Abstract
Background
As the human immunodeficiency virus (HIV) treatment landscape continues to evolve, the prolonged life expectancy and long-term exposure to antiretroviral drugs have modified the burden associated with living with HIV.
Objective
To better understand the current treatment and comorbidity burden in people living with HIV (PLWH).
Methods
Peer-reviewed systematic literature reviews (SLRs) between 2017 and 2020 that included US studies and examined drug adherence/pill burden, resistance burden, or comorbidities in PLWH were identified. Methods and findings were extracted for the overall studies and examined in the subset of US studies.
Results
Among 665 publications identified, 47 met the inclusion criteria (drug adherence/pill burden: 5; resistance: 3; comorbidities: 40). While antiretroviral drug adherence levels varied across SLRs, single-tablet regimens (STR) were associated with higher adherence versus multiple-tablet regimens. STRs were also associated with lower risk of treatment discontinuation, higher cost-effectiveness, and lower risk of hospitalization. Longer survival resulted in a high comorbidity burden, with non-AIDS causes accounting for 47% of deaths among PLWH in the US. HIV doubled the risk of cardiovascular disease and was associated with other health problems, including bone and muscle diseases, depression, and cancers. Several antiretroviral regimens were associated with chronic diseases, including cardiometabolic conditions. Lifetime HIV costs are substantially increasing, driven by antiretroviral, adverse event, and comorbidity treatment costs cumulated due to longer survival times.
Conclusions
There is a considerable burden associated with HIV and antiretroviral treatment, highlighting the benefits of less complex and safer regimens, and the unmet need for effective preventative interventions.
1. Introduction
In 2018, an estimated 1,173,900 individuals of 13 years of age and older were living with human immunodeficiency virus (HIV) in the United States (US), with 37,515 newly-diagnosed casesCitation1.
Treatment for HIV was revolutionized with the introduction of combination antiretroviral therapy (ART), which is effective at suppressing HIV replication, but is not curativeCitation2,Citation3. Nonetheless, effective combination ART has increased viral suppression rates, thereby decreasing HIV-related morbidity and mortality, and reducing the risk of sexual transmission of HIVCitation4. Combination ART regimens have historically comprised three active agents, including a backbone of two nucleoside/nucleotide reverse transcriptase inhibitors (NRTIs) and an additional drug from another drug class, such as non-nucleoside reverse transcriptase inhibitors (NNRTIs), integrase strand transfer inhibitors (INSTIs), or protease inhibitors (PIs)Citation4. Selected two-drug regimens are now part of the treatment armamentariumCitation5, and the use of single-tablet regimens (STRs) has been shown to improve adherence to treatment compared to multiple-tablet regimens (MTRs)Citation4,Citation6. Nevertheless, as a chronic disease that is incurable with ART, HIV is associated with a substantial burden, including the requirement for lifelong treatment and the risk of treatment resistanceCitation2,Citation7,Citation8. In addition, with the increased life expectancy and the long-term use of ART among people living with HIV (PLWH), the lifetime risk of developing non-AIDS comorbidities is on the riseCitation7.
While previous systematic literature reviews (SLRs) included publications on the burden associated with medication adherence and complexityCitation9–11, treatment resistanceCitation12,Citation13, and comorbiditiesCitation14,Citation15 among PLWH, none have comprehensively covered all these aspects of the disease and treatment burden in this population. Therefore, this SLR of SLRs was conducted to provide a comprehensive summary of the ART adherence and pill burden, antiretroviral resistance burden, and comorbidity burden in PLWH based on SLRs that included the US as one of the countries of interest.
2. Methods
2.1. Search strategy
A systematic search was conducted on 8 December 2020 through MEDLINE, MEDLINE In Process, and EMBASE. The search used a combination of terms relating to HIV and the outcomes of interest (see for the full search strategy).
Table 1. Search strategy.
2.2. Study selection
Two researchers independently conducted the selection process (RB and KM), and discrepancies were resolved through discussion with a third researcher (HR). Included studies met the following selection criteria: peer-reviewed SLRs (with or without meta-analysis), included patients diagnosed with HIV-1, evaluated at least one of the outcomes of interest (i.e. ART adherence and pill burden, antiretroviral resistance burden, or comorbidity burden), included studies on patients aged ≥ 9 years, and were published in English in 2017 or later. SLRs were excluded if the US was not part of the countries considered or if the regions covered were not specified. Conference abstracts, articles with data not reported or where the full text could not be found were excluded.
2.3. Data extraction
The following characteristics of the selected articles were extracted: study type (SLR or SLR in combination with meta-analysis), type of studies included in the SLR (e.g. retrospective, randomized controlled trial [RCT]), publication period covered, population(s) considered, and outcome(s) of interest assessed. In addition, the main findings related to the outcomes of interest were extracted. If available, study-level findings from the US studies included in each SLR were also reviewed. Data were compiled into an electronic spreadsheet and a narrative synthesis of the included studies was conducted. In this paper, the results from the SLRs were reported for the overall studies included. The findings of the US studies were reported when noteworthy or generally inconsistent with those of the overall studies.
3. Results
3.1. Study characteristics
The electronic database search identified 665 review abstracts. After the 2-level screening, 47 review articles were included in the final analysis ().
Figure 1. PRISMA diagram of study selection.
Abbreviations: ART, antiretroviral therapy; HIV, human immunodeficiency virus; PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-analysis; SLR, systematic literature review. US, United States.
aSLRs that included both non-US and US studies, with results at the study level presented (for the US studies), were not excluded.
bSLRs where the country was not specified overall or at the study level were excluded.
cOne scoping review included a US study in children aged 7–17 years. It was manually added to include potentially relevant information, despite the study population including children less than 9 years of age.
