ABSTRACT
Hepatitis B virus (HBV) vaccination is recommended for all susceptible chronic pre-hemodialysis and hemodialysis patients. This study assessed the immunogenicity of HBV vaccines (adjuvanted and non-adjuvanted) in chronic kidney disease patients vaccinated at the Hospital Clinic of Barcelona (Spain) between January 2007 and July 2012. In addition, the costs for the health system were evaluated accor-ding to the proportion of vaccine responders after receiving either vaccine. Patients receiving 3 doses of hepatitis B adjuvanted vaccine were 3 times more likely to seroconvert than patients immunized with non-adjuvanted vaccines, OR 3.56 (95% CI 1.84–6.85). This resulted in fewer patients requiring a second course of HBV vaccination and fewer outpatient visits, saving more than €9,500 per 100 patients. The higher immunogenicity of the adjuvanted HBV vaccine would counterbalance the lower costs associated with the non-adjuvanted vaccine.
Introduction
Chronic kidney disease patients are at high risk for hepatitis B virus (HBV) infection due to increased exposure to blood products, shared hemodialysis equipment, frequent skin breaches, and immunodeficiency.Citation1,2 Despite preventive measures to protect these patients against HBV infection, outbreaks continue to be reported in dialysis units.Citation3,4
HBV vaccination is recommended for all susceptible chronic pre-hemodialysis and hemodialysis patients, with regular monitoring of antibody levels to ensure they remain above 10 IU/ml.Citation5 Conventional HBV vaccines are poorly immunogenic in patients with renal insufficiency,Citation1 with low response rates and suboptimal antibody titers, and require frequent boosters to maintain protection.Citation6 The efficacy of conventional vaccines in chronic kidney disease patients has been reported as 55.4% one month after the third dose.Citation7 To improve the immunological response to HBV vaccination, patients should be vaccinated as soon as possible in the course of the renal disease.Citation8
A Hepatitis B vaccine adjuvanted with AS04 (Fendrix®, GlaxoSmithKline) has been licensed for use in this population in Europe since 2005. In a comparative clinical study in 165 pre-hemodialysis and hemodialysis patients, protective levels of specific humoral antibodies (antibodies against the hepatitis B surface antigen (anti-HBs) titers ≥ 10 IU/ml) were observed in 74.4% of Fendrix recipients (N = 82) one month after the third dose, compared with 52.4% of patients in the control group who received a double dose of a commercially available HBV vaccine (N = 83).Citation9 The adjuvanted vaccine has a good safety profile, with clinically-acceptable reactions similar to those of non-adjuvanted HBV vaccines.Citation10
In the region of Catalonia, Spain, the adjuvanted vaccine was acquired for the last time in 2010, and only non-adjuvanted HBV vaccines (Engerix-B® 20 µg, GlaxoSmithKline and HBVAXPRO® 40µg, Sanofi-Pasteur) have been acquired in succeeding years (coinciding with the economic crisis) for chronic kidney disease patients.
The objectives of this study were to assess the immunogenicity of HBV vaccination with the adjuvanted and non-adjuvanted vaccines in chronic kidney disease patients and to evaluate the economic costs for the health system according to the immunogenicity achieved after receiving either vaccine.
Results
Characteristics of the study population
A total of 267 patients with chronic kidney disease were inclu-ded in the analysis, a mean of 49 per year. The mean age of participants included was 68.1 y (SD 12.76) and 62.9% were male. Twenty patients presented immunocompromised conditions, 13 had a cancer diagnosis, of which 6 were kidney-related cancers. No patient presented HIV infection. Thirty-three patients were already in hemodialysis at the beginning of the vaccination schedule, and 2 patients who were not in hemodialysis started it before the administration of the third dose of vaccine. Other demographic and clinical characteristics of subjects by type of HBV received are shown in .
Table 1. Immunogenic response after the third dose of hepatitis B vaccine was administered in patients with chronic kidney disease.
Factors associated with response to the hepatitis B vaccine
Our results show that 51.7% of patients presented an immunological response after 3 doses. Proportion of immunogenicity shows differences between adjuvanted and non-adjuvanted vaccines (see ). Patients receiving 3 doses of hepatitis B adjuvanted vaccine were 3 times more likely to seroconvert than patients immunized with non-adjuvanted vaccines, OR 3.56 (95% CI 1.84–6.85) (see ). Only 43.3% of patients aged ≥ 65 y presented levels of anti-HBs ≥ 10 IU/mL, and had a worse response than those aged < 65, ORa 0.35 (95% CI 0.21–0.60). There were no significant differences in the immune response between immunocompromised and non-immunocompromised or between patients on hemodialysis or not.
