ABSTRACT
Background: The emergence of vaccine-associated paralytic poliomyelitis has become an ongoing burden of poliomyelitis. During this special period from OPV to IPV-only immunization schedule, we did a meta-analysis to compare the immunogenicity of sequential IPV and OPV versus IPV alone in healthy infants.
Methods: This systematic review and meta-analysis was registered at international prospective register of systematic reviews (PROSPERO), and the number was CRD42017054889. We performed it as described.
Results: Finally, 6 articles were qualified for our review. The results showed that seroconversion rates against all 3 serotype polioviruses were non-inferior and Geometric mean antibody titers (GMTs) were superior in sequential schedules compared with IPV-only schedule. Thus, the sequential vaccination schedules could induce a stronger immunogenicity.
Conclusions: To decrease vaccine-associated and vaccine-derived poliomyelitis, it is a reasonable option to select sequential schedules during this special transition from OPV to IPV-only immunization schedule, which coincides with the current WHO recommendations.
Introduction
Poliomyelitis, caused by wild poliovirus (type 1, 2 and 3), is an infectious disease threatening human health. Vaccination is the only effective way to eradicate poliomyelitis. With the introduction and widely use of live Oral Polio Vaccine (OPV),Citation1 which contains a live-attenuated vaccine-virus (Sabin virus), the global epidemic cases of poliomyelitis have led to a 99.99% sharply decrease (from about 350,000 cases in 1988 to 22 reported cases in 2017)Citation2 and the serotypes 2 and 3 of wild poliovirus have experienced an eradication.Citation3 Successful elimination is closer than ever before, and wild type 1 poliovirus remains the endemic strain which circulates only in Afghanistan, Pakistan and Nigeria.Citation4 Despite the achievements of OPV in reducing the spread of paralytic poliomyelitis, the emergence of vaccine-associated paralytic poliomyelitis (VAPP)Citation5 and vaccine-derived poliovirus (VDPV)Citation1 has become an ongoing burden caused by the use of live-attenuated vaccine strains.Citation6 VAPP is a rare but serious adverse event due to genetic mutations and recombination during the replication of attenuated OPV viruses in the gut. The vaccine viruses rapidly accumulate such mutations and genetic rearrangements, reverting to neurovirulence and transmissibility,Citation7 especially keen to infect patients with immunodeficiency.Citation8 In addition, VDPVs are genetic variants (OPV-derived polioviruses) with the potential to cause paralytic poliomyelitis and can transmit in regions with low population immunity.Citation9,Citation10 The nucleotide sequence of VDPV varies from the original OPV with the divergence >1%. However, in order to improve sensitivity for early prevention from its outbreaks, this threshold has been adjusted to 0.6% for type 2 VDPVs (VDPV2).Citation1
Trivalent OPV (tOPV) contains a mixture of live-attenuated polioviruses of all three serotypes, which has been the predominant vaccine against poliovirus used for routine immunization before April 2016. However, the type 2 component of tOPV has caused more than 90% of all cVDPV cases and up to 38% of all VAPP cases in recent years.Citation11 In addition, the burden related to poliomyelitis is mainly resulted from vaccine rather than wild poliovirus recently.Citation5 Therefore, the Global Polio Eradication Initiative (GPEI) implemented a switch from tOPV to bivalent OPV (bOPV) containing serotypes 1 and 3 poliovirus, and the switch means that cVDPV and VAPP related to type 2 vaccine components no long occur. To achieve the goal of eliminating VAPP and VDPVs completely requires introduction an inevitable but wise choiceCitation12: inactivated poliovirus vaccines (IPV). IPV is an inactivated but not a ‘live’ attenuated vaccine, which consists of all three wild-type poliovirus strains, so it carries no possibility to appear VAPP or VDPVs. IPV can induce human bodies to produce protective antibodies against all three types of poliovirus. In case of infection, these antibodies can prevent the virus spreading to central nervous system and protect against poliomyelitis. After OPV cessation, IPV will be the only vaccine available in routine vaccination schedule to eliminate the occurrence of VAPP and cVDVPs. GPEI is planning to quit OPV as soon as possible after Wild poliovirus (WPV) has been certified as eradicated.Citation11 The American Academy of Pediatrics (AAP) also recommended the all-IPV immunization schedule in early 2000.Citation13 The polio vaccination policy was changed from OPV to exclusive IPV successfully in the high standards of hygiene and sanitation areas such as United States,Citation14 Sweden, Iceland, Finland and the NetherlandsCitation15 and subsequently eliminated polio in these industrialized countries. An ever-increasing amount of industrialized, polio-free countries choose IPV as the vaccine. However, IPV-only schedules would not be sufficient to halt poliovirus transmission in regions of poor sanitary condition because of its absence of strong mucosal immunityCitation15 and close contacts immunization through a secondary spread.Citation16 Additionally, IPV is too expensive (over five times more expensive than OPV) for global use,Citation17 especially in developing countries, because administering the IPV requires sterile injection equipment and procedures, as well as trained health workers.Citation16,Citation18 However, it is inspiring that Gavi supports the Polio Endgame through the introduction of IPV into routine immunization programmes,Citation19 and there were more than 40 million children immunized with the help of Gavi (containing the poorest countries around the world) by the end of 2016.
