ABSTRACT
This article aims to explore the intellectual landscape of the study of monoclonal antibody (mAb), mainly to identify thematic trends, landmark articles and emerging trends involving mAb. This work is based on 4 sets of bibliographic records retrieved from the Web of Science. The final data set, consisting of 7,385 bibliographic records, was combined from the 4 individual data sets. This study explores the document co-citation clusters of 7,385 bibliographic records to identify the origin of mAb and the hot research specialty of this domain by applying CiteSpace software. We examined the mAb evolution from 4 perspectives: (1) Clusters of cited references regarding mAb; (2) Cited authors as contributors to mAb research; (3) Institutions participating in mAb research; and (4) Cited journals regarding mAb. The technical development, drug development and clinical applications of mAbs were analyzed. Through data analysis, we have identified the new directions for the exploration of mAbs, interactions between mAb technologies and diseases, and evolving global collaboration among institutions.
Introduction
Over the past 3 decades the development of monoclonal antibody (mAb) has made great progress. The first therapeutic mAb, Orthoclone OKT3, was commercialized in 1986, which is a murine mAb approved for prevention of kidney transplant rejection. Subsequently, chimeric mAbs were developed to overcome the drawbacks of murine mAb in the 1990s. Since 2002, humanized and fully human mAbs were successfully developed and approved for clinical applications.Citation1 In addition, the development of bispecifics and antibody drug conjugates (ADC) as additional forms of licensed antibodies has advanced significantly.Citation2 By the end of May 2016, 51 therapeutic mAb products have been approved in the US or Europe for the treatment of various diseases. Moreover, there were minibodies, nanobodies and other Ab forms as new R&D developments.Citation2 Until now, mAb has made a remarkable transformation from a scientific tool to a useful drug for human therapeutics.
With the technological development of mAbs, the mAb market has grown dramatically way. According to the statistics of Firestone, the global sales of mAbs was about US$98 billion in 2015, which is about 7 times that of the sales in 2005.Citation3 Approximately 43% of mAb sales were for the treatment of immune system diseases, while 35% of these sales were used for tumor therapy and 22% for anti-rejection.Citation3 The sales of mAbs accounted for over half of the total sales of all biopharmaceutical products.Citation3 As research and development investment on mAbs continues to grow, it is expected that mAb will lead the global biopharmaceutical market.Citation4
Based on the mechanism of action, therapeutic mAbs can be generally divided into 2 categories, e.g., those designed to modulate immune responses by directly target immune competent cells or molecules, and those designed to target cells or molecules not belonging to the classic immune system. Nevertheless, as a major product of immune cells and an important molecule to execute the effector function of immune cells, any given mAb inevitably has some immunoregulatory effect. In fact, the Fc region of mAbs binds to the receptors expressed by various immune cells, such as natural killer (NK) cells, monocytes, macrophages, and granulocytes, this can result in a modulation of immune responses.Citation2 Moreover, although not designed to target cells in the immune system, many mAbs still have the capacity to up- or down-regulate the activation of immune cells. Their immunoregulatory activities are known to contribute to their therapeutic effect.Citation5–8
The rapid growth of mAb literature presented challenges regarding how to identify emerging trends and new developments of mAb research. As the accumulated mAb literature is becoming extensive, a traditional expert review is not sufficient for providing a comprehensive and deep understanding of this topic. Moreover, the research and development of mAbs has become highly interdisciplinary, and thus it has become even more difficult to acquire a firm understanding of the entire mAb field. Some bibliometric surveys on mAbs have been conducted.Citation9–11 Through the use of statistical analysis, these studies have reported the main subjects and topics of mAb research by co-word analysis,Citation9 international research profiles about antibody drugsCitation11 and the different development stages.Citation10 However, a scientometric analysis is required to gain a deeper understanding of mAb research. In comparison with bibliometric research that focuses on the literature itself, scientometric analysis can quantitatively measure and analyze all aspects of the literature.Citation12 In particular, citation data analysis of scientometric research can facilitate the exploration of the field at issue by distinguishing various areas of research and its future directions.Citation13 Until now, very little scientometric research has been directed toward mAbs.
Thus, this article aims to explore the intellectual landscape of mAbs research through a scientometric analysis, mainly to identify thematic trends, landmark articles and emerging trends. In addition, the leading institutions of mAb research and development will also be studied.
In addition, based on the above analysis, we knew that not all mAbs belong to immunotherapeutic mAbs. In addition, we also found that a great preponderance and dominance of immunotherapeutic mAbs over simple-therapeutic mAbs exists in the field today. Therefore, it's difficult to separate immunotherapeutic mAbs from all mAbs, and thus we focus on the overall development of mAbs in this study.
This study is guided by a computational approach implemented using the CiteSpace software package. CiteSpace is a Java application for visualizing emerging trends and abrupt changes in the scientific literature.Citation14,15 This visual analytic system is readily accessible and specifically designed to meet the needs for generating a systematic review of a fast-moving and complex field.Citation16 One of the key features of CiteSpace is its ability to facilitate the detection and interpretation of emerging trends and transition patterns.Citation16 Therefore, it is expected that the application of CiteSpace can be used to identify and define the emerging trends and transition patterns of mAb research.
