancer subtypes were shown to vary by ethnicity, with the lowest proportion of HR+/HER2- observed among Korean-Americans (56.0%) compared to White-Americans (71.6%) and Chinese-Americans (65.9%)Citation3. Among patients with newly diagnosed breast cancer in Korea in 2015, 74% were estrogen receptor positive (ER+) and 63% were progesterone receptor positive (PR+)Citation3. Recent Korean National Health Insurance data suggested that 57% of all breast cancer patients were HR+Citation3.
The selection of treatment for locally advanced and metastatic breast cancer depends on the cancer subtype, menopausal status, prior treatment and extent of the disease. Systemic treatments such as ET and cytotoxic chemotherapy remain mainstream treatment options for the management of advanced HR + disease. Among international guidelines, ET is recommended for HR+/HER2- ABC with or without kinase inhibitors (cyclin-dependent kinase [CDK]: abemaciclib, palbociclib, ribociclib; mTOR: everolimus)Citation4. These ET include aromatase inhibitors (AI; non-steroidal: anastrozole, letrozole; steroidal: exemstane), selective estrogen receptor degraders (SERDs; fulvestrant) and selective estrogen receptor modulators (SERMs; tamoxifen, toremifene). Specific regimens are recommended based on receipt of ET in the previous year and menopausal status. Ovarian suppression (goserelin, leuprolide) or ovarian ablation is recommended with ET for pre-menopausal women who are HR+. Chemotherapy, generally as sequential single agents, is recommended for patients with visceral crisis or following progression or unacceptable toxicity after first-line ET.
Advances in the understanding of molecular heterogeneity and gene expression analysis over the past two decades have led to development of novel agents targeting patterns associated with specific cancer subtypes, offering increased progression-free and/or overall survival. Palbociclib was the first cyclin-dependent kinase (CDK) 4/6 inhibitor to be approved as a cancer therapy. The PALOMA-2 study showed that progression-free survival was longer with palbociclib plus letrozole than with letrozole alone in the initial treatment of postmenopausal women with HR+/HER2- ABCCitation5. Palbociclib, in combination with fulvestrant therapy, was also showed to prolong progression-free and overall survival in the PALOMA-3 studyCitation6,Citation7.
Abemaciclib, another CDK 4/6 inhibitor has been approved in the US for the treatment of women with HR+/HER2- ABC in combination with an aromatase inhibitor as initial ET-based therapy; or in combination with fulvestrant in patients with disease progression following ET; or as monotherapy in patients with disease progression following ET and prior chemotherapy in the metastatic setting. Abemaciclib has been shown to prolong overall survival (OS) and progression-free survival (PFS) in women with HR+/HER2- locally advanced or metastatic breast cancer who had progressed with ET (as neoadjuvant or adjuvant therapy or first-line treatment for metastatic disease; MONARCH-2) and PFS in women who had not received prior systemic treatment for advanced disease (MONARCH-3)Citation8,Citation9. In a single-arm phase 2 study (MONARCH-1), abemaciclib monotherapy showed promising clinical activity (overall response rate of 19.7%) and OS (median OS of 22.3 months) in a heavily pretreated population who had received prior ET and prior chemotherapy in the metastatic settingCitation8,Citation10,Citation11.
In addition, ribociclib with letrozole was also shown to achieve prolonged PFS in the MONALEESA-2 study women with HR+/HER2- ABCCitation12. The MONALEESA-7 study of ribociclib in addition to endocrine therapy (goserelin and either a nonsteroidal aromatase inhibitor or tamoxifen) showed improvement in OSCitation13, while the MONALEESA-3 study demonstrated improved PFS and OS with ribociclib in combination with fulvestrantCitation14,Citation15.
