ABSTRACT
In clinical practice, the administration of adjuvant chemotherapy (ACT) following tumor surgical resection raises a critical dilemma for stage II colon cancer (CC) patients. The prognostic features used to identify high-risk CC patients rely on the pathological assessment of tumor cells. Currently, these factors are considered for stratifying patients who may benefit from ACT at early CC stages. However, the extent to which these factors predict clinical outcomes (i.e. recurrence, survival) remains highly controversial, also uncertainty persists regarding patients’ response to treatment, necessitating further investigation. Therefore, an imperious need is to explore novel biomarkers that can reliably stratify patients at risk, to optimize adjuvant treatment decisions. Recently, we evaluated the prognostic and predictive value of Immunoscore (IS), an immune digital-pathology assay, in stage II CC patients. IS emerged as the sole significant parameter for predicting disease-free survival (DFS) in high-risk patients. Moreover, IS effectively stratified patients who would benefit most from ACT based on their risk of recurrence, thus predicting their outcomes. Notably, our findings revealed that digital IS outperformed the visual quantitative assessment of the immune response conducted by expert pathologists. The latest edition of the WHO classification for digestive tumor has introduced the evaluation of the immune response, as assessed by IS, as desirable and essential diagnostic criterion. This supports the revision of current cancer guidelines and strongly recommends the implementation of IS into clinical practice as a patient stratification tool, to guide CC treatment decisions. This approach may provide appropriate personalized therapeutic decisions that could critically impact early-stage CC patient care.
Introduction
Stage II colon cancer (CC) represents an early-stage of the disease wherein the tumor has not metastasized to lymph nodes or distant organs. This stage is featured by a high cure rate (approximately 80%) following surgical resection of the primary tumor.Citation1 Notably, stage II CC accounts for only 30% of all colon cancers with a relatively low rate of relapse. Traditionally, adjuvant chemotherapy (ACT) has been the standard treatment approach for patients with locally advanced tumors, particularly in stage III CC. The benefits of ACT following tumor resection translate into long-term survival rates ranging between 8.7% and 21.5%, contingent upon the T-and N- tumor stage classification.Citation2 However, the administration of ACT for stage II patients remains highly controversial, despite numerous clinical trials and meta-analyses.Citation3 Previous clinical trials administering ACT with fluoropyrimidines (5-fluorouracil) have only shown marginal benefits in high-risk patients,Citation4–8 (), complicating the approach to these patients and the assessment of the role and efficacy of ACT in the stage II setting.
Table 1. Key adjuvant chemotherapy trials in stage II colon cancer.
There exists a significant gap in knowledge regarding the predictive value of tumor features in stage II CC, which would enable the selection of patients most likely to benefit from ACT. Additionally, the correlations between tumor features and the appropriate chemotherapy regimen remain unclear. These issues have sparked debates regarding the patient population that would benefit from adjuvant therapy, as well as the optimal type of chemotherapy (monotherapy vs doublet therapy) that must be recommended and the survival endpoint to define.
This clinical situation underscores the urgent need for risk stratification methodologies to discern patients likely to derive significant benefit from post-operative chemotherapy, based on their risk of recurrence.
The classification of Stage II CC patients into high and low-risk groups is not definitely clear.
In clinical practice, patient risk stratification relies on various factors such as tumor size (T), lymph node involvement (N), tumor grading, and the necessity for emergency surgery. Unfortunately, these features lack the precision to consistently predict the risk of recurrence for each individual patient. Moreover, the early definition of high-risk stage II CC appears imperfect, as many patients with identified high-risk features do not experience tumor relapse.Citation9
Several multigene assays, including the ColDx assay,Citation10 Oncotype DxCitation11 and ColoPrint,Citation12 have been previously developed to assess recurrence and classify stage II patients into high and low-risk categories for potential ACT benefits,Citation10–14 However, their application in clinical routine has not been recommended due to insufficient evidence supporting their ability to identify patients who would benefit from ACTCitation15 (National Comprehensive Cancer Network (NCCN) clinical practice guidelines in oncology. Available at: https://www.nccn.org/professionals/physician_gls/pdf/gist.pdf).
The American Society of Clinical Oncology (ASCO)Citation16,Citation17 and the European Society for Medical Oncology (ESMO) guidelines did not advocate for the routine application of ACT in low-risk subgroups of stage II CC patients. This recommendation stems from a meta-analysis indicating that, on average, only 5% of patients who received ACT experienced a 5-year survival benefit.Citation17
In clinical settings, ACT is exclusively recommended for patients with stage II CC carrying high-risk features, despite the absence of significant benefit from randomized studies. According to ASCO and the National Comprehensive Cancer Network® (NCCN) reports, the current guidelines outline various pathological high-risk parameters, including one or more of the following prognostic indicators: T4 stage (being the grade of tumor invasion into the bowel wall), poorly differentiated histology (G3), deficient mismatch repair/microsatellite instability (dMMR/MSI), lympho-vascular and perineural invasion, emergency clinical presentation (bowel obstruction or perforation/Venous emboli), tumor budding and inadequate lymph node (LN) sampling (<12 nodes), Mucinous colloid type, sidednessCitation18 (). For patients with 1–7 retrieved LNs, the 5y-OS rate was 49.8%, increasing to 56.2% with 8–12 LNs, and further to 63.4% with > 13 LNs.Citation18 Currently, the main risk factors considered include T4 stage, lymph node number, grading, and microsatellite status. Stages IIB and IIC patients with T4 tumors, or those with lesions penetrating to the visceral peritoneum or invading the surrounding organ, are classified at higher risk of recurrence and are recommended ACT administration.Citation16 Conversely, stage IIA patients with other high-risk factors, including sampling of fewer than 12 lymph nodes, vascular emboli, lymphatic invasion, and perineural invasion (VELIPI), poorly or undifferentiated tumor grade, intestinal obstruction, tumor perforation, or grade BD3 tumor budding, may optionally receive ACT.Citation16 Furthermore, it has not been recommended for patients with mismatch repair deficiency/microsatellite instability (dMMR/MSI) tumors to be offered ACT. However, in case of combination of dMMR/MSI with high-risk factors, oxaliplatin-containing chemotherapy is recommended.Citation16 For patients eligible for adjuvant doublet chemotherapy, adjuvant oxaliplatin-containing chemotherapy may be administered for either 3- or 6- months.Citation19
Figure 1. (a) Multivariable analysis of risk parameters in stage II CC patients. The size of the box represents the importance (hazard ratio) of each parameter regarding prognosis, according to current pathological parameters. (b) Multivariable analysis of risk parameters in stage II CC patients. The size of the box represents the importance (hazard ratio) of each parameter regarding prognosis, according to immuno- pathological parameters. Immunoscore (the pink zone) occupies the highest hazard ratio and revealed as the sole significant parameter in multivariable analysis, therefore being the strongest and most significant risk-parameter in stage II CC. The plain blue line of the polygon represents significance in cox multivariable analysis, specifically referring to immunoscore, while the dotted blue lines of the boxes denote non-significance for all the cited risk parameters.
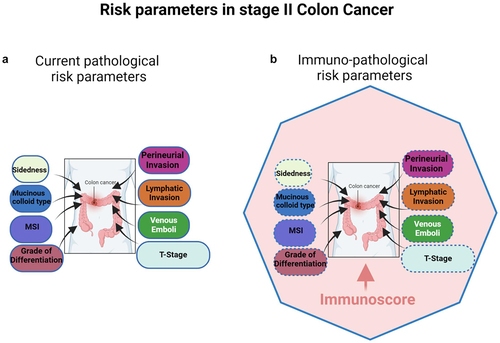
The co-existence of multiple risk factors may increase the risk of recurrence.Citation20 For example, the 5-year disease-free survival (DFS) rate stood at 74.8% for stage II patients presenting two or more risk factors, compared to 87.3% for those with only one risk factor.Citation19 Numerous clinical trials (), observational reports and retrospective analyses have investigated the role of ACT in high-risk stage II patients.Citation6,Citation21–24 Subgroup analyses were conducted on stage II CC patients who received FOLFOX as ACT.Citation25 Among the 899 patients diagnosed with stage II CC, 330 were classified as having low-risk disease and 569 as having high-risk disease. However, in high-risk patients, the application of FOLFOX did not yield statistically significant improvements in either DFS or overall survival (OS).Citation25
Further exploratory analyses on high-risk patients revealed that fluorouracil-based ACT could only improve survival by less than 5%,Citation26 exposing patients to the adverse effects of chemotherapy. Given the heterogeneity within stage II CC, accurate risk assessment and patient stratification are crucial factors in guiding adjuvant treatment decisions.Citation27
Several subgroup analyses of adjuvant trials have demonstrated that stage II disease with one or more prognostic features presents a relative long-term DFS benefit from ACT, similar to that observed in stage III patientsCitation22,Citation28–30 (Mosaic Trial, ). Nevertheless, unlike stage III, the unexpected lack of surrogacy of DFS for OS in stage II may be attributed to differences in disease biology.Citation31 The disparities observed among existing studies (Absence of ACT benefits vs relative benefits) may derive from variations in the baseline risks of the enrolled patient population. This has prompted the need to refine stratification strategies to accurately assess risks and predict treatment outcomes in individual stage II patients. This entails exploring improved predictive markers to identify patients who stand to benefit most from ACT.
