ABSTRACT
Europe is increasingly described as the region in the world with the least confidence in vaccination, and particularly in the safety of vaccines. The aim of this systematic literature review was to gather and summarise all peer-reviewed and grey literature published about determinants of Human Papillomavirus (HPV) vaccine hesitancy in Europe. Ten thematic categories were identified across the 103 articles which were included in the review. Participants from European studies most commonly reported issues with the quantity and quality of information available about HPV vaccination; followed by concerns about potential side effects of the vaccine; and mistrust of health authorities, healthcare workers, and new vaccines. Comparative analyses indicated that confidence determinants differed by country and population groups. This evidence supports the need to develop context-specific interventions to improve confidence in HPV vaccination and design community engagement strategies aiming to build public trust.
Introduction
Human Papillomavirus (HPV) vaccination was introduced into national immunisation programmes in all European Union (EU) countries, apart from Poland, between 2006 and 2018.Citation1
The HPV vaccine is mainly given to adolescent girls (9–18 years old) to prevent cervical cancer and/or genital warts, but sometimes also given to boys (e.g. Austria) or men who have sex with men (e.g. the United Kingdom (UK)) to prevent other HPV-induced cancers.Citation1 Coverage rates have been suboptimal in some EU countries, particularly in Eastern Europe but also in Ireland, France, and Denmark.Citation2 Romania had initiated a programme in 2008, but discontinued their HPV vaccination in 2014, due to very low acceptance.Citation1 These variations could partly be explained by contextual and implementation factors because the vaccine is currently delivered through schools or public or private health systems, depending on the country and immunisation programme. However, HPV vaccination coverage rates are also affected by healthcare worker (HCW) recommendations and public demand which are both known to be influenced by confidence in the vaccine.Citation3
In recent years, HPV vaccination has suffered from growing public distrust and criticism in Europe.Citation4 Vaccine hesitancy has been defined by the World Health Organization (WHO) SAGE working group as a behaviour influenced by issues of confidence, complacency, and convenience. Vaccine hesitancy does not always imply vaccine refusal, as hesitant individuals can accept certain vaccines but still have doubts about them.Citation5 European vaccine hesitancy can partly be attributed to a lack of confidence in vaccine safety, perceptions that vaccines do not work, distrust of information, perceived low risks of vaccine-preventable diseases, as well as a lack of trust in HCWs, authorities, and pharmaceutical companies.Citation4,Citation6
Many studies have been conducted in Europe and around the world to explore public confidence in HPV vaccination. Some reviews have tried to summarise these studies,Citation7-Citation10 but they have generally focused on a particular population group or outcome. The aim of this study was to systematically review all available literature on determinantsFootnote1 of HPV vaccine hesitancy for any population group in Europe. The specific objectives of the review were to understand determinants of HPV vaccine hesitancy in the EU, compare determinants of HPV vaccine hesitancy in different European Member States, and examine the importance of safety concerns around HPV vaccination.
Methods
Search strategy and inclusion/exclusion criteria
A search strategy was developed in OVID Medline and adapted for use across Embase, PsycINFO, Social Policy and Practice, and Global Health in November 2016. Keywords were drawn from the SAGE review on vaccine hesitancyCitation5 and reviewed by a panel of European experts, selected by the European Centre for Disease Prevention and Control (ECDC) as reviewers for this project. A grey literature search was simultaneously conducted across Open Grey, Web of Science, PsycEXTRA, and organisation websites (ECDC, WHO, the UK Department for International Development, and the Communication Initiative Network).
The selection criteria, developed from the research question by two researchers (EK, HL) and reviewed by the European experts, were broad enough to ensure access to as many studies as possible on determinants of HPV vaccine hesitancy in Europe. The search also focused on reasons for refusal or concern, public trust and confidence, perceptions, attitudes, and beliefs about HPV vaccination as the expression “vaccine hesitancy” was not commonly used before the WHO SAGE Working Group on Vaccine Hesitancy brought more attention to and usage of the term, as well as characterising and defining it.Citation11 Articles were excluded when they did not include results about hesitancy, confidence, or trust in HPV vaccination, for instance, articles focusing solely on reasons for accepting HPV vaccination, knowledge or awareness, or uptake or intentions to vaccinate (without reasons). While certain socio-economic determinants (i.e. age, income, education level) may be associated with HPV vaccination uptake or intentions, they were excluded from this study in order to retain focus on the less-studied, non-socio-economic determinants such as mistrust and uncertainty. Articles about all types of HPV vaccine were included.