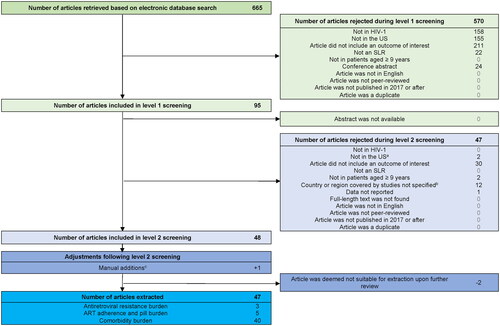
Five SLRs covered the ART adherence and pill burden in PLWHCitation9–12,Citation16, three SLRs covered treatment resistance in PLWHCitation12,Citation13,Citation17, and 40 SLRs focused on the HIV and/or ART-associated comorbidity burdenCitation14,Citation15,Citation18–55 (). These SLRs covered a range of study types, including observational studies in general, RCTs, prospective and retrospective studies, and cross-sectional and longitudinal studies. Publication periods covered in these SLRs varied across studies, including some that did not impose any publication period, and spanned up to 2020 (). While the retained SLRs mostly focused on adult PLWH, six also included adolescents (<18 years, excluding childrenCitation19; 13–18 yearsCitation15; ≥15 yearsCitation27,Citation32; ≥16 yearsCitation53; young people [age not specified]Citation49), one was conducted in children and adolescents (≤18 years)Citation22, and two in older adults (≥50 years)Citation14,Citation21, In addition, some of the SLRs focused on specific subpopulations of PLWH, such as prison inmatesCitation11, veteransCitation36, pregnant womenCitation43,Citation47,Citation54, premenopausal womenCitation35, menCitation33, and men who have sex with men (MSM)Citation52. The main findings of the included SLRs are detailed in .
Figure 2. Main findings related to HIV ART adherence and pill burden, antiretroviral resistance burden, and comorbidity burden.
Abbreviations: AE, adverse event; AIDS, acquired immunodeficiency syndrome; ART, antiretroviral therapy; CVD, cardiovascular disease; HCV, hepatitis C virus; HIV, human immunodeficiency virus; MTR, multiple-tablet regimen; NNRTI, non-nucleoside reverse transcriptase inhibitor; NRTI, nucleoside reverse transcriptase inhibitor; PI, protease inhibitor; PLWH, people living with HIV; STR, single-tablet regimen.
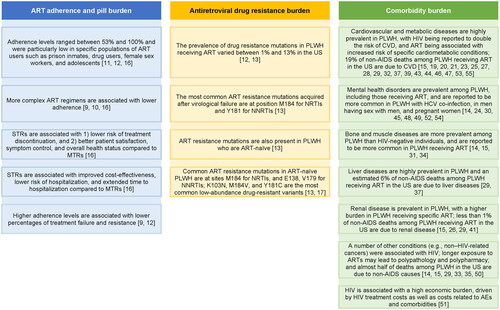
Table 2. Publication details of the included reviews.
3.2. Art adherence and pill burden
Different types of measures were used to report adherence to ART, including self-report, pill count, medication event monitoring system, medication possession ratio, proportion of days covered, and pharmacy/prescription refill rates. In addition, the threshold to determine adequate adherence levels varied across studies and was mostly either 90% or 95%Citation9,Citation11,Citation12.
Among the individual US studies included in the SLRs, ART adherence level estimates ranged between 74% and 98% based on pill countCitation16, and 53–99% based on self-reportCitation12. The individual studies included in these SLRs included various subpopulations, such as ART-naïveCitation56 and homeless and marginally housed individualsCitation57. In the specific population of prison inmates, the proportion of patients with ART adherence ≥ 95% was ∼54% (overall and in North America)Citation11, which the authors contrasted to other high-risk subgroups identified in literature outside of the SLR, such as drug users living with HIV (60%), female sex workers living with HIV (76%), and adolescents living with HIV (62%)Citation11.
PLWH using MTRs versus STRs were more likely to have lower adherence to ARTCitation9,Citation16, although some of the studies included in one SLR reported a non-significant associationCitation10. Odds ratios (ORs) for better adherence in patients using STRs versus MTRs ranged from 1.43 [9] to 1.96 [16] (p < .001 for both). This finding remained true when comparing STRs to once-daily MTRs (OR = 1.66, p = .002]) and to twice-daily MTRs (OR = 2.53, p = .02), separatelyCitation16. STRs were also reported to be associated with a lower risk of ART discontinuation (relative risk [RR] = 0.69, p = .05), incremental cost-effectiveness ratio for initial treatment of $26,383 per quality-adjusted life year, lower risk of hospitalization (HR = .71; 95% CI = .59–.86), extended time to hospitalization (median: 1,508 vs. 1,032 days; p = .004), and better patient satisfaction, symptom control, and overall health status, as compared to MTRsCitation16. Higher levels of adherence were associated with greater viral suppressionCitation9 and lower percentages of treatment failure and treatment resistanceCitation12.
3.3. Antiretroviral drug resistance in PLWH
The prevalence of drug resistance mutations in PLWH receiving ART varied between 1% and 13% in the USCitation12, and the prevalence of resistance acquired after virological failure was 23% for NRTI and 19% for NNRTI resistance mutations in North AmericaCitation13. The most frequent drug resistance mutation acquired after virological failure was at position M184 for NRTI (49% in North America) and at position Y181 for NNRTI (8% in North America)Citation13. In addition, the prevalence of pretreatment resistance in North America was estimated to be 6% for NRTI and 8% for NNRTI resistance mutations, based on studies with study periods spanning from 1995 to 2016Citation13.