We have recently demonstrated the robustness of the consensus Immunoscore (IS) assay in accurately stratifying stage II CC patients into IS-high and -low risk subgroups.Citation32,Citation33 Patients within these subgroups have shown significantly divergent clinical outcomes.Citation32,Citation33 IS is an immune digital pathology (DP)-based assay that quantifies CD3+ and CD8+ lymphocyte infiltration density in both the tumor core and invasive margin. Across all CC stages (I-III), IS assessment has shown a prognostic value significantly superior to all existing prognostic tumor parameters, including the classical AJCC/UICC TNM staging systemCitation34 (). Further studies have evaluated the potential of IS in predicting response to ACT. In line with earlier research,Citation34–37 our recent studies have consistently identified a correlation between a high IS and improved clinical outcomes in all stage II patients.Citation32,Citation33 Particularly, among stage II patients with microsatellite stability (MSS), a high IS was associated with improved DFS and reduced risk of recurrence. Notably, in high-risk stage II patients, IS accurately predicted recurrence risk with High IS exhibiting a remarkably similar recurrence risk to that of low-risk stage II patients. This risk was significantly lower than that observed in high-risk stage II patients with Low IS.Citation32 The high-risk stage II patients classified solely on histopathological features have shown different clinical outcomes. Consequently, the performance of IS contributes to improved patient stratification,Citation32 potentially guiding clinicians toward optimal treatment options for individual patients. Moreover, alongside IS, analysis of other immune cell subpopulations such as CD4 Th1 macrophages provides important additional information pertaining to patient survival and response to therapy.Citation38–40 Biomarkers related to specific genetic mutations or alterations within the tumor microenvironment (TME), such as KRAS, BRAF mutations, or microsatellite instability (MSI), hold prognostic significance, thus influencing treatment decisions.Citation41 For instance, patients with MSI-high tumors may respond favorably to immune checkpoint inhibitors.Citation42 Furthermore, biomarkers associated with inflammatory pathways or TME components, such as cytokines (TNF-α, IL-6, etc…), chemokines (CXCR1, CXCR2, CXCR4, CXCL8, CXCL12, etc.), NF-κB activation, and Cancer Associated-Fibroblasts (CAF) activation may offer prognostic insights and guide treatment strategies.Citation43–45 Moreover, tumor budding, a prognostic factor in stage II CRC, characterized by the presence of small clusters of undifferentiated tumor cells at the invasive front, is associated with aggressive tumor behavior and poor prognosis in colorectal cancer.Citation46,Citation47 These biomarkers along with many others play critical roles in guiding treatment decisions and predicting clinical outcomes for patients with colorectal cancer.
This review mainly delves into the critical aspects of risk assessment in stage II CC patients, with a particular emphasis on the significance of the IS biomarker in early-stage CC. It highlights the potential of IS as a robust DP tool for stratifying patients into different risk subgroups and discusses its impact on predicting clinical outcomes and guiding ACT decision-making.
The potential of the consensus IS in predicting survival and risk of relapse in early-stage colon cancer
Recently, the consensus IS has been evaluated in the tumors from two cohorts of 1885 AJCC/UICC-TNM stage I/II CC patients from Canada/USA (cohort 1) and Europe/Asia (cohort 2).Citation32 In these patients, immunity emerged as key determinant, surpassing all cancer cell-associated parameters (like grade of differentiation, T4 stage, venous emboli, lymphatic invasion, or perineural invasion (VELIPI)) in predicting outcomes (). Among Stage II patients (including Stage II, MSS Stage II, untreated Stage II, high-risk Stage II, and T4 tumors), the immune contexture measured by the IS was the most significant contributor to assess the DFS rate (72%) surpassing all other clinical parameters.Citation32
This study reports on the consensus IS as a powerful stratifier for stage II CC patients. The IS reliably diagnoses low immune cell infiltrated patients at risk of relapse, who would therefore benefit from ACT. Conversely, high-IS exhibited the lowest risk of recurrence across both cohorts comprising 1885 patients.
It has been also shown that in stages I/II, 5-year recurrence-free rates were 78.4% (95%-CI, 74.4–82.6), 88.1% (95%-CI, 85.7–90.4), 93.4% (95%-CI, 91.1–95.8) in low, intermediate, and high-IS, respectively (HR (Hi vs. Lo) = 0.27 (95%-CI, 0.18–0.41); p < 0.0001). Remarkably, the IS has emerged as the most potent predictor of survival among all other parameters, contributing to 60% of the predictive value. Thereby, IS was found significantly associated with survival in Stage II, high-risk Stage II, T4N0, and MSS patients. Furthermore, patients with high IS had prolonged time to recurrence (TTR) in T4N0 tumors even among those who did not receive chemotherapy. Cox multivariable analysis has revealed the IS as the sole remaining significant parameter for predicting DFS in high-risk stage II patients.Citation32 Conversely, patient gender, T-stage, VELIPI status, MSI status, sidedness, or differentiation grade did not emerge as significant prognostic indicators in the same analyses.Citation32
Notably, the association of IS with outcomes was independent (TTR: HR (Hi vs. Lo) = 0.29, (95%-CI, 0.17–0.50); p < 0.0001) of the T-stage, microsatellite instability-status (MSI), sidedness, and patient gender, as demonstrated in Cox multivariable analysis.Citation32,Citation33
This study provided critical evidence indicating that patients with high-pathological risk and a low IS face the highest risk of recurrence, suggesting potential benefits from ACT for this subgroup. Conversely, it was demonstrated that high-pathological risk patients with a high IS exhibit a recurrence risk comparable to that of low-pathological risk patients and could potentially avoid ACT and its associated toxicity.Citation32
Overall, these findings demonstrate the critical prognostic value of the IS in high-risk stage II CC and highly recommend the implementation of IS as a patient stratification assay into clinical practice to guide treatment decisions and enhance personalized care in early CC stages.
Prognostic and predictive value of IS and its association with ctDNA in stage II colorectal cancer
Circulating tumor DNA (ctDNA) is a recently identified prognostic factor and an emerging cancer biomarker that can guide treatment of stage II CC by avoiding the administration of unnecessary ACT without compromising recurrence-free survival.Citation48 Among ctDNA-positive patients who received adjuvant chemotherapy, a three-year recurrence-free survival rate of 86.4% was observed.Citation48 However, despite the availability of multiple ctDNA assays, recent developments have raised concerns regarding their efficacy and reliability. For instance, the Guardant Health company recently discontinued its cancer blood test in the COBRA trial and halted patient enrollment prematurely. This decision followed an interim analysis that revealed concerns about the test’s specificity, the study’s risky design, and the use of an older generation of minimal residual disease (MRD) assay. (https://www.medtechdive.com/news/guardant-abandons-cancer-blood-test-trial cobra/692786/)
Previously, we reported that a high density of CD3+, CD8+ and effector-memory T-cells associated with decreased VELIPI.Citation49 Since patients with VELIPI+ are more prone to have detectable ctDNA, we hypothesized a potential relationship between IS and ctDNA detection. To investigate this, we examined the roles of IS and ctDNA in guiding adjuvant treatment in Chinese early-stage colorectal cancer (CRC).Citation50 Our recent study demonstrated that IS-High patients, including clinically high-risk individuals, exhibited excellent clinical outcomes with no recurrences observed over a three-year follow-up period. Additionally, the 3-year DFS rates were 100% for IS-High, 76% for IS-Intermediate (IS-Int), and 47% for IS-Low (p < 0.001). Notably, IS exhibited strong prognostic value in stage II CRC patients. Multivariate Cox analysis underscored IS as the sole significant parameter associated with DFS, thus proving its clinical utility in prognostication and treatment decision-making.Citation50
This study further unveiled that IS-High patients exhibited the lowest rate of positive ctDNA, suggesting that this subgroup may be less likely to benefit from chemotherapy treatments. Intriguinly, among IS-High patients with positive ctDNA who did not receive adjuvant chemotherapy, none experienced relapse during the two-year follow-up period. Additionally, no instances of relapse were observed in IS-High patients, regardless of ctDNA status (ctDNA positive or negative cases). While post-operative ctDNA assay effectively identified patients at heightened risk of recurrence, it was IS that reliably determined patients at low risk of recurrence.Citation50 This underscores the complementary roles of ctDNA and IS assessment in guiding treatment decisions and prognostication in early-stage CRC.