The economic burden of cancer in Korea almost doubled between 2000 and 2010 placing significant financial burden on government and patientsCitation16. Analysis based on claims data of the National Health Insurance Service in Korea identified that breast cancer had the highest cost among cancer types in women with a cost of $1,044 million in 2015Citation17. Specifically in HR+/HER2- advanced breast cancer, a recent Korean study estimated per monthly per patient direct medical costs to increase from $870 to $3762 after disease progressionCitation18. The burden of breast cancer in terms of impaired self-reported health was found to be particularly unfavorable in advanced or metastatic breast cancer patients among all breast cancer patients in KoreaCitation19.
The aim of this research was to characterize current treatment patterns in ABC and HRU in Korea overall and within patients who had progressed with ET and patients with no prior systemic treatment. Understanding treatment patterns and related outcomes such as PFS and adverse event (AE) burden can, in turn, inform future health evaluations of new ABC treatments that estimate the cost-effectiveness or budgetary impact of adopting them in Korea.
Methods
Study design
This observational study included a retrospective review of medical charts to describe treatment patterns and health care resource utilization (HRU) in patients with locally advanced or metastatic breast cancer in Korea. The study data was collected via an online physician survey.
A total of 34 oncologists were recruited from an extensive list of a large proportion of oncologists who treat ABC in Korea. Invitations to the oncologists were sent out randomly until the minimum required sample size was achieved. Each oncologist reported anonymized data on 3 or 4 patients, resulting in a sample size of 109. The reasons for specifying the chart number per oncologists to up to four were to reduce physician burden, increase geographical representativeness, and limit cluster effect. Oncologists were asked to identify eligible patients from those meeting the inclusion/exclusion criteria, based on a randomly generated month of birth date to avoid selection bias, and review those patients’ charts to report anonymized patient-level information on treatments and outcomes. No subject-identifying data were collected on the forms.
The study was conducted in compliance with the protocol and the Code of Conduct of the World Association of Opinion and Marketing Research Professionals (https://www.esomar.org/). Ethics review was conducted by the New England (US) Institutional Review Board (IRB) (http://neirb.com/service-inquiry).
Study population
The target population included women ≥ 18 years old with HR+/HER2- locally advanced or metastatic breast cancer diagnosed between 1 January 2015 and 31 December 2017 (i.e. recurrence or de novo cases of ABC). Exclusion criteria included patients who have participated in a clinical trial for ABC, and patients with evidence of other prior or concurrent malignancy apart from ABC.
Subject groups
This was a non-comparative study; however, two sub-groups of patients were examined in a post-hoc analysis in addition to the total subject population.
Specifically, the patient group “progressed with ET” in the chart review included patients who were pre- or postmenopausal; had disease progression while receiving endocrine monotherapy (excluding fulvestrant) as first-line therapy post ABC diagnosis; an Eastern Cooperative Oncology Group (ECOG) Performance Status of 0 or 1; not received more than one ET; not received any prior chemotherapy for ABC; not received prior treatment with everolimus, or CDK 4 and 6 inhibitors; and no evidence or history of Central Nervous System (CNS) metastasis.
The patient group “no prior systemic treatment” in the chart review included subjects who: were postmenopausal; at the time of data abstraction, had not had disease progression while receiving endocrine monotherapy as first-line therapy post ABC diagnosis; had an ECOG of 0 or 1; had not received prior treatment with fulvestrant, everolimus, or CDK 4 and 6 inhibitors; and had no evidence or history of CNS metastasis.
Time periods
The index event was the diagnosis of ABC, which could be a de novo case or recurring disease. Adult women with HR+/HER2- ABC diagnosed between 1 January 2015 and 31 December 2017 were eligible for the study. Information prior to the index event (pre-period) were from the date of initial diagnosis of breast cancer. The observation period for each patient was from index date until date of data collection, December 2018, or death, whichever occurred earlier. Thus, patients were potentially observed for between 1 and 4 years, depending on their index date.
Data collected
The online data abstraction form was completed by the physicians based on a review of their medical charts. The data abstraction form included questions on physician characteristics, patient demographic characteristics, disease history, number and location of metastases at diagnosis of ABC, line of cancer directed systemic treatments (determined by the physician), reasons for treatment discontinuation, HRU and pre-specified grade 3 or higher AEs occurring during cancer directed systemic therapy.
