Abstract
Purpose
Discovery of falsified Symbicort 320/9 Turbohaler identified in the UK in 2013 demonstrated that falsified dry powder inhalers were also present in the European market. This work aimed to investigate the current situation of formoterol-containing dry powder inhalers in Europe and North Africa by assessing their aerodynamic performance profile.
Methods
A total of eight registered formoterol-based dry powder inhalers over the European and North African markets were involved in this study, including the reference drug Foradil. Samples were prepared using a multistage liquid impinger (MsLI) and further analyzed by a validated HPLC-UV method to determine the delivered and the fine particle doses (FPDs). This study also examined the impact of freezing-thawing cycles on sample stability in terms of analytical purpose handling.
Results
No substandard dry powder inhalers were identified among the medicinal products involved in this work. The delivered dose (DD) of assessed drugs varied from 8.33 to 9.69 µg, while the FPD was between 1.86 and 3.35 µg. As expected, this work confirmed that the capsule composition and the barrier properties of the primary packaging can affect the FPD of dry powder for inhalation use.
Conclusions
The FPD of products C and B was, respectively, 17.4 and 14.2% superior to Foradil, products D and H had the closest values compared to the original drug, and product F was 34.5% inferior. Additionally, this work showed that a high FPD can be achieved using HPMC capsules and moisture-impermeable primary packaging.
Introduction
Falsification of medicines has become an increasingly important issue in Europe over the past decades; it poses a real threat to public health. As described by the World Health Organization (WHO), falsified medical products are “medical products that deliberately/fraudulently misrepresent their identity, composition or source”; and substandard medical products are also called “out of specification, these are authorized medical products that fail to meet either their quality standards or their specifications, or both”Citation1. In September 2013, falsified Symbicort 320/9 Turbohaler was identified in the wholesale supply chain in the UK following the detection of batch number discrepancies. One year later falsified Symbicort 320/9 Turbohaler was discovered in GermanyCitation2. Symbicort 320/9 Turbohaler is a multidose dry powder inhaler developed for asthmatic patients via the pulmonary route. A unit dose contains 320 µg of budesonide and 9 µg of formoterol fumarate. The pulmonary administration route by inhalation has been typically used for local drug delivery in lung conditions, such as pulmonary infections, asthma and chronic obstructive pulmonary disease (COPD), more rarely for systemic therapiesCitation3–6. Active molecules indicated in local pulmonary treatments are generally administrated ranging from a few micrograms to milligrams, this corresponds to 0.05–10% of active pharmaceutical ingredient (API) in the powder formulationCitation7,Citation8. Nowadays, accurate delivery is commonly achieved via DPIsCitation8,Citation9. Furthermore, they are also propellant-free, environmentally friendly, easy-to-use and more stable than formulations in liquid form during storageCitation3,Citation4,Citation8. Indeed, in comparison to the first generation of pressurized metered-dose inhalers, DPIs have a different cradle-to-grave impact. Chlorofluorocarbons were formerly replaced by hydrofluoroalkane (HFA) in pressurized inhalers as the propellant to avoid the destruction of the ozone layer. Limiting the environmental impact, DPIs have a 10-fold smaller global warming potential or carbon footprint than the HFA inhalers because of the emission of HFA at each stage of the life cycle of these inhalersCitation10. DPIs exist in different formulation forms in the present pharmaceutical market, including single-dose, metered-dose and multi-unit-dose-based inhalersCitation5. The inhaler is a breath-activated device designed to release the formulated dry powder containing micronized API particles produced by spray-drying, to provide pharmacological effects in patients’ lungsCitation4–6,Citation8,Citation9,Citation11. The active substance may be associated with an appropriate carrier to improve its flowability and aerosol dispersionCitation7. High dispersion forces are needed to ensure the efficiency of the de-agglomeration and aerosolization process, and affect the delivery of high-dosed inhalable dry powder formulations to the lungsCitation4. Due to its low chemical reactivity, acceptable toxicological profile, and optimal flowability properties, lactose is mostly used as a carrier to build up DPIs formulations to facilitate the dispersion and aerosolization processesCitation4,Citation8,Citation11. Besides, considering the impact of production and storage conditions on the shelf-life of finished products, the capsule composition, form and packaging are also involved in these processes.