Table 2. Factors potentially associated with an immunogenic response after hepatitis B immunization in patients with chronic kidney disease.
Cost analysis
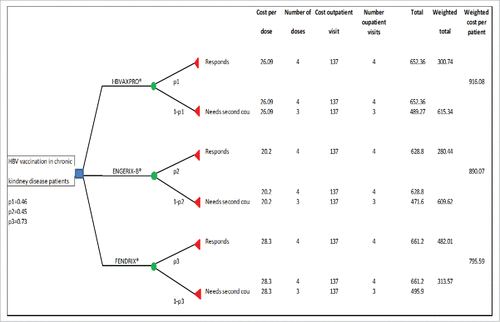
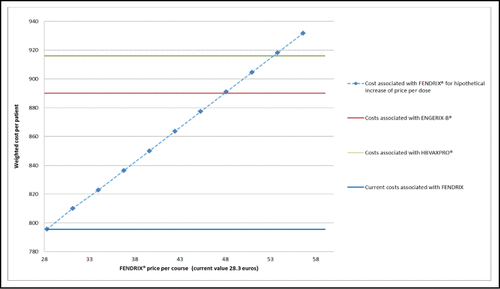
The lowest probability weighted cost per patient was the one associated with Fendrix® (€795.59), assessing the use of Fendrix® as the most convenient (). According to sensitivity analysis, EngerixX-B® or HBVAXPRO® would be more convenient than Fendrix®, should Fendrix® price per dose increase from the current value of 28.3 euros to about 48.11 or to 53 euros, respectively ().
Discussion
To our knowledge, this is one of the few studies to evaluate the economic costs associated with the type of HBV vaccine administered to chronic kidney disease patients. It is nested within the context of a change in the type of HBV vaccine acquired by the regional department of health, coinciding with the economic crisis in Spain. The results suggest that the decision to use non-adjuvanted, less immunogenic (and in this case, cheaper) HBV vaccines might also result in higher costs for the health system and for patients.
The differing seroconversion rates found in patients vaccinated with the adjuvant ASO4 vaccine and the non-adjuvanted HBV vaccines are consistent with previously reported studies.Citation11-13 The benefits in the immune response resulting from the use of the adjuvanted vaccine could also be augmented by including the longer persistence of anti-HBs antibody titers,Citation14 although this was not assessed in our study. As previously reported, older patients presented lower seroconversion rates, which were, however, higher with the adjuvanted vaccine.Citation15,16 Unlike other studies, we found no differences in the vaccine response according to the creatinine level or the hemodialysis status. This might be explained by the limited sample size of our study.
From an economic perspective, the differences translate into fewer patients requiring a second course of HBV vaccination and fewer outpatient visits, with a saving of more than €9,500 per 100 patients. Even with an increase of 70% in the adjuvanted vaccine price, the costs associated with this strategy were lower than those associated with non-adjuvanted vaccines.
Our study has some limitations. A higher sample size would have allowed us to obtain more robust conclusions and, perhaps, to determine other factors associated with the vaccine response. Secondly, there was no available information on previously-administered doses of vaccine with the hepatitis B component, although the age of the patients included suggests it is very unlikely that they had been vaccinated according to the Spanish routine immunization schedule. Thirdly, there was no patient follow-up, and therefore, the duration of antibody levels could not be assessed: this would potentially have added to the benefits of the adjuvanted vaccine. Fourthly, the cost per dose of vaccine and of outpatient visits in our hospital may differ between health care centers and vaccine prices may vary bet-ween countries and other time periods.
In conclusion, considering that patients not responding to the first 3 doses of the first HBV vaccine course will required at least 3 more doses, with the consequent outpatient visits, the accumulated costs of the non-adjuvanted and adjuvanted vaccines differ widely. The higher immunogenicity achieved with the adjuvanted HBV vaccine outweighs the lower costs associated with the non-adjuvanted vaccine.
Materials and methods
Study characteristics
We performed a retrospective study to assess the immunogenicity of adjuvanted and non-adjuvanted HBV vaccines in chronic kidney disease patients vaccinated at the Hospital Clinic of Barcelona (Spain) between January 2007 and July 2012.
Laboratory methods
Serological screening for HBsAg was made in all chronic kidney disease patients. The response to HBV vaccination was detected by measuring anti-HBs and was determined by enzyme immunoassay system using AUSAB AxSYM particles (ABBOTT®). Seroprotection was defined as anti-HBs titers ≥ 10 IU/mL. Patients not reaching this threshold were considered non-responders.