Systemic treatments included ET; chemotherapy; CDK inhibitors; mammalian target of rapamycin (mTOR) inhibitors; and ovarian suppression. Radiation therapy and surgery were also collected. The HRU collected included health care professional office visits, diagnostic and disease monitoring tests, blood transfusions, supportive care treatments, and hospitalizations. The pre-specified grade 3 or higher AEs included: alanine aminotransferase increased, anemia, spartate aminotransferase increased, asthenia/fatigue, diarrhea, dyspnea, gamma-glutamyl transferase increase, hyperglycemia, hypertension, infection, leukopenia, lymphopenia, nausea, neutropenia, and stomatitis.
Analytic methods
Descriptive statistics were used to characterize the study population and treatment patterns overall and within patients who had progressed with prior ET and patients with no prior systemic treatment. Systemic cancer-directed treatment lines were determined by the physician. The percentage of patients who had a pre-specified grade 3 or higher AE during first-line therapy was assessed. Annual rates and 95% confidence intervals for HRU were evaluated using Poisson models, which included an offset of patient observation time, to adjust for the variable patient follow-up time. Progression free survival from the commencement of first and second line therapy was assessed using Kaplan-Meier methods. Date of progression was defined as the earliest of: end of treatment if reason for discontinuation was progressive disease; death; and start of the next line of therapy. Patients not known to have progressed, were censored at their last contact date (e.g. last visit to the clinic).
Descriptive summary statistics for continuous variables included the number of subjects (n), mean, standard deviation (SD), median, interquartile range (IQR) and range. Descriptive summary statistics for categorical variables included frequency counts and percentages [n (%)]. No data imputation was undertaken.
Results
Physician and patient characteristics
All 34 physicians were medical oncologists based in Seoul, with 24 (71%) working in a tertiary hospital setting. The mean (SD) time physicians had been practicing in ABC was 11.6 years (3.64 years), and they reported treating a median (IQR) of 25 (20–30) patients with ABC per month.
The mean (SD) age of the 109 patients was 57.5 (9.39) years and ranged from 40 to 81 years. The majority of patients (94%) were not employed. A total of 33 patients met the subgroup criteria of having had progressed with prior ET and 52 patients met the subgroup criteria of having no prior systemic treatment. The mean number of metastatic sites was 1.8 overall, at the time of diagnosis of locally advanced or metastatic breast cancer; 1.4 and 1.6 within the patient group who progressed with ET and who had no prior systemic treatment, respectively ().
Table 1. Patient characteristics.
ABC treatment patterns
Patients use a variety of anti-cancer treatments since diagnosis of ABC including ET (N = 88 [81%]), chemotherapy (N = 49 [45%]), CDK 4 and 6 inhibitors and mTOR inhibitors, e.g. everolimus, palbociclib (N = 33 [30%]), ovarian suppression (N = 17 [16%]), as well as radiation (N = 11 [10%]) and surgery (N = 1 [1%]). Since ABC diagnosis (N = 47 [43%]), patients had two lines of systemic therapy regimens only; (N = 37 [34%]) had one line only; (N = 12 [11%]) had five or more lines; (N = 9 [8%]) had three lines only; and (N = 4 [4%]) had four lines only of systemic therapy regimens since diagnosis of ABC. The number of unique regimens in first-line treatment was 25 in the overall study population. The study population had 21 unique second-line regimens and 12 unique third-line regimens, respectively.