Based on these results combining IS with ctDNA assessment, a decision algorithm has been formulated for guiding adjuvant treatment strategies in stage II CC.Citation50 Firstly, patients with IS-High had the lowest risk of recurrence and may be spared from ACT. Secondly, patients with negative postoperative ctDNA and an intermediate or low IS demonstrated an intermediate risk of recurrence and therefore could be considered for ACT. Thirdly, patients with positive postoperative ctDNA and either an IS-Int or IS-Low status presented the highest risk of recurrence. Hence, they were strongly recommended for chemotherapy.
IS digital pathology outperforms expert pathologists’ quantitative visual assessment of immune response
For decades, traditional pathology practices have been instrumental in diagnosis and classification of cancer patients. However, recent landmark innovations in precision medicine have paved the way for the development of DP-based approaches for quantitative analyses. The advent of AI technologies in clinical settings has introduced significant benefits, enabling the exploration and retrieval of high-resolution quantitative information with accuracy and precision that surpasses human visual assessment. Notably, the quantitative evaluation of immune response was not considered a powerful biomarker in clinical pathology until the introduction of IS. This AI-assisted DP-immune assay has revealed the prognostic impact of the immune response in CC patients to improve treatment decision-making in early stages,Citation32–34,Citation50–52 In a study comparing immune response assessment in CC using IS (automated DP) and pathologist visual scoring (T-score), four pathologists evaluated tumor specimens from 50 early-stage CC patients and classified CD3+ and CD8+ stained slides.Citation51 While digital pathology IS proved highly reproducible,Citation34 agreement among pathologists was minimal to weak. Furthermore, a weak concordance between the two methods was observed, resulting in the misclassification of 48% of cases by pathologist scoring. Given the substantial disagreement in interpretation among pathologists, even after training, the IS is unlikely to be reliably reproduced using non-standardized methods.Citation51 It is rather highly recommended to use the reproducible and standardized consensus IS using digital pathology.Citation32–34,Citation52–56
In a recent multi-institutional evaluation study, we compared the performance of the consensus IS to a visual evaluation of the immune response performed by 10 expert pathologists on 540 hematoxylin – eosin (H&E) and CD3+/CD8+ stained slides from 270 randomly selected CC tumors. The IS performed with DP demonstrated high reproducibility and concordance.Citation52 However, in more than 92% of cases, pathologists’ T-score evaluations by 10 international expert pathologists were discordant with the IS assay.Citation52 Additionally, a disagreement between semi-quantitative visual assessment of T-score and the reference IS was observed in 91% and 96% of cases before and after training, respectively (Pathologists’ training consisted of providing them with 12 cases of CD3+ and CD8+ stained slides at the cutoff values for IS). The concordance index between pathologists and the digital IS was significantly weak in two- and three-category IS, according to the statistical analyses. Overall, these results demonstrated that the IS outperformed expert pathologists’ T-score evaluation in the clinical settingCitation52 ().
Figure 2. Agreements between pathologists’ T-score and the reference immunoscore are illustrated by Cohen’s kappa scores for 270 colon cancer patients, before and after supervised training. Kappa scores: none (0–0.2), weak (0.4–0.59), moderate (0.6–0.79), strong (0.8–0.9) and almost perfect (>0.9). IS digital pathology quantification showed almost perfect concordance (Cohen’s kappa K > 0.9) in the reproducibility. No concordance (Cohen’s kappa K < 0.25) was observed between TIL evaluated on H&E slides and immunoscore. A weak or minimal concordance (Cohen’s kappa K < 0.5) was observed between pathologists’ visual evaluation of stained CD3 and CD8 slides, both before and after training and with known IS cases. No concordance (Cohen’s kappa K < 0.12) was observed between pathologists’ visual evaluation of stained CD3 and CD8 slides, both before and after training. Each dot represents one observer. Each triangle illustrates evaluation of cases around the cut-points by one observer. Data points and kappa scores are previously described,Citation52 supplementary data.
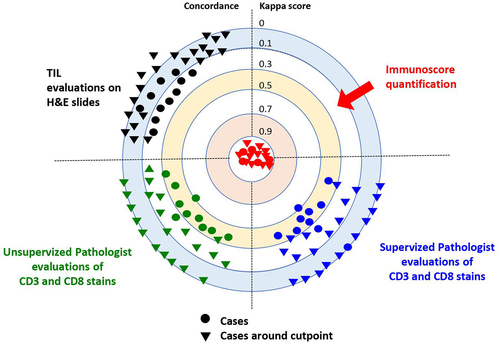
Based on our previous treatment decision-making algorithm, IS would impact treatment decision in up to 44% of stage II and 55% of stage III CC patients.Citation57 Besides, the non-concordance existing between pathologists’ visual T-scoring and IS would result in the misclassification of 70% of casesCitation52 (). Consequently, approximately 87.000 CC cases per year would likely be misclassified by pathologist scoring and may receive inadequate treatment.Citation52
Figure 3. A decision-making algorithm for predicting treatment and surveillance in stage II Low-risk and high risk for IS-Hi, -int and -lo patients’ categories. Immunoscore (IS) could impact treatment decision-making between 23 and 48% of the patients with stage II colon cancer. Immunoscore (IS) could impact surveillance decision-making for 48% of the patients with stage II colon cancer. Visual evaluation of T-score by pathologist would lead to 70% of non concordant cases, leading to inappropriate treatment and surveillance. Percentages derive from clinical decision-tree according to IS in stage II proposed in a prior study,Citation52 supplementary data.
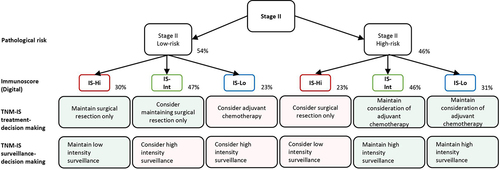
Therefore, these findings unveiled the potential of the IS as a reproducible assay for the quantitative analysis of tumor-infiltrated immune cells. This immune DP tool can improve diagnosis and patient stratification into prognostic recurrence groups, thereby guiding appropriate therapeutic decisions for CC patientsCitation51,Citation52 (). Furthermore, this research sheds light on the importance of implementing DP in clinical practice for the appropriate personalized treatment of CC patients and potentially other tumor types.
Discussion
The current literature is widely controversial regarding the classification of stage II CC patients at high risk and their potential benefit from ACT after surgery,Citation58–60 There are conflicting results and inappropriate staging guidelines,Citation61 making the administration of ACT for stage II CC quite inconclusive. Additionally, the absence of reliable biomarkers predicting response to chemotherapy and the lack of randomized trials demonstrating potential benefits of chemotherapy in high-risk Stage II CC patients significantly complicate treatment decisions in this patient population.
The recent international implementation of the consensus IS assay in stages I/II/III CCCitation34,Citation62 has demonstrated the reproducibility of DP and image analysis software. It has also revealed the significant prognostic value of the tumor immune contexture in assessing patients’ immune response and predicting clinical outcomes.Citation34,Citation39,Citation51,Citation52,Citation63,Citation64 The consensus IS has proven to be a powerful stratifier for Stage II patients, including Stage II, MSS Stage II, untreated Stage II, high-risk Stage II and T4 tumors.Citation32 Moreover, the IS permitted the accurate stratification of CC patients into low- and high-risk subgroups, identifying individuals with good prognostic features who may avoid adjuvant therapy.Citation32,Citation65
According to available guidelines, ACT is recommended for patients with high-risk features. However, our findings indicate that a major part of these patients (69.5%), who did not receive ACT but carry an IS-High, present a recurrence risk comparable to that of the low-risk patients.Citation32 Additionally, IS serves as a reliable prognostic marker to assess patients’ risk of recurrence. Therefore, IS plays a crucial role in identifying the subgroup of patients at high risk of relapse, guiding the need for critical care monitoring after surgery. Consequently, IS is highly recommended as a test for informing adjuvant treatment decisions in Stage II CC patients.Citation32
However, it’s essential to acknowledge the major limitation of this non-randomized study, which stems from its heterogeneous patient population obtained from 13 different countries.Citation32 Despite this limitation, our study evaluated the potential of IS in patients from diverse ethnicities and healthcare programs. Moving forward, further efforts are imperative to validate the IS assay in randomized clinical trials and to evaluate its predictive value for response to chemotherapy in these patients. Such endeavors are critical for advancing personalized treatment strategies and optimizing outcomes in Stage II CC patients.