Results
The intellectual landscape
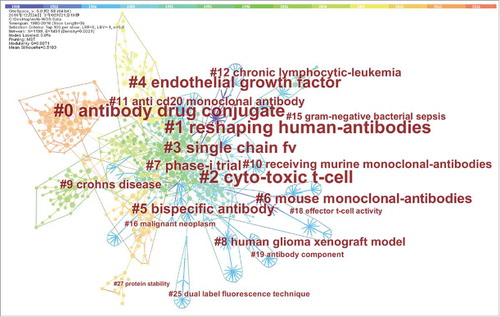
The intellectual landscape analysis was shown by a document co-citation network. This network was generated from the final data set. It contains 1,199 references from the top 100 most cited references per time slice between 1980 and 2016. As shown in Fig. 1, the node represents different cited references. The modularity Q 0.8871 and mean silhouette 0.5183 suggest a strong inter-cluster connection within the network and considerable partition of the network, which shows a significant network for intellectual landscape analysis.
The largest cluster in the visualization is #0 antibody drug conjugate, and followed by #1 reshaping human-antibodies and #2 cytotoxic t-cell. summarizes the top-cited terms derived from citing articles of the 10 largest clusters in the mAb domain.
Table 1. Top 10 largest clusters of panorama (time sorting).
shows the top 10 largest different clusters in the mAb domain. It accounted for 54.21% of the target references. The silhouette scores are all over 0.8, which correspond to a relatively reliable quality of these clusters. The publications years of articles covering mABs range from 1979 to 2010. Based on the development characteristics of the field, we can divide these clusters into 3 phases.
Table 2. References with the strongest citation bursts every year.
Table 3. The summary of the top 5 largest clusters.
The first phase (#8, #6, #1, #7, #2, #3): from the first mAb appearance to the fully human mAb application, mAb continue to be improved. The human glioma xenograft model was used to localize the production and characterization of mAbs.Citation17 Humanization is a remarkable progress of antibodies as therapeutic reagents. It's a technological breakthrough that has played a fundamental role in addressing the drawbacks of murine mAbs.Citation18 Since the first murine mAb was introduced to clinical practice until fully human mAbs were enabled, the drawbacks, including allergic reactions, anti-drug antibodies (ADAs), relatively short half-life in humans, poor recruiters of effector function, ADCC, and complement-dependent cytotoxicity (CDC), were improved stage by stage.Citation19-21 It is apparent that the humanization technology played a major role to increase the human proportion in mAbs.Citation22,23 Furthermore, with the advent of in vitro phage display technology and the transgenic mouse technology, the fully human mAbs were enabled.Citation24–28 This significantly reduced immunogenic potential and showed properties similar to those of human endogenous IgGs.Citation29 However, it also should be noted that the immunogenicity cannot be completely removed by any known technology as even fully human mAbs are immunogenic (anti-Id responses) in some of subjects following repeated administrations.
The second phase (#9, #4): the target expansion. Crohn's disease (CD) is a chronic inflammatory disorder of unknown etiology that can affect any portion of the gastrointestinal tract.Citation30 The proinflammatory cytokine of tumor necrosis factor α (TNF- α) plays an important role in the pathogenesis of CD.Citation31–33 Infliximab is a chimeric anti- TNF- α mAb that was approved by the US FDA in 1998, while adalimumab is a human anti- TNF- α mAb that was approved by the US in 2002. Both of them bind to TNF- α with high affinity, thereby neutralizing its biological activity.Citation34,35 Vascular endothelial growth factor (VEGF) is an important regulator of physiological angiogenesis during embryogenesis, skeletal growth and reproductive functions, the more implication is been used in pathological angiogenesis associated with tumors, intraocular neovascular disorders and other conditions.Citation36 There are 3 mAbs against VEGF had been approved by FDA. Bevacizumab, which is marketed with the brand name Avastin, was approved by the US FDA in 2004. It's the first mAb against VEGF and implicated in clinical activity against metastatic colorectal cancer.Citation37 Ranibizumab was approved for treating age-related macular degeneration in 2006. Ramucirumab was approved for treating non-small cell lung cancer.
The third phase (#5, #0): the innovation of mAb. Bispecific is a mAb-based therapeutic that interacts with 2 target antigens but not with mAb mixtures. Bispecifics have recently emerged as a novel and exciting way to target multiple antigens, or multiple epitopes within the same antigen.Citation38 The first bispecific catumaxomab (Removab) was approved in 2009 for treating malignant ascites. This bispecific simultaneously targets CD3 on T cells and epithelial cell adhesion molecule (EpCAM) on tumor cells to facilitate the killing of tumor cells.Citation39 The second bispecific blinatumomab (Blincyto) was approved in 2014 for the treatment of leukemia with targeting CD3 /CD19 T cells.Citation40 Antibody drug conjugate (ADC) combines a mAb with a highly potent cytotoxic chemical for the treatment of solid tumors. Thus far, only 3 ADCs have been approved for use in humans. The first ADC gemtuzumab ozogamicin (Mylotarg) was marketed in 2000 for the treatment of granulocytic leukemia, but withdrawn in 2010 as its efficacy did not differentiate from chemotherapy alone.Citation41 Brentuximab vedotin (Adcetris) was approved for the treatment of lymphoma in 2011 with targeting CD30. Ado-Trastuzumabemtansine (Kadcyla) was approved for the treatment of breast cancer in 2013 with targeting HER2.