3.4. Burden associated with comorbidities
Among the 40 SLRs that covered comorbidities in PLWH, 21 articles focused on cardiovascular and metabolic diseasesCitation15,Citation18–21,Citation23,Citation25,Citation27–29,Citation32,Citation36–39,Citation43,Citation44,Citation46,Citation47,Citation53,Citation55, nine on mental health disordersCitation14,Citation15,Citation24,Citation30,Citation45,Citation48,Citation49,Citation52,Citation54, five on bone and muscle diseasesCitation14,Citation15,Citation31,Citation34,Citation40, four on liver diseasesCitation15,Citation29,Citation37,Citation42, four on renal diseasesCitation15,Citation26,Citation29,Citation41, and six on other comorbiditiesCitation14,Citation15,Citation29,Citation33,Citation35,Citation50. Additionally, one SLR assessed hearing loss in children with HIVCitation22, and another one assessed the economic burden of HIV management and comorbidities in the USCitation51.
3.4.1. Cardiovascular and metabolic diseases
The global burden of HIV-associated cardiovascular disease (CVD) has tripled over the last two decades and is now responsible for 2.6 million disability-adjusted life years per annumCitation46. In the US, 29% of PLWH were estimated to have moderate-to-high cardiovascular riskCitation32, and 19% of non-AIDS deaths among PLWH receiving ART were attributable to CVD (13% globally)Citation29.
The prevalence of hypertension in PLWH varied between 20% and 25% globallyCitation20,Citation32,Citation53, with a prevalence of 42% in PLWH aged 50 and overCitation21 and of 30% among PLWH in the Americas WHO regionCitation20. The prevalence of diabetes in PLWH varied between 6%Citation44 and 7.24%Citation32, and of dyslipidemia between 22%Citation44 and 39.5%Citation32.
HIV was found to roughly double the risk of CVDCitation46, with greater odds of having acute myocardial infarction (AMI; OR = 1.87; 95% CI = 1.42–2.47)Citation44 and higher risk of myocardial infarction (MI; RR = 1.73; 95% CI = 1.44–2.08)Citation28, AMI (RR = 1.96; 95% CI = 1.48–2.57)Citation44, heart failure (RR = 1.7; 95% CI = 1.4–2.0), or any grade of diastolic dysfunction (RR = 3.0; 95% CI = 1.8–5.1)Citation27, compared to no HIV.
In addition, ART was reported to be associated with a higher prevalence of hypertension (34.7% in ART-experienced vs. 12.7% in ART-naïve PLWH)Citation53, increased odds of hypertensive disorders of pregnancy (OR range = 1.27–8.90)Citation43, increased risk of MI (RR = 1.80; 95% CI = 1.17–2.77)Citation28, and increased body mass index (BMI; effect size = 1.58 kg/m2; 95% CI = 1.36–1.81)Citation39. In some studies, recent exposure to abacavir was associated with an increased risk of developing CVD in general and AMI/MI in particularCitation23,Citation28. However, another SLR reported that while one observational study found that abacavir was associated with increased risk of MI, one meta-analysis of 26 trials found no association between abacavir use and risk of MICitation15. Exposure to PIs as a class was reported to be associated with increased risk of MICitation28, although the association was inconsistent across individual PI agents, with no association being found between atazanavir, saquinavir, or nelfinavir exposure and MI risk in the SLRs included in this studyCitation15,Citation28. PI exposure was also reported to be associated with increased risk of developing metabolic syndrome, but with a non-significant increase in risk of diabetesCitation25. PIs were also associated with a non-significant increase in risk of gestational diabetes mellitus (GDM) in pregnant women, except for studies that solely investigated the exposure to older PIs (i.e. those no longer widely used in the US), which reported a significant association with GDMCitation47. While efavirenz was associated with increased risk of cardiovascular eventsCitation15, exposure to efavirenz or nevirapine was not associated with a higher risk of MICitation28.
3.4.2. Mental health disorders
Depression was reported in 31% of PLWHCitation45 and 41% of those receiving ARTCitation48, and was more common in the subpopulations of PLWH with hepatitis C virus (HCV) co-infectionCitation30, MSMCitation52, and pregnant womenCitation54. The prevalence of lifetime suicidal ideation and suicidal attempt in young PLWH was estimated to be 24% and 13%, respectivelyCitation49. Alcohol use disorders were reported in 30% of PLWH, with a higher prevalence in developed (42%) versus developing countries (25%)Citation24. Additionally, approximately half of older PLWH were found to experience some degree of cognitive loss, with some progressing to dementiaCitation14.
3.4.3. Bone and muscle diseases
Bone and muscle diseases were reported to be significantly more prevalent in PLWH than HIV-negative individuals (osteopenia/osteoporosis at the lumbar spine: OR = 2.4; 95% Cl = 2.0–2.8, and at the hip: OR = 2.6; 95% Cl = 2.2–3.0Citation31; vertebral fractures: OR = 2.33; 95% CI = 1.37–3.85Citation34). ARTs in general and PIs in particular have been associated with higher prevalence of osteopenia/osteoporosisCitation31, tenofovir disoproxil fumarate (TDF) was associated with increased risk of fractureCitation15, and bone mineral density was reported to decrease during the first 2 years of ARTCitation14.
3.4.4. Liver diseases
The estimated prevalence of nonalcoholic fatty liver disease, nonalcoholic steatohepatitis, and significant fibrosis in mono-infected PLWH is 35.3%, 41.7%, and 21.7%, respectivelyCitation37, and it was estimated that 6% of non-AIDS deaths among PLWH receiving ART in the US were due to liver diseases (11% globally)Citation29.
3.4.5. Renal diseases
The prevalence of chronic kidney disease (CKD) in PLWH varied between 4.8% and 12.8%Citation26,Citation41, and it was estimated that less than 1% of non-AIDS deaths among PLWH receiving ART in the US (and globally) were due to renal diseaseCitation29. TDF and ritonavir-boosted atazanavir were associated with increased risk of CKD and the use of TDF and PIs was associated with increased risk of renal adverse events (AEs)Citation15.