No restrictions were made on study participants, settings, or publication year. Articles from any EU or European Economic Area (EEA) countries and language were included, although only English language search terms were used. Articles comparing different vaccines or countries from inside and outside Europe were included only if data for HPV vaccination and/or EU countries were included. Quantitative (observational cross-sectional studies) and qualitative studies were included, but articles without original data (i.e. commentaries or editorials) or those which had the following foci were excluded: safety or efficacy research, serologic or immunogenicity studies, pre-clinical trials, cost-benefit or cost-effectiveness analysis. Intervention studies without data about determinants of vaccine hesitancy were also excluded as this review focused only on determinants of vaccine hesitancy.
Selection, management, and analysis of articles
Articles were stored in Endnote X7 (Thomson Reuters) and after duplicates were removed, were screened independently by two (EK, CS) reviewers (title and abstract followed by full text appraisal). Reviewers met to discuss differences in selection by reviewing reasons for exclusion or inclusion until consensus was met. The number of articles included at various stages were summarised using the PRISMA chart. Data from articles in English, Spanish, French, or Italian was directly collected by EK and CS on a Microsoft Excel spreadsheet while articles in other European languages were first translated by experts at ECDC.
Methodologies for mixed-methods systematic literature reviews – which combine the synthesis and analysis of qualitative and quantitative research – are relatively new and developing rapidly.Citation12 There is currently insufficient methodological evidence on how to conduct mixed-methods reviews to identify and develop an understanding of health-related beliefs and/or behaviours. The methods for this study were therefore developed by combining and adapting methodologies from different types of mixed-methods reviews and are described in more details in the paragraph below.Citation12-Citation19
Data extraction and analysis were performed by one researcher (EK) and reviewed by two researchers (CJ, HL). Two separate descriptive summaries and analyses of the studies were undertaken: one qualitative and one quantitative. No integration of qualitative and quantitative studies was performed. Data from mixed methods studies were included in both analyses. Qualitative studies were analysed thematically: a set of relevant themes was developed inductively, into which each concern was categorised and analysed. No meta-analysis could be performed for the quantitative studies, due to the heterogeneity of the studies. Instead, a descriptive analysis was performed by reporting average proportions of participants with specific concerns about HPV vaccination. Due to some studies not presenting the raw data, each proportion was calculated by averaging proportions extracted from the studies. The quantitative studies identified through the systematic review were found to assess determinants of vaccine hesitancy by looking at i) concerns among participants who had refused (or would refuse) the HPV vaccine; ii) concerns among participants who had accepted (or would accept) the HPV vaccine; or iii) concerns among participants in general (those do not correspond to the sum of the two first groups but to studies that did not specify whether participants had refused or accepted the HPV vaccine). These three categories, summarised respectively as i) “hesitant” participants, ii) vaccine “favourable” participants and iii) “general” participants were created by the reviewer (EK) to sort data extracted from all studies, and to limit bias when reporting proportions based on different denominators. It is important to note that concerns or doubts were reported in all three groups, and hesitant individuals can be found in hesitant, favourable and general participants (see definition of hesitancy in the introduction).
Critical appraisal of studies was performed by one researcher (CJ) and checked by another (EK). The “Effective public health practice project quality assessment tool”Citation20 was used to appraise quantitative studies and the “Critical appraisal skills programme qualitative checklist”Citation21 was used for qualitative studies. Mixed methods studies were appraised using both of these tools. Studies were included regardless of the outcome of their appraisal.