The preexisting immune contexture in cancer patients, could be a driving determinant for different treatment decisions across various stages of the disease.Citation39,Citation63,Citation64,Citation66–71 The IS has shown predictive value in identifying patients who benefit from specific treatments, such as 6 months FOLFOX6, within both low- and high-risk pathological stages.Citation72 Consequently, IS assessment holds significant clinical relevance in both early- and late-stage CC.Citation34,Citation73–77
Moreover, it was lately revealed that stage II CRC patients with IS-Int and IS-Low with the lowest DFS rate could mostly benefit from ACT, while patients with IS-High should not receive ACT.Citation50 Thus, IS helps identifying patients with excellent prognostic features, who may be spared from chemotherapy. IS emerged as the most significant biomarker with robust clinical usefulness in adjuvant setting at early-stage CRC.Citation32,Citation50 In recent years, IS and postoperative ctDNA have demonstrated superior prognostic value compared to all other clinical parameters in early-stage CRC. Therefore, their implementation into clinical practice is highly recommended to guide adjuvant treatment strategies in early CRC.Citation50,Citation78 The preexisting adaptive immunity significantly impact cancer patients’ response to treatments.Citation74,Citation79,Citation80 Thus, the misclassification of stage II and III CC patients based on T-score evaluation could lead to improper treatment decision-making.Citation32,Citation52,Citation72,Citation81–83 Beside ACT, high-risk patients may also derive benefits from different oncology treatments.Citation64,Citation66,Citation67,Citation79,Citation84–97
Thereby, Stage II CC patients identified as high risk based on IS assessment could be misclassified in the low clinical risk-subgroup category.Citation52 This leads to inappropriate prediction of their risk of recurrence, resulting in inadequate surveillance for patients considered at high risk of recurrence. This would negatively impact patient health as they may be under-treated, which delays the detection of early symptoms of tumor relapse. Similarly, IS-High stage II CC patients wrongly classified as low T-score by visual examination may be erroneously recommended for ACT, unnecessary exposing them to potential side-effects and toxicity.Citation52
As previously discussed, the IS provided highly consistent quantitative analysis of tumor-infiltrated immune cells. This revealed the potential of this immune pathology tool for a better diagnosis and stratification of cancer patients into reliable prognostic groups, based on their immune parameters. According to recent findings, IS quantification of CD3+ and CD8+ cells on a whole digital slide section could avoid potential misclassification of cancer patients, often seen with traditional visual assessment methods.Citation52 Therefore, standardized IS assay outperforms visual immune response assessment by expert pathologists in predicting the risk of relapse in stage II CC.Citation51,Citation52 These results raise the importance of implementing DP to improve cancer diagnosis and provide appropriate personalized therapeutic decisions that would critically impact patient care management in early-stage CC.Citation51,Citation52
7Herein, it is also critical to address the challenge posed by single-point tumor biopsy, which inherently fails to capture the full spectrum of tumor heterogeneity, given the diverse cellular compositions and genetic mutations present at different regions of the tumor. This limitation can result in potential mischaracterization and development of inadequate treatment strategies. To overcome this challenge, novel imaging modalities have emerged as promising alternatives. Techniques such as radiolabelled anti-CD8 imaging and radiomics-based AI provide valuable insights into the immune microenvironment and enable the extraction of quantitative features from medical images, enhancing the ability to comprehensively understand tumor heterogeneity.Citation98 These innovative approaches not only could guide clinicians toward more effective and targeted interventions in precision medicine but also highlight the importance of addressing the limitations of traditional biopsy methods. In the realm of combining radiotherapy and immunotherapy, imaging biomarkers and radiomics offer noninvasive tools for assessing the entire disease, including its spatial heterogeneity and temporal changes. Molecular imaging technologies, like positron emission tomography and single-photon emission computed tomography, show promise in tumor detection, predict immunotherapy responses and assess risk parameters.Citation99 Also, a radiomic signature can predict disease-free survival in stage II and III CC, serving as a valuable supplement for risk stratification in these patients.Citation100 Therefore, the identification of novel imaging biomarkers holds the key to developing personalized treatment plans and addressing challenges in the field of immunotherapy and radio-immunotherapy combinations. Future strategies using these imaging techniques may also contribute to the early stratification of patients, improving the overall management of immunotherapy challenges faced by oncologists. However, it is imperative to acknowledge that the limitations attributed to single-point tumor biopsy are predominantly applicable in the context of Stage IV metastatic cancers and do not extend to early-stage cancers. In such instances, where the primary tumor is surgically excised, pathologists have the opportunity to stain the entire tumor slide. Consequently, IS is performed on the whole tumor slide from the resected primary tumor, with all positive cells meticulously counted via digital pathology. This method yields a comprehensive, objective, and quantitative density of adaptive immune cells within the tumor.Citation34,Citation39,Citation53–56,Citation101–103
The prognostic value of IS has been extensively addressed in a variety of cancer types,Citation39,Citation71,Citation101,Citation104–110 demonstrating its robust prognostic value regardless of the tumor stage. By stratifying patients according to their tumor immune components, IS can sort individuals at risk and also predict their survival outcomes and treatments. Moreover, the consensus IS has been validated worldwide for its superior prognostic value compared to the classical TNM staging system, even enhancing the prognostication provided by TNM.Citation34
For the first time, the immune response criterion has been introduced into the latest (5th) edition of the WHO classification of digestive tumors, as essential and desirable biomarker. WHO classification of CRC now recommends the inclusion of cytotoxic T-cell densities evaluated in the center and the invasive margin of the tumor, which is performed by the consensus IS. Furthermore, the IS was introduced into the 2020 European ESMOCitation27 and into the 2021 Pan-Asian adapted ESMO Clinical Practice GuidelinesCitation111 to refine prognosis and thus adjust the chemotherapy decision-making process.Citation27,Citation111 Given its potent ability to predict cancer recurrence, IS emerged as a novel paradigm for guiding cancer treatment. Additionally, the latest findings may significantly improve risk stratification in stage II CC with the implementation of IS. Thus, integrating IS into clinical practice could help identifying patients who may benefit from ACT.
Overall, this research argues for the revision of the current cancer guidelines (National Comprehensive Cancer Network® (NCCN®), College of American Pathologists (CAP), and AJCC/UICC-TNM) to introduce the consensus IS into the clinical prognostic features of cancer patients.
Ultimately, it would be fundamental to implement recent technological advances in AI and the powerful stratification systems into clinical settings to guide clinical decisions and improve treatment outcomes for cancer patients.
Disclosure statement
JG has patents associated with the immune prognostic biomarkers and immunotherapies. JG is co-founder of HalioDx biotech company, a Veracyte company and has part-time employment at Veracyte. Immunoscore® a registered trademark from the National Institute of Health and Medical Research (INSERM) licensed to Veracyte.
Additional information
Funding
References
- Rebuzzi SE, Pesola G, Martelli V, Sobrero AF. Adjuvant chemotherapy for stage II colon cancer. Cancers Basel. 2020;12(9):2584. doi:10.3390/cancers12092584. PMID: 32927771.
- Sobrero AF, Puccini A, Shi Q, Grothey A, Andre T, Shields AF, Souglakos I, Yoshino T, Iveson T, Ceppi M, et al. A new prognostic and predictive tool for shared decision making in stage III colon cancer. Eur J Cancer. 2020;138:182–11. doi:10.1016/j.ejca.2020.07.031. PMID: 32892120.
- Fang SH, Efron JE, Berho ME, Wexner SD. Dilemma of stage II colon cancer and decision making for adjuvant chemotherapy. J Am Coll Surg. 2014;219:1056–1069. doi:10.1016/j.jamcollsurg.2014.09.010. PMID: 25440029.
- Petrelli F, Labianca R, Zaniboni A, Lonardi S, Galli F, Rulli E, Rosati G, Corallo S, Ronzoni M, Cardellino GG, et al. Assessment of duration and effects of 3 vs 6 months of adjuvant chemotherapy in high-risk stage II colorectal cancer: a subgroup analysis of the TOSCA randomized clinical trial. JAMA Oncol. 2020;6:547–551. doi:10.1001/jamaoncol.2019.6486. PMID: 32053133.
- Efficacy of adjuvant fluorouracil and folinic acid in colon cancer. International multicentre pooled analysis of colon cancer trials (IMPACT) investigators. Lancet. 1995;345(8955):939–944. doi:10.1016/S0140-6736(95)90696-7. PMID: 7715291.
- Efficacy of adjuvant fluorouracil and folinic acid in B2 colon cancer. International multicentre pooled analysis of B2 colon cancer trials (IMPACT B2) investigators. JCO. 1999;17(5):1356–1363. doi:10.1200/JCO.1999.17.5.1356. PMID: 10334519.
- Figueredo A, Charette ML, Maroun J, Brouwers MC, Zuraw L. Adjuvant therapy for stage II colon cancer: a systematic review from the cancer care ontario program in evidence-based care’s gastrointestinal cancer disease site group. J Clin Oncol. 2004;22:3395–3407. doi:10.1200/JCO.2004.03.087. PMID: 15199087.
- Quasar Collaborative G, Gray R, Barnwell J, McConkey C, Hills RK, Williams NS, Kerr DJ. Adjuvant chemotherapy versus observation in patients with colorectal cancer: a randomised study. Lancet. 2007;370(9604):2020–2029. doi:10.1016/S0140-6736(07)61866-2. PMID: 18083404.
