ABSTRACT
The intestinal microbiota may contribute to the development of obesity by affecting host lipid metabolism and insulin sensitivity. To investigate the effects of microbiota manipulation on ex vivo basal and β-adrenergically-stimulated lipolysis in human adipocytes, 36 obese men were randomized to amoxicillin (broad-spectrum antibiotic), vancomycin (narrow-spectrum antibiotic) or placebo treatment (7 d, 1500 mg/d). Before and after treatment, ex vivo adipose tissue lipolysis was assessed under basal conditions and during stimulation with the non-selective β-agonist isoprenaline using freshly isolated mature adipocytes. Gene (targeted microarray) and protein expression were analyzed to investigate underlying pathways.
Antibiotics treatment did not significantly affect basal and maximal isoprenaline-mediated glycerol release from adipocytes. Adipose tissue β-adrenoceptor expression or post-receptor signalling was also not different between groups. In conclusion, 7 d oral antibiotics treatment has no effect on ex vivo lipolysis in mature adipocytes derived from adipose tissue of obese insulin resistant men.
Introduction
The obese, insulin resistant state is characterized by an increased fat storage in adipose and non-adipose tissue. A reduced catecholamine-induced lipolysis may contribute to the maintenance of increased adipose tissue stores, and is related to a reduced β-adrenoceptor expression and post-receptor changes including decreased lipase activation [Citation1,Citation2].
The intestinal microbiota and its products have been indicated to contribute to the development of obesity and related metabolic complications [Citation3–Citation5], by influencing metabolic processes in the host. Previous studies suggested that in addition to effects on skeletal muscle and liver metabolism, microbial products might affect lipid storage [Citation6,Citation7] and lipolysis [Citation8,Citation9] in the adipose tissue. External alterations of the gut microbiota composition (i.e. via administration of antibiotics), might alter circulating levels of these microbial products and thereby change host metabolic processes such as lipolysis [Citation9]. For example, microbiota-derived angiopoietin-like protein 4 (ANGPTL4) and short-chain fatty acids (SCFA) have been indicated to be involved in these changes of lipid turnover. ANGPTL4 promoted the intracellular lipolytic response to fasting and catecholamines in murine adipocytes [Citation10]. In line, human in vivo studies showed that plasma ANGPTL4 concentrations were positively associated with fasting levels of free fatty acids (FFA) and adipose tissue lipolysis [Citation11]. Oral or rectal administration of SCFA, in particular acetate, significantly decreased plasma FFA concentrations in humans [Citation12–Citation14], and treatment of murine 3T3-L1 adipocytes with SCFA reduced fasting and catecholamine-mediated lipolysis, via activation of the G-protein-coupled receptor GPR43 [Citation15]. Finally, also gut-derived bile acids (BA) [Citation16,Citation17], lipopolysaccharides and glucagon-like peptide 1 (GLP-1) [Citation18], might affect adipose tissue lipid metabolism, but the underlying mechanisms are unknown.
While these data indicate that direct administration of microbial products (i.e. SCFA or GLP-1 agonists) may indeed affect AT metabolism, it remains to be determined whether modulation of the gut microbiota may provide a strategy to target adipose tissue lipolysis and potentially improve obesity-related metabolic impairments. To this end, we collected adipose tissue biopsies of obese, insulin resistant humans after microbiota manipulation using broad spectrum (amoxicillin; AMOX) and narrow-spectrum (vancomycin; VANCO) antibiotics for 7 days [Citation19], In the collected human adipocytes, we assessed ex vivo basal and β-adrenergically stimulated lipolysis. Targeted gene and protein expression analyses were performed to investigate potential underlying mechanisms that link the gut microbiota with host metabolic processes.
Materials and methods
Study participants
The current research was conducted as part of a larger clinical trial on the effect of gut microbiota manipulation on host metabolism [Citation19], In total, 22 overweight and obese (BMI 25–35 kg × m2) Caucasian men between 35–70 years were included in the present study. Detailed inclusion and exclusion criteria have previously been described [Citation19]. All subjects gave written informed consent before participation in this trail. The protocol was reviewed and approved by the local Medical Ethical Committee of Maastricht University Medical Centre+. All procedures were according to the declaration of Helsinki (revised version, October 2008, Seoul, South Korea).