Since the discovery of falsified Symbicort 320/9 Turbohaler in the UK, no cases of substandard or falsified inhaler formulations have been reported to European health authorities. Statistically, due to their high profitability, the pharmaceuticals most affected by falsification or poor quality are antibiotics, antiretrovirals, antimalarials, anticancer drugs and vaccines. Despite the limited data available on the inhalers, the risk of poor quality or falsified products is perhaps low but still present, therefore this work aimed to investigate the current situation of formoterol-containing inhalers in Europe and North Africa. It also extended the examination of potential falsified or substandard DPIs to a different formulation: capsule-based DPIs. More specifically, this work focused on formoterol fumarate delivered by Aerolizer. Formoterol is a long-acting ß-agonist indicated in asthma and COPD treatmentsCitation3,Citation6. It can be administrated in monotherapy or combined with another active molecule within the same formulation, e.g. in combination with an inhaled corticosteroid (ICS) when the ICS monotherapy can no longer control asthmaCitation3,Citation6.
To date, Aerolizer is the standard DPI and the most widely used in formoterol deliveryCitation3. Different from other multidose DPIs, such as Easyhaler, Novolizer and Turbohaler available on the market as well, Aerolizer is a capsule-based inhaler. Admittedly, the aerodynamic performance of DPIs is not only dependent on the dry powder formulation and the inhaler type. Furthermore, the proportion, size and shape of particles play a critical role in the powder dispersion, aerosolization and pulmonary deposition, therefore, these differences may cause variations in the delivered dose (DD) and the fine particle dose (FPD)Citation5,Citation7,Citation8,Citation12. Meanwhile, in the case of capsule-based inhalers, the impact of the capsule composition on the aerodynamic profile is also demonstratedCitation5.
The falsification of medicines is by far an isolated concern of European authorities. According to the WHO, on the global market of falsified and substandard medicines, around 42% of cases are reported from AfricaCitation1. In the meantime, several studies have revealed falsified pharmaceutical products on the African market, for instance, falsified chloroquine phosphate-based formulations in the COVID-19 context, artemether-lumefantrine, and quinine formulations in malaria treatment or preventionCitation13–15. Consequently, this study focused on pharmaceuticals equipped with capsule-based devices (Aerolizer-like inhalers). A total of eight capsule-based DPI formulations containing formoterol fumarate commercialized within Europe and Africa were assessed in terms of aerodynamic performance including the DD and FPD profiles and compared to the reference drug Foradil. The assays were carried out using the standard procedures of the European Pharmacopeia (Ph. Eur.) 0671 and 2.9.18 to determine, respectively, the DD and the FPD employing a multistage liquid impinger (MsLI), also known as apparatus C. Samples were then analyzed by a validated HPLC-UV method to evaluate the DD and the FPD.
In parallel, this work also examined the impact of freezing-thawing cycles on the sample solution stability after storage at −20 °C to evaluate the capability of reanalysis in routine activities.
Materials and methods
Chemicals and reagents
Eight registered drug products (including DPI capsules and device) A (Foradil, Novartis), B (Formoterol AL, Aliud Pharma), C (Formagal, Laboratories SMB), D (Formoterol Biogaran, Biogaran), E (Formoterol Mylan, Mylan), F (Broncotec, Tecnimede Group), G (Formoterol Aldo-Union, Aldo-Union) and H (Formoterol Stada, Stada) were selected to carry out the study. They were purchased on local European and African markets. Products A, C and F are commercialized in Europe and Africa, while the others are only in Europe.
Only analytical grade reagents and HPLC grade solvents were used. Formoterol fumarate dihydrate was purchased from Industriale Chimica (Saronno, Italy). Acetonitrile HPLC grade and methanol HPLC grade were purchased from Biosolve B.V. (Dieuze, France) and J.T. Baker (Gliwice, Poland) respectively. Phosphoric acid 85% and potassium dihydrogen phosphate were provided by Merck (Darmstadt, Germany). Milli-Q water was obtained by Milli-Q Plus 185 system.
Instrumentation and chromatographic conditions
Recovered samples were analyzed on an Alliance 2695 HPLC System (Waters, Milford, MA) coupled with a PDA 2998 detector (Waters). LC separation was achieved on an RP-C8 stationary phase LiChrospher 60 RP Select B (125 × 4 mm i.d. − 5 µm). The mobile phase consisted of a mixture of phosphate buffer at pH 2.7 and acetonitrile (75:25, v/v) at a flow rate of 1.0 mL/min. The injection volume was 200 µL. The column temperature was set at 30 °C and the autosampler temperature at 10 °C. The total run time and retention time were 8 min and approximately 3.5 min respectively. The PDA was set at 254 nm for formoterol detection.