- Babcock BD, Aljehani MA, Jabo B, Choi AH, Morgan JW, Selleck MJ, Luca F, Raskin E, Reeves ME, Garberoglio CA, et al. High-risk stage II colon cancer: not all risks are created equal. Ann Surg Oncol. 2018;25:1980–1985. doi:10.1245/s10434-018-6484-8. PMID: 29675762.
- Kennedy RD, Bylesjo M, Kerr P, Davison T, Black JM, Kay EW, Holt RJ, Proutski V, Ahdesmaki M, Farztdinov V, et al. Development and independent validation of a prognostic assay for stage II colon cancer using formalin-fixed paraffin-embedded tissue. J Clin Oncol. 2011;29:4620–4626. doi:10.1200/JCO.2011.35.4498. PMID: 22067406.
- Kelley RK, Venook AP. Prognostic and predictive markers in stage II colon cancer: is there a role for gene expression profiling? Clin Colorectal Cancer. 2011;10:73–80. doi:10.1016/j.clcc.2011.03.001. PMID: 21859557.
- Maak M, Simon I, Nitsche U, Roepman P, Snel M, Glas AM, Schuster T, Keller G, Zeestraten E, Goossens I, et al. Independent validation of a prognostic genomic signature (ColoPrint) for patients with stage II colon cancer. Annals Surg. 2013;257:1053–1058. doi:10.1097/SLA.0b013e31827c1180. PMID: 23295318.
- Gray RG, Quirke P, Handley K, Lopatin M, Magill L, Baehner FL, Beaumont C, Clark-Langone KM, Yoshizawa CN, Lee M, et al. Validation study of a quantitative multigene reverse transcriptase–polymerase chain reaction assay for assessment of recurrence risk in patients with stage II colon cancer. J Clin Oncol. 2011;29(35):4611–4619. doi:10.1200/JCO.2010.32.8732. PMID: 22067390.
- Salazar R, Roepman P, Capella G, Moreno V, Simon I, Dreezen C, Lopez-Doriga A, Santos C, Marijnen C, Westerga J, et al. Gene expression signature to improve prognosis prediction of stage II and III colorectal cancer. J Clin Oncol. 2011;29:17–24. doi:10.1200/JCO.2010.30.1077. PMID: 21098318.
- Varghese A. Chemotherapy for stage II colon cancer. Clin Colon Rectal Surg. 2015;28:256–261. doi:10.1055/s-0035-1564430. PMID: 26648796.
- Baxter NN, Kennedy EB, Bergsland E, Berlin J, George TJ, Gill S, Gold PJ, Hantel A, Jones L, Lieu C, et al. Adjuvant therapy for stage II colon cancer: ASCO guideline update. J Clin Oncol. 2022;40:892–910. doi:10.1200/JCO.21.02538. PMID: 34936379.
- Benson AB 3rd, Schrag D, Somerfield MR, Cohen AM, Figueredo AT, Flynn PJ, Krzyzanowska MK, Maroun J, McAllister P, Van Cutsem E, et al. American society of clinical oncology recommendations on adjuvant chemotherapy for stage II colon cancer. J Clin Oncol. 2004;22(16):3408–3419. doi:10.1200/JCO.2004.05.063. PMID: 15199089.
- Costas-Chavarri A, Nandakumar G, Temin S, Lopes G, Cervantes A, Cruz Correa M, Engineer R, Hamashima C, Ho GF, Huitzil FD, et al. Treatment of patients with early-stage colorectal cancer: ASCO resource-stratified guideline. J Glob Oncol. 2019;5(5):1–19. doi:10.1200/JGO.18.00214. PMID: 30802158.
- Iveson TJ, Sobrero AF, Yoshino T, Souglakos I, Ou FS, Meyers JP, Shi Q, Grothey A, Saunders MP, Labianca R, et al. Duration of adjuvant doublet chemotherapy (3 or 6 months) in patients with high-risk stage II colorectal cancer. J Clin Oncol. 2021;39:631–641. doi:10.1200/JCO.20.01330. PMID: 33439695.
- Bockelman C, Engelmann BE, Kaprio T, Hansen TF, Glimelius B. Risk of recurrence in patients with colon cancer stage II and III: a systematic review and meta-analysis of recent literature. Acta Oncol. 2015;54(1):5–16. doi:10.3109/0284186X.2014.975839. PMID: 25430983.
- Booth CM, Nanji S, Wei X, Peng Y, Biagi JJ, Hanna TP, Krzyzanowska MK, Mackillop WJ. Adjuvant chemotherapy for stage II colon cancer: practice patterns and effectiveness in the general population. Clin Oncol (R Coll Radiol). 2017;29(1):e29–e38. doi:10.1016/j.clon.2016.09.001. PMID: 27663601.
- Gill S, Meyerhardt JA, Arun M, Veenstra CM. Translating IDEA to practice and beyond: managing stage II and III colon cancer. Am Soc Clin Oncol Educ Book. 2019;39(39):226–235. doi:10.1200/EDBK_237443. PMID: 31099666.
- Iveson T, Boyd KA, Kerr RS, Robles-Zurita J, Saunders MP, Briggs AH, Cassidy J, Hollander NH, Tabernero J, Haydon A, et al. 3-month versus 6-month adjuvant chemotherapy for patients with high-risk stage II and III colorectal cancer: 3-year follow-up of the SCOT non-inferiority RCT. Health Technol Assess. 2019;23:1–88. doi:10.3310/hta23640. PMID: 31852579.
- Yamazaki K, Yamanaka T, Shiozawa M, Manaka D, Kotaka M, Gamoh M, Shiomi A, Makiyama A, Munemoto Y, Rikiyama T, et al. Oxaliplatin-based adjuvant chemotherapy duration (3 versus 6 months) for high-risk stage II colon cancer: the randomized phase III ACHIEVE-2 trial. Ann Oncol. 2021;32:77–84. doi:10.1016/j.annonc.2020.10.480. PMID: 33121997.
- Tournigand C, Andre T, Bonnetain F, Chibaudel B, Lledo G, Hickish T, Tabernero J, Boni C, Bachet JB, Teixeira L, et al. Adjuvant therapy with fluorouracil and oxaliplatin in stage II and elderly patients (between ages 70 and 75 years) with colon cancer: subgroup analyses of the multicenter international study of oxaliplatin, fluorouracil, and leucovorin in the adjuvant treatment of colon cancer trial. J Clin Oncol. 2012;30(27):3353–3360. doi:10.1200/JCO.2012.42.5645. PMID: 22915656.
- O’Connor ES, Greenblatt DY, LoConte NK, Gangnon RE, Liou JI, Heise CP, Smith MA. Adjuvant chemotherapy for stage II colon cancer with poor prognostic features. J Clin Oncol. 2011;29:3381–3388. doi:10.1200/JCO.2010.34.3426. PMID: 21788561.
- Argilés G, Tabernero J, Labianca R, Hochhauser D, Salazar R, Iveson T, Laurent-Puig P, Quirke P, Yoshino T, Taieb J, et al. Localised colon cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2020;31:1291–1305. doi:10.1016/j.annonc.2020.06.022. PMID: 32702383.
- Andre T, Boni C, Navarro M, Tabernero J, Hickish T, Topham C, Bonetti A, Clingan P, Bridgewater J, Rivera F, et al. Improved overall survival with oxaliplatin, fluorouracil, and leucovorin as adjuvant treatment in stage II or III colon cancer in the MOSAIC trial. J Clin Oncol. 2009;27:3109–3116. doi:10.1200/JCO.2008.20.6771. PMID: 19451431.
- Bastos DA, Ribeiro SC, de Freitas D, Hoff PM. Combination therapy in high-risk stage II or stage III colon cancer: current practice and future prospects. Ther Adv Med Oncol. 2010;2(4):261–272. doi:10.1177/1758834010367905. PMID: 21789139.
- Moertel CG, Fleming TR, Macdonald JS, Haller DG, Laurie JA, Tangen CM, Ungerleider JS, Emerson WA, Tormey DC, Glick JH, et al. Intergroup study of fluorouracil plus levamisole as adjuvant therapy for stage II/Dukes’ B2 colon cancer. JCO. 1995;13(12):2936–2943. doi:10.1200/JCO.1995.13.12.2936. PMID: 8523058.
- Naxerova K, Reiter JG, Brachtel E, Lennerz JK, van de Wetering M, Rowan A, Cai T, Clevers H, Swanton C, Nowak MA, et al. Origins of lymphatic and distant metastases in human colorectal cancer. Science. 2017;357:55–60. doi:10.1126/science.aai8515. PMID: 28684519.
- Mlecnik B, Lugli A, Bindea G, Marliot F, Bifulco C, Lee JJ, Zlobec I, Rau TT, Berger MD, Nagtegaal ID, et al. Multicenter international study of the consensus immunoscore for the prediction of relapse and survival in early-stage colon cancer. Cancers Basel. 2023;15(2):418. doi:10.3390/cancers15020418. PMID: 36672367.