Results
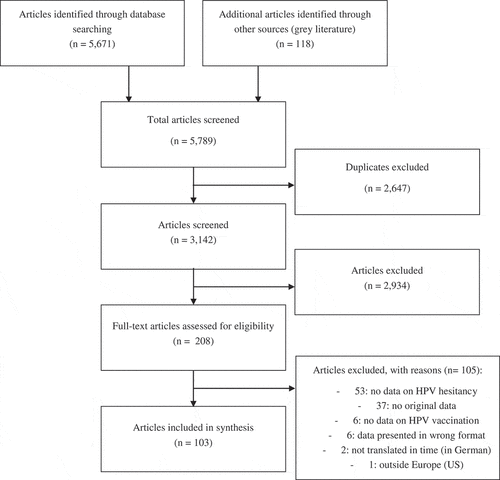
The systematic literature review yielded 3,143 unique peer-reviewed and grey literature articles, of which 2,934 were excluded after title and abstract review based on the inclusion and exclusion criteria. From the 209 full text articles screened, 103 were included in the final analysis (see ).
Twenty out of the 31 qualitative studies were found to be of “good” quality (vs. 6/31 “reasonable” and 5/31 “insufficient”); and 1/65 studies with quantitative data were assessed as “strong” (vs. 25/65 “moderate” and 39/65 “weak”) (see supplementary material 1). From the mixed methods studies, 0/7 were assessed as “strong” for their quantitative sections (vs. 2/7 “moderate” and 5/7 “weak”), while 2/7 were as assessed as “good” for their qualitative sections (vs. 3/7 “reasonable” and 2/7 “insufficient”).
Most studies were conducted with parents (34/103, 10 of which were with mothers only) and HCWs (22/103); and reported results from the UK (28/103), Italy (12/103), France (10/103), and Sweden (10/103). Readers should exert caution when looking at the data, as studies included in the review were conducted both before or after the introduction of the vaccine (from 2005–2016), which could have influenced some public opinions and therefore might not be representative of current public perceptions in countries. The years in which study data were collected are added in the results section wherever country-specific data is presented (if data collection year was not available, the publication year is instead presented). More information about study characteristics, including whether the study was conducted before or after the vaccine was introduced, are available in supplementary material 2.
Determinants of HPV vaccine hesitancy in Europe
Ten thematic categories of determinants of HPV vaccine hesitancy in Europe were identified across the literature: i) information issues, ii) concerns about vaccine safety, iii) issues of trust, iv) effectiveness of the vaccine, v) influencers, vi) issues related to sexual behaviour, vii) against all or too many vaccines, viii) access barriers, ix) perceived need for the vaccine and risk of disease, and x) fear of injections. These are discussed in more detail in the following sub-sections. The themes most frequently identified in qualitative studies () were: concerns about potential side effects of HPV vaccination (37 studies with qualitative data), beliefs that information about the vaccine is insufficient and inadequate (31), and issues related to the sexual health aspects of the vaccine (22). The categories of concerns raised by the highest average proportions of hesitant participants across all quantitative studies in the review () were: perceived insufficient and/or inadequate information and knowledge about the vaccine (average of 44.2% of participants across all studies), fear of perceived side effects (43.3%), mistrust of health authorities, any type of doctor and new vaccines (39.7%), and doubts about the effectiveness of the vaccine (33.7%). Fear of needles and injections (9.4%) and low perceived need for the vaccine or low risk of HPV/cervical cancer (14.1%) were the categories least often reported by hesitant participants across all studies.
Figure 2. Average proportions of hesitant participants who reported certain categories of determinants of HPV vaccine hesitancy, by country.
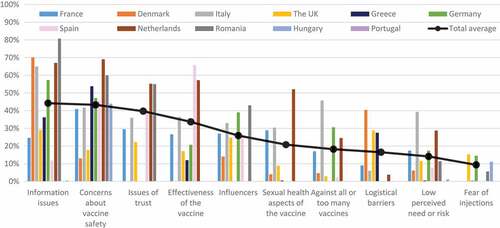
Differences between countries () and population groups () were identified, although care should be taken when looking at these differences throughout the paper as they could be due to differences in study design, variable definitions, and participant selection. For instance, overall results for a specific theme such as perceived lack of information could reflect the views of a particular population if more studies were conducted with this group. While perceived insufficient and inadequate information about HPV vaccination was found to be the most important theme in studies with hesitant participants in Romania, Denmark, Italy, Germany, and the UK, fear about potential vaccine side effects predominated the studies in the Netherlands, Greece, Hungary, and France. In Spanish studies, the most commonly reported theme was doubt about the effectiveness of the vaccine.
