ABSTRACT
Aim: To conduct a systematic review of the economic evaluations (EE) of HBV vaccination, taking also into account the studies published in the new millennium.Methods: An extensive scientific literature review was conducted using two electronic medical journal databases: Scopus and PubMed engines for published studies on EE of HBV vaccination.Results: 22 articles were reviewed, 9, 5 and 8 cost-effectiveness, cost-benefit and cost-utility analysis, respectively. Studies were mainly concerning EE of universal vaccination (UV), mostly with regards to low or low-medium income countries. For high income countries, EE were focused on the possible implementation of HBV vaccination in particular settings, such as diabetic, renal and other chronic conditions care, as well as infectious diseasesUV has usually a very good cost-effectiveness ratio (80%), ranging from cost-saving (China) or few Euro per LY/QALY gained (in Thailand, and Vietnam) to 630.00$/QALY in USA (Asian and Pacific Islands) Moreover, EE of HBV vaccination are favorable in the infectious diseases field as well as for chronic conditions. In relation to diabetes the studies gave controversial results.Conclusion: This systematic review highlighted the importance of introducing HBV vaccination not only for infant UV program but also for other settings in which patients are people affected by communicable and non-communicable diseases.
Introduction
It is well recognized that Hepatitis B virus (HBV) is one of world's most common blood-borne viral infection, chronically infecting hundreds of million people worldwide.Citation1 Moreover, there is evidence that it is responsible for substantial morbidity and mortality and is together with HCV a leading cause of hepatocellular carcinoma.Citation1 It is estimated that nearly 2 billion people worldwide have been infected with HBV; of these, 360 million are chronically infected, and among them 600000 individuals die each year from HBV associated liver cirrhosis or hepatocellular carcinoma.Citation2
According to DrummondCitation3 the economic evaluation can be defined as the comparative analysis of alternative courses of action in terms of both their costs and consequences, and the first attempts to consider economic issues related to HBV vaccination date back to early ‘80,Citation4 and particular attention was posed on health care personnel (HCP) (i.e., renal dialysis, blood transfusion centers, clinical biology laboratories, surgery, anesthesia, closed psychiatric institutions and others), finding that the financial investment has proved economically beneficial for the insurance fund. In fact, we must recognized that, since HBV is transmitted primarily through blood and sexual routes, in a way that is similar to HIV and hepatitis C virus (HCV),Citation5 it must be considered an occupational hazard for HCPs.Citation6 Moreover, according to Di Giuseppe et al Citation7 HCPs are at risk of blood-borne, airborne, and droplet-spread transmission of infectious agents, and this is particularly true due to frequent and often intensive occupational exposures, among which it can be included percutaneous injury, contact with mucous membranes, or nonintact skin with blood or other potentially infectious bodily fluids.Citation8-10 On the other hand, HCPs can also act as potential paths of nosocomial transmission of several infections to patients and other close contacts.
Beutels underlined that in areas of low, intermediate and high endemicity of HBV infection, the universal vaccination seems justifiable on the basis of economic evaluation. However, he noted that the accuracy of the models has improved over the years, even if some improvements have been made, further steps are required, especially concerning transparency, completeness and comparability of analyses.Citation11 Furthermore, Tu et al. very recently reviewed this issue applied to developing and less developed countries, finding that the almost totality of the studies regarding the implementation of universal immunization against HBV is capable to reduce the level of endemicity of hepatitis B, and probably cost effective in many settings.Citation12
As far as concerns infant vaccination importance, it is well known that infants infected with hepatitis B virus (HBV) face the risk of developing severe complications Citation13 and childhood chronic HBV infection prevalence has been markedly reduced in those counties where vaccination policies have been put in place Citation14-17
In accordance with the World Health Organization Citation18 recommendations, all infants should receive their first dose of the hepatitis B vaccine immediately after birth, preferably within 24 hours.
The availability of a vaccine against HBV infection let the disease effectively preventable in a safe and effective way since 1982.Citation19 As of 2011, hepatitis B vaccine has been incorporated in 179 countries' national infant immunization programs, and about 69% of the 2008 birth cohort received all three doses of the vaccine.Citation19 Nevertheless, while the vaccination coverage of three doses at the world level is 75%, many differences do exist between countries and macro-areas (), with lower coverage in South-East Asia (57%) and African (71%) WHO regions.
Table 1. HBV Vaccination coverage estimates, by vaccine and World Health Organization (WHO) region — worldwide, 2011.
At the beginning of the ‘90 Bloom et al.Citation20 found that the strategy of universal newborn vaccination alone is cost-effective (incremental cost-per-year-of-life saved of $3.332), and the same results were found by Holliday and Faulds.Citation21 However, other studies found that mass vaccination of adolescents were more cost-effective Citation22 or at least as cost-effective as the universal vaccination for infants, over a wide range of assumptions,Citation23 but no cost-saving.Citation24
Further, it was clear that the majority of the cost of HBV vaccination program is due to the cost of the vaccine (more than three fourth of the cost of introducing HBV vaccine).Citation25,26
Mass vaccination for health care workers was an issue since the availability of HBV vaccination, and this option was considered more beneficial than costly,Citation27-29 and now must be considered all over the world also for ethical and social, as well as for economic aspects.Citation30 There is also evidence that preventing the transmission of HBV infections in dialysis centers is possible with the administration of HBV vaccine to susceptible patients and staff.Citation31
Essentially, the need to go beyond the infant universal vaccination, i.e. to vaccinate older age groups, including adolescents and adults, is determined by the burden of disease (HBV infection) in a specific country.Citation32 Italy was one of the first countries (1991) that, in order to reduce the HBV related pathologies in the Italian population, implemented a multi-level approach, including the universal vaccination of newborn babies, 12-year old adolescents and high risk groups.Citation33 The effect of this vaccination strategy was impressive, reaching two main goals, i.e., protecting susceptible people from the infection caused by the hepatitis B virus and reducing the virus circulation in the population.Citation34
Thus, the objective of this paper is to conduct a systematic review of the economic evaluations of HBV vaccination, taking also into account the studies published in the new millennium.
Results
Data extraction
The information extracted were: references, publication year and type of analyses, alternatives, nation/perspective, sample, efficacy measures/cost measure and results. The characteristics of each study are shown in Table 2.
Identification of relevant research
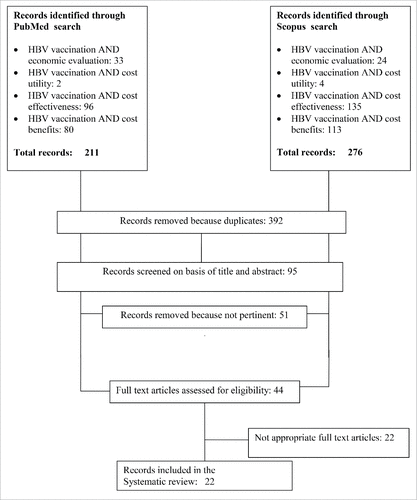
Using the aforementioned inclusion criteria the following articles were found (see Fig 1. Flow-chart):
211 articles for Pubmed search;
276 articles for Scopus search;
A total of 487 articles were found for all strings, of which 392 were removed because were duplicates in two or all search engines. Moreover, 51 articles were excluded because they were not relevant. At the end of the evaluation, 44 articles met the pre-determined criteria described above, and 22 were included in the systematic review process.Citation35-56
Type of economic evaluation of the included studies
The 22 articles reviewed are shown in .