- Mlecnik B, Torigoe T, Bindea G, Popivanova B, Xu M, Fujita T, Hazama S, Suzuki N, Nagano H, Okuno K, et al. Clinical performance of the consensus immunoscore in colon cancer in the Asian population from the multicenter international SITC study. Cancers Basel. 2022;14(18):4346. doi:10.3390/cancers14184346. PMID: 36139506.
- Pages F, Mlecnik B, Marliot F, Bindea G, Ou FS, Bifulco C, Lugli A, Zlobec I, Rau TT, Berger MD, et al. International validation of the consensus immunoscore for the classification of colon cancer: a prognostic and accuracy study. Lancet. 2018;391(10135):2128–2139. doi:10.1016/S0140-6736(18)30789-X. PMID: 29754777.
- Galon J, Costes A, Sanchez-Cabo F, Kirilovsky A, Mlecnik B, Lagorce-Pages C, Tosolini M, Camus M, Berger A, Wind P, et al. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science. 2006;313(5795):1960–1964. doi:10.1126/science.1129139. PMID: 17008531.
- Mlecnik B, Tosolini M, Kirilovsky A, Berger A, Bindea G, Meatchi T, Bruneval P, Trajanoski Z, Fridman WH, Pages F, et al. Histopathologic-based prognostic factors of colorectal cancers are associated with the state of the local immune reaction. J Clin Oncol. 2011;29(6):610–618. doi:10.1200/JCO.2010.30.5425. PMID: 21245428.
- Pages F, Kirilovsky A, Mlecnik B, Asslaber M, Tosolini M, Bindea G, Lagorce C, Wind P, Marliot F, Bruneval P, et al. In situ cytotoxic and memory T cells predict outcome in patients with early-stage colorectal cancer. J Clin Oncol. 2009;27(35):5944–5951. doi:10.1200/JCO.2008.19.6147. PMID: 19858404.
- Nalio Ramos R, Missolo-Koussou Y, Gerber-Ferder Y, Bromley CP, Bugatti M, Nunez NG, Tosello Boari J, Richer W, Menger L, Denizeau J, et al. Tissue-resident FOLR2+ macrophages associate with CD8+ T cell infiltration in human breast cancer. Cell. 2022;185(7):1189–1207 e1125. doi:10.1016/j.cell.2022.02.021. PMID: 35325594.
- Bruni D, Angell HK, Galon J. The immune contexture and immunoscore in cancer prognosis and therapeutic efficacy. Nat Rev Cancer. 2020;20(11):662–680. doi:10.1038/s41568-020-0285-7. PMID: 32753728.
- Galon J, Sudarshan C, Ito S, Finbloom D, O’Shea JJ. IL-12 induces IFN regulating factor-1 (IRF-1) gene expression in human NK and T cells. J Immunol. 1999;162(12):7256–7262. doi:10.4049/jimmunol.162.12.7256. PMID: 10358173.
- Marisa L, de Reynies A, Duval A, Selves J, Gaub MP, Vescovo L, Etienne-Grimaldi MC, Schiappa R, Guenot D, Ayadi M, et al. Gene expression classification of colon cancer into molecular subtypes: characterization, validation, and prognostic value. PLOS Med. 2013;10(5):e1001453. doi:10.1371/journal.pmed.1001453. PMID: 23700391.
- Ghidini M, Fusco N, Salati M, Khakoo S, Tomasello G, Petrelli F, Trapani D, Petrillo A. The emergence of immune-checkpoint inhibitors in colorectal cancer therapy. Curr Drug Targets. 2021;22:1021–1033. doi:10.2174/1389450122666210204204415. PMID: 33563194.
- Chen X, Song E. Turning foes to friends: targeting cancer-associated fibroblasts. Nat Rev Drug Discov. 2019;18:99–115. doi:10.1038/s41573-018-0004-1. PMID: 30470818.
- Li H, Courtois ET, Sengupta D, Tan Y, Chen KH, Goh JJL, Kong SL, Chua C, Hon LK, Tan WS, et al. Reference component analysis of single-cell transcriptomes elucidates cellular heterogeneity in human colorectal tumors. Nat Genet. 2017;49:708–718. doi:10.1038/ng.3818. PMID: 28319088.
- Nigam M, Mishra AP, Deb VK, Dimri DB, Tiwari V, Bungau SG, Bungau AF, Radu AF. Evaluation of the association of chronic inflammation and cancer: insights and implications. Biomed Pharmacother. 2023;164:115015. doi:10.1016/j.biopha.2023.115015. PMID: 37321055.
- Lugli A, Zlobec I, Berger MD, Kirsch R, Nagtegaal ID. Tumour budding in solid cancers. Nat Rev Clin Oncol. 2021;18:101–115. doi:10.1038/s41571-020-0422-y. PMID: 32901132.
- Mitrovic B, Schaeffer DF, Riddell RH, Kirsch R. Tumor budding in colorectal carcinoma: time to take notice. Mod Pathol. 2012;25:1315–1325. doi:10.1038/modpathol.2012.94. PMID: 22790014.
- Tie J, Cohen JD, Lahouel K, Lo SN, Wang Y, Kosmider S, Wong R, Shapiro J, Lee M, Harris S, et al. Circulating tumor DNA analysis guiding adjuvant therapy in stage II colon cancer. N Engl J Med. 2022;386:2261–2272. doi:10.1056/NEJMoa2200075. PMID: 35657320.
- Pages F, Berger A, Camus M, Sanchez-Cabo F, Costes A, Molidor R, Mlecnik B, Kirilovsky A, Nilsson M, Damotte D, et al. Effector memory T cells, early metastasis, and survival in colorectal cancer. N Engl J Med. 2005;353(25):2654–2666. doi:10.1056/NEJMoa051424. PMID: 16371631.
- Wang F, Lu S, Cao D, Qian J, Li C, Zhang R, Wang F, Wu M, Liu Y, Pan Z, et al. Prognostic and predictive value of immunoscore and its correlation with ctDNA in stage II colorectal cancer. Oncoimmunology. 2023;12:2161167. doi:10.1080/2162402X.2022.2161167. PMID: 36632564.
- Boquet I, Kassambara A, Lui A, Tanner A, Latil M, Lovera Y, Arnoux F, Hermitte F, Galon J, Catteau A. Comparison of immune response assessment in colon cancer by immunoscore (automated digital pathology) and pathologist visual scoring. Cancers Basel. 2022;14(5):1170. doi:10.3390/cancers14051170. PMID: 35267475.
- Willis J, Anders RA, Torigoe T, Hirohashi Y, Bifulco C, Zlobec I, Mlecnik B, Demaria S, Choi WT, Dundr P, et al. Multi-institutional evaluation of pathologists’ assessment compared to Immunoscore. Cancers Basel. 2023;15(16):4045. doi:10.3390/cancers15164045. PMID: 37627073.
- Galon J, Lanzi A. Immunoscore and its introduction in clinical practice. Q J Nucl Med Mol Imaging. 2020;64:152–161. doi:10.23736/S1824-4785.20.03249-5. PMID: 32107902.
- Lanzi A, Pages F, Lagorce-Pages C, Galon J. The consensus immunoscore: toward a new classification of colorectal cancer. Oncoimmunology. 2020;9:1789032. doi:10.1080/2162402X.2020.1789032. PMID: 32934885.
- Marliot F, Lafontaine L, Galon J. Immunoscore assay for the immune classification of solid tumors: technical aspects, improvements and clinical perspectives. Methods Enzymol. 2020;636:109–128. doi:10.1016/bs.mie.2019.07.018. PMID: 32178816.
- Marliot F, Pages F, Galon J. Usefulness and robustness of immunoscore for personalized management of cancer patients. Oncoimmunology. 2020;9:1832324. doi:10.1080/2162402X.2020.1832324. PMID: 33194318.
- Pages F, Taieb J, Laurent-Puig P, Galon J. The consensus immunoscore in phase 3 clinical trials; potential impact on patient management decisions. Oncoimmunology. 2020;9:1812221. doi:10.1080/2162402X.2020.1812221. PMID: 32939329.
- Kumar A, Kennecke HF, Renouf DJ, Lim HJ, Gill S, Woods R, Speers C, Cheung WY. Adjuvant chemotherapy use and outcomes of patients with high-risk versus low-risk stage II colon cancer. Cancer. 2015;121:527–534. doi:10.1002/cncr.29072. PMID: 25332117.
- Lewis C, Xun P, He K. Effects of adjuvant chemotherapy on recurrence, survival, and quality of life in stage II colon cancer patients: a 24-month follow-up. Support Care Cancer. 2016;24:1463–1471. doi:10.1007/s00520-015-2931-2. PMID: 26349575.
- Verhoeff SR, van Erning FN, Lemmens VE, de Wilt JH, Pruijt JF. Adjuvant chemotherapy is not associated with improved survival for all high-risk factors in stage II colon cancer. Int J Cancer. 2016;139:187–193. doi:10.1002/ijc.30053. PMID: 26914273.