Overall, the most common first-, second- and third-line systemic therapy after diagnosis of ABC were letrozole ± palbociclib (N = 56 [51%]), endocrine therapy ± everolimus (N = 30 [42%]) or ChT (N = 28 [39%]), and ChT (N = 17 [68%]), respectively (). In patients who progressed with ET and received treatment (N = 33 first-line, N = 30 s-line), the most common respective first-and second-line treatments were letrozole (N = 27 [82%]), and exemestane + everolimus (N = 9 [30%]). In patients with no prior systemic treatment and received treatment (N = 52 first-line, N = 20 s-line), the most common respective first-and second-line treatments were letrozole + palbociclib (N = 22 [42%]), and endocrine therapy (N = 9 [45%]) or ChT (N = 8 [40%]).
Table 2. Most common treatments (>10% of patients) after diagnosis of ABC.
Overall, the main reason for discontinuation from first-line systemic therapy was disease progression (N = 65 [60%]), with 31 (28%) patient having ongoing first-line therapy at the end of the observation period. Similar distributions were observed for second and third-line therapy.
Adverse events
Overall, the percentage of patients with at least one of the selected grade 3 or higher adverse event during first-line therapy was higher in patients on a chemotherapy based regimen (27 of 29 [93%]) compared to an endocrine based therapy (27 of 74 [39%]).
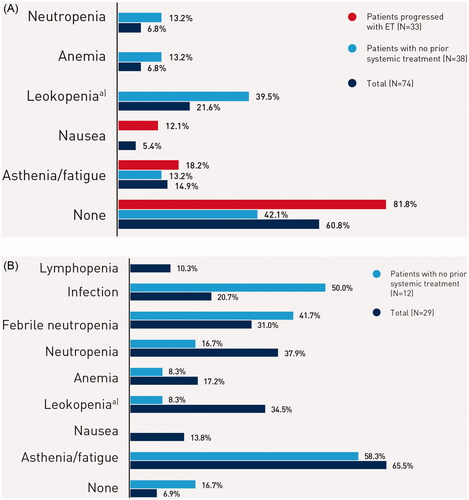
The most commonly reported severe (grade 3 or higher) adverse events during first-line were asthenia/fatigue (N = 19 [66%]), neutropenia (N = 11 [38%]), leukopenia (N = 10 [34%]), and febrile neutropenia (N = 9 [31%]), for patients on a chemotherapy based regimen; and leukopenia (N = 16 [22%]), and asthenia/fatigue (N = 11 [15%]) for patients on an endocrine based therapy ().
Figure 1. (A) Most common severe adverse events (>10% of patients) during treatment in patients on endocrine based therapy. Notes: Percentages may add to more than 100% as patients may be counted in more than one category. Abbreviation. SAE, severe adverse event (grade 3 or higher). a)Leukopenia was not necessarily reported separately if neutropenia or febrile neutropenia is also reported. (B) Most common severe adverse events (>10% of patients) during treatment in patients on chemotherapy based regimen. Notes: Percentages may add to more than 100% as patients may be counted in more than one category. Abbreviation. SAE, severe adverse event (grade 3 or higher). a)Leukopenia was not necessarily reported separately if neutropenia or febrile neutropenia is also reported.
Health care resource utilization
Overall, HRU included oncologist visits, at an average rate of 9.27 (95%CI: 8.87, 9.69) visits per patient-year, and hospitalizations, with an annual rate of 0.44 (95%CI: 0.36, 0.54) per patient-year, and a mean (SD) length of stay of 14.3 (10.32) days (). Among the diagnostic and disease monitoring tests, nearly all patients received at least one; chest X-ray 103 (94.5%), bone scan 106 (97.2%), tumor markers 104 (95.4%), and CT 108 (99.1%), while the majority of patients also received at least one PET 91 (83.5%), MRI 69 (63.3%), and ultrasound 65 (57.8%). The most common palliative and supportive care treatments included antiemetic drugs, pain medications, antibiotics, bone-modifying agents, and growth factors ().
Table 3. Health care resource utilization after diagnosis of ABC.
Table 4. Palliative and supportive care treatments after diagnosis of ABC.