Study design
Study participants were randomized (double-blind) to the oral intake of AMOX, VANCO or placebo (PLA) for 7 consecutive days (1500 mg/d.). Participants were asked to maintain their habitual physical activity pattern and dietary habits (monitored by 3-day food diaries)throughout the study. An abdominal subcutaneous adipose tissue biopsy was taken under local anaesthesia under fasted conditions before and after 7 days antibiotics treatment for ex vivo characterization of adipocyte lipolysis, targeted microarray and Western Blot analyses, as described in more detail below. To ensure proper systemic and gastrointestinal clearance of antibiotics, a 2-day washout period was taken into account before the adipose tissue biopsies were collected.
Ex vivo adipocyte lipolysis
A part of the adipose tissue biopsy (∼500 mg) was used for the isolation of mature adipocytes following collagenase digestion in DMEM-Ham's F12 at 37°C [Citation20]. The resulting suspension was filtered through a 200 µm filter and adipocytes were washed with DMEM-Ham's F12 to eliminate collagenase [Citation21]. Following washing, isolated mature adipocytes were counted in a 2 uL drop under a light microscope. The average of 10 drops (2 independent investgators) was used to estimate total cell number in the suspension. Isolated mature adipocytes were diluted in DMEM-Ham's F12 supplemented with 3% bovine serum albumin for lipolysis assays (≈ 5000–10000 cells/incubation) and incubated with increasing concentrations of isoprenaline (ISO, a non-selective β-adrenergic agonist; 10−10 – 10−3 M) in duplicate at a finale volume of 100 µl for 3 h at 37°C. Following incubation, 60 µl cell-free aliquots of the infranatant were collected for glycerol determination (lipolysis index) using the EnzyChrome™ Adipolysis assay kit (Gentaur, Eersel, The Netherlands)[Citation21]. Glycerol release was expressed per cell number and relative to baseline, as lipolysis index.
Adipose tissue gene expression analyses
RNA was extracted from frozen AT (∼500 mg) using Trizol chloroform extraction (Invitrogen, Cergy Pontoise, France). Next, total RNA (100 ng per sample) was labeled by Whole-Transcript Sense Target Assay and hybridized to human whole-genome Affymetrix Gene 1.1 ST arrays targeting 19793 unique genes (Affymetrix, Santa Clara, CA, USA). Quality control and data analysis have been described in detail previously [Citation22]. Relevant lipolysis related genes were selected from the total microarray as published in the clinical trail where the current study is part of (GEO GSE76003) [Citation23].
Adipose tissue protein expression
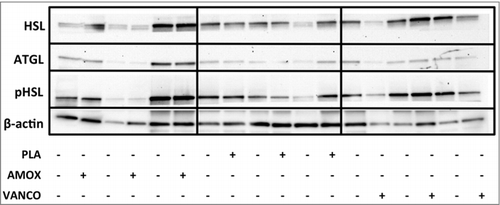
Adipose tissue biopsies (100–300 mg) were ground to a fine powder under liquid nitrogen and homogenized in 200–600 µL RIPA buffer supplemented with a protease/phosphatase inhibitor cocktail (Cell Signalling Technology Europe, Leiden, The Netherlands). Lysates were vortexed for 5 min at room temperature and centrifuged at 14.000 rpm for 30 min at 10°C. Infranatant was carefully transferred to new tubes. Protein concentrations were determined using the Pierce® BCA protein assay kit (Santa Cruz Biotechnology Inc., Heidelberg, Germany). Samples were stored at −80°C prior to analysis. 25 µg protein was separated on Any kD SDS-PAGE gels (Bio-Rad Laboratories, Veenendaal, The Netherlands) and subsequently blotted semi-dry onto nitrocellulose membrane using Trans-Blot® Turbo Transfer System (Bio-Rad Laboratories, Veenendaal, The Netherlands). Following transfer, membranes were blocked for 1 h in blocking buffer (Tris-buffered saline with 0.1% Tween 20 (TBS-T), 5% nonfat dry milk). The membranes were incubated overnight at 4°C with primary antibodies diluted in blocking buffer. Next, membranes were incubated with the corresponding secondary antibodies for 1 h at room temperature. The primary antibodies used were adipose triglyderide lipase (ATGL) (#2138), total HSL (#4107) and Phospho-HSL Ser563 (corresponding to human Ser552), a major protein kinase A (PKA) target (#4139), all from Cell Signaling [Citation24]. β-actin was used as loading control (Santa Cruz, #sc-47778, 1:1000 dilution). Secondary antibodies were horse-radish peroxidase-conjugated-IgG α-swine or α-rabbit (DAKO, Heverlee, Belgium). Antigen-antibody complexes were visualized by chemiluminescence using SuperSignal™ West Femto extended Duration Substrate (Life Technologies, Gent, Belgium). Visualization and analysis was performed using a Chemidoc XRS system (Bio-Rad Laboratories, CA, USA) and Quantity One software.