3.4.6. Other comorbidities
HIV was associated with various additional conditions. Higher rates of non-HIV-related cancers and more frequent severe weight loss, exhaustion, and low physical activity were reported in older PLWH (aged > 50 years) compared with non-infected older people (aged > 50 years)Citation14. A higher prevalence of amenorrhea in premenopausal women living with HIV compared to HIV-negative controls was also reportedCitation35. While longer exposure to ART was associated with lower risk of AIDS-defining cancers, use of PIs was associated with higher risk of non-AIDS-defining cancersCitation15. Additionally, it was found that older age (> 50 years) and each added year on ART may lead to polypathology (defined as the simultaneous occurrence of two or more defined diseases) and polypharmacy (defined as the use of four or more medications)Citation14. Finally, the overall proportion of non-AIDS causes of death in PLWH was estimated to be equal to 35% globally and 47% in the USCitation29.
3.4.7. Economic burden of HIV management and comorbidities
The estimated total lifetime cost of HIV in the US (in 2017 USD) increased from $1,246,810 in 1996 to $1,673,510 in 2018, driven by antiretroviral drug and AE costs (35% increase) and comorbidity treatment costs (e.g. 180% increase for CVD and 174% for CKD). However, costs of HIV management, including costs of inpatient care, emergency department and outpatient visits, opportunistic infections prophylaxis, HIV testing, and non-HIV medication, decreased as HIV patients approached general population survival ratesCitation51.
The total costs of HIV treatment and disease management ranged from $254 to $6,608 (in 2017 USD) per-patient-per-month (PPPM)Citation51. The mean per-event costs for AEs ranged up to $31,545 for MI. The mean per-event costs for opportunistic infections ranged between $8,495 and $13,036. Lastly, the mean PPPM costs for CVD management, CKD management, and fracture/osteoporosis were $5,898, $6,108, and $4,365, respectivelyCitation51.
4. Discussion
This SLR summarized the burden associated with ART adherence and complexity, treatment resistance, and comorbidities among PLWH based on SLRs that included the US as part of the countries of interest.
Achieving adequate adherence was shown to be especially challenging in certain subpopulations, such as prison inmates, sex workers, drug users, and adolescentsCitation11. Poorer adherence has also been associated in the literature with other patient factors such as female gender, Black/non-white race, low education, poverty, and unemploymentCitation58,Citation59. Evidence from outside of the SLR has additionally demonstrated an association between multiple comorbidities and decreased ART adherenceCitation60, with patients citing poor understanding of health conditions, concern regarding comorbidities, and complex regimens as barriers to treatment adherenceCitation61. Relatedly, MTRs were shown to be associated with lower adherence and worse clinical and economic outcomes, including higher rates of treatment resistance and treatment failureCitation9,Citation12,Citation16. These associations are all the more plausible because MTRs are more prone to adherence patterns that may increase the risk of failure with resistance, such as variable adherence to different components of an ART regimenCitation62. Indeed, the prevalence of treatment resistance observed in PLWH was non-negligible. Given the potential for cross-resistanceCitation63,Citation64 and the important associated clinical burdenCitation65, less complex ART regimens containing agents with higher resistance barriers are important to improve adherence and reduce the chances of treatment failureCitation63.
HIV patients living longer due to successful ART resulted in a high comorbidity burden, with the proportion of non-AIDS causes of death in PLWH estimated to be equal to 35% globally and 47% in the USCitation29. HIV was reported to double the risk of CVD and to be associated with several other comorbidities, including bone and muscle diseases and depressionCitation34,Citation40,Citation45,Citation46. In older patients, HIV was additionally associated with severe weight loss, low physical activity, and non-HIV-related cancersCitation14. The long-term use of ART was shown to further increase the risk of developing cardiovascular, metabolic, bone, liver, and renal diseasesCitation15,Citation28,Citation31,Citation39. Each of these comorbidities may ultimately impact quality-of-life (QoL) negatively, so much so that QoL has been proposed as a “fourth 90” target in the Joint United Nations Program on HIV/AIDS (UNAIDS) 90-90-90 goals for HIV testing and treatment, specifically that 90% of PLWH with viral suppression have good health-related QoLCitation66. As the HIV population continues to live longer and with more comorbidities, equalization of QoL with persons without HIV will be essential, in addition to closure of the current gaps in comorbidity-free years of lifeCitation67. In this regard, the establishment of specialized HIV clinics may be one way to help improve management of the aging HIV population, among other treatment-related initiatives. Indeed, implementation of a clinic dedicated to PLWH older than 50 years has led to the initiation of specialized care pathways and new joint HIV/specialty clinics, with ongoing research activities to evaluate and improve issues related to polypharmacy and comorbidities among the elderly populationCitation68–71.
As an SLR of SLRs, more recent articles were not covered by the SLRs included. Indeed, other studies in the literature reported lower prevalence rates of drug resistance to PIs and INSTIs relative to NRTIs and NNRTIsCitation72,Citation73, and suggested that starting with regimens with higher genetic barriers to resistance in the first line may help to improve the long-term success of ARTCitation74. Furthermore, some studies in the literature reported a few additional findings on the comorbidity burden related to HIV and ART. For example, frailty and neurocognitive impairment were recently shown to be prevalent in PLWH and to strongly predict poor health outcomes in PLWH ≥ 40 years of age in the USCitation75. The burden of cancer among PLWH in the US was reported to shift from AIDS-defining cancers to non-AIDS-defining cancers, such as prostate and lung cancer, regardless of ART receivedCitation76. Notably, non-AIDS-defining cancers are now the most common tumors in PLWH in the Veterans Healthcare SystemCitation77, and younger ages at cancer diagnosis were observed in PLWH compared with the general population in North AmericaCitation78. Additionally, recent studies reported that INSTIs were associated with higher weight gain than NNRTI or PI agentsCitation79–81, and with an increased incidence of DM diagnoses following treatment initiationCitation82,Citation83 and cumulative use of ritonavir-boosted darunavir has been found to be associated with progressively increasing risk of CVDCitation84. MSM of all races and ethnicities, Blacks, Latinx, people who inject drugs, and transgender individuals have been identified by the Centers for Disease Control and Prevention as populations of greatest risk of HIV infectionCitation85. These high-risk groups are also associated with a higher HIV burden, such as higher barriers to HIV care, stigma, and lack of social supportCitation86, and higher risk of cardiovascular and metabolic diseasesCitation87 and coinfectionCitation88. Moreover, there are gender differences in the prevalence of some comorbidities, possibly mediated by differences in systemic immune activation and inflammation, that need to be better understoodCitation89,Citation90.