Considering those disadvantages for IPV-only schedule, it is difficult to abolish OPV completely and feasible for phased removal of OPV. The sequential IPV-OPV / OPV-IPV vaccination schedules may play a significant role during the transition, although the WHO strategy is to substitute OPV with exclusive IPV for protection against polio ultimately.Citation20 It is consistent with the recommendations of WHO position paper,Citation21,Citation22 which suggests staged withdrawal of OPV beginning with type 2 component and global introduction at least one dose of IPV into routine vaccination schedules. Under these circumstances, policy makers need to know if such sequential schedules are competent to prevent poliovirus transmission. There was only one studyCitation23 directly assessed the immunogenicity of sequential schedules compared with IPV-only schedule, but median titres comparison for each group was not be implemented. Therefore, it was necessary for further study on this topic. Our meta-analysis is an integrated review, which compared the humoral immunity responds by not only seroconversion rate but also GMTs against poliovirus of those two schedules. In this review we aimed to determine whether sequential vaccination programs could be with a high seroconversion and successfully protect against polio infection in this special period. Thus, it could accelerate an evidence-based decision-making process.
Results
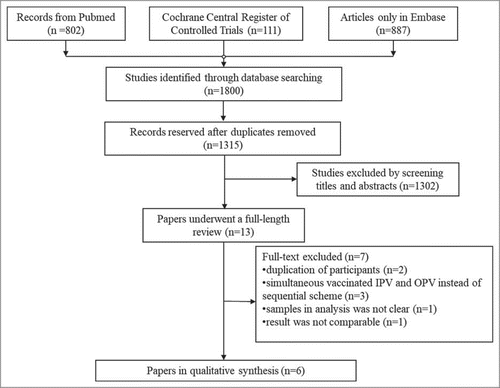
Our search strategy yielded 1800 potentially relevant publications, and there were 1315 records reserved after duplicates removed. Afterwards, we eliminated 1302 articles by screening titles and abstracts for not matching inclusion criteria. Ultimately, 13 papers underwent a full-length review, and of those, 6 studiesCitation23–Citation28 were qualified for our meta-analysis. The ineligible records contained 2 researchesCitation29,Citation30 which included duplication of participants with their own other papers; 3 publicationsCitation31–Citation33 in which the vaccination schedule was simultaneous IPV and OPV instead of the sequential scheme. In addition, there was an articleCitation34 without a clear amount of samples involved in analysis (its withdrawal rate was not shown). And the results of another oneCitation35 were not comparable, because its sequential vaccination groups were inoculated more than the IPV-only group ().
Methodological quality of qualified studies
Quality assessment of two included randomized controlled trials (RCTs)Citation23,Citation25 is shown in . They were both rated as “high” methodological quality. However, the four included case series were all rated as “poor”, because the follow-up rates of three studiesCitation24,Citation27,Citation28 were not up to 90% and the other were never reported or explained.Citation26
Table 1. Methodological quality of included RCTs.
Study characteristics
As displayed in , of 6 qualified studies were published in recent 5 years and the remaining 2 studiesCitation24,Citation25 were published earlier. A half studies were performed in China and the others came from different countries. There were only 2 RCTsCitation23,Citation25 reported their study period. Moreover, participants were infants aged below 13 weeks in 5 studies. Although the vaccination time varied from study to study, all participants had completed their vaccination program within 24 weeks. The blood collection time detailed was one month after every participant fulfilled the vaccination schedule. Furthermore, sequential IPV and OPV schedules in our selected studies included one or two doses of IPV followed by two or one dose of OPV.
Table 2. Study characteristics.
Neutralizing antibody seroconversion
As described in selected studies, it was considered that seroconversion presented a positive and protective neutralizing antibody level if titers ≧1:8. Both sequential IPV/OPV and IPV-only groups developed very high seroconversion rates with almost 100% against serotypes 1 and 3 one month after the full vaccination schedules and without significant heterogeneity between groups (). Besides, seroconversion rates against serotype 2 were both 100%, but it should be noted that a study of O'Ryan et al.Citation23 was not included, because they used bOPV without type 2 poliovirus component. In summary, both sequential IPV and OPV schedules and IPV alone could induce strong serum immunogenicity in healthy infants.
Table 3. Meta-analysis for seroconversion rates between groups.