Table 2. Characteristics of the selected studies by year of publication and types of economic analysis.
The classification of the studies is based on the type of economic evaluation:
CEA (cost-effectiveness analysis) was performed in 9 economic evaluation studies.Citation36,38-40,43,44,47,50,53
CBA (cost-benefit analysis) was evaluated by 5 studies.Citation35,37,46,48,51
CUA (cost-utility analysis) was considered in 8 studies.Citation41,42,44,49,52,54-56
The population and the countries considered were different in the studies: 5 analyses were conducted in Europe (Bulgaria, Germany, Ireland and UK), 8 in America (all in the USA), 7 in Asia (China, Iran, South Korea Taiwan, Thailand, Vietnam), 1 in Africa (Mozambique) and 1 in Oceania (Australia).
Sample and results
The majority of the studies were concerning economic analysis of universal vaccination (12; 54.5%), mostly with regards to low or low-medium income countries (7; 58.3%). Moreover, it is interesting to note that in studies on high income countries, the economic evaluations were focused on the possible implementation of HBV vaccination in particular settings, such as diabetic, renal and other chronic conditions care, as well as infectious diseases (Sexually transmitted diseases, HIV counseling, HCV patients, injection drug users).
So the results of this systematic review are heterogeneous. Concerning the universal vaccination, this has usually a very good cost-effectiveness ratio (80%), ranging from cost-saving (China) Citation56 or few Euro per LY/QALY gained (in Thailand, and Vietnam) Citation40,52 to 630.00 $ /QALY in USA (Asian and Pacific Islands).Citation42
There is clear evidence that all infants should receive their first dose of hepatitis B vaccine as soon as possible after birth, preferably within 24 hours.Citation2
As suggested by WHO the universal HBV vaccination represents a comprehensive approach to eliminating HBV transmission that considers infections acquired perinatally and during early childhood, but it is important to consider also other settings that involve different pattern of transmissions and different populations (teenagers and adults).Citation2 In fact, as far as concerns the other settings, the economic evaluations of HBV vaccination are favorable in the infectious diseases field as well as for chronic conditions. The HBV vaccination could be of great interest and impact in settings that involve injection drug users (IDUs) and jails, where HBV infections could be present and rapidly spreading,Citation57-58 alone or together to other viruses (HIV, HCV). In this cases, the accelerated vaccination schedule could easily improve hepatitis B vaccination adherence among IDUs Citation59 as well as the use of the standard vaccine regimen (0, 1, 6 months) is capable to may induce higher levels of antibody to the virus comparable to those induced by regimens of four injections of either standard or double doses.Citation60
In relation to diabetes the economic evaluations gave controversial results, reflecting what happens for the clinical recommendation. In 2011, the Advisory Committee on Immunization Practices (ACIP) recommended that all previously unvaccinated adults aged between 19 and 59 y with diabetes mellitus (both type 1 and 2) should be vaccinated against hepatitis B as soon as possible after a diagnosis of diabetes is made (recommendation category A). On the other hand, data on the risk for hepatitis B among adults aged 60 y and over are less robust, so that this group of patients could be vaccinated at the discretion of the treating clinician, only after the assessment of the risk and the likelihood of an adequate immune response to vaccination.Citation61 Concerning the economic evaluations, while Kuan et al Citation55 found an acceptable incremental C/E (12.613$/QALY), this did not happen for Hoerger et al.Citation54
As far as concerns the quality of these economic evaluations, using the weighted scale of Drummond, very high scores were found (median 91.5; range: 53–98) with the vast majority of the papers (90.9%) with score over 80 ().
Discussion
In this systematic review a wide range of results in terms of economic evaluations of HBV vaccination was found, especially regarding the way for measuring the efficacy/effectiveness. From the methodological point of view, cost-utility and cost-effectiveness analysis are almost equally represented. In order to be more adherent to commonly used cost-utility analysis performed in the biomedical field, future studies will address better the way in incorporating a quality weighted measure of effectiveness, such as QALY (Quality Adjusted Life Years). This kind of studies will be of crucial importance in order to convince decision makers to implement the HBV vaccination programs in the public sector.
Moreover, in this review we found five single studies in which costs and effectiveness were measured in the same way (money), and this is in line with the actual trends of the economic evaluations that use less frequently cost-benefit analysis.
Concerning possible limitation of this study, it must be recognized that there is a lack of information in many countries/continents, considering that a single study exists coming from Africa and no one from Southern and Central America. However, this is not surprising, given the very low number of randomized clinical trial conducted in these continents.Citation62-68
Some final considerations must be underlined. Since Hepatitis B infection and related diseases are considered an important hazard for the general population and for many workers, not only in the health sector, the economic impact is relevant.Citation69-70
There is evidence that the virus transmission usually occurs through blood as well as blood products, and other body fluids (saliva, semen, et cetera). Different situations could be the case of transmission, such as sexual intercourse, illicit drug use with shared needles, above all heroin, blood transfusions or use of blood products. Moreover, HBV infection is one of the mail virus transmitted by medical practices, such as surgical intervention, dental care, especially in cases where infection control precautions are inadequate.Citation71-80
The infections sustained by HBV mainly affect the liver, causing acute and chronic diseases, such as liver infections and cirrhosis.Citation73
It is now well known that chronic infections with HBV hepatitis C virus (HCV) are also considered the main cause of liver cancer and for this fact classified by the International Agency for Research on Cancer (IARC) as carcinogenic to humans (group 1).Citation74
According to WHO forecast, the infections caused by HBV will be the third cause of death for infectious diseases in 2030 in the industrialized countries.Citation75 Viral hepatitis, and among which those caused by HBV, knows no borders.Citation76
The epidemiology of HBV related pathologies is continuously changing. As a typical example the waste management field is a field of increasing interest, considering the vulnerability of recyclable general waste collectors to HBV infection, as well as the medical waste handlers. There is sufficient evidence that witnesses HBV immunization, as well as post-exposure protection of medical waste handlers, in addition to proper management of medical waste by the health authorities, might reduce the risk of acquiring infectious agents by this type of workers in a significant way.Citation77 Additionally, we must consider in this field also that the need to use HBV vaccine prophylaxis in this category of workers is important for avoid possible medical legal litigations.Citation78-79
In this field the importance of public health policies that address the health and safety of this socially vulnerable population is very high.Citation80-82
A very recent systematic review highlights that waste workers need to be vaccinated against HBV infection taking into account these workers are at risk of acquiring this infection through the exposure to potentially infected waste.Citation83
The HBV vaccination has also an indirect effect on the epidemiology of blood transmitted diseases (BTD). In fact, there is evidence that significant improvements in the screening process of BTD of both donors and household contacts is fundamental in order to minimize the infectious risk.Citation84-85
Moreover, a very good field for reducing the HBV burden through the HBV vaccination is concerning men who have sex with men, and Commercial sex workers, among which there exist a disproportionate burden of HBV infections.Citation86-95
The WHO recommendations include the offer the rapid HBV vaccination regimen for persons who inject drugs.Citation96-97
As far as concerns one of the strengths of this review, a gap has been filled, since it was shown the use of economic evaluations in highly endemic areas, that was lacking in previous reviews,Citation11 and this is very important to raise both public awareness of the effectiveness and economic attractiveness of universal immunization against HBV.Citation52
Conclusion
In conclusion, this systematic review highlighted the importance of introducing HBV vaccination not only for infant universal vaccination program but also for other settings in which patients are people affected by communicable and non-communicable diseases.