The rates for nearly all HRU categories were higher in patients taking chemotherapy compared to those taking ET therapy as first-line therapy post ABC diagnosis. The annual rates of HRU which were higher within the chemotherapy compared to the ET group included: ultrasound, chest X-ray, bone scans, CTs, MRIs, oncologist visits, surgeon visits, pain or palliative physician, blood transfusions, accident and emergency visits, and hospitalizations. Of note, the annual rate of hospitalizations was 1.47 for all patients in the chemotherapy group compared to 0.04 for all patients in the ET group, while length of stay per hospitalization was also longer in the chemotherapy group (15.22 vs. 9.33 days, respectively).
Progression-free survival
Overall, the median first-line progression-free survival, was 9.2 months (95% CI: 6.9, 11.0). Of the 109 patients who commenced first-line therapy, i.e. all patients per the study inclusion criteria, 76 (69.7%) were defined as having disease progression, the remaining 33 (30.3%) patients were censored. For the 72 patients who commenced second-line therapy, only 25 (34.7%) discontinued treatment, and therefore the median progression-free survival was not evaluable, as at least 50% of patients need to have progression in order to assess median progression-free survival.
Discussion
This study presents a recent overview of current treatment patterns and HRU observed between 2015 and 2018 in patients with ABC in the real world setting in Korea. Results quantified a substantial burden of ABC in terms of treatments and health resources required, including visits and hospitalizations especially among those patients who received chemotherapy, which were consistent with the severity of ABC. Studies on health care resource utilization in breast cancer in Korea are very limited and, as such, this chart review fills a gap for such evidence. However, one previous study on claims data based direct medical costs had a similar finding in terms of identifying inpatient care as a key cost driver and quantifying a substantial cost increase in more severe patients who experienced disease progressionCitation18.
The findings also showed that treatment patterns in real world practice reflected clinical guidelines published internationally and in KoreaCitation4,Citation16,Citation20,Citation21 in terms of systemic treatments such as ET and chemotherapy options dominating ABC care with ovarian suppression with ET used in a certain portion of pre-menopausal patients.
While a diverse and high number of unique regimens were shown to characterize treatment patterns, there was a clear difference by line of treatment with letrozole ± palbociclib the most common first-line, ET ± everolimus combination or chemotherapy dominating second-line, and chemotherapy dominating third-line treatments, respectively. However, chemotherapy use remained high even within first-line prescribing.
Current treatment patterns suggest an unmet need for new medications, including for the overall patient population and sub-groups based on prior treatment (those who had progressed with prior ET and those with no prior systemic treatment), that lead to reduction in high rate of chemotherapy use and frequent severe AEs associated with them. This unmet need was underlined with a striking finding that the prevalence of severe adverse (grade 3 or higher) events during first-line systemic therapy after ABC diagnosis in real world setting was reported to be over double in patients undergoing chemotherapy treatment compared to ET-based treatments. In addition, HRU was also substantially higher in patients who received chemotherapy based regimens as first-line treatment with hospitalization burden found particularly high.
With the very recent introduction and reimbursement coverage of several medications, treatment patterns may have or likely to change in the future. Namely, while palbociclib obtained regulatory approval in 2016, its reimbursement for first line prescribing in November 2017 and second line prescribing in June 2020 may have altered prescribing patterns since the chart review data collection. In addition, ribociclib and abemaciclib both got approval in 2019 and reimbursement coverage in 2020.
Some further limitations of this study need to be highlighted. The study included physician and patients from Seoul hospitals only, where breast cancer treatment in Korea is centered. Therefore, the generalizability of the results to other regions within or outside Korea may be limited. The sample size of patients within each of the subgroups was small and therefore the finding within the subgroups should be interpreted with caution. In addition, as recent patient data was collected and the observation period was therefore limited, overall survival was not possible to report in this study.