GMTs
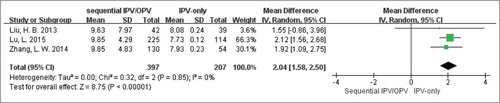
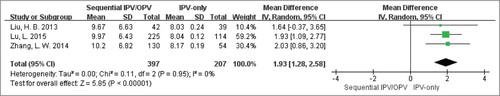
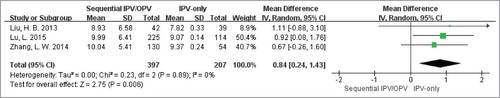
Afterwards, we performed meta-analysis to compare the immunogenicity between such two groups through the quantitative index GMTs when data was available (two authorsCitation23,Citation24 were contacted, but didn't provided useful information.). Ultimately, there were three eligible studiesCitation26–Citation28 performed in China included. As well known, antibody titer is exponentially increased, so it is unsuitable for direct meta-analysis. It was the value that the logarithm of the raw data to base 2 being used in our further analysis. As shown in , the sequential vaccination schedules (IPV followed by OPV) could induce much stronger serotype 1 antibody titers than IPV-only scheme. After exponential conversion, the mean difference (95%CI) was 671.76 (639.40, 704.12). And there was no significant heterogeneity existed between the two groups with Cochran Q test P = 0.85; I2 = 0%. Similarly, the meta-analysis forest plots of GMTs in serotype 2 () and serotype 3 () were also finished, which produced the same results as type 1: the weighted GMTs were higher in the sequential group compared with IPV-only group, and the mean differences were 731.50 (604.48, 858.52) and 378.92 (293.73, 464.10) respectively. And there was no great heterogeneity in above two analyses across groups (Cochran Q test P = 0.95; I2 = 0% as well as Cochran Q test P = 0.89; I2 = 0%).
Subgroup analysis
As mentioned, both sequential IPV/OPV and IPV-only groups induced very strong immune response and without great heterogeneity against all 3 serotypes after completing vaccination programs. Therefore, it was no need and significance for the further subgroup analysis by seroconversion rates. However, considering only three articles could be used for GMTs meta-analysis, those articles were all performed in China and the vaccination procedures were all IPV followed by OPV at the same age, there was no further subgroup analysis performed in this review. We summarized the GMTs of diverse sequential vaccination series (IPV-OPV-OPV vs. IPV-IPV-OPV) in these researches. We found that GMTs were very high after above both vaccination regimens, whereas for the former program, they were slightly higher against serotype 1, and slightly lower against serotype 2 and 3.
Adverse event
As showed in our eligible studies, both sequential IPV/OPV and IPV-only vaccination strategies were well tolerated, with no fatality. Although several infants experienced adverse events and considered to be related to studying vaccines, none aggressive VAPP or VDPV was reported. The relevant adverse events included irritability, fever, local reaction (pain and induration at the vaccination site) and so on.
Discussion
To our knowledge, there was no meta-analysis previously to compare the immunogenicity of sequential IPV and OPV schedules with IPV-only schedules in healthy infants. Compared with IPV-only schedules, we found that seroconversion rates against all 3 serotype polioviruses were not inferior in sequential schedules, and the proportions after three doses were close or up to 100% with no statistical difference between groups. However, the GMTs of seroconversion reached higher levels in sequential schedules than in IPV-only schedules. Thus, sequential schedules could induce a stronger immunogenicity.
With the success in global eradication of wild poliovirus, evidence-based strategies for polio endgame should be agreed on and implemented. Currently, the burden related to poliomyelitis is mainly caused by vaccine rather than wild poliovirus.Citation36 The Polio Eradication and Endgame Strategic Plan 2013–2018 set by GPEI recommended that it was necessary to introduce at least one dose of IPV worldwide in their routine immunization programs and eventually withdrew all OPV by 2019 in order to eliminate all poliomyelitis, including VAPP, and consistently keep eradication of all polioviruses.Citation37 Sequential IPV and OPV immunization schedules seem to optimal option during this transition period. Our meta-analysis also provides an important clinical evidence for the stronger immunogenicity of sequential schedules than IPV-only schedules. To speed up the universal IPV introduction, it is necessary to develop more-affordable IPV options.Citation38 Possible approaches include developing microneedle patch or intradermal device, using fractional IPV dose, reducing the quantity of IPV doses in routine immunization, using adjuvanted vaccines to reduce doses of IPV and optimizing the production process (using of non-infectious seed strain materials). Of course, this is also a GPEI's multi-pronged research agenda to meet this objective. Resik et al.Citation39,Citation40 demonstrated that fractional dose of IPV (fIPV) delivery using needle-free injector devices is feasible and highly accepted. And subsequent study hasCitation41 revealed that a single fractional dose (1/5 of the full dose) of IPV provides lower seroconversion rates compared with a single full dose, but after 2 fractional doses the rates are non-inferior to those after 2 full doses. In addition, 2 fractional doses of IPV, given intradermally at 6 and 14 weeks, provide higher seroconversion rates than a single full dose given at 14 weeks. Besides, Rivera and his colleaguesCitation42 found that three aluminium hydroxide adjuvanted vaccines with different doses (1/3, 1/5 and 1/10) of IPV were non-inferior to IPV in percentage of seroconversion. In combination with the results of our meta-analysis, future RCTs can consider exploring humoral and intestinal immunity of those more-affordable IPV options in sequential schedules. Meanwhile, their safety also needs to be closely concentrated on.