HBV infection causes worldwide a high burden in terms of costs, both direct and indirect.Citation98-120
In accordance with Beutels Citation11 it must be recognized that the role of economic evaluations of HBV vaccination in the decision-making process could be important, but the economic evaluation is only one of several elements that can have an influence on the introduction of a specific vaccine policy in a given country, including issues such as medical, epidemiological, organizational and ethical aspects. There is sufficient evidence that, after the cases of HPV,Citation121-123 influenza and pneumococcal vaccinations,Citation124-125 there is the need for a full health technology assessment (HTA) Citation126 also for HBV vaccination. As suggested by NICE international,Citation127 HTA should always be part of the priority-setting process, and this is particularly true in the field of vaccinations, in order to offer efficient and equitable allocation of health care in the field of prevention.
Materials and methods
Identification of relevant studies
A scientific literature review was conducted considering the period 2000–2013 and using two electronic medical journal databases: Scopus and PubMed engines for published studies on economic evaluations of HBV vaccination.
The keywords used were the following:
“HBV,” “vaccination,” “cost-effectiveness,” “cost utility,” “cost-effectiveness,” “cost benefit” and “economic evaluation.”
Search criteria and the flow-chart of the results are summarized in Figure 1.
No date and language restrictions were applied for the selection of the papers published. Further, all studies focused on the economic evaluation of HBV vaccination were selected, without any limitation of population and country.
The review process, including search and selection (identification, screening, eligibility of included studies) was performed according to the PRISMA criteria Citation128 (Fig. 1).
In the selection process, abstracts were initially read to identify potentially eligible full text papers which were then retrieved and assessed in order to decide on the final inclusion.
Articles were examined and were excluded if:
the research was based on considering costs only;
studies were not pertaining to HBV vaccination;
the full text was not available.
If the electronic databases outcomes overlapped, all duplicate articles were eliminated.
The weighted Drummond's checklist Citation129 was used to assess the quality of the economic evaluations.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
References
- Venook AP, Papandreou C, Furuse J, de Guevara LL. The incidence and epidemiology of hepatocellular carcinoma: a global and regional perspective. Oncologist 2010; 15 (Suppl 4):5-13; PMID:21115576; http://dx.doi.org/10.1634/theoncologist.2010-S4-05
- WHO. Revised WHO position paper on hepatitis B vaccine, Oct 2009 – the abridged version - (draft 22 Sept 2009). Available from: http://www.who.int/immunization/HepB_position_paper_oct09_summary.pdf
- Drummond MF. Principles of economic appraisal in health care Oxford University Press, Oxford, 1980
- Lahaye D, Strauss P, Baleux C, van Ganse W. Cost-benefit analysis of hepatitis-B vaccination. Lancet 1987; 2(8556):441-3; PMID:2887738; http://dx.doi.org/10.1016/S0140-6736(87)90971-8
- Liguori G, Gallé F, Marinelli P. Epidemiology of hepatitis C virus infection in the world, Europe, Italy and Campania: an overview. Ital J Public Health 2004; 1(1–2):40-6
- WHO. Hepatitis B (Fact sheet N°204). Available from: http://www.who.int/mediacentre/factsheets/fs204/en/ (updated July 2013)
- Di Giuseppe G, Nobile CG, Marinelli P, Angelillo IF. A survey of knowledge, attitudes, and behavior of Italian dentists toward immunization. Vaccine 2007; 25(9):1669-75; PMID:17129642; http://dx.doi.org/10.1016/j.vaccine.2006.10.056
- Piazza M. Experimental Viral Hepatitis. Charles C. Thomas Pubbl. Illinois, 1969
- Piazza M, Guadagnino V, Picciotto L, Borgia G, Nappa S. Contamination by hepatitis B surface antigen in dental surgeries. Br Med J (Clin Res Ed). 1987 Aug 22; 295(6596):473-4; PMID:3117175
- Piazza M. Universal hepatitis B vaccination. Lancet Infect Dis 2008 Feb; 8(2):88-9; PMID:18222158; http://dx.doi.org/10.1016/S1473-3099(08)70005-7
- Beutels P. Economic evaluations of hepatitis B immunization: a global review of recent studies (1994–2000). Health Econ 2001; 10(8):751-74; PMID:11747055; http://dx.doi.org/10.1002/hec.625
- Tu HA, Woerdenbag HJ, Kane S, Riewpaiboon A, van Hulst M, Postma MJ. Economic evaluations of hepatitis B vaccination for developing countries. Expert Rev Vaccines 2009; 8(7):907-20; PMID:19538116; http://dx.doi.org/10.1586/erv.09.53
- Zhang W, Guo Z, Zhang L, Liu Z, Li J, Ji Z, Xu R, Zhao N, Li F, Chen X, et al. Maternal immunization promotes the immune response of neonates towards hepatitis B vaccine. J Viral Hepat 2013 Dec; 20(12):875-81; PMID:24304457; http://dx.doi.org/10.1111/jvh.12103
- Park NH, Chung YH, Lee HS. Impacts of vaccination on hepatitis B viral infections in Korea over a 25-year period. Intervirology 2010; 53(1):20-8; PMID:20068337; http://dx.doi.org/10.1159/000252780
- Luo Z, Li L, Ruan B. Impact of the implementation of a vaccination strategy on hepatitis B virus infections in China over a 20-year period. Int J Infect Dis 2012 Feb; 16(2):e82-8; PMID:22178658; http://dx.doi.org/10.1016/j.ijid.2011.10.009
- Mao B, Patel MK, Hennessey K, Duncan RJ, Wannemuehler K, Soeung SC. Prevalence of chronic hepatitis B virus infection after implementation of a hepatitis B vaccination program among children in three provinces in Cambodia. Vaccine 2013Sep 13; 31(40):4459-64; PMID:23684825; http://dx.doi.org/10.1016/j.vaccine.2013.05.009
- Nguyen TH, Vu MH, Nguyen VC, Nguyen LH, Toda K, Nguyen TN, Dao S, Wannemuehler KA, Hennessey KA. A reduction in chronic hepatitis B virus infection prevalence among children in Vietnam demonstrates the importance of vaccination. Vaccine 2013; 32(2):217-22