Hepatitis B immunization protocol
In non-immune patients (HBsAg negative), 4 doses of HBV vaccine were recommended (0, 1, 2, 6 months regimen). The vaccines used during the study period were Engerix-B® (GlaxoSmithKline (2 × 20 μg)), HBVAXPRO® (Sanofi-Pasteur (40 μg)) and Fendrix® (GlaxoSmithKline (20 μg)), which includes the AS04 adjuvant. Only patients who received 3 or more doses of HBV vaccine were included in the analysis.
Approximately one month after the third dose, a blood sample was obtained. For responders, a fourth dose was recommended 6 months after the first. For non-responders, the immunization schedule was reinitiated with 3 further doses followed by anti-HBs determination. If the patient again presented anti-HBs < 10 IU/mL, more doses were not recommended.
Data collection and analysis
Variables were limited to information recorded in the medical records, including sex, creatinine level at initiation of the hepatitis B vaccination schedule, reported immunocompromised conditions (cancer, chemotherapy treatment, HIV), hemodialysis status, type of vaccine administrated (adjuvanted or non-adjuvanted), dates of administration of HBV vaccines, among others. The post-vaccination anti-HBs level was the main endpoint. Vaccine safety-related variables were not collected, since a safety assessment was not an objective of this study.
To evaluate factors independently associated with seroconversion after hepatitis B vaccination, we performed univariate and multivariable logistic regression analyses. The statistical analysis was made using the SPSS® v18.0 statistical package. Statistical significance was established as a p-value < 0.05.
For the cost analysis we compared costs per patient associated with the 3 vaccination strategies by developing a decision tree (). Costs associated with each vaccination strategy were the price of each vaccine plus the cost of outpatient visits. (For vaccines we used official prices for 2011–2012 in Catalonia (Spain) of HBV vaccines. These were €28.30 per dose for Fendrix®, €26.09 per dose for HBVAXPRO® and €10.10 per dose for Engerix-B® (for which, 2 doses were administered at each visit and thus the total comparable cost was €20.20 per visit).Citation17 The cost of an outpatient medical visit at HCB was €137.Citation18 The 3 strategies differed for the probability of needing a second vaccination course. Such probability was given by the immunogenic response after the third doses of each hepatitis B vaccine.
One way sensitivity analysis was performed on Fendrix price per dose.
Ethical considerations
Patient records/information were anonymized prior to analysis. The study was approved by the HCB Clinical Research Ethics Committee (HCB/2015/0040).
Abbreviations
| anti-HBs | = | antibodies against the hepatitis B surface antigen |
| CI | = | confidence interval |
| HBsAg | = | hepatitis B surface antigen |
| HBV | = | hepatitis B virus |
| HCB | = | Hospital Clinic of Barcelona |
| IU/ml | = | international units/milliliter |
| OR | = | odds ratio |
| SD | = | standard deviation |
Disclosure of potential conflicts of interest
JMB has collaborated in educational activities supported by GlaxoSmithKline and Sanofi Pasteur MSD, Novartis and Pfizer, and has participated as an investigator in clinical trials sponsored by GlaxoSmithKline and Sanofi Pasteur MSD.
AV has collaborated in educational activities supported by Sanofi Pasteur MSD.
The remaining authors report no conflict of interest.
Acknowledgments
The authors wish to thank Valentín Calvente, Consolación Diez, and Joan Sánchez for their collaboration.