Taken together, the current findings show the substantial burden of HIV and long-term use of ART, including the risk of treatment resistance and development of comorbidities, which highlights the benefits of ART agents with lower toxicity as well as the need for a preventative intervention for HIV-1. Indeed, significant progress has been made in the use of antiretroviral drugs for HIV prevention, but major challenges remainCitation91. An effective HIV-1 vaccine would further alleviate the clinical and economic burden of HIV, especially in the subpopulations experiencing a higher burden of disease; however, the quest for an effective HIV vaccine has achieved little success so farCitation92.
4.1. Limitations
The current findings should be interpreted in the context of some limitations. Differences in study selection criteria, countries/regions covered, and methods used at the SLR level, along with differences in designs, subpopulations of PLWH, and ARTs considered across the studies included in the SLRs may have influenced the conclusions drawn. This review is also subject to any limitations of the included SLRs, including if inclusion and exclusion criteria were poorly specified, if some studies were missed, or if there were any errors in the extraction, analysis, and synthesis of the findings. Additionally, despite a thorough search strategy, some relevant SLRs may have been missed. The search was limited to articles published in English, potentially excluding some that are relevant to the global population of PLWH. Recent findings that were not yet summarized in an SLR may have been missed as well. Also, findings from this study reporting a relationship between ART and specific comorbidities or medical events should not be interpreted as a causal relationship, but as an association. Causality would need to be further evaluated on a case-by-case basis. Lastly, the included SLRs may have covered overlapping studies.
5. Conclusions
This SLR of SLRs reveals substantial burden associated with HIV and long-term use of ART, highlighting the benefits of antiretroviral agents with lower toxicity and higher resistance barriers, less complex regimens, as well as ways to bridge current gaps in HIV prevention strategies. Further research is needed to assess the potential impact of the use of a preventative HIV vaccine on the clinical and economic burden of HIV and its related comorbidities.
Transparency
Declaration of funding
Financial support for this research was provided by Janssen Scientific Affairs, LLC (JSA). The study sponsor was involved in several aspects of the research, including the study design, the interpretation of data, the writing of the manuscript, and the decision to submit the manuscript for publication.
Declaration of financial/other relationships
BOT has served as a paid consultant to ViiV Healthcare, GSK, Gilead, Merck, and JSA. HR and MHL are employees of Analysis Group, Inc., a consulting company that has provided paid consulting services to JSA, which funded the development and conduct of this study and manuscript. RB and KM were employees of Analysis Group, Inc. at the time of study conduct.
PD is an employee of JSA and stockholder of Johnson & Johnson.
Author contributions
HR, MHL, RB, and KM contributed to study conception and design, literature search, and data analysis and interpretation. BOT and PD contributed to study conception and design, and data analysis and interpretation. All authors reviewed and approved the final content of this manuscript.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Acknowledgements
Medical writing support was provided by a professional medical writer, Christine Tam, an employee of Analysis Group, Inc.
Data availability statement
All data included in the study are publicly available or available for purchase through the journal or publisher.
References
- Centers for Disease Control and Prevention (CDC). Epidemiology of HIV. 2020; [cited 2020 December 14]. Available from: https://www.hiv.uw.edu/go/screening-diagnosis/epidemiology/core-concept/all.
- Deeks SG, Overbaugh J, Phillips A, et al. HIV infection. Nat Rev Dis Primers. 2015;1:15035.
- Ghosn J, Taiwo B, Seedat S, et al. HIV. Lancet. 2018;392(10148):685–697.
- Cutrell J, Bedimo R. Single-Tablet regimens in the treatment of HIV-1 infection. Fed Pract. 2016;33(Suppl 3):24S–30S.
- Department of Health & Human Services. Guidelines for the use of antiretroviral agents in adults and adolescents with HIV. 2021; p. 1–454. Available from: https://clinicalinfo.hiv.gov/en/guidelines/hiv-clinical-guidelines-adult-and-adolescent-arv/whats-new-guidelines
- Truong WR, Schafer JJ, Short WR. Once-daily, single-tablet regimens for the treatment of HIV-1 infection. P T. 2015;40(1):44–55.
- Rodriguez-Penney AT, Iudicello JE, Riggs PK, et al. Co-morbidities in persons infected with HIV: increased burden with older age and negative effects on health-related quality of life. AIDS Patient Care STDS. 2013;27(1):5–16.
- Zhou S, Martin K, Corbett A, et al. Total daily pill burden in HIV-infected patients in the Southern United States. AIDS Patient Care STDS. 2014;28(6):311–317. PMCPMC4180528.
- Altice F, Evuarherhe O, Shina S, et al. Adherence to HIV treatment regimens: systematic literature review and meta-analysis. Patient Prefer Adherence. 2019;13:475–490.
- Pantuzza LL, Ceccato M, Silveira MR, et al. Association between medication regimen complexity and pharmacotherapy adherence: a systematic review. Eur J Clin Pharmacol. 2017;73(11):1475–1489.
- Uthman OA, Oladimeji O, Nduka C. Adherence to antiretroviral therapy among HIV-infected prisoners: a systematic review and meta-analysis. AIDS Care. 2017;29(4):489–497.