Figure 3. Average proportions of hesitant participants who reported certain categories of determinants of HPV vaccine hesitancy, by population groups.
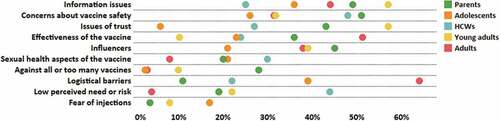
Concerns about potential side effects of HPV vaccination were commonly reported by hesitant parents and HCWs in studies but came second for studies with hesitant adolescents. Adolescents included in studies mostly reported dissatisfaction with the quality and quantity of information available about HPV vaccination. This was the second most important issue for hesitant parents included in studies. For studies with hesitant HCWs, the second most commonly reported theme was a low perceived need for the vaccine or a low perceived risk of HPV and/or cervical cancer.
Information issues
Quantitative results
Insufficient knowledge or information, and beliefs that the information available is unclear, biased and/or inadequate were identified in almost all articles reviewed. An average of 44% of hesitant participants from quantitative studies reported that there was insufficient information available about HPV vaccination, and/or that their own knowledge was insufficient,Citation22-Citation41 particularly in studies in Romania (2015, 81%),Citation30 the Netherlands (2009–2011, 67%),Citation26,Citation28 and Denmark (2010, 70%).Citation35 This was also reported by 53% of general study participantsCitation42-Citation51 and 11% of favourable study participants.Citation25 Additionally, 92% of hesitant parents in a study from 2009 from the Netherlands believed information about the vaccine was biased and unclear,Citation26 while 32% of general parents in a study from 2012 in ItalyCitation45 and 18% of general parents in a study from 2009 in the NetherlandsCitation44 believed information was unreliable. General parents in studies in the UK (2005) and Italy (2007) most frequently reported a need for more information about HPV vaccination efficacy (74%),Citation50,Citation51 followed by vaccine safety (48%),Citation50 and duration of protection (10%).Citation50
Qualitative results
In qualitative studies, a need for clearer, more transparent, and unbiased information about HPV vaccinationCitation52-Citation69 was identified by some participants, together with the need for more verbal, interactive communication.Citation52,Citation55,Citation57,Citation65,Citation69 Additionally, some participants recommended providing information in schools, for instance by organising peer discussion groups with vaccinated girlsCitation53,Citation55-Citation57,Citation61,Citation62,Citation67,Citation69; while others preferred social media or communication methods that allow more discretion.Citation52-Citation54,Citation62,Citation69
Concerns about vaccine safety
Quantitative results
Concerns about the safety of HPV vaccination and potential long- or short-term side effects were reported by an average of 43% of hesitant study participants.Citation23-Citation29,Citation31,Citation32,Citation34-Citation38,Citation40,Citation42,Citation46,Citation49,Citation70-Citation81 Fear of side effects was reported by an average of 52% of general study participantsCitation51,Citation82-Citation97 and 17% of favourable study participants.Citation25,Citation37,Citation71 Concerns about side effects were particularly prevalent among hesitant participants from studies in the Netherlands (2009–2011, 69%),Citation26,Citation28 Romania (2010, 60%),Citation79 and Greece (2005–2014, 54%).Citation31,Citation34,Citation40,Citation42,Citation78
Qualitative results
Almost all qualitative articles described concerns about potential side effects of HPV vaccination,Citation52-Citation57,Citation59,Citation60,Citation62,Citation64,Citation66,Citation69,Citation98-Citation107 often described as fears of long-term side effects not yet identified through trials.Citation54,Citation55,Citation59,Citation60,Citation62,Citation64,Citation66,Citation69,Citation81,Citation98-Citation104,Citation106 In qualitative studies, the most commonly reported perceived side effect by parents was infertility.Citation52,Citation57,Citation59,Citation60,Citation65,Citation100,Citation102,Citation106 Other perceived side effects included: autoimmune complications,Citation56,Citation57,Citation64,Citation99,Citation104,Citation108 resistance of the virus to vaccines and/or treatment,Citation99,Citation102,Citation105 cancer,Citation52menstrual complications,Citation65 HPV infection,Citation59 and death.Citation65