References
- García-Agudo R, Aoufi Rabih S, Araque Torres P, Dolores Fraga Fuentes M, Carlos Valenzuela Gámez J, Mancha Ramos J, María Tenías Burillo J. Efficacy of a hepatitis B vaccination schedule with two cycles of four double doses of conventional vaccine and four doses of adjuvanted vaccine in chronic kidney disease patients evaluated for renal transplantation. Transplant Proc 2012; 44:2532-4; PMID:23146445; http://dx.doi.org/10.1016/j.transproceed.2012.09.046
- Burdick R, Bragg-Gresham J, Woods J, Hedderwick S, Kurokawa K, Combe C, Saito A, LaBrecjque J, Port F, Young E. Patterns of hepatitis B prevalence and seroconversion in hemodialysis units from three continents : The DOPPS. Kidney Int 2003; 63:2222-9; PMID:12753311; http://dx.doi.org/10.1046/j.1523-1755.2003.00017.x
- Thompson ND, Perz JF, Moorman AC, Holmberg SD. Nonhospital health care-associated hepatitis B and C virus transmission: United States, 1998–2008. Ann Intern Med 2009; 150:33-9; PMID:19124818; http://dx.doi.org/10.7326/0003-4819-150-1-200901060-00007
- Finelli L, Miller JT, Tokars JI, Alter MJ, Arduino MJ. National surveillance of dialysis-associated diseases in the United States, 2002. Semin Dial 2005; 18:52-61; PMID:15663766; http://dx.doi.org/10.1111/j.1525-139X.2005.18108.x
- Advisory Committee on Immunization Practices (ACIP). December 2012 [Internet]. Guidel. Vaccinating. Kidney Dial. Patients Patients with Chronic Kidnewy Dis 2012; :1-12. Available from: http://www.cdc.gov/vaccines/pubs/downloads/dialysis-guide-2012.pdf
- Martin P, Friedman LS. Chronic viral hepatitis and the management of chronic renal failure. Kidney Int 1995; 47:1231-41; PMID:7637252; http://dx.doi.org/10.1038/ki.1995.177
- Agencia Española de Medicamentos y Productos Sanitarios (AEMPS). Ficha Técnica Engerix-B. [Internet]. 2012; Available from: http://www.aemps.gob.es/cima/fichasTecnicas.do?d-1338201-e=3&6578706f7274=1&met-odo=buscar&metodo=buscar&especial-idad=FENDRIX
- Grzegorzewska AE. Hepatitis B vaccination in chronic kidney disease: review of evidence in non-dialyzed patients. Hepat Mon 2012; 12:e7359; PMID:23326280; http://dx.doi.org/10.5812/hepatmon.7359
- European Medicines Agency (EMA). European Public Reports (EPARs) for authorized medical products for human use. Fendrix. [Internet]. Available from: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Summary_for_the_public/human/0005-50/WC500021703.pdf
- Beran J. Safety and immunogenicity of a new hepatitis B vaccine for the protection of patients with renal insufficiency including pre-haemodialysis and haemodialysis patients Safety and immunogenicity of a new hepatitis B vaccine for the protection of patients wit. Expert Opin Biol Ther 2008; 8:235-47; PMID:18194079; http://dx.doi.org/10.1517/14712598.8.2.235
- Tong NKC, Beran J, Kee SA, Miguel JL, Sánchez C, Bayas JM, Vilella A, de Juanes JR, Arrazola P, Calbo-Torrecillas F, et al. Immunogenicity and safety of an adjuvanted hepatitis B vaccine in pre-hemodialysis and hemodialysis patients. Kidney Int 2005; 68:2298-303; PMID:16221232; http://dx.doi.org/10.1111/j.1523-1755.2005.00689.x
- Kong NCT, Beran J, Kee SA, Miguel JL, Sánchez C, Bayas JM, Vilella A, Calbo-Torrecillas F, López de Novales E, Srinivasa K, et al. A new adjuvant improves the immune response to hepatitis B vaccine in hemodialysis patients. Kidney Int 2008; 73:856-62; PMID:18160963; http://dx.doi.org/10.1038/sj.ki.5002725
- Surquin M, Tielemans C, Nortier J, Jadoul M, Peeters P, Ryba M, Roznovsky L, Domán J, Barthelemy X, Crasta PD, et al. Anti-HBs antibody persistence following primary vaccination with an investigational AS02(v)-adjuvanted hepatitis B vaccine in patients with renal insufficiency. Hum Vaccin 2011; 7:913-8; PMID:21892006; http://dx.doi.org/10.4161/hv.7.9.16225
- Watson B, West DJ, Chilkatowsky A, Piercy S, Ioli VA. Persistence of immunologic memory for 13 years in recipients of a recombinant hepatitis B vaccine. Vaccine 2001; 19:3164-8; PMID:11312012; http://dx.doi.org/10.1016/S0264-410X(01)00019-6
- Melappioni M, Baldassari M, Baldini S, Radicioni R, Panichi N. Use of immunomodulators (thymopentine) in hepatitis B vaccine in elderly patients undergoing chronic hemodialysis. Nephron 1992; 61:358-9; PMID:1386910; http://dx.doi.org/10.1159/000186942
- Pichichero ME. Improving vaccine delivery using novel adjuvant systems. Hum Vaccin 2008; 4:262-70; PMID:18398303; http://dx.doi.org/10.4161/hv.4.4.5742
- Departament de Salut. Generalitat de Catalunya. Precios oficiales concursos Vacuna Hepatitis B. Barcelona: 2014.
- Hospital Clínic de Barcelona. Tarifario Hospital Clínic de Barcelona. Barcelona: 2012.