There was no comparison between such two schedules about their intestinal immunogenicity, which is reflected by the amount of virus shed and duration of shedding after challenged with corresponding serotype OPV. However, several earlier studies demonstrated that IPV used alone can only provide weak intestinal immunity,Citation43,Citation44 but can achieve a substantial boost in intestinal immunity in the children under 5 years old who received mixed IPV and OPV vaccination program.Citation45,Citation46 This finding indicated that sequential schedules were more likely to play an important role in accelerating elimination and preventing outbreaks of poliomyelitis. However, further high-quality studies are needed to support this.
There was no case of poliomyelitis induced by naturally circulating wild-type 2 poliovirus having been reported for more than 15 years. But with the withdrawal of type 2 components in tOPV, IPV should be added to bOPV for a sequential vaccination schedule in order to protect new birth cohorts from outbreaks of poliomyelitis. This measure has already been implemented in some regions, containing Chilean,Citation23 Latin American,Citation46 IndiaCitation47 and Pakistan,Citation48 and it has been testified inducing excellent intestinal and humoral immune response against type 2 poliovirus. Furthermore, Sáez-Llorens et al.Citation20 found that there was an alternative option to use a novel monovalent high-dose inactivated poliovirus type 2 vaccine (mIPV2HD) for primary protection and outbreak response during the poliomyelitis eradication phase in area at risk of type 2 poliovirus. Because the seroconversion rates and median antibody titers against serotype 2 were both significantly higher in mIPV2HD group than in standard IPV group. To make policy decisions more flexible in choosing a vaccination schedule to eliminate type 2 related VAPP and VDPVs, more investigations are needed for further confirmation.
This meta-analysis had some limitations. There were several variations from the previous plan listed below: 1) it is no longer an ineligibility criterion that the participants administered with other vaccines in the progress of research, because it was unreasonable to quit other routine vaccine regimens during this study. 2) Considering slightly loosening selecting criterion did not affect the quality of review,Citation49 case series were also selected in this review rather than just RCTs on account of lacking sufficient eligible RCTs, which suggests that more high-quality studies are required to further confirmation. 3) As mentioned earlier, in-depth subgroup analyzes were not conducted due to the limited literature data selected in our review; so more high-quality researches are indispensable for obtaining more accurate conclusions.
In conclusion, seroconversion rates against all 3 serotype polioviruses were non-inferior and GMTs were superior in sequential IPV and OPV schedules compared with IPV-only schedules. In order to decrease vaccine-associated and vaccine-derived poliomyelitis and take into account the challenges faced with complete withdrawal OPV, it is a reasonable option to select sequential IPV and OPV schedules during this special transition.
Methods
Search methods
This systematic review and meta-analysis was registered at the international prospective register of systematic reviews (PROSPERO), and the number was CRD42017054889. A comprehensive literature retrieval of the Pubmed, Embase (only articles in this database) and Cochrane Central Register of Controlled Trials (CENTRAL) was conducted on November 14th, 2016. The search strategy was without any methods filters and not limited by language and date. We used the search strategy for pubmed below, and adapted it for other databases. Search terms included medical subject headings (MeSH) and free words found in title or abstract. “Poliovirus Vaccine, Inactivated” was our first MeSH, studies identified were combined using the set operator “or” with its free words (Inactivated Poliovirus Vaccine /Vaccine, Inactivated Poliovirus /Salk vaccine /Vaccine, Salk). The second MeSH: “Poliovirus Vaccine, Oral”, which were combined with its free words (Oral Poliovirus Vaccine /Vaccine, Oral Poliovirus / Sabin Vaccine / Vaccine, Sabin) in the same way. Afterwards, we combined the two groups of terms with the operational character “and” to complete our literature search.
Selection criteria
Eligibility criteria were established in advance by the authors and were shown as follows: 1) Studies in which the populations were healthy infants who were not in morbid states and of which the outcomes contained proportion of seroconversion to the three poliovirus serotypes were included. 2) We selected the publications with both sequential IPV-OPV or OPV-IPV schedules and IPV-only vaccination schedule. 3) Only studies with each group no less than 10 participants were selected to minimize bias inevitably related to individual small case series. 4) Only English or Chinese articles were not being removed as well. 5) In addition, RCTs and case series but only completed publications were included in this review.