Emerging trends and research front of mAb: Articles with citation burst
There are a total of 532 references that have citation bursts. We summarized the ones with the strongest burst in the group of articles that started to burst at the same year in . As shown in , it summarized the 28 high citation burst references. The contents include the cited paper, the strength of the citation burst, the time frame of the citation burst, the global citation on Web of Science and the summary. According to the research characteristics and chronological order, we can divide these 28 high citation burst references into 4 different categories
From 1982 to 1987, the initial development of mAbs. The emergence of hybridoma technology directly contributed to the development of mAb, and the in vitro production of murine mAbs from hybridomas was described in 1975.Citation42,43 At that stage, mAbs were mainly used as clinical therapeutics. Murine mAb was the first generation of mAbs. The application of murine mAb in human cancer provided a possible approach for cancer treatment in humans with immunotherapeutics. However, the drawback of ADCC was very significant from murine mAbs.Citation44 It seriously affects the development prospects of mAb.Citation20
From 1988 to 1993, mAb research was in a rapid development stage. Chimeric mAbs, humanized mAbs and human mAbs were developed to overcome the inherent immunogenicity and reduce effector function of murine mAbs in humans. With genetic engineering techniques, the drawbacks of murine mAbs have been satisfactorily resolved. Chimeric mAbs and humanized mAbs performed better than murine mAb in clinical trial efficacy of kinetics and immune response. Furthermore, human mAbs exhibited the best performance.
From 1994 to 2005, the application of mAbs in cancer diagnosis, detection and therapy have made important progress. Many new therapeutic areas have been developed. Meanwhile, the blockbuster drugs rituximab, infliximab and bevacizumab were approved. In addition, it demonstrated that the target CD20, TNFα, EGFR, VEGF and HER2 were suitable therapeutic targets.
From 2007 to 2014, new technologies and applications were developed for mAbs. The emergence of engineered antibody fragments and single-domain antibodies (nanobodies), bispecifics and ADCs were developed to improve the performance of mAbs. Particularly, bispecifics are expected to revolutionize mAb therapy with the progress of the immune cell re-targeting and synergistic efficacy through engagement of multiple targets.Citation45 The development of bispecific and ADC were significant. The development of ADCs are significant. Much research is focused on the ADC design, especially in terms of the optimization of the ratio of cytotoxin molecules per mAb. In addition, the prophylaxis or treatment of human or avian influenza infections and HIV were the new directions explored at this stage.
In general, we can summarize the development of mAbs as proceeding through 4 stages. In the first stage, researchers attempted to find new treatment approaches for serious diseases through clinical trials. The first generation mAbs were developed, but they exhibited serious drawbacks, which limited their clinical applications. In the second stage, a new generation of mAbs was developed to overcome these drawbacks. During the third stage, researchers mainly focused on targeting diseases. Researchers explored the effectiveness of these treatments on the different targets. During the fourth stage, researchers attempted to develop new technologies and applications for mAb.
Leading institutions
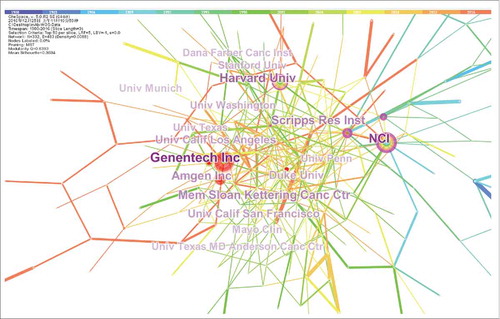
Figure 2 shows the top institutions on publishing number label. There are a total of 332 institutions in the institutional cooperation network. These include 176 universities (53.01%), 67 enterprises (20.18%), 66 research institutes (19.88%), and 23 hospitals (6.93%). As the thickness of a ring reflects the number of publications, we can determine the frequency of publications for each institution. As Fig. 2 shows, Genentech (Genentech Inc.) stands out with the largest circle, but the earliest research on mAbs was not performed at Genentech. A large number of publications were produced by researchers at Genentech in recent years, which were followed by the number of publications produced by researchers at NCI (National Cancer Institute) and Harvard University.
Actually, there are 62 clusters in the institutional cooperation network. summarizes the top 5 clusters. The composition of the largest cluster #0 includes 70.45% universities, 13.64% enterprises, 9.09% research institutes and 6.82% hospitals. Universities account for the main component, the proportion of enterprise and research institute are similar in these clusters. This implies that the collaboration among universities is characterized densely and the collaboration between universities and enterprises or research institutes is the key relationship.
Top cited journals
depicts the high specialty from the view of top cited journals. This includes the top 20 journals and all had a cited frequency of over 1,400. Based on the categories of the journals, it is apparent that immune system diseases, tumor therapy and anti-rejection are the central research themes involving mAbs.
Table 4. Top 20 cited journals.
Discussion and conclusion
In this study, we explored the structure and dynamics of citation networks and thematic trends involving mAbs. We examined the mAbs from multiple perspectives: (1) Clusters of cited references in mAbs; (2) Cited authors as contributors to mAbs; (3) Institutions of mAbs; and (4) Cited journals of mAbs.
Based on the analysis of 9,827 bibliographic records, we found that research on mAbs has mainly been focused on their technical development, drug development and clinical application. With the integration of hybridomas, genetic engineering techniques, phage display techniques, transgenic mouse strains, the emergence of engineered antibody fragments, and single-domain antibodies (nanobodies) in mAbs, it appears that therapeutic mAbs will become more accessible than ever before.
During the early stage, research was concentrated on human mAbs, with particular emphasis on their transformation from scientific tools to powerful human therapeutics. The Hybridomas technique is the basic and original technology for mAbs. However, the serious drawbacks of murine mAbs have severely limited their development. Genetic engineering techniques are used to graft the entire antigen-specific variable domain of a mouse Ab onto the constant domains of a human mAb.Citation72 This effectively addressed the problem of immunogenicity, but the ADAs of chimeric mAbs still cannot be ignored. Therefore, the grafting of murine hypervariable regions onto a human mAb framework was performed via genetic engineering techniques to further improve their effectiveness.Citation73 The application of a phage display technique and transgenic mouse strains technique enabled the use of fully human mAbs. The transformation from scientific tools to powerful human therapeutics achieved landmark success. The transformation process with related technologies and its practical effect assessment were the research front. These can be verified by the cited references clusters from 1982 to 1993.