Calculations and statistical analysis
The EC50 was determined using logistic conversion of each dose-response curve as described previously [Citation25]. The negative logarithm of the EC50 value (pD2) was defined as the β-adrenergic sensitivity. ANOVA was applied to compare subject characteristics at baseline. Treatment effects within and between groups were tested using repeated-measures ANOVA. Individual genes on the microarray were defined as changed when p < 0.05 for the differential fold change after compared to before intervention, as determined by ANOVA. Data are expressed as mean ± SEM. Statistical analysis was performed using SPSS version 20.0 (Chicago, IL, USA). P < 0.05 was considered to be statistically significant.
Results
Subject characteristics
Baseline subject characteristics are shown in . No significant differences were present between the PLA and VANCO group and the PLA and AMOX group.
Table 1. Baseline Characteristics.
Ex vivo lipolysis
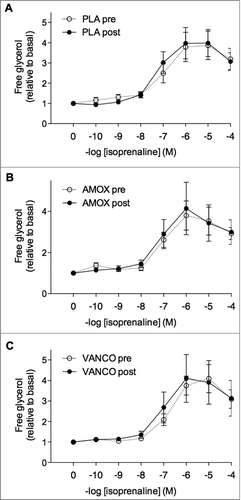
We investigated the effect of oral antibiotics treatment on ex vivo basal and β-adrenergically mediated lipolysis in freshly isolated mature adipocytes. Basal glycerol release, expressed per number of cells, was not altered following treatment (ANOVA P = 0.757, ). In addition, maximal ISO-mediated lipolytic response (adjusted for cell number) was comparable between groups (P = 0.591, ). The half-maximal effective concentration (EC50) and pD2 (-logEC50) values for ISO, which were calculated based on the dose-response curves (), were also not significantly different between groups (P = 0.124 and P = 0.057 respectively).
Table 2. Basal and maximal ISO- mediated lipolytic response in SCAT adipocytes before and after intervention, compared to placebo.
Figure 1. Dose-response curves for ISO-mediated lipolytic response in human mature adipocytes derived from the SCAT before and after intervention. Lipolysis (glycerol release in the medium) is expressed compared to baseline, following incubation with increasing concentrations ISO (10−10 to 10−4 mol/l) before (circles) and after (triangles) 7 d treatment with placebo (panel A), AMOX (B) or VANCO (C), n = 22.
β-adrenoceptor expression and lipase activation
Next, targeted microarray and Western Blot analyses on adipose tissue biopsies were performed to determine whether the improved β-adrenoceptor responsiveness following VANCO was related to changes in receptor expression and activation of major lipolytic proteins. The microarray data revealed no significant changes in adrenoceptor expression or post-receptor signalling after VANCO treatment (). In accordance with these findings, total protein content of ATGL, HSL and the phosphorylation status (on Ser563) of HSL were not significantly altered following VANCO treatment (A-C and ).
Figure 2. Gene expression profiling of lipolysis-related genes in adipose tissue before and after intervention, compared to placebo. This heat map depicts fold changes (FC) observed after compared to before AMOX (A), VANCO (V) and PLA (P) intervention. Data are derived from n = 15 individuals.
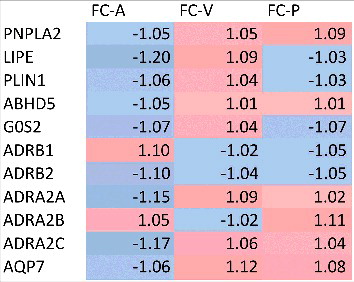
Figure 3. Quantitative analysis of the Western blots of HSL (A), ATGL (B) and phosphorylated HSL on Ser563 (corresponding to human Ser552) (C). Pre (white bars) and post (black bars) intervention data are normalized for the loading control β-actin. Values are given as mean ± SEM (n = 5 for PLA, n = 5 for VANCO and n = 6 for AMOX).
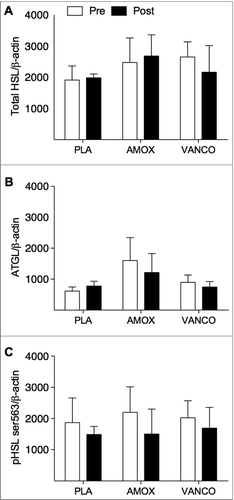
Figure 4. Representative Western Blot for lipolytic markers in human adipose tissue. Membranes were probed with antibodies directed against total ATGL, total HSL, phosphorylated HSL (pHSL) on Ser563 (corresponding to human Ser552) and β-actin was used as a loading control. A subset of 3 subjects per group is shown.