Study selection and Data collection
Two independent reviewers (Wen Yin and Guihua Tang) screened the title, abstract or full text and determined their eligibility of retrieved literatures using predefined inclusion criteria. Discrepancies were resolved through consultation and discussion, asking for guidance, if necessary.
Data were extracted using a pretested and standardized data extraction form by the above two reviewers respectively. We have tried our best to contact the corresponding authors for the unpublished GMTs of qualified studies. If there were any differences, a supplementary discussion to consensus was carried out. Information collected included demographic information; vaccination schedules; blood sampling date; seroconversion rates and antibody titers; and adverse events related to vaccination.
Methodological quality assessment
Methodological qualities of Randomized controlled trials were assessed using the Cochrane risk of bias tool.Citation50,Citation51 The items were evaluated including blinding, sequence generation, allocation sequence concealment, selective outcome reporting, dealing with incomplete outcome data and other potential threats to validity. They were rated as high, low or unclear risks respectively. Whereas case series were appraised using the 8 guidance criterions from the Centre for Reviews and Dissemination checklistCitation52 (). Researches were identified as “good” quality if matched all listed criteria, they were considered to be “satisfactory” when the answer is “yes” to checklists 2 and 4–7, and rated as “poor” if the answer is “no” to any criteria listed for “satisfactory”. Results of methodological quality appraisal did not affect the eligibility of the studies.
Table 4. Quality assessment items for case series.
Statistical methods
The immunogenicity of diverse polio vaccination program was calculated and judged by the rates of seroconversion and GMTs. We analyzed the data with Review Manager (version 5.3) and used the Mantel-Haenszel method to calculate odd ratios with 95% confidence intervals (CI) to summarize the seroconversion rates and GMTs of the three serotype poliovirus respectively as well as predefined subgroups across trials. Considering the most conservative (a more correct) estimate will be given, we performed this meta-analysis based on the random-effects model. Heterogeneity among studies or subgroups was evaluated using the Cochran Q test and the I2 statistic, while Cochrane Q < 0.10 or I2 > 50% was considered to significant heterogeneity.
Conflicts of interest
None.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
References
- Lopalco P. Wild and vaccine-derived poliovirus circulation, and implications for polio eradication[J]. Epidemiol Infect. 2017;145(3):413–9.
- WHO. Poliomyelitis. [accessed 2018 Apr 10]. http://www.who.int/mediacentre/factsheets/fs114/en/.
- Shaghaghi M, Shahmahmoodi S, Abolhassani H, Soleyman-Jahi S, Parvaneh L, Mahmoudi S, Chavoshzadeh Z, Yazdani R, Zahraei SM, Ebrahimi M, et al. Vaccine-derived polioviruses and children with primary immunodeficiency, Iran, 1995–2014[J]. Emerg Infect Dis. 2016;22(10):1712–9. doi:10.3201/eid2210.151071.
- Sepúlveda J. Global health: Towards polio eradication[J]. Nature. 2017;547(7664):411–2.
- Platt LR, Estivariz CF, Sutter RW. Vaccine-associated paralytic poliomyelitis: a review of the epidemiology and estimation of the global burden[J]. J Infect Dis. 2014;210 Suppl 1:S380–9. doi:10.1093/infdis/jiu184.
- Pliaka V, Kyriakopoulou Z, Markoulatos P. Risks associated with the use of live-attenuated vaccine poliovirus strains and the strategies for control and eradication of paralytic poliomyelitis[J]. Expert Rev Vaccines. 2012;11(5):609–28. doi:10.1586/erv.12.28.
- Korotkova E, Gmyl A, Yakovenko M, Ivanova O, Eremeeva T, Kozlovskaya L, Shakaryan A, Lipskaya G, Parshina I, Loginovskikh N, et al. A cluster of Paralytic Poliomyelitis cases due to transmission of slightly Diverged Sabin 2 Vaccine Poliovirus[J]. J Virol. 2016;90(13):5978–88.
- Foiadelli T, Savasta S, Battistone A, Kota M, Passera C, Fiore S, Bino S, Amato C, Lozza A, Marseglia G, et al. Nucleotide variation in Sabin type 3 poliovirus from an Albanian infant with agammaglobulinemia and vaccine associated poliomyelitis[J]. BMC Infect Dis. 2016;16:277.
- Etsano A, Damisa E, Shuaib F, Nganda G, Enemaku O, Usman S, Adeniji A, Jorba J, Iber J, Ohuabunwo C, et al. Environmental isolation of circulating Vaccine-Derived Poliovirus after interruption of Wild Poliovirus Transmission – Nigeria, 2016[J]. MMWR Morb Mortal Wkly Rep. 2016;65(30):770–3.
- Pons-Salort M, Burns C, Lyons H, Blake I, Jafari H, Oberste M, Kew O, Grassly N. Preventing Vaccine-Derived Poliovirus emergence during the Polio Endgame[J]. PLoS Pathog. 2016;12(7):e1005728.