At a later stage, researchers attempted to expand the therapeutic usage of mAbs and enhance their effectiveness. The prevention of kidney transplant rejection was the first application of mAbs, which was followed by the treatment of colorectal carcinoma, breast cancer, gastric cancer, lung cancer, Crohn's disease and other tumors. The treatment of diseases associated with immune disorders, tumor and transplant rejection were the mainly therapeutic area of mAbs. Moreover, bispecifics and ADCs are novel mAb-based therapeutics which are expected to revolutionize mAb therapy with the significant development in clinical. Bispecifics and ADCs are likely to expand the spectrum of disease areas, and will hopefully be able to address significant untreated diseases. In addition, the prophylaxis or treatment of human or avian influenza infections and HIV represented new directions of mAbs research. These can be verified by the cited references clusters from 1994 to 2014.
From the analysis of articles with citation burst, we also find the evidence of interactions between technology development and diseases treatment. At first, researchers chose the targets in the process of the use of mAbs for treating tumors. However, when researchers found a variety of targets, they attempted to explore what diseases can be treated with specific targets.
This is a scientometric analysis to explore the intellectual landscape of the study of monoclonal antibodies (mAbs), however, some research limitations are notable. Firstly, in this study, we only chose bibliographic records of Web of Science as analysis sample. We realized that PubMed has the most extensive coverage of scientific literature on biomedicine, but the references are not available. Therefore, we only retried Web of Science core database to obtain uniform references. In the future research, we will try to integrate scientific literature of PubMed database to the analysis, the co-occurrence keywords are a promising direction. Secondly, this study only focused on the bibliographic records, but did not involve cross analysis with the R&D projects of drug development. A future study could extend the current findings to incorporate a cross analysis of mAb research with the data from the discovery stage research to availability on the market.
In a summary, this study analyzed the structure and the evolution trend of the mAb field over time through an analysis of scientific publications and document co-citation clusters using the CiteSpace program. Our findings have revealed that mAb research and development has reached a mature and diversified stage. While researchers have more opportunities to utilize accumulated mAb science and technology to develop therapeutic mAbs, they need to further specify their research target and integrate interdisciplinary technologies to achieve breakthroughs in mAb research.
Method
Data collection
For data collection, we first used literature search strategies by considering mAb types. Now there are 4 types of mAbs, including murine mAbs (suffix: -omab), chimeric mouse-human antibodies (suffix: -ximab), humanized mAbs (suffix: -zumab), and human mAbs (suffix: -umab).Citation2,45 Moreover, bispecifics and ADCs are the emerging mAb-based therapeutics and have been an important component of mAbs.Citation2,45 Accordingly, we constructed 4 different search strategies. DA was developed to retrieve murine mAbs articles as the data set DA, while DB was developed to retrieve chimeric mouse-human antibodies as the data set DB. Meanwhile, DC was developed to retrieve humanized mAbs as the data set DC, DD was developed to retrieve human mAbs as the data set DD, DE was developed to retrieve bispecifics as the data set DE, and DF was developed to retrieve ADCs as the data set DF. All of the data sets were retrieved by a topic search (TS) on the ISI Web of Science Core Citation Database with a time span ranging from 1980/01/01 to 2016/04/09. summarized the search strategies.
Table 5. The search strategies used in the database.
As there were duplicate bibliographic records in the 4 individual data sets, we cleared the duplicates with the “remove duplicates” function of native CiteSpace. Finally, we combined all the 4 individual data sets together as the final data set DABCD for the scientometric analysis, which included 7,385 bibliographic records. summarizes the final data sets collected.
Table 6. A summary of the data sets collected.
Data analysis
This study used several scientometric and visual analytic methods with CiteSpace. The time span of the bibliographic record is approximately 37 y. We specify 3 y as the length of a single time slice. The Minimize Spinning Tree (MST) was chosen for network pruning.
This study mainly focused on a document co-citation network and an institutional cooperation network based on the scientific literature. The top 100 most cited references per time slice were selected to map the document co-citation network in a standard graph view. In the document co-citation network, the link indicates how frequently 2 articles are cited together by other articles in a data set, such as the data set DABCD. The interconnectivity nodes can be aggregated into clusters. The quality of the document co-citation network is measured by the modularity Q score from 0 to 1. A well-structured network would thus exhibit a high modularity.Citation74 The homogeneity of the clusters is measured by the silhouette score ranging from −1 to 1. If the silhouette score is closer to 1, it would correspond to a highly distinct or homogenous group in comparison with other clusters.
A different cluster represents a unique and distinct specialty or a different thematic concentration. This clustering analysis can identify the salient conceptual structures. Based on the concept of burst detection, it can analyze the thematic trends.Citation75 Citation bursts of papers imply that the appearance of the paper increases sharply with reference to its peers, and it used to identify emerging trends in terms of highly cited landmark articles.Citation76 Persson stated that “the citing articles form a research front, and the cited articles constitute an intellectual base.”Citation77 However, another scholar claimed that the features of a research front are the emerging trends and abrupt changes.Citation14 Therefore, when we explored the high frequency of cited references among document co-citation network, it is of practical significance to highlight the salient theme and contributors of the mAb research field, and provides evidence to explain how the focus of a domain changes over time.
The institutional cooperation network can be used to trace the collaboration among a variety of institutions. These institutions are the main body of bibliographic record. In other words, these institutions are the main body of mAb technical activity. By analyzing the institutional cooperation network, we can gain a further understanding of the cooperation among different institutions, even the cooperation contents and cooperation directions. In additional, analysis of highly cited journals helps to provide an understanding of the main application areas.