Discussion
The present study investigated whether short-term manipulation of the microbiota by means of 7 days antibiotic treatment alters adipose tissue lipolysis in humans. We demonstrated that oral administration of VANCO or AMOX for 7 days has no major effect on ex vivo β-adrenergic sensitivity in adipocytes derived from obese insulin resistant men. We have previously reported that VANCO but not AMOX treatment markedly reduced microbiota diversity and altered its composition, predominantly by decreasing gram-positive Firmicutes [Citation19]. In the current sub-cohort, microbiota changes were comparable (data not shown). VANCO treatment for 7 days decreased the abundance of Clostridium clusters XIVa and IV, which was accompanied by a reduced conversion of primary to secondary BAs, and a lower concentration of fecal SCFA [Citation19]. Interestingly, SCFA and BA receptors (i.e. GPR43 and FXR) are expressed in adipose tissue, and both SCFA and BA have been able to modulate fasting and β-adrenoceptor-mediated lipolysis in murine and human adipocytes [Citation15,Citation26]. indicating a possible role for BA and SCFA signaling in catecholamine-induced lipolysis. A decreased HSL phosphorylation may underlie this anti-lipolytic effect of SCFA[Citation27]. However, in our study, we were not able to detect changes of adipose tissue β-adrenoceptor expression upon 7 days VANCO treatment. In line, we did neither observe any changes in downstream signalling, including ATGL and HSL protein content, nor HSL phosphorylation.
It should however be mentioned that HSL phosphorylation at Ser563 may not affect catalytic activity directly [Citation28]. Moreover, since biopsies were taken after an overnight fast, we cannot draw any conclusions regarding changes in adipose tissue lipase activation and translocation of lipid droplet-associated proteins following β-adrenergic stimulation. Therefore, it would be good for future studies to take into account all major HSL phosphorylation sites to get a more complete indication of in vivo HSL activation. In addition, ex vivo lipolysis was measured in adipocytes derived from abdominal subcutaneous adipose tissue, which might respond differently to external perturbations than visceral adipose tissue. The lipolytic activity is higher in visceral than the presently examined subcutaneous depot [Citation29]. However, subcutaneous adipose tissue is by far the body's largest fat depot, and an important mass effect of its lipolytic action on whole body metabolism, is therefore, more likely. Another limitation of this study is that the gene and protein expression data reflect the total adipose tissue profile rather than cell membrane or lipid droplet-specific expression. Future studies should therefore address underlying mechanisms of microbiota effects on β-adrenergic sensitivity of lipolysis in more detail and on the longer term. Several studies have indicated that a long-term or more frequent perturbation of the microbiota composition may have more pronounced effects on metabolic health than short-term manipulation. For this reason, it is important to emphasize that the present study does not exclude an important role for the gut microbiota in AT lipolysis in obese subjects. This is supported by our previous study showing that 7 days VANCO treatment increased AT expression of genes involved in pathways related to peroxisome-proliferator activated receptor (PPAR)-signaling and of genes encoding proteins involved in fatty acid degradation [Citation19].
In summary, we demonstrated that short-term manipulation of the gut microbiota by VANCO treatment has no major effects on ex vivo β-adrenergic sensitivity in isolated mature adipocytes derived from adipose tissue of obese, insulin resistant patients. Future research is needed to establish whether long-term changes of the gut microbiota composition might affect adipose tissue lipolysis and adipose tissue function in the host.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Financial disclosure
The research is funded by TI Food and Nutrition, a public-private partnership on pre-competitive research in food and nutrition. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Author contributions
D.R. and J.J. wrote the manuscript; D.R., J.J., and E.C. contributed to data acquisition; J.J., G.G., J.P. and E.B. designed the study and analysed the data; E.B. had the primary responsibility for the final content. All authors revised the content of the manuscript, read and approved the manuscript for publication.
Trial registration
ClinicalTrials.gov, NCT02241421.