- WHO. Hepatitis B (Fact sheet N°204). Available from: http://www.who.int/mediacentre/factsheets/fs204/en/ (updated July 2013)
- CDC. Global Routine Vaccination Coverage, 2011. Morbidity and Mortality Weekly Report (MMWR) 2012; 61(43):883-885
- Bloom BS, Hillman AL, Fendrick AM, Schwartz JS. A reappraisal of hepatitis B virus vaccination strategies using cost-effectiveness analysis. Ann Intern Med 1993; 118(4):298-306; PMID:8420448; http://dx.doi.org/10.7326/0003-4819-118-4-199302150-00009
- Holliday SM, Faulds D. Hepatitis B vaccine: a pharmacoeconomic evaluation of its use in the prevention of hepatitis B virus infection. Pharmacoeconomics 1994; 5(2):141-71; PMID:10146907; http://dx.doi.org/10.2165/00019053-199405020-00008
- Antoñanzas F, Garuz R, Rovira J, Antón F, Trinxet C, Navas E, Salleras L. Cost-effectiveness analysis of hepatitis B vaccination strategies in Catalonia, Spain. Pharmacoeconomics 1995; 7(5):428-43; http://dx.doi.org/10.2165/00019053-199507050-00007
- Margolis HS, Coleman PJ, Brown RE, Mast EE, Sheingold SH, Arevalo JA. Prevention of hepatitis B virus transmission by immunization. An economic analysis of current recommendations. JAMA 1995; 274:1202-9; http://dx.doi.org/10.1001/jama.1995.03530150025029
- Van Damme P, Tormans G, Beutels P, Van Doorslaer E. Hepatitis B prevention in Europe: a preliminary economic evaluation. Vaccine 1995; 13(S1):54-7; PMID:7762278; http://dx.doi.org/10.1016/0264-410X(95)80053-G
- Hicks RA, Cullen JW, Jackson MA, Burry VF. Hepatitis B virus vaccine. Cost-benefit analysis of its use in a children's hospital. Clin Pediatr (Phila) 1989; 28(8):359-65; PMID:2527103; http://dx.doi.org/10.1177/000992288902800805
- Edmunds W, Dejene A, Mekonnen Y, Haile M, Alemnu W, Nokes D. The cost of integrating hepatitis B virus vaccine into national immunization programmes: a case study from Addis Ababa. Health Policy Plan 2000; 15(4):408-16; PMID:11124244; http://dx.doi.org/10.1093/heapol/15.4.408
- Fernández Barboza R, Rivero D, Echeverría B, Machado IV. Cost-benefits of vaccination against hepatitis B in hospital personnel in Venezuela. Bol Oficina Sanit Panam 1991; 111(1):16-23
- Hatziandreu EJ, Hatzakis A, Hatziyannis S, Kane MA, Weinstein MC. Cost-effectiveness of hepatitis-B vaccine in Greece. A country of intermediate HBV endemicity. Int J Technol Assess Health Care 1991; 7(3):256-62; PMID:1834601; http://dx.doi.org/10.1017/S026646230000564X
- Pennie RA, O'Connor AM, Dulberg CS, Bottiglia A, Manga P, Kang CY. Low-cost hepatitis B vaccine improves uptake among self-paying health-care students. J Med Virol 1992; 37(1):48-53; PMID:1535653; http://dx.doi.org/10.1002/jmv.1890370108
- La Torre G, Saulle R, Unim B. Hepatitis B immunization in health care workers: needs and opportunities. Hepatitis Monthly 2011; 11(8):664-5; PMID:22140393; http://dx.doi.org/10.5812/kowsar.1735143X.1504
- Fabrizi F, Di Filippo S, Marcelli D, Guarnori I, Raffaele L, Crepaldi M, Erba G, Locatelli F. Recombinant hepatitis B vaccine use in chronic hemodialysis patients. Long-term evaluation and cost-effectiveness analysis. Nephron 1996; 72(4):536-43; PMID:8730417; http://dx.doi.org/10.1159/000188935
- La Torre G, De Vito E, Langiano E, Petta P, Colarossi G, Cipriani L, Tucciarone M, Ricciardi G. Epidemiology of hepatitis C virus antibodies in blood donors from the province of Latina, Italy. Eur J Epidemiol 2003; 18(7):691-4; PMID:12952144; http://dx.doi.org/10.1023/A:1024817417635
- Da Villa G, Sepe G. Immunization programme against hepatitis B virus infection in Italy: cost-effectiveness. Vaccine 1999; 17:1734-8; PMID:10194831; http://dx.doi.org/10.1016/S0264-410X(98)00414-9
- La Torre G, Nicolotti N, de Waure C, Chiaradia G, Specchia ML, Mannocci A, Ricciardi W. An assessment of the effect of hepatitis B vaccine in decreasing the amount of hepatitis B disease in Italy. Virology J 2008; 5:84; http://dx.doi.org/10.1186/1743-422X-5-84
- Wiewióra-Pilecka D. Cost-benefit analysis of the Polish hepatitis B prevention Programme. Vaccine 2000; 18:S52-4; http://dx.doi.org/10.1016/S0264-410X(99)00465-X
- Harris A, Yong K, Kermode M. An economic evaluation of universal infant vaccination against hepatitis B virus using a combination vaccine (Hib-HepB): a decision analytic approach to cost effectiveness. Aust N Z J Public Health 2001; 25(3):222-9; PMID:11494989; http://dx.doi.org/10.1111/j.1467-842X.2001.tb00566.x
- Yang BM, Paik SW, Hahn OS, Yi DH, Choi MS, Payne S. Economic evaluation of the societal costs of hepatitis B in South Korea. J Gastroenterol Hepatol 2001; 16:301-8; PMID:11339422; http://dx.doi.org/10.1046/j.1440-1746.2001.02443.x
- Saab S, Weston SR, Ly D, Brezina M, Yee HF, Jr, Han SH, Gitnick G. Comparison of the cost and effectiveness of two strategies for maintaining hepatitis B immunity in hemodialysis patients. Vaccine 2002; 20(25–26):3230-5; PMID:12163275; http://dx.doi.org/10.1016/S0264-410X(02)00249-9
- Adibi P, Rezailashkajani M, Roshandel D, Behrouz N, Ansari S, Somi MH, Shahraz S, Zali MR. An economic analysis of premarriage prevention of hepatitis B transmission in Iran. BMC Infect Dis 2004 Sep 4; 4:31; PMID:15347430; http://dx.doi.org/10.1186/1471-2334-4-31
- Vimolket T, Poovorawan Y. An economic evaluation of universal infant Vaccination strategies against Hepatitis B in Thailand: an analytic decision approach to Cost-effectiveness. Southeast Asian J Trop Med Public health 2005; 36(3):693-9; PMID:16124440
- Kim SY, Billah K, Lieu TA, Weinstein MC. Cost effectiveness of hepatitis B vaccination at HIV counseling and testing sites. Am J Prev Med 2006; 30(6):498-506; PMID:16704944; http://dx.doi.org/10.1016/j.amepre.2006.01.017