Disclosure of potential conflicts of interest
The authors declare that there is no conflict of interests regarding the publication of this paper.
Funding
This work was supported by the University of Macau (MYRG2016–00055-ICMS-QRCM).
References
- Liu JKH. The history of monoclonal antibody development: Progress, remaining challenges and future innovations. Ann Med Surg 2014; 3(4):113-6; https://doi.org/10.1016/j.amsu.2014.09.001
- Weiner GJ. Building better monoclonal antibody-based therapeutics. Nat Rev Cancer 2015; 15:361-70; PMID:25998715; https://doi.org/10.1038/nrc3930
- Firestone Inventing. The Global Map of Monoclonal Antibody Drugs 2016 Report. Hangzhou, China: Firestone; 2016. Available at: http://www.hsmap.com/precision_medical_detail.html?pm_child=%E6%8A%97%E4%BD%93%E8%8D%AF%E7%89%A9&report_id=115&report_name=%E7%81%AB%E7%9F%B3%E5%8F%91%E5%B8%83-%E6%8A%97%E4%BD%93%E8%8D%AF%E7%89%A9%E5%85%A8%E7%90%83%E5%9C%B0%E5%9B%BE
- Ecker DM, Jones SD, Levine HL. The therapeutic monoclonal antibody market. MAbs 2015; 7(1):9-14; PMID:25529996; https://doi.org/10.4161/19420862.2015.989042
- Scott AM, Wolchok JD, Old LJ. Antibody therapy of cancer. Nat Rev Cancer 2012; 12:278-87; PMID:22437872; https://doi.org/10.1038/nrc3236
- Rimawi MF, Schiff R, Osborne Ck. Targeting HER2 for the treatment of breast cancer. Annu Rev Med 2015; 66:111-28; PMID:25587647; https://doi.org/10.1146/annurev-med-042513-015127
- Lavaud P, Andre F. Strategies to overcome trastuzumab resistance in HER2-overexpressing breast cancers: focus on new data from clinical trials. BMC Med 2014; 12:132; PMID:25285786; https://doi.org/10.1186/s12916-014-0132-3
- Yarden Y, Sliwkowski MX. Untangling the ErbB signalling network. Nat Rev Mol Cell Bio 2001; 2:127-37; https://doi.org/10.1038/35052073
- Li HC, Wang M, Xu PY. Analysis of monoclonal antibody research literatures in the world. J Prev Med Inf 2010; 26(4):298-304
- Ma LF, Chen LB, Zhang HL, Tian L. Monoclonal antibody analysis based on bibliometrics. J Med Informatics 2010; 31(11):41-45. Chinese
- Gao YH, Xu SJ, Diao TX. Advances in research of antibody drugs. Chinese J New Drugs 2014; 23(20):2414-7. Chinese
- Hood W, Wilson C. The literature of bibliometrics, scientometrics, and informetrics. Scientometrics 2001; 52(2):291-314; https://doi.org/10.1023/A:1017919924342
- Small H. The relationship of information science to the social sciences: a co-citation analysis. Inf Process Manag 1981; 17(1):39-50; https://doi.org/10.1016/0306-4573(81)90040-6
- Chen CM. CiteSpace II: Detecting and visualizing emerging trends and transient patterns in scientific literature. J Am Soc Inf Sci Technol 2006; 57(3):359-77; https://doi.org/10.1002/asi.20317
- Chen CM, Hu ZG, Liu SB, Tseng H. Emerging trends in regenerative medicine: a scientometric analysis in CiteSpace. Expert Opin Biol Ther 2012; 12(5):593-608; PMID:22443895; https://doi.org/10.1517/14712598.2012.674507
- Chen CM, Dubin R, Kim MC. Emerging trends and new developments in regenerative medicine: a scientometric update (2000-2014). Expert Opin Biol Ther 2014; 14(9):1295-17; PMID:25077605; https://doi.org/10.1517/14712598.2014.920813
- Wikstrand CJ, McLendon RE, Bullard DE, Fredman P, Svennerholm L, Bigner DD. Production and characterization of two human glioma xenograft-localizing monoclonal antibodies. Cancer Res 1986; 46(11):5933-40; PMID:3756930
- Almagro JC, Fransson J. Humanization of antibodies. Front Biosci 2008; 13:1619-33; PMID:17981654
- Ober RJ, Radu CG, Ghetie V, Ward ES. Differences in promiscuity for antibody-FcRn interactions across species: implications for therapeutic antibodies. Int Immunol 2001; 13:1551-9; PMID:11717196; https://doi.org/10.1093/intimm/13.12.1551
- Stern M, Herrmann R. Overview of monoclonal antibodies in cancer therapy: present and promise. Crit Rev Oncol Hematol 2005; 54:11-29; PMID:15780905; https://doi.org/10.1016/j.critrevonc.2004.10.011
- Presta LG. Engineering of therapeutic antibodies to minimize immunogenicity and optimize function. Adv Drug Deliv Rev 2006; 58:640-56; PMID:16904789; https://doi.org/10.1016/j.addr.2006.01.026
- Morrision SL, Johnson MJ, Herzenberg LA, Oi VT. Chimeric human antibody molecules: mouse antigen-binding domains with human constant region domains. Proc Natl Acad Sci USA 1984; 81:6851-5; PMID:6436822; https://doi.org/10.1073/pnas.81.21.6851