Preparation of calibration and quality control solutions
Formoterol fumarate dihydrate was weighed and dissolved in methanol to obtain a stock solution of 250 µg/mL. This was further diluted to prepare the calibration curve at concentrations of 25, 100, 250, 500 and 1000 ng/mL. The concentration of independent quality control (QC) solution was set at 250 ng/mL, the center of the dosing range. QC solution allowed verifying the preparation trueness of calibration solutions, so-called system suitability. The calibration and QC solutions were prepared from two independent weighings and then diluted in the dilution solvent composed of milli-Q water and methanol (75:25, v/v). The recovery rate was calculated and must be included between 90 and 110%.
Sample preparation
Three independent repetitions were performed on one batch for each studied drug to determine independently the DD and the FPD, using the MsLI (apparatus C) connected to a breath simulator. Samples were prepared in Milli-Q water and methanol (75:25, v/v) as dilution solvent.
This study focused on the evaluation of DD in reference to procedure 0671 from European Pharmacopeia; however, the assay of uniformity of DD was not included in this work. FPD was determined according to procedure 2.9.18 described in European Pharmacopeia. A total of 10 capsules were needed and vacuumed per test. The applied flow rate through the apparatus was set at 100 L/min. All assays were performed under standardized and controlled temperature and humidity conditions.
Impact of freezing-thawing cycles
The assessment of the impact of freezing-thawing cycles on the stability would allow us to examine and estimate the capacity of reanalysis in case of a detection of an out-of-specification result. Management of sample stability is therefore an important component in routine analysis.
Focusing on this purpose, the evaluation of stability was carried out using samples of product B. Samples were preserved at −20 °C after preparation. Twenty-four hours later, they were injected into HPLC for the first analysis (first cycle), and 7 d after for the second analysis (second cycle). The results of the DD were afterward compared.
Results
System suitability test
The system suitability test aims to verify that the analytical system conforms to the intended application. The recovery rate of the QC solution is included in the predetermined acceptation range from 90 to 110%. It was shown that the observations were homogenous, and no signal drift was observed.
Delivered dose
The main goals in inhalation are on the one hand to maximize the DD and the FPD and; on the other hand, to achieve a reproducible API deposition in the lungsCitation8,Citation9. DD corresponds to the amount of API leaving the inhaler and the capsule during oral inhalation. Based on the results obtained from HPLC-UV analysis, the DD from the 8 registered drugs was comprised between 8.33 and 9.69 µg, corresponding to products D and H, respectively, as mentioned in .
Table 1. Delivered dose of 8 studied drugs (n = 3).
Fine particle dose
FPD is defined as the mass of particles with an aerodynamic diameter inferior to 5 µm, theoretically able of reaching the lungs. Thus, it consists of the respirable dose which is critical and determining to achieve efficient treatments by inhalationCitation12. FPD was determined through Copley Inhaler Testing Data Analysis Software version 3.10 (CITDAS, Copley, Nottingham, UK) from the data generated by the HPLC-UV analysis. summarizes the average FPDs calculated from the three tests of each studied drug, they ranged from 1.86 µg for product F to 3.35 µg for product C. The aerodynamic profile of a capsule-based DPI formulation, so its FPD results in interactions between a multitude of factors such as the device, the particle characteristics, the powder formulation, the capsule, the storage conditions, etc. Meanwhile, this study investigated the impacts of the capsule composition and the packaging type on the FPD. The related information was found in the summary of product characteristics.
Table 2. Fine particle dose of 8 studied drugs (n = 3).
All evaluated drugs contain 12 µg of formoterol fumarate and are equipped with an Aerolizer like inhaler. Nevertheless, they differ generally in the capsule composition, and the packaging. Among eight studied products, two capsule types were identified, in gelatin or hydroxypropylmethylcellulose (HPMC). The latter is particularly suitable for DPIs, thanks to its low water content and its reduced brittleness. These capsules are less sensitive to storage conditions than gelatin capsulesCitation5. Indeed, cracking and fracturing are observed in capsules made of gelatin after piercing and inhalation simulation. The moisture undoubtedly facilitates dry powder’s agglomeration and so decreases the FPDCitation5. In fact, the primary packaging aims to protect the dry powder from atmospheric humidity. They exist in different types: plastic bottles and various blister types, e.g. aluminum/aluminum (Alu/Alu) blister, polyvinyl chloride/polyvinylidene chloride/aluminum (PVC/PVDC/Alu) blister.