- GPEI-Global Polio Eradication Initiative. [accessed 2018 Apr 12]. http://polioeradication.org/.
- Patel M, Zipursky S, Orenstein W, Garon J, Zaffran M. Polio endgame: the global introduction of inactivated polio vaccine[J]. Expert Rev Vaccines. 2015;14(5):749–62. doi:10.1586/14760584.2015.1001750.
- Abramson JS, Baker CJ, Fisher MC, Gerber MA, Meissner HC, Murray DL, Overturf GD, Prober CG, Rennels MB, Saari TN, et al. Prevention of poliomyelitis: Recommendations for use of only inactivated poliovirus vaccine for routine immunization[J]. Pediatrics. 1999;104(6):1404–6. doi:10.1542/peds.104.6.1404.
- Alexander LN, Seward JF, Santibanez TA, Pallansch MA, Kew OM, Prevots DR, Strebel PM, Cono J, Wharton M, Orenstein WA, et al. Vaccine policy changes and epidemiology of poliomyelitis in the United States[J]. Jama. 2004;292(14):1696–701.
- Parker EPK, Molodecky NA, Pons-Salort M, O'Reilly KM, Grassly NC. Impact of inactivated poliovirus vaccine on mucosal immunity: Implications for the polio eradication endgame[J]. Expert Rev Vaccines. 2015;14(8):1113–23. doi:10.1586/14760584.2015.1052800.
- Kraan H, van der Stel W, Kersten G, Amorij JP. Alternative administration routes and delivery technologies for polio vaccines[J]. Expert Rev Vaccines. 2016;15(8):1029–40. doi:10.1586/14760584.2016.1158650.
- Hamidi A, Bakker WAM. Innovative IPV from attenuated Sabin poliovirus or newly designed alternative seed strains[J]. Pharm Pat Anal. 2012;1(5):589–99. doi:10.4155/ppa.12.70.
- Sheikh MA, Makokha F, Hussein AM, Mohamed G, Mach O, Humayun K, Okiror S, Abrar L, Nasibov O, Burton J, et al. Combined use of inactivated and oral poliovirus vaccines in refugee camps and surrounding communities – Kenya, December 2013[J]. MMWR Morb Mortal Wkly Rep. 2014;63(11):237–41. http://www.embase.com/search/results?subaction=viewrecord&from=export&id=L372961210.
- Gavi, the Vaccine Alliance: Gavi's impact. [accessed 2018 Apr 12]. http://www.gavi.org/support/nvs/inactivated-polio-vaccine.
- Saez-Llorens X, Clemens R, Leroux-Roels G, Jimeno J, Clemens SA, Weldon WC, Oberste MS, Molina N, Bandyopadhyay AS. Immunogenicity and safety of a novel monovalent high-dose inactivated poliovirus type 2 vaccine in infants: a comparative, observer-blind, randomised, controlled trial[J]. Lancet Infect Dis. 2016;16(3):321–30. doi:10.1016/S1473-3099(15)00488-0.
- Organization WH. Polio vaccines: WHO position paper – March, 2016[J]. Wkly Epidemiol Rec. 2016;91(12):145–68.
- Organizition WH. Introduction to inactivated polio vaccine and switch from trivalent to bivalent oral poliovirus vaccine worldwide, 2013–2016[J]. Wkly Epidemiol Rec. 2015;90(27):337–43. http://www.embase.com/search/results?subaction = viewrecord&from = export&id = L606055009.
- Faden H, Modlin JF, Thoms ML, McBean AM, Ferdon MB, Ogra PL. Comparative evaluation of immunization with live attenuated and enhanced-potency inactivated trivalent poliovirus vaccines in childhood: systemic and local immune responses[J]. J Infect Dis. 1990;162(6):1291–7. http://www.embase.com/search/results?subaction = viewrecord&from = export&id = L21001210.
- Asturias EJ, Dueger EL, Omer SB, Melville A, Nates SV, Laassri M, Chumakov K, Halsey NA. Randomized trial of inactivated and live polio vaccine schedules in Guatemalan infants[J]. J Infect Dis. 2007;196(5):692–8. http://onlinelibrary.wiley.com/o/cochrane/clcentral/articles/656/CN-00703656/frame.html.
- Liu HB, Lin JP, Wu YH, Liang H. Serological responses following primary immunization with poliomyelitis vaccine by various schedules[J]. Chinese Journal of Biologicals. 2013;26(11):1641–3. http://www.embase.com/search/results?subaction = viewrecord&from = export&id = L372150217.