- Hutton DW, Tan D, So SK, Brandeau ML. Cost-effectiveness of screening and vaccinating Asian and Pacific Islander adults for hepatitis B. Ann Intern Med 2007; 147(7):460-9; PMID:17909207; http://dx.doi.org/10.7326/0003-4819-147-7-200710020-00004
- Jakiche R, Borrego ME, Raisch DW, Gupchup GV, Pai MA, Jakiche A. The cost-effectiveness of two strategies for vaccinating US veterans with hepatitis C virus infection against hepatitis A and hepatitis B viruses. Am J Med Sci 2007; 333(1):26-34; PMID:17220691; http://dx.doi.org/10.1097/00000441-200701000-00004
- Tilson L, Thornton L, O'Flanagan D, Johnson H, Barry M. Cost effectiveness of hepatitis B vaccination strategies in Ireland: an economic evaluation. Eur J Public Health 2007; 18(3):275-82; PMID:18160389; http://dx.doi.org/10.1093/eurpub/ckm123
- Hu Y, Grau LE, Scott G, Seal KH, Marshall PA, Singer M, Heimer R. Economic evaluation of delivering hepatitis B vaccine to injection drug users. Am J Prev Med 2008; 35(1):25-32; PMID:18541174; http://dx.doi.org/10.1016/j.amepre.2008.03.028
- Miriti MK, Billah K, Weinbaum C, Subiadur J, Zimmerman R, Murray P, Gunn R, Buffington J. Economic benefits of hepatitis B vaccination at sexually transmitted disease clinics in the US. Public Health Rep 2008; 123(4):504-13; PMID:18763413
- Hung HF, Chen THH. Probabilistic cost-effectiveness analysis of the long-term effect of universal hepatitis B vaccination: An experience from Taiwan with high hepatitis B virus infection and Hepatitis B e Antigen positive prevalence. Vaccine 2009; 27:6770-6; PMID:19735755; http://dx.doi.org/10.1016/j.vaccine.2009.08.082
- Fischinger JM, Stephan B, Wasserscheid K, Eichler H, Gärtner BC. A cost-benefit analysis of blood donor vaccination as an alternative to additional DNA testing for reducing transfusion transmission of hepatitis B virus. Vaccine 2010; 28(49):7797-802; PMID:20875488; http://dx.doi.org/10.1016/j.vaccine.2010.09.037
- Siddiqui MR, Gay N, Edmunds WJ, Ramsay M. Economic evaluation of infant and adolescent hepatitis B vaccination in the UK. Vaccine 2011; 29(3):466-75; PMID:21073988; http://dx.doi.org/10.1016/j.vaccine.2010.10.075
- Klingler C, Thoumi AI, Mrithinjayam VS. Cost-effectiveness analysis of an additional birth dose of Hepatitis B vaccine to prevent perinatal transmission in a medical setting in Mozambique. Vaccine 2012; 31(1):252-9; PMID:22902676; http://dx.doi.org/10.1016/j.vaccine.2012.08.007
- Savova A, Petrova G, Gotseva A, Kurcatova A, Koguharova M. Economic analysis 20 years after the introduction of universal HBV immunisation in Bulgaria. Biotechnol Biotechnol Eq 2012; 26(1):2811-6; http://dx.doi.org/10.5504/BBEQ.2011.0141
- Tu HAT, de Vries R, Woerdenbag HJ, Li SC, Le HH, van Hulst M, Postma MJ. Cost-Effectiveness Analysis of Hepatitis B Immunization in Vietnam: Application of Cost-Effectiveness Affordability Curves in Health Care Decision Making. Value in Health Regional Issues 2012; 1:7-14; http://dx.doi.org/10.1016/j.vhri.2012.03.007
- Chen SC, Toy M, Yeh JM, Wang JD, Resch S. Cost-effectiveness of augmenting universal hepatitis B vaccination with immunoglobin treatment. Pediatrics 2013; 131(4):e1135-43; PMID:23530168; http://dx.doi.org/10.1542/peds.2012-1262
- Hoerger TJ, Schillie S, Wittenborn JS, Bradley CL, Zhou F, Byrd K, Murphy TV. Cost-effectiveness of hepatitis B vaccination in adults with diagnosed diabetes. Diabetes Care 2013; 36(1):63-9; PMID:22933435; http://dx.doi.org/10.2337/dc12-0759
- Kuan RK, Janssen R, Heyward W, Bennett S, Nordyke R. Cost-effectiveness of hepatitis B vaccination using HEPLISAV™ in selected adult populations compared to Engerix-B→ vaccine. Vaccine 2013; 31(37):4024-32; PMID:23707166; http://dx.doi.org/10.1016/j.vaccine.2013.05.014
- Lu SQ, McGhee SM, Xie X, Cheng J, Fielding R. Economic evaluation of universal newborn hepatitis B vaccination in China. Vaccine 2013; 31(14):1864-9; PMID:23384752; http://dx.doi.org/10.1016/j.vaccine.2013.01.020
- La Torre G, Vezzo D, Arcese R, Bongiovanni C, Capelli G, Ricciardi G. Occurrence and correlates of HIV and Hepatitis B/C virus infections among prisoners of Southern Lazio, Italy. Ital J Public Health 2004; 1:33-9
- SeyedAlinaghi SA, Kheirandish P, Karami N, Salem S, Shirzad H, Jahani MR, SeyedAhmadian MR, Valiollahi P, Hosseini M, Mohraz M, McFarland W. High prevalence of chronic hepatitis B infection among injection drug users in Iran: the need to increase vaccination of adults at risk. Acta Med Iran 2010 Jan–Feb; 48(1):58-60; PMID:21137671
- Hwang LY, Grimes CZ, Tran TQ, Clark A, Xia R, Lai D, Troisi C, Williams M. Accelerated hepatitis B vaccination schedule among drug users: a randomized controlled trial. J Infect Dis 2010 Nov 15; 202(10):1500-9; PMID:20936979; http://dx.doi.org/10.1086/656776
- Chaiklang K, Wipasa J, Chaiwarith R, Praparattanapan J, Supparatpinyo K. Comparison of Immunogenicity and Safety of Four Doses and Four Double Doses vs. Standard Doses of Hepatitis B Vaccination in HIV-Infected Adults: A Randomized, Controlled Trial. PLoS One 2013 Nov 12; 8(11):e80409; PMID:24265819; http://dx.doi.org/10.1371/journal.pone.0080409
- Centers for Disease Control and Prevention (CDC). Use of hepatitis B vaccination for adults with diabetes mellitus: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep 2011 Dec 23; 60(50):1709-11; PMID:22189894
- Simani OE, Leroux-Roels G, François G, Burnett RJ, Meheus A, Mphahlele MJ. Reduced detection and levels of protective antibodies to hepatitis B vaccine in under 2-year-old HIV positive South African children at a paediatric outpatient clinic. Vaccine 2009 Jan 1; 27(1):146-51; PMID:18940220; http://dx.doi.org/10.1016/j.vaccine.2008.10.004
- Hodgson A, Forgor AA, Chandramohan D, Reed Z, Binka F, Bevilacqua C, Boutriau D, Greenwood B. A phase II, randomized study on an investigational DTPw-HBV/Hib-MenAC conjugate vaccine administered to infants in Northern Ghana. PLoS One 2008 May 14; 3(5):e2159; PMID:18478093; http://dx.doi.org/10.1371/journal.pone.0002159