However, there is no particular reason to believe that these limitations would alter the key overall conclusions of this study. Future research is warranted on how treatment patterns and health resource utilization differ in further sub-groups of ABC patients of particular interest, such as those with poor prognostic factors, e.g. ECOG > 0, existence of visceral metastases, and/or endocrine resistance. In addition, with the emergence of new treatments and updates to clinical guidelines, changes in treatments and HRU should be monitored over time to assess their impact on health outcomes and health care budgets in Korea. Furthermore, the assessment of the impact of new products on the cost-effectiveness profile of first and subsequent line treatments in HR+/HER2- patients in Korea would be interesting to explore as similar research has been conducted elsewhereCitation22,Citation23.
Additional research is also warranted on resource utilization and costs faced by patients directly. Studies from Korea, similar to other countries, suggest that “catastrophic health expenditures” present a substantial problem for households with members who are severely illCitation24,Citation25. While costs beyond health care are important from the patient and the societal perspective, such data collection would require surveying patients beyond the limitation of a retrospective chart review.
Conclusion
This study fills in a gap of evidence on current treatment patterns and HRU in patients with ABC in the real world setting in Korea. Results highlight a substantial unmet need for new medications that lead to reduction in chemotherapy use and associated AE burden, including for the overall patient population and sub-groups based prior treatment status, such as those who had progressed with ET and patients with no prior systemic treatment. Detailed results can inform future health economic evaluations of new ABC treatments that estimate the cost-effectiveness or budgetary impact of adopting them in Korea.
Transparency
Declaration of funding
Eli Lilly funded the study.
Declaration of financial/other interests
DN, SYL, and DHK are employees of Eli Lilly. Sam Colman and AS are employees of Covance that received funding from Eli Lilly.
Author contributions
DN: Conceptualization, Methodology, Reviewing, Project Supervision.
SYL: Conceptualization, Reviewing.
DHK: Conceptualization, Reviewing.
AS: Conceptualization, Methodology, Writing, Project Supervision.
SC: Conceptualization, Methodology, Formal Analysis, Writing.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Acknowledgements
None reported.
References
- Jung KW, Won YJ, Kong HJ, et al. Cancer statistics in Korea: incidence, mortality, survival, and prevalence in 2015. Cancer Res Treat. 2018;50(2):303–316.
- Korea Central Cancer Registry and National Cancer Center. Annual report of cancer statistics in Korea in 2015. http://ncc.re.kr/downloadByFileUrl.ncc?path=/downloadFiles/eng/Annual2017.
- Parise CA, Caggiano V. Breast cancer survival defined by the ER/PR/HER2 subtypes and a surrogate classification according to tumor grade and immunohistochemical biomarkers. J Cancer Epidemiol. 2014;2014:469251.
- NCCN. NCCN Clinical practice guidelines in oncology: Breast Cancer. Version 1.2018 – March 20, 2018.
- Finn RS, Martin M, Rugo HS, et al. Palbociclib and letrozole in advanced breast cancer. N Engl J Med. 2016;375(20):1925–1936.
- Turner NC, Slamon DJ, Ro J, et al. Overall survival with palbociclib and fulvestrant in advanced breast cancer. N Engl J Med. 2018;379(20):1926–1936.
- Cristofanilli M, Rugo HS, Im SA, et al. Overall survival with palbociclib and fulvestrant in women with HR+/HER2- ABC: updated exploratory analyses of PALOMA-3, a double-blind, phase 3 randomized study. Clin Cancer Res. 2022;2022;clincanres.0305.2022.
- Johnston S, Martin M, Leo AD, et al. MONARCH 3 final PFS: a randomized study of abemaciclib as initial therapy for advanced breast cancer. NPJ Breast Cancer. 2019;5:5.
- Sledge GW, Jr., Toi M, Neven P, et al. MONARCH 2: Abemaciclib in combination with fulvestrant in women with HR+/HER2- advanced breast cancer who had progressed while receiving endocrine therapy. J Clin Oncol. 2017;35(25):2875–2884. Sep 1
- Dickler MN, Tolaney SM, Rugo HS, et al. MONARCH 1, a phase II study of abemaciclib, a CDK4 and CDK6 inhibitor, as a single agent, in patients with refractory HR(+)/HER2(–) metastatic breast cancer. Clin Cancer Res. 2017;23(17):5218–5224.