- Amin MB, Edge S, Greene F, Byrd DR, Brookland RK, Washington MK, Gershenwald JE, Compton CC, Hess KR, Sullivan DC, et al. The AJCC TNM cancer staging manual. 8th ed. USA, New York: Springer Book.; 2017. p. 849–854.
- Pages F, Mlecnik B, Galon J. Quantifying immunoscore performance – authors’ reply. Lancet. 2018;392(10158):1624–1625. doi:10.1016/S0140-6736(18)32385-7. PMID: 30496076.
- Galon J, Angell HK, Bedognetti D, Marincola FM. The continuum of cancer immunosurveillance: prognostic, predictive, and mechanistic signatures. Immunity. 2013;39:11–26. doi:10.1016/j.immuni.2013.07.008. PMID: 23890060.
- Galon J, Bruni D. Approaches to treat immune hot, altered and cold tumours with combination immunotherapies. Nat Rev Drug Discov. 2019;18:197–218. doi:10.1038/s41573-018-0007-y. PMID: 30610226.
- Galon J, Hermitte F, Mlecnik B, Marliot F, Bifulco CB, Lugli A, Nagtegaal ID, Hartmann A, Van den Eynde M, Roehrl MHA, et al. Immunoscore clinical utility to identify good prognostic colon cancer stage II patients with high-risk clinico-pathological features for whom adjuvant treatment may be avoided. J Clin Oncol. 2019;37(4_suppl):487–487. doi:https://dx.doi.org/10.1200/JCO.2019.37.4_suppl.487. PMID: WOS:000489107600493.
- Galluzzi L, Vacchelli E, Fridman WH, Galon J, Sautes-Fridman C, Tartour E, Zucman-Rossi J, Zitvogel L, Kroemer G. Trial watch: monoclonal antibodies in cancer therapy. Oncoimmunology. 2012;1:28–37. doi:10.4161/onci.1.1.17938. PMID: 22720209.
- Vacchelli E, Eggermont A, Galon J, Sautes-Fridman C, Zitvogel L, Kroemer G, Galluzzi L. Trial watch: monoclonal antibodies in cancer therapy. Oncoimmunology. 2013;2:e22789. doi:10.4161/onci.22789. PMID: 23482847.
- Antoniotti C, Rossini D, Pietrantonio F, Catteau A, Salvatore L, Lonardi S, Boquet I, Tamberi S, Marmorino F, Moretto R, et al. Upfront FOLFOXIRI plus bevacizumab with or without atezolizumab in the treatment of patients with metastatic colorectal cancer (AtezoTRIBE): a multicentre, open-label, randomised, controlled, phase 2 trial. Lancet Oncol. 2022;23(7):876–887. doi:10.1016/S1470-2045(22)00274-1. PMID: 35636444.
- Ghiringhelli F, Bibeau F, Greillier L, Fumet JD, Ilie A, Monville F, Lauge C, Catteau A, Boquet I, Majdi A, et al. Immunoscore immune checkpoint using spatial quantitative analysis of CD8 and PD-L1 markers is predictive of the efficacy of anti- PD1/PD-L1 immunotherapy in non-small cell lung cancer. EBioMedicine. 2023;92:104633. doi:10.1016/j.ebiom.2023.104633. PMID: 37244159.
- Hijazi A, Antoniotti C, Cremolini C, Galon J. Light on life: immunoscore immune-checkpoint, a predictor of immunotherapy response. Oncoimmunology. 2023;12:2243169. doi:10.1080/2162402X.2023.2243169. PMID: 37554310.
- Scholler N, Perbost R, Locke FL, Jain MD, Turcan S, Danan C, Chang EC, Neelapu SS, Miklos DB, Jacobson CA, et al. Tumor immune contexture is a determinant of anti-CD19 CAR T cell efficacy in large B cell lymphoma. Nat Med. 2022;28:1872–1882. doi:10.1038/s41591-022-01916-x. PMID: 36038629.
- Pagès F, André T, Taieb J, Vernerey D, Henriques J, Borg C, Marliot F, Ben Jannet R, Louvet C, Mineur L, et al. Prognostic and predictive value of the immunoscore in stage III colon cancer patients treated with oxaliplatin in the prospective IDEA France PRODIGE-GERCOR cohort study. Ann Oncol. 2020;31:921–929. doi:10.1016/j.annonc.2020.03.310. PMID: 32294529.
- Fridman WH, Dieu-Nosjean MC, Pagès F, Cremer I, Damotte D, Sautès-Fridman C, Galon J. The immune microenvironment of human tumors: general significance and clinical impact. Cancer Microenviron. 2013;6:117–122. doi:10.1007/s12307-012-0124-9. PMID: 23108700.
- Galon J, Bruni D. Tumor immunology and tumor evolution: intertwined histories. Immunity. 2020;52:55–81. doi:10.1016/j.immuni.2019.12.018. PMID: 31940273.
- Kirilovsky A, Marliot F, El Sissy C, Haicheur N, Galon J, Pages F. Rational bases for the use of the immunoscore in routine clinical settings as a prognostic and predictive biomarker in cancer patients. Int Immunol. 2016;28:373–382. doi:10.1093/intimm/dxw021. PMID: 27121213.
- Pages F, Galon J, Fridman WH. The essential role of the in situ immune reaction in human colorectal cancer. J Leukoc Biol. 2008;84(4):981–987. doi:10.1189/jlb.1107773. PMID: 18559950.
- Taube JM, Galon J, Sholl LM, Rodig SJ, Cottrell TR, Giraldo NA, Baras AS, Patel SS, Anders RA, Rimm DL, et al. Implications of the tumor immune microenvironment for staging and therapeutics. Mod Pathol. 2018;31:214–234. doi:10.1038/modpathol.2017.156. PMID: 29192647.
- Naidoo M, Gibbs P, Tie J. ctDNA and adjuvant therapy for colorectal cancer: time to Re-invent our treatment paradigm. Cancers Basel. 2021;13(2):346. doi:10.3390/cancers13020346. PMID: 33477814.
- Aranda F, Vacchelli E, Obrist F, Eggermont A, Galon J, Sautes-Fridman C, Cremer I, Henrik Ter Meulen J, Zitvogel L, Kroemer G, et al. Trial watch: toll-like receptor agonists in oncological indications. Oncoimmunology. 2014;3:e29179. doi:10.4161/onci.29179. PMID: 25083332.
- Bindea G, Mlecnik B, Angell HK, Galon J. The immune landscape of human tumors: implications for cancer immunotherapy. Oncoimmunology. 2014;3:e27456. doi:10.4161/onci.27456. PMID: 24800163.
- Mlecnik B, Bifulco C, Bindea G, Marliot F, Lugli A, Lee JJ, Zlobec I, Rau TT, Berger MD, Nagtegaal ID, et al. Multicenter international society for immunotherapy of cancer study of the consensus immunoscore for the prediction of survival and response to chemotherapy in stage III colon cancer. J Clin Oncol. 2020;38:3638–3651. doi:10.1200/jco.19.03205. PMID: 32897827.
- Sinicrope FA, Shi Q, Catteau A, Poage GM, Zemla TJ, Mlecnik B, Benson AB, Gill S, Goldberg RM, Kahlenberg MS, et al. Immunoscore is prognostic in low-risk and high-risk stage III colon carcinomas treated with adjuvant infusional fluorouracil, leucovorin, and oxaliplatin in a phase III trial. JCO Precis Oncol. 2022;6:e2200010. doi:10.1200/PO.22.00010. PMID: 35952316.
- Sinicrope FA, Shi Q, Hermitte F, Zemla TJ, Mlecnik B, Benson AB, Gill S, Goldberg RM, Kahlenberg MS, Nair SG, et al. Contribution of immunoscore and molecular features to survival prediction in stage III colon cancer. JNCI Cancer Spectr. 2020;4:kaa023. doi:10.1093/jncics/pkaa023. PMID: 32455336.
- Bloy N, Buqué A, Aranda F, Castoldi F, Eggermont A, Cremer I, Sautès-Fridman C, Fucikova J, Galon J, Spisek R, et al. Trial watch: naked and vectored DNA-based anticancer vaccines. Oncoimmunology. 2015;4:e1026531. doi:10.1080/2162402x.2015.1026531. PMID: 26155408.
- Buque A, Bloy N, Aranda F, Castoldi F, Eggermont A, Cremer I, Fridman WH, Fucikova J, Galon J, Marabelle A, et al. Trial watch: immunomodulatory monoclonal antibodies for oncological indications. Oncoimmunology. 2015;4:e1008814. doi:10.1080/2162402X.2015.1008814. PMID: 26137403.
- Galluzzi L, Vacchelli E, Eggermont A, Fridman WH, Galon J, Sautes-Fridman C, Tartour E, Zitvogel L, Kroemer G. Trial watch: adoptive cell transfer immunotherapy. Oncoimmunology. 2012;1:306–315. doi:10.4161/onci.19549. PMID: 22737606.