- Montesano R. Hepatitis B immunization and hepatocellular carcinoma: The Gambia Hepatitis Intervention Study. J Med Virol 2002 Jul; 67(3):444-6; PMID:12116042; http://dx.doi.org/10.1002/jmv.10093
- Whittle HC, Maine N, Pilkington J, Mendy M, Fortuin M, Bunn J, Allison L, Howard C, Hall A. Long-term efficacy of continuing hepatitis B vaccination in infancy in two Gambian villages. Lancet 1995 Apr 29; 345(8957):1089-92; PMID:7715343; http://dx.doi.org/10.1016/S0140-6736(95)90822-6
- Excler JL, Yvonnet B, Gaye Y, Monnereau A, Mangin JL, Schlumberger M, Mireux F, Gaye AB, Sarr LC. Inclusion of hepatitis B vaccination in the Expanded Program of Immunization: feasibility study in the medical region of Kolda (Senegal). Sante 1995 Jan–Feb; 5(1):37-42; PMID:7894828
- Coursaget P, Deciron F, Tortey E, Barin F, Chiron JP, Yvonnet B, Diouf C, Denis F, Diop-Mar I, Correa P, et al. Immune response to hepatitis B vaccine in infants and newborns: control trial in an endemic area (Senegal). IARC Sci Publ 1984; (63):319-35; PMID:6242150
- Espinoza F, Tregnaghi M, Gentile A, Abarca K, Casellas J, Collard A, Lefevre I, Jacquet JM. Primary and booster vaccination in Latin American children with a DTPw-HBV/Hib combination: a randomized controlled trial. BMC Infect Dis 2010 Oct 15; 10:297; PMID:20950456; http://dx.doi.org/10.1186/1471-2334-10-297
- Gunson RN, Shouval D, Roggendorf M, Zaaijer H, Nicholas H, Holzmann H, de Schryver A, Reynders D, Connell J, Gerlich WH, et al. European Consensus Group. Hepatitis B virus (HBV) and hepatitis C virus (HCV) infections in health care workers (HCWs): guidelines for prevention of transmission of HBV and HCV from HCW to patients. J Clin Virol 2003 Aug; 27(3):213-30; PMID:12878084; http://dx.doi.org/10.1016/S1386-6532(03)00087-8
- Roman∫ L, Paladini S, Van Damme P, Zanetti AR. The worldwide impact of vaccination on the control and protection of viral hepatitis B. Dig Liver Dis 2011; 43 Suppl 1:S2-7; http://dx.doi.org/10.1016/S1590-8658(10)60685-8
- Teshale EH, Hepatitis B. In Centers For Disease Control and Prevention. CDC Health InFormation For International Travel 2012, OxfordUniversity Press, NewYork, NY, USA, 2012, available at http://wwwnc.cdc.gov/travel/yellowbook/2012/chapter-3-infectious-diseases-related-to-travel/hepatitis-b.htm
- Garnett GP. The theoretical impact and cost-effectiveness of vaccines that protect against sexually transmitted infections and disease. Vaccine 2014 Mar 20; 32(14):1536-42; PMID:24606635; http://dx.doi.org/10.1016/j.vaccine.2013.11.007
- Ieluzzi D, Covolo L, Donato F, Fattovich G. Progression to cirrhosis, hepatocellular carcinoma and liver-related mortality in chronic hepatitis B patients in Italy. Dig Liver Dis 2014; 46(5):427-32; PMID:24548819
- International Agency for Research on Cancer. Biological agents. Volume 100B. A review of human carcinogens. In IARC Monographs on the Evaluation of Carcinogenic Risks to Humans, pp. 93-133, World Health Organization, Geneva, Switzerland, 2012, available at http://monographs.iarc.fr/ENG/Monographs/vol100B/index.php
- World Health Organization. “Viral hepatitis. Report by the secretariat,” in Proceedings of the 63rd World Health Assembly March 2010
- Hatzakis A, Wait S, Bruix J, Buti M, Carballo M, Cavaleri M, Colombo M, Delarocque-Astagneau E, Dusheiko G, Esmat G, et al. The state of hepatitis B and C in Europe: report from the hepatitis B and C summit conference. J Viral Hepat 2011; 18 Suppl 1:1-16; PMID:21824223; http://dx.doi.org/10.1111/j.1365-2893.2011.01499.x
- Franka E, El-Zoka AH, Hussein AH, Elbakosh MM, Arafa AK, Ghenghesh KS. Hepatitis B virus and hepatitis C virus in medical waste handlers in Tripoli, Libya. J Hosp Infect 2009; 72(3):258-61; PMID:19443080; http://dx.doi.org/10.1016/j.jhin.2009.03.019
- Squeri R, La Fauci V, Sindoni L, Cannav∫ G, Ventura Spagnolo E. Study on hepatitis B and C serologic status among municipal solid waste workers in Messina (Italy). J Prev Med Hyg 2006 Sep; 47(3):110-3; PMID:17217188
- Dounias G1, Kypraiou E, Rachiotis G, Tsovili E, Kostopoulos S. Prevalence of hepatitis B virus markers in municipal solid waste workers in Keratsini (Greece). Occup Med (Lond) 2005 Jan; 55(1):60-3; PMID:15699092; http://dx.doi.org/10.1093/occmed/kqi007
- Hatzakis A, Van Damme P, Alcorn K, Gore C, Benazzouz M, Berkane S, Buti M, Carballo M, Cortes Martins H, Deuffic-Burban S, et al. The state of hepatitis B and C in the Mediterranean and Balkan countries: report from a summit conference. J Viral Hepat 2013; 20 Suppl 2:1-20; PMID:23827008; http://dx.doi.org/10.1111/jvh.12120
- Marinho TA, Lopes CL, Teles SA, Matos MA, Matos MA, Kozlowski AG, Oliveira MP, Silva AM, Martins RM. Epidemiology of hepatitis B virus infection among recyclable waste collectors in central Brazil. Rev Soc Bras Med Trop 2014; 47(1):18-23; PMID:24603732; http://dx.doi.org/10.1590/0037-8682-0177-2013
- Rachiotis G, Papagiannis D, Markas D, Thanasias E, Dounias G, Hadjichristodoulou C. Hepatitis B virus infection and waste collection: prevalence, risk factors, and infection pathway. Am J Ind Med 2012; 55(7):650-5; PMID:22544469; http://dx.doi.org/10.1002/ajim.22057
- Corrao CR, Del Cimmuto A, Marzuillo C, Paparo E, La Torre G. Association between waste management and HBV among solid municipal waste workers: a systematic review and meta-analysis of observational studies. Scientific World J 2013 Oct 9; 2013:692083; http://dx.doi.org/10.1155/2013/692083
- Hâţu G, Brumboiu MI, Gorgan IN, Bocşan IS. Romanian blood donors screening: is it really necessary and/or mandatory? Rev Med Chir Soc Med Nat Iasi 2013; 117(3):741-6