- Jones PT, Dear PH, Foote J, Neuberger MS, Winter G. Replacing the complementarity-determining regions in a human antibody with those from a mouse. Nature 1986; 321:522-5; PMID:3713831; https://doi.org/10.1038/321522a0
- McCafferty J, Griffiths AD, Winter G, Chiswell DJ. Phage antibodies: filamentous phage displaying antibody variable domains. Nature 1990; 348:552-4; PMID:2247164; https://doi.org/10.1038/348552a0
- Winter G, Griffiths AD, Hawkins RE, Hoogenboom HR. Making antibodies by phage display technology. Annu Rev Immunol 1994; 12:433-55; PMID:8011287; https://doi.org/10.1146/annurev.iy.12.040194.002245
- Jostock T, Vanhove M, Brepoels E, Gool RV, Daukandt M, Wehnert A, Hegelsom RV, Dransfield D, Sexton D, Devlin M, et al. Rapid generation of functional human IgG antibodies derived from Fab-on-phage display libraries. J Immunol Methods 2004; 289:65-80; PMID:15251413; https://doi.org/10.1016/j.jim.2004.03.014
- Lonberg N, Taylor LD, Harding FA, Trounstine M, Higgins KM, Schramm SR, Kuo CC, Mashayekh R, Wymore K, Mccabe JG, et al. Antigen-specific human antibodies from mice comprising four distinct genetic modifications. Nature 1994; 368:856-9; PMID:8159246; https://doi.org/10.1038/368856a0
- Green LL, Hardy MC, Maynard-Currie CE, Tsuda H, Louie DM, Mendez MJ, Abderrahim H, Noguchi M, Smith DH, Zeng Y, et al. Antigen-specific human monoclonal antibodies from mice engineered with human Ig heavy and light chain YACs. Nat Genet 1994; 7:13-21; PMID:8075633; https://doi.org/10.1038/ng0594-13
- Baker MP, Reynolds HM, Lumicisi B, Bryson CJ. Immunogenicity of protein therapeutics: the key causes, consequences and challenges. Self Nonself 2010; 1:314-22; PMID:21487506; https://doi.org/10.4161/self.1.4.13904
- Hanauer SB, Feagan BG, Lichtenstein GR, Mayer LF, Schreiler S, Colombel JF, Rachmilewitz D, Wolf DC, Olson A, Bao WH, et al. Maintenance infliximab for Crohn's disease: the ACCENT I randomised trial. Lancet 2002; 359:1541-9; PMID:12047962; https://doi.org/10.1016/S0140-6736(02)08512-4
- Kindler V, Sappino A, Grau GE, Piguet PF, Vassalli P. The inducing role of tumor necrosis factor in the development of bactericidal granulomas during BCG infection. Cell 1989; 56:731-40; PMID:2647299; https://doi.org/10.1016/0092-8674(89)90676-4
- Beese EJ, Michie CA, Nicholls SW, Murch SH, Williams CB, Domizio P, Walker-Smith JA, Macdonald TT. Tumor necrosis factor alphaproducing cells in the intestinal mucosa of children with inflammatory bowel disease. Gastroenterology 1994; 106:1455-66; PMID:8194690; https://doi.org/10.1016/0016-5085(94)90398-0
- Schreiber S, Nikolaus S, Hampe J, Hamling J, Koop I, Groessner B, Lochs H, Raedler A. Tumor necrosis factor α and interleukin 1ß in relapse of Crohn's disease. Lancet 1999; 353:459-61; PMID:9989717; https://doi.org/10.1016/S0140-6736(98)03339-X
- Knight DM, Trinh H, Le J, Siegel S, Shealy D, McDonough M, Scallon B, Moore MA, Vilcek J, Daddona P, et al. Construction and initial characterization of a mouse-human chimeric anti-TNF antibody. Mol Immunol 1993; 30:1443-53; PMID:8232330; https://doi.org/10.1016/0161-5890(93)90106-L
- Hanauer SB, Sandborn WJ, Rutgeerts P, Fedorak RN, Lukas M, Maclntosh D, Panaccione R, Wolf D, Pollack P. Human anti-tumor necrosis factor monoclonal antibody (adalimumab) in Crohn's disease: the CLASSIC I trial. Gastroenterology 2006; 130:323-33; PMID:16472588; https://doi.org/10.1053/j.gastro.2005.11.030
- Ferrara N, Gerber HP, LeCouter J. The biology of VEGF and its receptors. Nat Med 2003; 9:669-76; PMID:12778165; https://doi.org/10.1038/nm0603-669
- Hurwitz H, Fehrenbacher L, Novotny W, Cartwright T, Hainsworth J, Heim W, Berlin J, Baron A, Griffing S, Holmgren E, et al. Bevacizumab plus Irinotecan, Fluorouracil, and Leucovorin for Metastatic Colorectal Cancer. N Engl J Med 2004; 350:2335-42; PMID:15175435; https://doi.org/10.1056/NEJMoa032691
- Pedersen MW, Jacobsen HJ, Koefoed K, Hey A, Pyke C, Haurum JS, Kragh M. Sym004: a novel synergistic anti–epidermal growth factor receptor antibody mixture with superior anticancer efficacy. Cancer Res 2010; 70(2):588-97; PMID:20068188; https://doi.org/10.1158/0008-5472.CAN-09-1417
- Linke R, Klein A, Seimetz D. Catumaxomab: clinical development and future directions. mAbs 2010; 2(2):129-36; PMID:20190561; https://doi.org/10.4161/mabs.2.2.11221