- Diallo M, Adekpedjou R, Ahouada C, et al. Impact of pre-antiretroviral therapy CD4 counts on drug resistance and treatment failure: a systematic review. AIDS Rev. 2020;22(2):78–92.
- Vannappagari V, Ragone L, Henegar C, et al. Prevalence of pretreatment and acquired HIV-1 mutations associated with resistance to lamivudine or rilpivirine: a systematic review. Antivir Ther. 2019;24(6):393–404.
- Bhatta M, Nandi S, Dutta N, et al. HIV care among elderly population: systematic review and meta-analysis. AIDS Res Hum Retroviruses. 2020;36(6):475–489.
- Chou R, Dana T, Grusing S, et al. Screening for HIV infection in asymptomatic, nonpregnant adolescents and adults: updated evidence report and systematic review for the US preventive services task force. JAMA. 2019;321(23):2337–2348.
- Clay PG, Yuet WC, Moecklinghoff CH, et al. A meta-analysis comparing 48-week treatment outcomes of single and multi-tablet antiretroviral regimens for the treatment of people living with HIV. AIDS Res Ther. 2018;15(1):17.
- Mbunkah HA, Bertagnolio S, Hamers RL, et al. Low-abundance drug-resistant HIV-1 variants in antiretroviral drug-naive individuals: a systematic review of detection methods, prevalence, and clinical impact. J Infect Dis. 2020;221(10):1584–1597.
- Biadgo B, Ambachew S, Abebe M, et al. Gestational diabetes mellitus in HIV-infected pregnant women: a systematic review and meta-analysis. Diabetes Res Clin Pract. 2019;155:107800.
- Bigna JJ, Nansseu JR, Noubiap JJ. Pulmonary hypertension in the global population of adolescents and adults living with HIV: a systematic review and meta-analysis. Sci Rep. 2019; May 24 9(1):7837.
- Bigna JJ, Ndoadoumgue AL, Nansseu JR, et al. Global burden of hypertension among people living with HIV in the era of increased life expectancy: a systematic review and meta-analysis. J Hypertens. 2020;38(9):1659–1668.
- Dakum P, Kayode GA, Abimiku A, et al. Prevalence of hypertension among patients aged 50 and older living with human immunodeficiency virus. Medicine. 2019;98(15):e15024.
- Dawood G, Klop D, Olivier E, et al. Nature and extent of hearing loss in HIV-infected children: a scoping review. Int J Pediatr Otorhinolaryngol. 2020;134:110036.
- Dorjee K, Choden T, Baxi SM, et al. Risk of cardiovascular disease associated with exposure to abacavir among individuals with HIV: a systematic review and meta-analyses of results from 17 epidemiologic studies. Int J Antimicrob Agents. 2018;52(5):541–553.
- Duko B, Ayalew M, Ayano G. The prevalence of alcohol use disorders among people living with HIV/AIDS: a systematic review and meta-analysis. Subst Abuse Treat Prev Policy. 2019;14(1):52.
- Echecopar-Sabogal J, D’Angelo-Piaggio L, Chaname-Baca DM, et al. Association between the use of protease inhibitors in highly active antiretroviral therapy and incidence of diabetes mellitus and/or metabolic syndrome in HIV-infected patients: a systematic review and meta-analysis. Int J STD AIDS. 2018;29(5):443–452.
- Ekrikpo UE, Kengne AP, Bello AK, et al. Chronic kidney disease in the global adult HIV-infected population: a systematic review and meta-analysis. PLoS One. 2018;13(4):e0195443.
- Erqou S, Lodebo BT, Masri A, et al. Cardiac dysfunction among people living with HIV: a systematic review and Meta-Analysis. JACC Heart Fail. 2019;7(2):98–108.
- Eyawo O, Brockman G, Goldsmith CH, et al. Risk of myocardial infarction among people living with HIV: an updated systematic review and meta-analysis. BMJ Open. 2019;9(9):e025874.
- Farahani M, Mulinder H, Farahani A, et al. Prevalence and distribution of non-AIDS causes of death among HIV-infected individuals receiving antiretroviral therapy: a systematic review and meta-analysis. Int J STD AIDS. 2017;28(7):636–650.
- Fialho R, Pereira M, Rusted J, et al. Depression in HIV and HCV co-infected patients: a systematic review and meta-analysis. Psychol Health Med. 2017;22(9):1089–1104.
- Goh SSL, Lai PSM, Tan ATB, et al. Reduced bone mineral density in human immunodeficiency virus-infected individuals: a meta-analysis of its prevalence and risk factors. Osteoporos Int. 2018;29(3):595–613.
- Grand M, Bia D, Diaz A. Cardiovascular risk assessment in people living with HIV: a systematic review and meta-analysis of real-life data. Curr HIV Res. 2020;18(1):5–18.
- Huntingdon B, Muscat DM, de Wit J, et al. Factors associated with erectile dysfunction among men living with HIV: a systematic review. AIDS Care. 2020;32(3):275–285.
- Ilha T, Comim FV, Copes RM, et al. HIV and vertebral fractures: a systematic review and metanalysis. Sci Rep. 2018;8(1):7838.
- King EM, Albert AY, Murray MCM. HIV and amenorrhea: a meta-analysis. AIDS. 2019;33(3):483–491.
- Masenga SK, Hamooya BM, Nzala S, et al. Patho-immune mechanisms of hypertension in HIV: a systematic and thematic review. Curr Hypertens Rep. 2019;21(7):56.
- Maurice JB, Patel A, Scott AJ, et al. Prevalence and risk factors of nonalcoholic fatty liver disease in HIV-monoinfection. AIDS. 2017;31(11):1621–1632.