- Scognamiglio P, Girardi E, Fusco M, Piselli P, Spena SR, Maione C, Pisanti FA, Serraino D. Collaborating Study Group. Lack of implementation of Hepatitis B Virus (HBV) vaccination policy in household contacts of HBV carriers in Italy. BMC Infect Dis 2009; 9:86; PMID:19500412; http://dx.doi.org/10.1186/1471-2334-9-86
- Pitasi MA, Bingham TA, Sey EK, Smith AJ, Teshale EH. Hepatitis B Virus (HBV) Infection, Immunity and Susceptibility Among Men Who Have Sex with Men (MSM), Los Angeles County, USA. AIDS Behav 2013; 18 Suppl 3:248-55
- Wang C, Wang Y, Huang X, Li X, Zhang T, Song M, Wu L, Du J, Lu X, Shao S, et al. Prevalence and factors associated with hepatitis B immunization and infection among men who have sex with men in Beijing, China. PLoS One 2012; 7(10):e48219; PMID:23133573; http://dx.doi.org/10.1371/journal.pone.0048219
- MacKellar DA, Valleroy LA, Secura GM, McFarland W, Shehan D, Ford W, LaLota M, Celentano DD, Koblin BA, Torian LV, et al. Young Men's Survey Study Group. Two decades after vaccine license: hepatitis B immunization and infection among young men who have sex with men. Am J Public Health 2001; 91(6):965-71; PMID:11392942; http://dx.doi.org/10.2105/AJPH.91.6.965
- Carobene M1, Bolcic F, Farías MS, Quarleri J, Avila MM. HIV, HBV, and HCV molecular epidemiology among trans (transvestites, transsexuals, and transgender) sex workers in Argentina. J Med Virol 2014; 86(1):64-70; PMID:24123155; http://dx.doi.org/10.1002/jmv.23805
- Johnston LG, Vaillant TC, Dolores Y, Vales HM. HIV, hepatitis B/C and syphilis prevalence and risk behaviors among gay, transsexuals and men who have sex with men, Dominican Republic. Int J STD AIDS 2013; 24(4):313-21; PMID:23970664; http://dx.doi.org/10.1177/0956462412472460
- Hahné S, van Houdt R, Koedijk F, van Ballegooijen M, Cremer J, Bruisten S, Coutinho R, Boot H. Selective hepatitis B virus vaccination has reduced hepatitis B virus transmission in the Netherlands. PLoS One 2013 Jul 29; 8(7):e67866; http://dx.doi.org/10.1371/journal.pone.0067866
- Taketa K, Ikeda S, Suganuma N, Phornphutkul K, Peerakome S, Sitvacharanum K, Jittiwutikarn J. Differential seroprevalences of hepatitis C virus, hepatitis B virus and human immunodeficiency virus among intravenous drug users, commercial sex workers and patients with sexually transmitted diseases in Chiang Mai, Thailand. Hepatol Res 2003; 27(1):6-12; PMID:12957200; http://dx.doi.org/10.1016/S1386-6346(03)00163-3
- Schuelter-Trevisol F, Custódio G, Silva AC, Oliveira MB, Wolfart A, Trevisol DJ. HIV, hepatitis B and C, and syphilis prevalence and coinfection among sex workers in Southern Brazil. Rev Soc Bras Med Trop 2013; 46(4):493-7; PMID:23681430; http://dx.doi.org/10.1590/0037-8682-1364-2013
- Praseeda SD, Anuradha D, Jayanthi SS. A Study on the HBV and the HCV Infections in Female Sex Workers and their Co-Infection with HIV. J Clin Diagn Res 2013; 7(2):234-7; PMID:23543505
- Kassak K, Mahfoud Z, Kreidieh K, Shamra S, Afifi R, Ramia S. Hepatitis B virus and hepatitis C virus infections among female sex workers and men who have sex with men in Lebanon: prevalence, risk behaviour and immune status. Sex Health 2011 Jun; 8(2):229-33; PMID:21592438; http://dx.doi.org/10.1071/SH10080
- World Health Organization. Guidance on Prevention of Viral Hepatitis B and C Among People Who Inject Drugs. Geneva, 2012 Jul. Available from: http://www.ncbi.nlm.nih.gov/books/NBK144127/
- Walsh N, Verster A, Rodolph M, Akl EA. WHO guidance on the prevention of viral hepatitis B and C among people who inject drugs. Int J Drug Policy 2014; S0955-3959(14):00012-7
- Arama V, Leblebicioglu H, Simon K, Zarski JP, Niederau C, Habersetzer F, Vermehren J, Bludzin W, Jinga M, Ulusoy S, et al. the AI; European Longitudinal Chronic Hepatitis B Study Group. Chronic hepatitis B monitoring and treatment patterns in five European countries with different access and reimbursement policies. Antivir Ther 2013; 19(3):245-57
- Owusu-Edusei K, Jr, Chesson HW, Gift TL, Tao G, Mahajan R, Ocfemia MC, Kent CK. The estimated direct medical cost of selected sexually transmitted infections in the United States, 2008. Sex Transm Dis 2013; 40(3):197-201; PMID:23403600; http://dx.doi.org/10.1097/OLQ.0b013e318285c6d2
- Marcellin P, Arama V, Leblebicioglu H, Zarski JP, Zeuzem S, Mauss S, Sieklucki J, Acalovschi M, Usluer G, Klauck I, et al. the AI; Longitudinal Study Group. Chronic hepatitis B treatment initiation and modification patterns in five European countries: a 2-year longitudinal, non-interventional study. Antivir Ther 2013; 19(3):235-43; PMID:23574686
- Lu J, Xu A, Wang J, Zhang L, Song L, Li R, Zhang S, Zhuang G, Lu M. Direct economic burden of hepatitis B virus related diseases: evidence from Shandong, China. BMC Health Serv Res 2013; 13:37; PMID:23368750; http://dx.doi.org/10.1186/1472-6963-13-37
- Colombo GL, Gaeta GB, Vigan∫ M, Di Matteo S. A cost-effectiveness analysis of different therapies in patients with chronic hepatitis B in Italy. Clinicoecon Outcomes Res 2011; 3:37-46; PMID:21935331; http://dx.doi.org/10.2147/CEOR.S16655
- Yang BM, Kim DJ, Byun KS, Kim HS, Park JW, Shin S. The societal burden of HBV-related disease: South Korea. Dig Dis Sci 2010; 55(3):784-93; PMID:19333757; http://dx.doi.org/10.1007/s10620-009-0786-4
- Yang BM, Kim CH, Kim JY. Cost of chronic hepatitis B infection in South Korea. J Clin Gastroenterol 2004; 38(10 Suppl 3):S153-7; PMID:15602164; http://dx.doi.org/10.1097/00004836-200411003-00007
- Ong SC, Lim SG, Li SC. How big is the financial burden of hepatitis B to society? A cost-of-illness study of hepatitis B infection in Singapore. J Viral Hepat 2009; 16(1):53-63; PMID:19192158; http://dx.doi.org/10.1111/j.1365-2893.2008.01042.x
- Hsieh CR, Kuo CW. Cost of chronic hepatitis B virus infection in Taiwan. J Clin Gastroenterol 2004; 38(10 Suppl 3):S148-52; PMID:15602163; http://dx.doi.org/10.1097/00004836-200411003-00006