- Teachey DT, Rheingold SR, Maude SL, Zugmaier G, Barrett DM, Seif AE, Nichols KE, Suppa EK, Kalos M, Berg RA, et al. Cytokine release syndrome after blinatumomab treatment related to abnormal macrophage activation and ameliorated with cytokine-directed therapy. Blood 2013; 121:5154-7; PMID:23678006; https://doi.org/10.1182/blood-2013-02-485623
- Webb S. Pharma interest surges in antibody drug conjugate. Nat Biotechnol 2011; 29:297-8; PMID:21478833; https://doi.org/10.1038/nbt0411-297
- Kohler G, Milstein C. Continuous cultures of fused cells secreting antibody of predefined specificity. Nature 1975; 256:495-7; PMID:1172191; https://doi.org/10.1038/256495a0
- Milstein C, Cuello AC. Hybrid hybridomas and their use in immunohistochemistry. Nature 1983; 305:537-40; PMID:6137772; https://doi.org/10.1038/305537a0
- Sgro C. Side-effects of a monoclonal antibody, muromonab CD3/orthoclone OKT3: a bibliographic review. Toxicology 1995; 105(1):23-9; PMID:8638282; https://doi.org/10.1016/0300-483X(95)03123-W
- Buss N APS, Henderson SJ, McFarlane M, Shenton JM, Haan LD. Monoclonal antibody therapeutics: history and future. Curr Opin Pharmacol 2012; 12:615-22; PMID:22920732; https://doi.org/10.1016/j.coph.2012.08.001
- Herlyn DM, Steplewski Z, Herlyn MF, Koprowski H. Inhibition of growth of colorectal carcinoma in nude mice by monoclonal antibody. Cancer Res 1980; 40:717-21; PMID:7471090
- Milstein C, Cuello AC. Hybrid hybridomas and their use in immunohistochemistry. Nature 1983; 305:537-40; PMID:6137772; https://doi.org/10.1038/305537a0
- Staerz UD, Kanagawa O, Bevan MJ. Hybrid antibodies can target sites for attack by T cells. Nature 1985; 314:628-31; PMID:2859527; https://doi.org/10.1038/314628a0
- Lanzavecchia A, Scheidegger D. The use of hybrid hybridomas to target human cytotoxic T lymphocytes. Eur J Immunol 1987; 17(1):105-11; PMID:3102250; https://doi.org/10.1002/eji.1830170118
- Brennan M, Davison PF, Paulus H. Preparation of bispecific antibodies by chemical recombination of monoclonal immunoglobulin G1 fragments. Science 1985; 229(4708):81-3; PMID:3925553; https://doi.org/10.1126/science.3925553
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970; 227:680-5; PMID:5432063; https://doi.org/10.1038/227680a0
- LoBuglio AF, Wheeler RH, Trang J, Haynes A, Rogers K, Harvey EB, Ghrayeb J, Khazaeli MB. Mouse/human chimeric monoclonal antibody in man: kinetics and immune response. Proc Natl Acad Sci USA 1989; 86:4220-4; PMID:2726771; https://doi.org/10.1073/pnas.86.11.4220
- Nitta T, Yagita H, Okumura K, Sato K, Ishii S. Preliminary trial of specific targeting therapy against malignant glioma. Lancet 1990; 335(8686):368-71; https://doi.org/10.1016/0140-6736(90)90205-J
- Ziegler EJ, Fisher Jr CJ, Sprung CL, Straube RC, Sadoff JC, Foulke GE, Wortel CH, Fink MP, Dellinger RP, Teng NNH, et al. Treatment of gram-negative bacteremia and septic shock with HA-1A human monoclonal antibody against endotoxin: a randomized, double-blind, placebo-controlled trial. N Engl J Med 1991; 324(7):429-36; PMID:1988827; https://doi.org/10.1056/NEJM199102143240701
- Bird RE, Hardman KD, Jacobson JW, Johnson S, Kaufman BM, Lee SM, Lee T, Pope SH, Riordan GS, Whitlow M. Single-chain antigen-binding proteins. Science 1988; 242(4877):423-6; PMID:3140379; https://doi.org/10.1126/science.3140379
- Bolhuis RL, Lamers CH, Goey SH, Eggermont AM, Trimbos JB, Stoter G, Lanzavecchia A, di Re E, Miotti S, Raspagliesi F. Adoptive immunotherapy of ovarian carcinoma with bs-MAb-targeted lymphocytes: a multicenter study. Int J Cancer 1992; 7:78-81
- Riethmuller G, Gruber R, Schneider-Gadicke E, Schlimok G, Schmiegel W, Raab R, Hoffken K, Gruber R, Pichlmaier H, Hirche H, et al. Randomised trial of monoclonal antibody for adjuvant therapy of resected Dukes' C colorectal carcinoma. Lancet 1994; 343(8907):1177-83; PMID:7909866; https://doi.org/10.1016/S0140-6736(94)92398-1
- Winter G, Griffiths AD, Hawkins RE, Hoogenboom HR. Making antibodies by phage display technology. Annu Rev Immunol 1994; 12:433-55; PMID:8011287; https://doi.org/10.1146/annurev.iy.12.040194.002245
- Targan SR, Hanauer SB, Deventer SJH, Mayer L, Present DH, Braakman T, DeWoody KL, Schaible TF, Rutgeerts PJ. A short-term study of Chimeric Monoclonal Antibody cA2 to Tumor Necrosis Factor α for Crohn's Disease. N Engl J Med 1997; 337:1029-36; PMID:9321530; https://doi.org/10.1056/NEJM199710093371502