- Nansseu JR, Bigna JJ, Kaze AD, et al. Incidence and risk factors for prediabetes and diabetes mellitus among HIV-infected adults on antiretroviral therapy: a systematic review and meta-analysis. Epidemiology. 2018;29(3):431–441.
- Olawepo JO, Pharr JR, Cross CL, et al. Changes in body mass index among people living with HIV who are new on highly active antiretroviral therapy: a systematic review and meta-analysis. AIDS Care. 2021;33(3):326–336.
- Oliveira VHF, Borsari AL, Webel AR, et al. Sarcopenia in people living with the human immunodeficiency virus: a systematic review and meta-analysis. Eur J Clin Nutr. 2020;74(7):1009–1021.
- Park J, Zuniga JA. Chronic kidney disease in persons living with HIV: a systematic review. J Assoc Nurses AIDS Care. 2018;29(5):655–666.
- Pires LB, Rocha R, Vargas D, et al. Non-alcoholic fatty liver disease in patients infected with human immunodeficiency virus: a systematic review. Rev Assoc Med Bras. 2020;66(1):81–86.
- Premkumar A, Dude AM, Haddad LB, et al. Combined antiretroviral therapy for HIV and the risk of hypertensive disorders of pregnancy: a systematic review. Pregnancy Hypertens. 2019;17:178–190.
- Rao SG, Galaviz KI, Gay HC, et al. Factors associated with excess myocardial infarction risk in HIV-infected adults: a systematic review and meta-analysis. J Acquir Immune Defic Syndr. 2019;81(2):224–230.
- Rezaei S, Ahmadi S, Rahmati J, et al. Global prevalence of depression in HIV/AIDS: a systematic review and meta-analysis. BMJ Support Palliat Care. 2019;9(4):404–412.
- Shah ASV, Stelzle D, Lee KK, et al. Global burden of atherosclerotic cardiovascular disease in people living with HIV: systematic review and Meta-Analysis. Circulation. 2018;138(11):1100–1112.
- Soepnel LM, Norris SA, Schrier VJ, et al. The association between HIV, antiretroviral therapy, and gestational diabetes mellitus. AIDS. 2017;31(1):113–125.
- Tao J, Vermund SH, Qian HZ. Association between depression and antiretroviral therapy use among people living with HIV: a meta-analysis. AIDS Behav. 2018;22(5):1542–1550.
- Tsegay L, Ayano G. The prevalence of suicidal ideation and attempt among young people with HIV/AIDS: a systematic review and meta-analysis. Psychiatr Q. 2020;91(4):1291–1304.
- Vancampfort D, Mugisha J, De Hert M, et al. Sedentary behavior in people living with HIV: a systematic review and meta-analysis. J Phys Act Health. 2017;14(7):571–577.
- Ward T, Sugrue D, Hayward O, et al. Estimating HIV management and comorbidity costs among aging HIV patients in the United States: a systematic review. J Manag Care Spec Pharm. 2020;26(2):104–116.
- Xiao L, Qi H, Wang YY, et al. The prevalence of depression in men who have sex with men (MSM) living with HIV: a meta-analysis of comparative and epidemiological studies. Gen Hosp Psychiatry. 2020;66:112–119.
- Xu Y, Chen X, Wang K. Global prevalence of hypertension among people living with HIV: a systematic review and meta-analysis. J Am Soc Hypertens. 2017;11(8):530–540.
- Zhu QY, Huang DS, Lv JD, et al. Prevalence of perinatal depression among HIV-positive women: a systematic review and meta-analysis. BMC Psychiatry. 2019;19(1):330. 30
- Mulè G, Mulè G, Tranchida V, et al. Aortic stiffness in HIV infection with and without antiretroviral therapy. A meta-analysis of observational studies. Art Res. 2020;26(1):13–20.
- Buscher A, Hartman C, Kallen MA, et al. Impact of antiretroviral dosing frequency and pill burden on adherence among newly diagnosed, antiretroviral-naive HIV patients. Int J STD AIDS. 2012;23(5):351–355.
- Bangsberg DR, Ragland K, Monk A, et al. A single tablet regimen is associated with higher adherence and viral suppression than multiple tablet regimens in HIV + homeless and marginally housed people. AIDS. 2010;24(18):2835–2840.
- Benson C, Wang X, Dunn KJ, et al. Antiretroviral adherence, drug resistance, and the impact of social determinants of health in HIV-1 patients in the US. AIDS Behav. 2020;24(12):3562–3573.
- Geter A, Sutton MY, Armon C, et al. Disparities in viral suppression and medication adherence among women in the USA, 2011–2016. AIDS Behav. 2019;23(11):3015–3023.
- Cantudo Cuenca M, Cantudo Cuenca M, Blanquez Martínez D, et al. CP-032 prevalence of comorbidities and effect on ART adherence in HIV-infected patients. Eur J Hosp Pharm. 2014;21(Suppl 1):A13.1–A13.
- Monroe AK, Rowe TL, Moore RD, et al. Medication adherence in HIV-positive patients with diabetes or hypertension: a focus group study. BMC Health Serv Res. 2013;13:488.
- Gardner EM, Burman WJ, Maravi ME, et al. Selective drug taking during combination antiretroviral therapy in an unselected clinic population. J Acquir Immune Defic Syndr. 2005;40(3):294–300.
- Beyrer C, Pozniak A. HIV drug resistance – an emerging threat to epidemic control. N Engl J Med. 2017;377(17):1605–1607.
- Hurt CB, Sebastian J, Hicks CB, et al. Resistance to HIV integrase strand transfer inhibitors among clinical specimens in the United States, 2009–2012. Clin Infect Dis. 2014;58(3):423–431.
- Emu B, Fessel J, Schrader S, et al. Phase 3 study of ibalizumab for multidrug-resistant HIV-1. N Engl J Med. 2018;379(7):645–654.