- Lee TA, Veenstra DL, Iloeje UH, Sullivan SD. Cost of chronic hepatitis B infection in the United States. J Clin Gastroenterol 2004; 38(10 Suppl 3):S144-7; PMID:15602162; http://dx.doi.org/10.1097/00004836-200411003-00005
- Butler JR, Pianko S, Korda RJ, Nguyen S, Gow PJ, Roberts SK, Strasser SI, Sievert W. The direct cost of managing patients with chronic hepatitis B infection in Australia. J Clin Gastroenterol 2004; 38(10 Suppl 3):S187-92; PMID:15602169; http://dx.doi.org/10.1097/00004836-200411003-00012
- Arnold E, Yuan Y, Iloeje U, Cook G. Cost-effectiveness analysis of entecavir versus lamivudine in the first-line treatment of Australian patients with chronic hepatitis B. Appl Health Econ Health Policy 2008; 6(4):231-46; PMID:19382822; http://dx.doi.org/10.1007/BF03256136
- Gagnon YM, Levy AR, Iloeje UH, Briggs AH. Treatment costs in Canada of health conditions resulting from chronic hepatitis B infection. J Clin Gastroenterol 2004; 38(10 Suppl 3):S179-86; PMID:15602168; http://dx.doi.org/10.1097/00004836-200411003-00011
- Kalantari H, Davari M, Akbari M, Hejazi SM, Kalantari M, Zakerin S, Shahshahan Z. The estimation of direct medical costs of treating patients with chronic hepatitis B and C in Iran. Int J Prev Med 2012; 3(3):191-6; PMID:22448312
- Hu M, Chen W. Assessment of total economic burden of chronic hepatitis B (CHB)-related diseases in Beijing and Guangzhou, China. Value Health 2009; 12 Suppl 3:S89-92; PMID:20586991; http://dx.doi.org/10.1111/j.1524-4733.2009.00636.x
- Zhiqiang G, Zhaohui D, Qinhuan W, Dexian C, Yunyun F, Hongtao L, Iloeje UH. Cost of chronic hepatitis B infection in China. J Clin Gastroenterol 2004; 38(10 Suppl 3):S175-8; PMID:15602167; http://dx.doi.org/10.1097/00004836-200411003-00010
- Wiens A, Lenzi L, Venson R, Pedroso ML, Correr CJ, Pontarolo R. Economic evaluation of treatments for chronic hepatitis B. Braz J Infect Dis 2013; 17(4):418-26; PMID:23849851; http://dx.doi.org/10.1016/j.bjid.2012.12.005
- Park JY, Heo J, Lee TJ, Yim HJ, Yeon JE, Lim YS, Seo MJ, Ahn SH, Lee MS. A novel estimation of the relative economic value in terms of different chronic hepatitis B treatment options. PLoS One 2013; 8(3):e57900; PMID:23536775; http://dx.doi.org/10.1371/journal.pone.0057900
- Hulstaert F, Schwierz C, Nevens F, Thiry N, Gamil M, Colle I, Van de Sande S, Horsmans Y. Should chronic hepatitis B be treated as early as possible? Int J Technol Assess Health Care 2013; 29(1):35-41;; PMID:23298548; http://dx.doi.org/10.1017/S0266462312000736
- Robotin M, Patton Y, Kansil M, Penman A, George J. Cost of treating chronic hepatitis B: comparison of current treatment guidelines. World J Gastroenterol 2012; 18(42):6106-13;; PMID:23155339; http://dx.doi.org/10.3748/wjg.v18.i42.6106
- He J, Bowen JM, Xie F, Goeree R. Cost-effectiveness analysis of antiviral treatments for HBeAg-positive chronic hepatitis B in Canada. Value Health 2012; 15(6):894-906; PMID:22999140; http://dx.doi.org/10.1016/j.jval.2012.06.005
- Husic-Selimovic A, Vukobrat-Bijedic Z, Bevanda M, Mesihovic R, Zerem E, Ahmetagic S, Trbojevic S, Verhaz A, Kezic Z, Zildzic M, et al. Associations of gastroenterology and hepatology of Bosnia and Herzegovina. Diagnosis and treatment of chronic viral hepatitis B and C: doctrinary approach. Med Arh 2012; 66(3 Suppl 1):56-69; http://dx.doi.org/10.5455/medarh.2012.66.s56-s68
- Toy M, Onder FO, Idilman R, Kabacam G, Richardus JH, Bozdayi M, Akdogan M, Kuloglu Z, Kansu A, Schalm S, et al. The cost-effectiveness of treating chronic hepatitis B patients in a median endemic and middle income country. Eur J Health Econ 2012; 13(5):663-76; PMID:22815098; http://dx.doi.org/10.1007/s10198-012-0413-8
- La Torre G, de Waure C, Chiaradia G, Mannocci A, Capri S, Ricciardi W. The Health Technology Assessment of bivalent HPV vaccine Cervarix in Italy. Vaccine 2010; 28(19):3379-84; PMID:20197141; http://dx.doi.org/10.1016/j.vaccine.2010.02.080
- Ricciardi W, Dirodi B, Bonanni P, Capri S, Castiglia P, Gabutti G, Gasparini R, Giorgi Rossi P, Grilli G, La Torre G. Methodological aspects of clinical and economic impact of vaccine interventions and HTA. Focus on HPV vaccination. Ann Ig 2011; 23(5):419-34; PMID:22403995
- La Torre G, de Waure C, Chiaradia G, Mannocci A, Capri S, Bamfi F, Ricciardi W. Guidance for future HTA applications to vaccines: the HPV lesson. Hum Vaccin 2011; 7(9):900-4; PMID:21865880; http://dx.doi.org/10.4161/hv.7.9.16084
- Turner D, Wailoo A, Nicholson K, Cooper N, Sutton A, Abrams K. Systematic review and economic decision modelling for the prevention and treatment of influenza A and B. Health Technol Assess 2003; 7(35):iii-iv, xi-xiii, 1-170; http://dx.doi.org/10.3310/hta7350
- Sehatzadeh S. Influenza and pneumococcal vaccinations for patients with chronic obstructive pulmonary disease (COPD): an evidence-based review. Ont Health Technol Assess Ser 2012; 12(3):1-64
- La Torre G, de Waure C, Chiaradia G, Mannocci A, Specchia ML, Nicolotti N, Ricciardi W. The future of best investing in vaccines: the Health Technology Assessment approach. Vaccine 2008; 26(13):1609-10; PMID:18289744; http://dx.doi.org/10.1016/j.vaccine.2008.01.009
- Chalkidou K, Marten R, Cutler D, Culyer T, Smith R, Teerawattananon Y, Cluzeau F, Li R, Sullivan R, Huang Y, et al. Health technology assessment in universal health coverage. Lancet 2013; 382(9910):e48-9; PMID:24360390; http://dx.doi.org/10.1016/S0140-6736(13)62559-3
- Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, Clarke M, Devereaux PJ, Kleijnen J, Moher D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. Ital J Public Health 2009; 4:94-131
- La Torre G, Nicolotti N, De Waure C, Ricciardi W. Development of a weighted scale to assess the quality of cost-eectiveness studies and an application to the economic evaluations of tetravalent HPV vaccine. J Public Health (Springer) 2010; 19 (2):103-11; http://dx.doi.org/10.1007/s10389-010-0377-z