- Present DH, Rutgeerts P, Tagan S, Hanauer SB, Mayer L, Hogezand RAV, Podolsky DK, Sands BE, Braakman T, DeWoody KL, et al. Infliximab for the treatment of fistulas in patients with Crohn's disease. N Engl J Med 1999; 340:1398-405; PMID:10228190; https://doi.org/10.1056/NEJM199905063401804
- McLaughlin P, Grillo-Lopez AJ, Link BK, Levy R, Czuczman MS, Williams ME, Heyman MR, Bence-Bruckler I, White CA, Cabanillas F, et al. Rituximab chimeric anti-CD20 monoclonal antibody therapy for relapsed indolent lymphoma: half of patients respond to a four-dose treatment program. J Clin Oncol 1998; 16(8):2825-33; PMID:9704735
- Rutgeerts P, D'Haens G, Targan S, Vasiliauskas E, Hanauer SB, Present DH, Mayer L, Hogezand RAV, Braakman T, DeWoody KL, et al. Efficacy and safety of retreatment with anti–tumor necrosis factor antibody (infliximab) to maintain remission in Crohn's disease. Gastroenterology 1999; 117(4):761-9; PMID:10500056; https://doi.org/10.1016/S0016-5085(99)70332-X
- Clynes RA, Towers TL, Presta LG, Ravetch JV. Inhibitory Fc receptors modulate in vivo cytoxicity against tumor targets. Nat Med 2000; 6:443-6; PMID:10742152; https://doi.org/10.1038/74704
- Baert F, Noman M, Vermeire S, Assche GV, D'Haens G, Carbonez A, Rutgeerts P. Influence of immunogenicity on the long-term efficacy of infliximab in Crohn's disease. N Engl J Med 2003; 348:601-8; PMID:12584368; https://doi.org/10.1056/NEJMoa020888
- Holliger P, Hudson PJ. Engineered antibody fragments and the rise of single domains. Nat Biotechnol 2005; 23:1126-36; PMID:16151406; https://doi.org/10.1038/nbt1142
- Zeidler R, Reisbach G, Wollenberg B, Lang S, Chaubal S, Schmitt B, Lindhofer H. Simultaneous activation of T cells and accessory cells by a new class of Intact Bispecific Antibody Results in efficient tumor cell killing. J Immunol 1999; 163(3):1246-52; PMID:10415020
- Phillips GDL, Li GM, Dugger DL, Crocker LM, Parsons KL, Mai E, Blattler WA, Lambert JM, Chari RVJ, Lutz RJ, et al. Targeting HER2-positive breast cancer with Trastuzumab-DM1, an Antibody–Cytotoxic drug conjugate. Cancer Res 2008; 68(22):9280-90; PMID:19010901; https://doi.org/10.1158/0008-5472.CAN-08-1776
- Younes A, Bartlett NL, Leonard JP, Kennedy DA, Lynch CM, Sievers EL, Forero-Torres A. Brentuximab Vedotin (SGN-35) for Relapsed CD30-Positive Lymphomas. N Engl J Med 2010; 363:1812-21; PMID:21047225; https://doi.org/10.1056/NEJMoa1002965
- Doronina SO, Toki BE, Torgov MY, Mendelsohn BA, Cerveny CG, Chace DF, DeBlanc RL, Gearing P, Bovee TD, Siegall CB, et al. Development of potent monoclonal antibody auristatin conjugates for cancer therapy. Nat Biotechnol 2003; 21:778-84; PMID:12778055; https://doi.org/10.1038/nbt832
- Junutula JR, Raab H, Clark S, Bhakta S, Leipold DD, Weir S, Chen Y, Simpson M, Tsai SP, Dennis MS, et al. Site-specific conjugation of a cytotoxic drug to an antibody improves the therapeutic index. Nat Biotechnol 2008; 26(8):925-36; PMID:18641636; https://doi.org/10.1038/nbt.1480
- Strop P, Liu SH, Dorywalska M, Delaria K, Dushin RG, Tran TT, Ho WH, Farias S, Casas MG, Abdiche Y, et al. Location Matters: Site of conjugation modulates stability and pharmacokinetics of antibody drug conjugates. Chem Biol 2013; 20(2):161-7; PMID:23438745; https://doi.org/10.1016/j.chembiol.2013.01.010
- Morrison SL, Johnson MJ, Herzenberg LA, Oi VT. Chimeric human antibody molecules: mouse antigen-binding domains with human constant region domains. Proc Natl Acad Sci USA 1984; 81:6851-5; PMID:6436822; https://doi.org/10.1073/pnas.81.21.6851
- Jones PT, Dear PH, Foote J, Neuberger MS, Winter G. Replacing the complementarity-determining regions in a human antibody with those from a mouse. Nature 1986; 321:522-5; PMID:3713831; https://doi.org/10.1038/321522a0
- Chen CM, Chen Y, Horowitz M, Hou HY, Liu ZY, Pellegrino D. Towards an explanatory and computational theory of scientific discovery. J Informetr 2009; 3:191-209; https://doi.org/10.1016/j.joi.2009.03.004
- Chen CM, Song IY, Yuan XJ, Zhang J. The thematic and citation landscape of data and knowledge engineering (1985-2007). Data Knowl Eng 2008; 67:234-59; https://doi.org/10.1016/j.datak.2008.05.004
- Chen CM, Dubin R, Kim MC. Orphan drugs and rare diseases: a scientometric review (2000-2014). Expert Opin Orphan Drugs 2014; 2(7):709-24; https://doi.org/10.1517/21678707.2014.920251
- Person O. The intellectual base and research fronts of JASIS 1986-1990. JASIS 1994; 45(1):31-8; https://doi.org/10.1002/(SICI)1097-4571(199401)45:1<31::AID-ASI4>3.0.CO;2-G