- Iribarren K, Bloy N, Buqué A, Cremer I, Eggermont A, Fridman WH, Fucikova J, Galon J, Špíšek R, Zitvogel L, et al. Trial watch: immunostimulation with toll-like receptor agonists in cancer therapy. Oncoimmunology. 2016;5:e1088631. doi:10.1080/2162402x.2015.1088631. PMID: 27141345.
- Pol J, Bloy N, Buque A, Eggermont A, Cremer I, Sautes-Fridman C, Galon J, Tartour E, Zitvogel L, Kroemer G, et al. Trial watch: peptide-based anticancer vaccines. Oncoimmunology. 2015;4:e974411. doi:10.4161/2162402X.2014.974411. PMID: 26137405.
- Pol J, Buqué A, Aranda F, Bloy N, Cremer I, Eggermont A, Erbs P, Fucikova J, Galon J, Limacher JM, et al. Trial watch—oncolytic viruses and cancer therapy. Oncoimmunology. 2016;5(2):e1117740. doi:10.1080/2162402x.2015.1117740. PMID: 27057469.
- Senovilla L, Vacchelli E, Garcia P, Eggermont A, Fridman WH, Galon J, Zitvogel L, Kroemer G, Galluzzi L. Trial watch: DNA vaccines for cancer therapy. Oncoimmunology. 2013;2:e23803. doi:10.4161/onci.23803. PMID: 23734328.
- Vacchelli E, Aranda F, Bloy N, Buqué A, Cremer I, Eggermont A, Fridman WH, Fucikova J, Galon J, Spisek R, et al. Trial watch—immunostimulation with cytokines in cancer therapy. Oncoimmunology. 2016;5(2):e1115942. doi:10.1080/2162402x.2015.1115942. PMID: 27057468.
- Vacchelli E, Aranda F, Obrist F, Eggermont A, Galon J, Cremer I, Zitvogel L, Kroemer G, Galluzzi L. Trial watch: immunostimulatory cytokines in cancer therapy. Oncoimmunology. 2014;3:e29030. doi:10.4161/onci.29030. PMID: 25083328.
- Vacchelli E, Bloy N, Aranda F, Buqué A, Cremer I, Demaria S, Eggermont A, Formenti SC, Fridman WH, Fucikova J, et al. Trial watch: immunotherapy plus radiation therapy for oncological indications. Oncoimmunology. 2016;5:e1214790. doi:10.1080/2162402x.2016.1214790. PMID: 27757313.
- Vacchelli E, Galluzzi L, Eggermont A, Galon J, Tartour E, Zitvogel L, Kroemer G. Trial watch: immunostimulatory cytokines. Oncoimmunology. 2012;1(4):493–506. doi:10.4161/onci.20459. PMID: 22754768.
- Vacchelli E, Galluzzi L, Fridman WH, Galon J, Sautes-Fridman C, Tartour E, Kroemer G. Trial watch: chemotherapy with immunogenic cell death inducers. Oncoimmunology. 2012;1:179–188. doi:10.4161/onci.1.2.19026. PMID: 22720239.
- Vacchelli E, Martins I, Eggermont A, Fridman WH, Galon J, Sautes-Fridman C, Tartour E, Zitvogel L, Kroemer G, Galluzzi L. Trial watch: peptide vaccines in cancer therapy. Oncoimmunology. 2012;1:1557–1576. doi:10.4161/onci.22428. PMID: 23264902.
- Vacchelli E, Senovilla L, Eggermont A, Fridman WH, Galon J, Zitvogel L, Kroemer G, Galluzzi L. Trial watch: chemotherapy with immunogenic cell death inducers. Oncoimmunology. 2013;2:e23510. doi:10.4161/onci.23510. PMID: 23687621.
- Sun R, Henry T, Laville A, Carre A, Hamaoui A, Bockel S, Chaffai I, Levy A, Chargari C, Robert C, et al. Imaging approaches and radiomics: toward a new era of ultraprecision radioimmunotherapy? J Immunother Cancer. 2022;10(7):e004848. doi:10.1136/jitc-2022-004848. PMID: 35793875.
- Wu M, Zhang Y, Zhang Y, Liu Y, Wu M, Ye Z. Imaging-based biomarkers for predicting and evaluating cancer immunotherapy response. Radiol Imaging Cancer. 2019;1(2):e190031. doi:10.1148/rycan.2019190031. PMID: 33778682.
- Yao X, Sun C, Xiong F, Zhang X, Cheng J, Wang C, Ye Y, Hong N, Wang L, Liu Z, et al. Radiomic signature-based nomogram to predict disease-free survival in stage II and III colon cancer. Eur J Radiol. 2020;131:109205. doi:10.1016/j.ejrad.2020.109205. PMID: 32871292.
- Angell HK, Bruni D, Barrett JC, Herbst R, Galon J. The immunoscore: colon cancer and beyond. Clin Cancer Res. 2020;26:332–339. doi:10.1158/1078-0432.ccr-18-1851. PMID: 31413009.
- Galon J, Bruni D. The role of the immune infiltrate in distinct cancer types and its clinical implications: lymphocytic infiltration in colorectal cancer. Cancer Treat Res. 2020;180:197–211. doi:10.1007/978-3-030-38862-1_7. PMID: 32215871.
- Marliot F, Chen X, Kirilovsky A, Sbarrato T, El Sissy C, Batista L, Van den Eynde M, Haicheur-Adjouri N, Anitei MG, Musina AM, et al. Analytical validation of the immunoscore and its associated prognostic value in patients with colon cancer. J Immunother Cancer. 2020;8:e000272. doi:10.1136/jitc-2019-000272. PMID: 32448799.
- Anitei MG, Zeitoun G, Mlecnik B, Marliot F, Haicheur N, Todosi AM, Kirilovsky A, Lagorce C, Bindea G, Ferariu D, et al. Prognostic and predictive values of the immunoscore in patients with rectal cancer. Clin Cancer Res. 2014;20:1891–1899. doi:10.1158/1078-0432.CCR-13-2830. PMID: 24691640.
- Berghoff AS, Fuchs E, Ricken G, Mlecnik B, Bindea G, Spanberger T, Hackl M, Widhalm G, Dieckmann K, Prayer D, et al. Density of tumor-infiltrating lymphocytes correlates with extent of brain edema and overall survival time in patients with brain metastases. Oncoimmunology. 2016;5:e1057388. doi:10.1080/2162402X.2015.1057388. PMID: 26942067.
- El Sissy C, Kirilovsky A, Lagorce Pages C, Marliot F, Custers PA, Dizdarevic E, Sroussi M, Castillo-Martin M, Haicheur N, Dermani M, et al. International validation of the immunoscore biopsy in patients with rectal cancer managed by a watch-and-wait strategy. J Clin Oncol. 2023. doi:10.1200/JCO.23.00586. JCO2300586; PMID: 37788410.
- El Sissy C, Kirilovsky A, Van den Eynde M, Musina AM, Anitei MG, Romero A, Marliot F, Junca A, Doyen J, Mlecnik B, et al. A diagnostic biopsy-adapted immunoscore predicts response to neoadjuvant treatment and selects patients with rectal cancer eligible for a watch-and-wait strategy. Clin Cancer Res. 2020;26:5198–5207. doi:10.1158/1078-0432.ccr-20-0337. PMID: WOS:000576795700019.
- Nassif EF, Mlecnik B, Thibault C, Auvray M, Bruni D, Colau A, Compérat E, Bindea G, Catteau A, Fugon A, et al. The immunoscore in localized urothelial carcinoma treated with neoadjuvant chemotherapy: clinical significance for pathologic responses and overall survival. Cancers. 2021;13(3):494. doi:10.3390/cancers13030494. PMID: doi:10.3390/cancers13030494.
- Rapoport BL, Galon J, Nayler S, Mlecnik B, Fugon A, Benn CA, Martel M, Cronje T, Smit T, Moosa F, et al. Tumour infiltrating lymphocytes in breast cancer: high levels of CD3, CD8 cells and immunoscore (R) are associated with pathological CR in patients receiving neo-adjuvant chemotherapy. Ann Oncol. 2020;31:S31–S32. doi:10.1016/j.annonc.2020.03.180. PMID: WOS:000538879300042.
- Zhao Z, Zhao D, Xia J, Wang Y, Wang B. Immunoscore predicts survival in early-stage lung adenocarcinoma patients. Front Oncol. 2020;10:691. doi:10.3389/fonc.2020.00691. PMID: 32457841.
- Yoshino T, Argilés G, Oki E, Martinelli E, Taniguchi H, Arnold D, Mishima S, Li Y, Smruti BK, Ahn JB, et al. Pan-asian adapted ESMO clinical practice guidelines for the diagnosis treatment and follow-up of patients with localised colon cancer. Ann Oncol. 2021;32:1496–1510. doi:10.1016/j.annonc.2021.08.1752. PMID: 34411693.