summarizes the data of FPDs, the capsule composition, and the packaging type of each studied drug. Based on generated results, products B and C provided the highest FPD values. These dry powder formulations are both contained in HPMC capsules and packaged in high-density polyethylene (HDPE) bottle equipped with a polypropylene (PP) screw cap containing silica gel as a desiccant agent to absorb the moisture in the container. They are followed by products E and D which only differ in the packaging that uses an Alu/Alu blister which is impermeable to gas and water vapor. Comparatively, the products utilizing either gelatin capsules or a water-permeable blister, for instance, polyamide (PA), PVC, and PVDC had a lower FPD, as shown in . Indeed, the lowest FPD values were observed in products F and G which both employ gelatin capsules and PVC/PVDC/Alu blister as primary packaging. Moreover, the impact of the capsule composition was highlighted by product H: using HPMC capsules rather than the gelatin-based ones can offer a greater FPD compared to products F and G. Although products A, F, and G utilize gelatin capsules, a higher FPD was achieved with product A because, unlike to other two products, it is packaged in a PA/Alu/PVC/Alu blister that is almost completely moisture-impermeableCitation16.
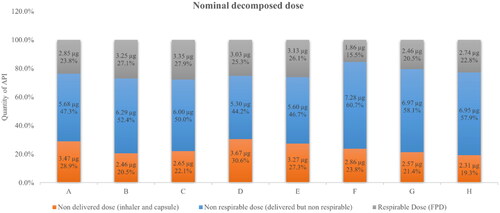
Nominal decomposed dose
The nominal dose of 12 µg formoterol fumarate DPI capsules can be virtually decomposed into three components: non-DD trapped in the inhaler device and the capsule, non-respirable dose theoretically not deposited in lungs, and respirable dose or FPD capable to reach patients’ lungs for pharmacological effects. The non-respirable dose consists of the difference between the DD and the respirable dose.
As shown in , the three components varied considerably among the eight studied pharmaceuticals. Out of a nominal dose of 12 µg, 19.3–30.6% of formoterol was retained in the device and the capsule, 44.2–60.7% left the device and the capsule but cannot arrive successfully in the lungs. Only 15.5–27.9% of formoterol was capable to reach the lungs.
Assessment of the impact of freezing-thawing cycles on solution sample stability
The DD values of samples of product B stored at −20 °C differed approximately 1% after the first and second freezing-thawing cycles after, respectively, 24 h and 7 d (see ). This finding added to our knowledge and demonstrated that the formoterol solution samples are stable at −20 °C for at least 7 d and can be reanalyzed after thawing within a week. A slight variation of 1% between the DD results of two freezing-thawing cycles is consistent with the validation data of the analytical method.
Table 3. Evaluation of freezing-thawing cycles’ impact on sample stability (n = 3).
Discussions and conclusions
The recovery rate of the calibration curve and the QC solution was comprised in the range of acceptance from 90 to 110%. Therefore, the calibration solutions were accurately prepared. The system’s suitability was proved, and no signal drift was observed, so the analyses were carried out properly on a daily basis during the entire study.
Eight registered capsule-based DPI formulations from the European and African pharmaceutical markets have been assessed in this study. All of them have a unit dose of 12 µg of formoterol fumarate. They were evaluated and compared in terms of DD and FPD, including the original drug Foradil. Three independent assays were performed for each drug to determine independently three DD as well as three FPD values. Regarding the European guidelines, identical in vitro aerodynamic performance has to be demonstrated between the original and generic DPIs to prove comparable pulmonary deposition characteristics. In general, a variation within 20% of DD and FPD is acceptable for genericsCitation17,Citation18.
No substandard DPI was identified in those under investigation. An FPD of approximately 30–40% of the nominal dose is usually expected in classic DPI formulations. Among eight studied drugs, as anticipated, a minor fraction (inferior to 30% of the nominal dose) of formoterol was theoretically deposited in the lungs and could have pharmacological effects locally. Consequently, most API was lost; either trapped in the inhaler and the capsule during inhalation or arrived in the digestive tract due to the particle aerodynamic diameter greater than 5 µm. In terms of respirable dose (or FPD), i.e. the dose being able to reach the lungs and have its local pharmaceutical effects for the treatment of asthma or COPD, the reference drug Foradil had an average formoterol FPD of 2.85 µg. Compared to the latter, the FPDs of products C and B were, respectively, 17.4 and 14.2% superior to Foradil, while products D and H had the closest values compared to the princeps. On the basis of the results, all DPIs differed less than 20% from the reference product FPD, except Product F which was 34.5% lower than the original product. By contrast, the DD was not significantly different between the original and generic DPIs. In conclusion, the nature of capsules and the barrier properties of primary packaging affect undeniably the FPD and the pulmonary deposition. It was noticed that the highest FPD values are reached by the registered formoterol DPI formulations whose dry powder is contained in HPMC capsules and primary packaging with a low water vapor transmission rate or a low permeability to moisture such as Alu/Alu blister and HDPE bottle with a PP screw cap containing a desiccant. In contrast, due to the non-optimal water vapor barrier properties of gelatin capsules and PVC/PVDC/Alu blister, the FPD was considerably reduced.