- Lazarus JV, Safreed-Harmon K, Barton SE, et al. Beyond viral suppression of HIV – the new quality of life frontier. BMC Med. 2016;14(1):94.
- Marcus JL, Leyden WA, Alexeeff SE, et al. Comparison of overall and comorbidity-free life expectancy between insured adults with and without HIV infection, 2000–2016. JAMA Netw Open. 2020;3(6):e207954.
- Mazzitelli M, Branca Isabel P, Muramatsu T, et al. FRAX assessment in people ageing with HIV. HIV Med. 2022;23(1):103–108.
- Mazzitelli M, Milinkovic A, Pereira B, et al. Polypharmacy and evaluation of anticholinergic risk in a cohort of elderly people living with HIV. AIDS. 2019;33(15):2439–2441.
- Pereira B, Mazzitelli M, Milinkovic A, et al. Evaluation of a clinic dedicated to people aging with HIV at Chelsea and Westminster Hospital: results of a 10-Year experience. AIDS Res Hum Retroviruses. 2022;38(3):188–197.
- Pereira B, Mazzitelli M, Milinkovic A, et al. Use of coronary artery calcium scoring to improve cardiovascular risk stratification and guide decisions to start statin therapy in people living with HIV. J Acquir Immune Defic Syndr. 2020;85(1):98–105.
- Mbhele N, Chimukangara B, Gordon M. HIV-1 integrase strand transfer inhibitors: a review of current drugs, recent advances and drug resistance. Int J Antimicrob Agents. 2021;57(5):106343.
- World Health Organization. HIV Drug Resistance Report. 2021. p. 1–138.
- Gunthard HF, Calvez V, Paredes R, et al. Human immunodeficiency virus drug resistance: 2018 recommendations of the international antiviral Society-USA panel. Clin Infect Dis. 2019;68(2):177–187.
- Erlandson KM, Perez J, Abdo M, et al. Frailty, neurocognitive impairment, or both in predicting poor health outcomes among adults living with human immunodeficiency virus. Clin Infect Dis. 2019;68(1):131–138.
- Shiels MS, Islam JY, Rosenberg PS, et al. Projected cancer incidence rates and burden of incident cancer cases in HIV-Infected adults in the United States through 2030. Ann Intern Med. 2018;168(12):866–873.
- Sigel K, Park L, Justice A. HIV and cancer in the veterans health administration system. Semin Oncol. 2019;46(4–5):334–340.
- Shiels MS, Althoff KN, Pfeiffer RM, et al. HIV infection, immunosuppression, and age at diagnosis of Non-AIDS-Defining cancers. Clin Infect Dis. 2017;64(4):468–475.
- Bourgi K, Jenkins CA, Rebeiro PF, et al. Weight gain among treatment-naive persons with HIV starting integrase inhibitors compared to non-nucleoside reverse transcriptase inhibitors or protease inhibitors in a large observational cohort in the United States and Canada. J Int AIDS Soc. 2020;23(4):e25484.
- Chow W, Donga P, Côté-Sergent A, et al. An assessment of weight change associated with the initiation of a protease or integrase strand transfer inhibitor in patients with human immunodeficiency virus. Curr Med Res Opin. 2020;36(8):1313–1323.
- Emond B, Rossi C, Côté-Sergent A, et al. Weight change and predictors of weight change among patients initiated on darunavir/cobicistat/emtricitabine/tenofovir alafenamide or bictegravir/emtricitabine/tenofovir alafenamide: a real-world retrospective study. J Health Econ Outcomes Res. 2021;8(1):88–98.
- Rebeiro PF, Jenkins CA, Bian A, et al. Risk of incident diabetes mellitus, weight gain, and their relationships with integrase inhibitor-based initial antiretroviral therapy among persons with HIV in the US and Canada. Clin Infect Dis. 2020;73(7):e2234–e2242.
- Asundi A, Olson A, Jiang W, et al. 946. Risk factors and metabolic implications of integrase inhibitor associated weight gain. Open Forum Infectious Diseases. 2020;7(Supplement_1):S505–S506.
- Ryom L, Lundgren JD, El-Sadr W, et al. Cardiovascular disease and use of contemporary protease inhibitors: the D:A: D international prospective multicohort study. Lancet HIV. 2018;5(6):e291–e300.
- Centers for Disease Control and Prevention (CDC). HIV Surveillance Report – Diagnoses of HIV Infection in the United States and Dependent Areas, 2019. 2019. p. 1–123.
- Geter A, Sutton MY, Hubbard McCree D. Social and structural determinants of HIV treatment and care among black women living with HIV infection: a systematic review: 2005–2016. AIDS Care. 2018;30(4):409–416.
- Levy M, Greenberg A, Hart R, et al. High burden of metabolic comorbidities in a citywide cohort of HIV outpatients: evolving health care needs of people aging with HIV in Washington, DC. HIV Med. 2017;18(10):724–735.
- Chu PL, Santos GM, Vu A, et al. Impact of syndemics on people living with HIV/AIDS in San Francisco. Oral abstract presented at the 2012 International AIDS Conference; 2012 Jul 22–27; Washington, DC.
- Branas F, Sanchez-Conde M, Carli F, et al. Sex differences in people aging with HIV. J Acquir Immune Defic Syndr. 2020;83(3):284–291.
- Raghavan A, Rimmelin DE, Fitch KV, et al. Sex differences in select non-communicable HIV-Associated comorbidities: exploring the role of systemic immune activation/inflammation. Curr HIV/AIDS Rep. 2017;14(6):220–228.
- Pyra MN, Haberer JE, Hasen N, et al. Global implementation of PrEP for HIV prevention: setting expectations for impact. J Int AIDS Soc. 2019;22(8):e25370.
- Rios A. Fundamental challenges to the development of a preventive HIV vaccine. Curr Opin Virol. 2018;29:26–32.