- Zhang LW, Xu Y, Xing HY, Wang HH, Yuan XH, Zhu ZL. Evaluation of immune effects of primary and booster immunizations with inactivated and oral polio vaccines by various sequential programs in Changping District, Beijing City, China[J]. Chinese Journal of Biologicals. 2014;27(10):1283–7. http://www.embase.com/search/results?subaction = viewrecord&from = export&id = L601832225.
- Lu L, Li X, Zhang H, Liu D, Zhang Z, Wang H, Liu F, Ning Z, Li J, Pang X. Immunogenicity and persistence from different 3-dose schedules of live and inactivated polio vaccines in Chinese infants[J]. Vaccine. 2015;33(36):4653–8.
- O'Ryan M, Bandyopadhyay AS, Villena R, Espinoza M, Novoa J, Weldon WC, Oberste MS, Self S, Borate BR, Asturias EJ, et al. Inactivated poliovirus vaccine given alone or in a sequential schedule with bivalent oral poliovirus vaccine in Chilean infants: a randomised, controlled, open-label, phase 4, non-inferiority study[J]. Lancet Infect Dis. 2015;15(11):1273–82. doi:10.1016/S1473-3099(15)00219-4.
- Lu L, Li XM, Liu DL, Zhang HR, Zhang ZJ, Wang HH, Liu F, Ning ZQ, Zhang LW, Chu P, et al. Study of immunogenicity after primary vaccination by different sequential program of inactivated poliovirus vaccine and oral poliovirus vaccine[J]. Zhonghua yu Fang yi Xue za Zhi. 2012;46(6):510–3. http://www.embase.com/search/results?subaction = viewrecord&from = export&id = L366400664.
- Faden H, Duffy L, Sun M, Shuff C. Long-term immunity to poliovirus in children immunized with live attenuated and enhanced-potency inactivated trivalent poliovirus vaccines[J]. J Infect Dis. 1993;168(2):452–4.
- Greenwood BM, Hall AJ, Rowe MG, Whittle HC, George M, Al-Ghassani AAK, Elbualy M, Malankar PG, Suleiman AJM, Clements GB, et al. Combined immunization of infants with oral and inactivated poliovirus vaccines: results of a randomized trial in The Gambia, Oman, and Thailand. Bull World Health Organ. 1996;74(3):253–68. http://onlinelibrary.wiley.com/o/cochrane/clcentral/articles/252/CN-00405252/frame.html.
- Robertson SE. Combined immunization of infants with oral and inactivated poliovirus vaccines: Results of a randomized trial in the Gambia, Oman, and Thailand[J]. J Infect Dis. 1997;175(2 SUPPL.):S215–S27. http://www.embase.com/search/results?subaction = viewrecord&from = export&id = L27053513.
- Sutter RW, Suleiman AJ, Malankar PG, Mehta FR, Medany MA, Arif MA, Linkins RW, Pallansch MA, El-Bualy MS, Robertson SE. Sequential use of inactivated poliovirus vaccine followed by oral poliovirus vaccine in Oman[J]. J Infect Dis. 1997;175 Suppl1:S235–40. http://www.embase.com/search/results?subaction = viewrecord&from = export&id = L27053515.
- Modlin JF, Halsey NA, Thoms ML, Meschievitz CK, Patriarca PA. Humoral and mucosal immunity in infants induced by three sequential inactivated poliovirus vaccine-live attenuated oral poliovirus vaccine immunization schedules. Baltimore Area Polio Vaccine Study Group[J]. J Infect Dis. 1997;175 Suppl 1:S228–34. http://www.embase.com/search/results?subaction = viewrecord&from = export&id = L27053514.
- Anand A, Zaman K, Estivariz CF, Yunus M, Gary HE, Weldon WC, Bari TI, Steven Oberste M, Wassilak SG, Luby SP, et al. Early priming with inactivated poliovirus vaccine (IPV) and intradermal fractional dose IPV administered by a microneedle device: A randomized controlled trial[J]. Vaccine. 2015;33(48):6816–22. doi:10.1016/j.vaccine.2015.09.039.
- Bandyopadhyay AS, Garon J, Seib K, Orenstein WA. Polio vaccination: Past, present and future[J]. Future Microbiol. 2015;10(5):791–808. doi:10.2217/fmb.15.19.
- Polio Eradication and Endgame Strategic Plan 2013-“2018. [accessed 2018 Apr 15]. http://www.polioeradication.org/Portals/0/Document/Resources/.
- Okayasu H, Sutter RW, Jafari HS, Takane M, Aylward RB. Affordable inactivated poliovirus vaccine: strategies and progress[J]. J Infect Dis. 2014;210 Suppl 1:S459–64.
- Resik S, Tejeda A, Mach O, Sein C, Molodecky N, Jarrahian C, Saganic L, Zehrung D, Fonseca M, Diaz M, et al. Needle-free jet injector intradermal delivery of fractional dose inactivated poliovirus vaccine: Association between injection quality and immunogenicity [J]. Vaccine. 2015;33(43):5873–7. http://onlinelibrary.wiley.com/o/cochrane/clcentral/articles/739/CN-01170739/frame.html.