- Rugo HS, Tolaney SM, Cortés J, et al. Abstract CT044: MONARCH 1: final overall survival analysis of a phase 2 study of abemaciclib, a CDK4 and CDK6 inhibitor, as monotherapy, in patients with HR+/HER2- breast cancer, after chemotherapy for advanced disease. Cancer Research. 2017;77(13_Supplement):CT044–CT44.
- Hortobagyi GN, Stemmer SM, Burris HA, et al. Updated results from MONALEESA-2, a phase III trial of first-line ribociclib plus letrozole versus placebo plus letrozole in hormone receptor-positive, HER2-negative advanced breast cancer. Ann Oncol. 2018;29(7):1541–1547.
- Im SA, Lu YS, Bardia A, et al. Overall survival with ribociclib plus endocrine therapy in breast cancer. N Engl J Med. 2019;381(4):307–316.
- Slamon DJ, Neven P, Chia S, et al. Phase III randomized study of ribociclib and fulvestrant in hormone receptor-positive, human epidermal growth factor receptor 2-negative advanced breast cancer: MONALEESA-3. J Clin Oncol. 2018;36(24):2465–2472.
- Slamon DJ, Neven P, Chia SKL, et al. Updated overall survival (OS) results from the phase III MONALEESA-3 trial of postmenopausal patients (pts) with HR+/HER2- advanced breast cancer (ABC) treated with fulvestrant (FUL) ± ribociclib (RIB). J Clin Oncol. 2021;39(15_suppl):1001–1001.
- Lee KS, Chang HS, Lee SM, et al. Economic burden of cancer in Korea during 2000–2010. Cancer Res Treat. 2015;47(3):387–398.
- Kim YA, Lee YR, Park J, et al. Socioeconomic burden of cancer in korea from 2011 to 2015. Cancer Res Treat. 2020;52(3):896–906.
- Park SK, Park JA, Yang SY, et al. The economic impact of disease progression and death in hormone receptor positive (HR+), human epidermal growth factor receptor 2 negative (HER2-) advanced breast cancer patients: using Korean nationwide health insurance claims data. Curr Med Res Opin. 2020;36(11):1825–1833.
- Kim SH, Jo MW, Ock M, et al. Estimation of health state utilities in breast cancer. Patient Prefer Adherence. 2017;11:531–536.
- Han A, Lee KE, Lee HK, et al. Meeting highlights: the first korean breast cancer treatment consensus conference. J Breast Cancer. 2014;17(4):308–313.
- Lee S, Park IH, Park S, et al. Meeting highlights: the second consensus conference for breast cancer treatment in korea. J Breast Cancer. 2017;20(3):228–233.
- Giuliani J, Bonetti A. Palbociclib or ribociclib in First-Line treatment in patients with hormone receptor-positive/human epidermal receptor 2-negative advanced or metastatic breast cancer? A perspective based on pharmacologic costs. Clin Breast Cancer. 2019;19(4):e519–e521.
- Giuliani J, Bonetti A. The introduction of a third CDK4/6 inhibitor does not change the cost-effectiveness profile in first and subsequent-lines after progression or relapse during previous endocrine therapy in patients with hormone receptor positive (HR+)/human epidermal receptor-2 negative (HER-2) advanced or metastatic breast cancer. J Oncol Pharm Pract. 2020;26(6):1486–1491.
- Lee JE, Shin HI, Do YK, et al. Catastrophic health expenditures for households with disabled members: evidence from the korean health panel. J Korean Med Sci. 2016;31(3):336–344.
- Shin SM, Lee HW. Comparison of out-of-pocket expenditure and catastrophic health expenditure for severe disease by the health security system: based on end-stage renal disease in South Korea. Int J Equity Health. 2021;20(1):6.