Undoubtedly, the FPD has a considerable clinical impact because it is the effective dose exercising beneficial effects. If the FPD is too low, an insufficient respirable dose could cause less effective or ineffective treatment. Although too high an FPD is rather uncommon, it could also lead to toxicity.
Transparency
Declaration of funding
This work was supported by the Pharmaceutical Analytical Chemistry Laboratory from the University of Liege.
Declaration of financial/other relationships
No potential conflict of interest was reported by the authors.
The peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Author contributions
YZ conducted the study from sample preparation to data interpretation, and wrote the manuscript. CH designed experiments and supervised this work. CH and PH contributed to the review of the manuscript. All authors read and approved the final manuscript.
Data availability statement
Upon a reasonable request, the datasets of this study are available from the CONTACT.
References
- WHO. Global surveillance and monitoring system for substandard and falsified medicinal products. 2017. Available from: https://apps.who.int/iris/bitstream/handle/10665/326708/9789241513425-eng.pdf?ua=1
- Morris C. The threat from falsified medicines. 2015. Available from: http://www.unitrans-us.com/newsroom/Attachments_Uploads/2b-Enforcementactivities-Chris-Morris.pdf
- Otto-Knapp R, Conrad F, Hösch S, et al. Efficacy and safety of formoterol delivered through the novolizer®, a novel dry powder inhaler (DPI) compared with a standard DPI in patients with moderate to severe asthma. Pulm Pharmacol Ther. 2008;21(1):47–53.
- Ambrus R, Benke E, Farkas Á, et al. Novel dry powder inhaler formulation containing antibiotic using combined technology to improve aerodynamic properties. Eur J Pharm Sci. 2018;123:20–27.
- Wauthoz N, Hennia I, Ecenarro S, et al. Impact of capsule type on aerodynamic performance of inhalation products: a case study using a formoterol-lactose binary or ternary blend. Int J Pharm. 2018;553(1–2):47–56.
- Suzuki ÉY, Simon A, da Silva AL, et al. Effects of a novel roflumilast and formoterol fumarate dry powder inhaler formulation in experimental allergic asthma. Int J Pharm. 2020;588:119771.
- Guchardi R, Frei M, John E, et al. Influence of fine lactose and magnesium stearate on low dose dry powder inhaler formulations. Int J Pharm. 2008;348(1–2):10–17.
- Rahimpour Y, Kouhsoltani M, Hamishehkar H. Alternative carriers in dry powder inhaler formulations. Drug Discov Today. 2014;19(5):618–626.
- Islam N, Gladki E. Dry powder inhalers (DPIs)-a review of device reliability and innovation. Int J Pharm. 2008;360(1–2):1–11.
- Jeswani HK, Azapagic A. Environmental impacts of healthcare and pharmaceutical products: Influence of product design and consumer behaviour. J Clean Prod. 2020;253:119860.
- Noriega-Fernandes B, Malmlöf M, Nowenwik M, et al. Dry powder inhaler formulation comparison: study of the role of particle deposition pattern and dissolution. Int J Pharm. 2021;607;121025.
- Yaqoubi S, Chan HK, Nokhodchi A, et al. A quantitative approach to predicting lung deposition profiles of pharmaceutical powder aerosols. Int J Pharm. 2021;602:120568.
- Ciza PH, Sacre PY, Waffo C, et al. Comparing the qualitative performances of handheld NIR and raman spectrophotometers for the detection of falsified pharmaceutical products. Talanta. 2019;202:469–478.
- Ciza PH, Sacre PY, Kanyonyo MR, et al. Application of NIR handheld transmission spectroscopy and chemometrics to assess the quality of locally produced antimalarial medicines in the democratic republic of Congo. Talanta Open. 2021;3:100025.
- Waffo Tchounga CA, Sacre PY, Ciza P, et al. Composition analysis of falsified chloroquine phosphate samples seized during the COVID-19 pandemic. J Pharm Biomed Anal. 2021;194:113761.
- Pedrosa de Oliveira D, Costa JSR, Oliveira-Nascimento L. Sustainability of blisters for medicines in tablet form. Sustain Chem Pharm. 2021;21:100423.
- European Medicines Agency. Guideline on the pharmaceutical quality of inhalation and nasal products. Amsterdam, Netherlands: European Medicines Agency; 2006.
- European Medicines Agency. Points to consider on the requirements for clinical documentation for Orally Inhaled Products (OIP). Amsterdam, Netherlands: European Medicines Agency; 2004.