- Resik S, Tejeda A, Mach O, Fonseca M, Diaz M, Alemany N, Garcia G, Hung LH, Martinez Y, Sutter R. Immune responses after fractional doses of inactivated poliovirus vaccine using newly developed intradermal jet injectors: a randomized controlled trial in Cuba[J]. Vaccine. 2015;33(2):307–13. doi:10.1016/j.vaccine.2014.11.025.
- Anand A, Molodecky NA, Pallansch MA, Sutter RW. Immunogenicity to poliovirus type 2 following two doses of fractional intradermal inactivated poliovirus vaccine: A novel dose sparing immunization schedule[J]. Vaccine. 2017;35(22):2993–8.
- Rivera L, Pedersen R, Peña L, Olsen K, Andreasen L, Kromann I, Nielsen PI, Sørensen C, Dietrich J, Bandyopadhyay AS, et al. Immunogenicity and safety of three aluminium hydroxide adjuvanted vaccines with reduced doses of inactivated polio vaccine (IPV-Al) compared with standard IPV in young infants in the Dominican Republic: a phase 2, non-inferiority, observer-blinded, randomised, and controlled dose investigation trial[J]. Lancet Infect Dis. 2017;17(7):745–53.
- Galindo M, Lago PM, Cáceres V, Landaverde M, Sutter R. Randomized, placebo-controlled trial of inactivated poliovirus vaccine in Cuba[J]. N Engl J Med. 2007;356(15):1536–44. doi:10.1056/NEJMoa054960.
- John J, Giri S, Karthikeyan A, Lata D, Jeyapaul S, Rajan A, Kumar N, Dhanapal P, Venkatesan J, Mani M, et al. The duration of intestinal Immunity after an inactivated Poliovirus Vaccine Booster Dose in Children immunized with Oral Vaccine: A randomized Controlled Trial[J]. J Infect Dis. 2017;215(4):529–36.
- John J, Giri S, Karthikeyan AS, Iturriza-Gomara M, Muliyil J, Abraham A, Grassly NC, Kang G. Effect of a single inactivated poliovirus vaccine dose on intestinal immunity against poliovirus in children previously given oral vaccine: an open-label, randomised controlled trial[J]. Lancet. 2014;384(9953):1505–12. http://onlinelibrary.wiley.com/o/cochrane/clcentral/articles/495/CN-01022495/frame.html.
- Asturias EJ, Bandyopadhyay AS, Self S, Rivera L, Saez-Llorens X, Lopez E, Melgar M, Gaensbauer JT, Weldon WC, Oberste MS, et al. Humoral and intestinal immunity induced by new schedules of bivalent oral poliovirus vaccine and one or two doses of inactivated poliovirus vaccine in Latin American infants: an open-label randomised controlled trial[J]. Lancet. 2016;388(10040):158–69. http://onlinelibrary.wiley.com/o/cochrane/clcentral/articles/932/CN-01167932/frame.html.
- Sutter RW, Bahl S, Deshpande JM, Verma H, Ahmad M, Venugopal P, Rao JV, Agarkhedkar S, Lalwani SK, Kunwar A, et al. Immunogenicity of a new routine vaccination schedule for global poliomyelitis prevention: an open-label, randomised controlled trial[J]. Lancet. 2015;386(10011):2413–21. http://onlinelibrary.wiley.com/o/cochrane/clcentral/articles/547/CN-01108547/frame.html.
- Saleem AF, Mach O, Quadri F, Khan A, Bhatti Z, Rehman NU, Zaidi S, Weldon WC, Oberste SM, Salama M, et al. Immunogenicity of poliovirus vaccines in chronically malnourished infants: a randomized controlled trial in Pakistan[J]. Vaccine. 2015;33(24):2757–63. doi:10.1016/j.vaccine.2015.04.055.
- Fleischmann-Struzek C, Goldfarb DM, Schlattmann P, Schlapbach LJ, Reinhart K, Kissoon N. The global burden of paediatric and neonatal sepsis: a systematic review[J]. Lancet Respir Med. 2018;6(3):223–30.
- Higgins JP, Green S. Assessing risk of bias in included studies. http://handbook.cochrane.org/. [updated March 2011].
- Tang G, Yin W, Liu W. Is frozen fecal microbiota transplantation as effective as fresh fecal microbiota transplantation in patients with recurrent or refractory Clostridium difficile infection: A meta-analysis?[J]. Diagn Microbiol Infect Dis. 2017;88(4):322–9.
- Chambers DRM, Woolacott N. Not only randomized controlled trials, but also case series should be considered in systematic reviews of rapidly developing technologies[J]. J Clin Epidemiol. 2009;62(12):1253–60.e4.