References
- Jocken JW, Langin D, Smit E, Saris WH, Valle C, Hul GB, Holm C, Arner P, Blaak EE. Adipose triglyceride lipase and hormone-sensitive lipase protein expression is decreased in the obese insulin-resistant state. The Journal of clinical endocrinology and metabolism. 2007 Jun;92(6):2292-9. doi: 10.1210/jc.2006-1318
- Ryden M, Jocken J, van Harmelen V, Dicker A, Hoffstedt J, Wiren M, Blomqvist L, Mairal A, Langin D, Blaak E, et al. Comparative studies of the role of hormone-sensitive lipase and adipose triglyceride lipase in human fat cell lipolysis. American journal of physiology Endocrinology and metabolism. 2007 Jun;292(6):E1847–55. doi: 10.1152/ajpendo.00040.2007
- Greenhill C. Obesity: Gut microbiota, host genetics and diet interact to affect the risk of developing obesity and the metabolic syndrome. Nature reviews Endocrinology. 2015 Sep 8:11.
- Sommer F, Backhed F. The gut microbiota–masters of host development and physiology. Nature reviews Microbiology. 2013 Apr;11(4):227–38. doi: 10.1038/nrmicro2974
- Janssen AW, Kersten S. The role of the gut microbiota in metabolic health. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2015 Aug;29(8):3111–23. doi: 10.1096/fj.14-269514
- Backhed F, Ding H, Wang T, Hooper LV, Koh GY, Nagy A, Semenkovich CF, Gordon JI. The gut microbiota as an environmental factor that regulates fat storage. Proceedings of the National Academy of Sciences of the United States of America. 2004 Nov 2;101(44):15718–23. doi: 10.1073/pnas.0407076101
- Backhed F, Manchester JK, Semenkovich CF, Gordon JI. Mechanisms underlying the resistance to diet-induced obesity in germ-free mice. Proceedings of the National Academy of Sciences of the United States of America. 2007 Jan 16;104(3):979–84. doi: 10.1073/pnas.0605374104
- Heimann E, Nyman M, Degerman E. Propionic acid and butyric acid inhibit lipolysis and de novo lipogenesis and increase insulin-stimulated glucose uptake in primary rat adipocytes. Adipocyte. 2015 Apr-Jun;4(2):81–8. doi: 10.4161/21623945.2014.960694
- Jocken JWE, Gonzalez Hernandez MA, Hoebers NTH, van der Beek CM, Essers YPG, Blaak EE, et al. Short-Chain Fatty Acids Differentially Affect Intracellular Lipolysis in a Human White Adipocyte Model. Frontiers in endocrinology. 2017;8:372.
- Gray NE, Lam LN, Yang K, Zhou AY, Koliwad S, Wang JC. Angiopoietin-like 4 (Angptl4) protein is a physiological mediator of intracellular lipolysis in murine adipocytes. The Journal of biological chemistry. 2012 Mar 9;287(11):8444–56. doi: 10.1074/jbc.M111.294124
- Staiger H, Haas C, Machann J, Werner R, Weisser M, Schick F, Machicao F, Stefan N, Fritsche A, Häring HU. Muscle-derived angiopoietin-like protein 4 is induced by fatty acids via peroxisome proliferator-activated receptor (PPAR)-delta and is of metabolic relevance in humans. Diabetes. 2009 Mar;58(3):579–89. doi: 10.2337/db07-1438
- Laurent C, Simoneau C, Marks L, Braschi S, Champ M, Charbonnel B, Krempf M. Effect of acetate and propionate on fasting hepatic glucose production in humans. European journal of clinical nutrition. 1995 Jul;49(7):484–91.
- Fernandes J, Vogt J, Wolever TM. Intravenous acetate elicits a greater free fatty acid rebound in normal than hyperinsulinaemic humans. European journal of clinical nutrition. 2012 Sep;66(9):1029–34. doi: 10.1038/ejcn.2012.98
- Crouse JR, Gerson CD, DeCarli LM, Lieber CS. Role of acetate in the reduction of plasma free fatty acids produced by ethanol in man. Journal of lipid research. 1968 Jul;9(4):509–12.
- Ge H, Li X, Weiszmann J, Wang P, Baribault H, Chen JL, Tian H, Li Y. Activation of G protein-coupled receptor 43 in adipocytes leads to inhibition of lipolysis and suppression of plasma free fatty acids. Endocrinology. 2008 Sep;149(9):4519–26. doi: 10.1210/en.2008-0059
- Abdelkarim M, Caron S, Duhem C, Prawitt J, Dumont J, Lucas A, Bouchaert E, Briand O, Brozek J, Kuipers F, et al. The farnesoid X receptor regulates adipocyte differentiation and function by promoting peroxisome proliferator-activated receptor-gamma and interfering with the Wnt/beta-catenin pathways. The Journal of biological chemistry. 2010 Nov 19;285(47):36759–67. doi: 10.1074/jbc.M110.166231
- Rizzo G, Disante M, Mencarelli A, Renga B, Gioiello A, Pellicciari R, Fiorucci S. The farnesoid X receptor promotes adipocyte differentiation and regulates adipose cell function in vivo. Molecular pharmacology. 2006 Oct;70(4):1164–73. doi: 10.1124/mol.106.023820
- Nogueiras R, Perez-Tilve D, Veyrat-Durebex C, Morgan DA, Varela L, Haynes WG, et al. Direct control of peripheral lipid deposition by CNS GLP-1 receptor signaling is mediated by the sympathetic nervous system and blunted in diet-induced obesity. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2009 May 6;29(18):5916–25. doi: 10.1523/JNEUROSCI.5977-08.2009
- Reijnders D, Goossens GH, Hermes GD, Neis EP, van der Beek CM, Most J, et al. Effects of Gut Microbiota Manipulation by Antibiotics on Host Metabolism in Obese Humans: A Randomized Double-Blind Placebo-Controlled Trial. Cell metabolism. 2016 Jul 12;24(1):63–74. doi: 10.1016/j.cmet.2016.06.016
- Jocken JW, Goossens GH, Popeijus H, Essers Y, Hoebers N, Blaak EE. Contribution of lipase deficiency to mitochondrial dysfunction and insulin resistance in hMADS adipocytes. International journal of obesity. 2016 Mar;40(3):507–13. doi: 10.1038/ijo.2015.211
- Verboven K, Hansen D, Moro C, Eijnde BO, Hoebers N, Knol J, Bouckaert W, Dams A, Blaak EE, Jocken JW. Attenuated atrial natriuretic peptide-mediated lipolysis in subcutaneous adipocytes of obese type 2 diabetic men. Clinical science. 2016 Jul 01;130(13):1105–14. doi: 10.1042/CS20160220
- Konings E, Timmers S, Boekschoten MV, Goossens GH, Jocken JW, Afman LA, Müller M, Schrauwen P, Mariman EC, Blaak EE. The effects of 30 days resveratrol supplementation on adipose tissue morphology and gene expression patterns in obese men. International journal of obesity. 2014 Mar;38(3):470–3. doi: 10.1038/ijo.2013.155
- Augustin HG, Koh GY, Thurston G, Alitalo K. Control of vascular morphogenesis and homeostasis through the angiopoietin-Tie system. Nature reviews Molecular cell biology. 2009 Mar;10(3):165–77. doi: 10.1038/nrm2639
- Lorente-Cebrian S, Kulyte A, Heden P, Naslund E, Arner P, Ryden M. Relationship between site-specific HSL phosphorylation and adipocyte lipolysis in obese women. Obesity facts. 2011;4(5):365–71. doi: 10.1159/000334036
- Ostman J, Arner P, Kimura H, Wahrenberg H, Engfeldt P. Influence of fasting on lipolytic response to adrenergic agonists and on adrenergic receptors in subcutaneous adipocytes. European journal of clinical investigation. 1984 Oct;14(5):383–91. doi: 10.1111/j.1365-2362.1984.tb01199.x
- Al-Lahham SH, Roelofsen H, Priebe M, Weening D, Dijkstra M, Hoek A, Rezaee F, Venema K, Vonk RJ. Regulation of adipokine production in human adipose tissue by propionic acid. European journal of clinical investigation. 2010 May;40(5):401–7. doi: 10.1111/j.1365-2362.2010.02278.x
- Aberdein N, Schweizer M, Ball D. Sodium acetate decreases phosphorylation of hormone sensitive lipase in isoproterenol-stimulated 3T3-L1 mature adipocytes. Adipocyte. 2014 Apr 1;3(2):121–5. doi: 10.4161/adip.27936
- Roepstorff C, Vistisen B, Donsmark M, Nielsen JN, Galbo H, Green KA, Hardie DG, Wojtaszewski JF, Richter EA, Kiens B. Regulation of hormone-sensitive lipase activity and Ser563 and Ser565 phosphorylation in human skeletal muscle during exercise. The Journal of physiology. 2004 Oct 15;560(Pt 2):551–62. doi: 10.1113/jphysiol.2004.066480
- Arner P. Differences in lipolysis between human subcutaneous and omental adipose tissues. Annals of medicine. 1995 Aug;27(4):435–8. doi: 10.3109/07853899709002451