In France, mothers and HCWs in studies conducted between 2007–2010 discussed rumours about Hepatitis B vaccination allegedly causing multiple sclerosis and some expressed worries HPV vaccination could have similar side effects.Citation22,Citation81,Citation107,Citation109 In Sweden, study participants in 2012 worried about the safety of HPV vaccination due to reports of narcolepsy following H1N1 vaccination.Citation56
Issues of trust
Quantitative results
Mistrust of health authorities was reported by an average of 47% of general participants from studies in Sweden (2007),Citation110 Hungary (2009),Citation72 and the UK (2006)Citation91 as well as 55% of hesitant participants from studies in France (2015)Citation74 and the Netherlands (2009).Citation26 Additionally, 12% of general practitioners (GPs) in general in a study conducted between 2007–2010 in France reported mistrust because of “excessive marketing” around HPV vaccination.Citation22
Mistrust of doctors was raised by 41% of hesitant parents in a 2009 study from the Netherlands,Citation26 and 45% of general parents and young adults in studies from Hungary (2009)Citation72 and the UK (2006).Citation91
Mistrust of new vaccines and concerns about the relative newness of the HPV vaccine was reported by an average of 36% of hesitant study participants.Citation22,Citation27,Citation45,Citation74,Citation75,Citation107,Citation111,Citation112 In French studies, concerns about the newness of the vaccine, reported by general GPs, decreased with time but remained important: from 42% in 2007Citation22,Citation107 to 37% in 2010Citation22 and 30% in 2015.Citation74 Mistrust of new vaccines was reported only by 9% of general study participants in 2010 in GermanyCitation95 and 1% of favourable study participants in Italy in 2013.Citation111
Qualitative results
Beliefs that governments were not transparent,Citation57,Citation59 influenced by vaccine manufacturersCitation52,Citation98 or were withholding information about side effectsCitation65,Citation98 were reported in some qualitative studies. Some participants shared concerns about transparency,Citation52,Citation59 commercial influences from pharmaceutical companies,Citation52,Citation98,Citation104,Citation113 and perceptions that doctors were dismissive and deliberately withheld information about side effects.Citation52,Citation65,Citation98,Citation104 Mistrust of pharmaceutical companies due to their underlying profit-making motives and doubts about the trustworthiness of vaccine trials and safety claims were also reported in studies in Bulgaria (2009–2014),Citation98,Citation104 Romania (2010–2012),Citation52 Sweden (2010, 2012),Citation55,Citation56 Ireland (2012),Citation63 the Netherlands (2008),Citation60 Spain (2014),Citation98 and the UK (2014).Citation98
Finally, conspiracy theories about the vaccine allegedly contributing to reducing world population or being an experiment on young girls were reported in studies in Bulgaria (2010–2013),Citation104 Romania (2010–2012),Citation52 Sweden (2014),Citation57 the Netherlands (2015),Citation65 and the UK (2007–2016).Citation59,Citation100,Citation105
Effectiveness of the vaccine
Quantitative results
Across all studies, an average of 34% of hesitant participants were found to doubt the effectiveness of the vaccine,Citation23,Citation24,Citation26,Citation32,Citation34,Citation37,Citation73-Citation75,Citation77,Citation114 as well as 39% of general participantsCitation43,Citation46,Citation82,Citation84-Citation86,Citation96 and 3% of favourable participants.Citation26,Citation37 Perceived low vaccine effectiveness was particularly prevalent among hesitant participants in studies in Spain (2013, 66%),Citation112 the Netherlands (2009, 57%),Citation26 and Italy (2006–2014, 37%).Citation73,Citation75,Citation114 Additionally, 41% of hesitant study participantsCitation74,Citation112 and 31% of general study participantsCitation84,Citation86,Citation97 doubted the length of protection of the vaccine.
Qualitative results
In addition to worries about the duration of effectiveness of the vaccine,Citation22,Citation52,Citation55–Citation57,Citation60,Citation64,Citation67,Citation99,Citation104,Citation105,Citation108,Citation109,Citation115 participants in qualitative studies also reported concerns that the vaccine did not protect against all types of HPV.Citation22,Citation58,Citation101,Citation105,Citation109,Citation115,Citation116
Influencers
Quantitative results
While an average of 30% of hesitant parents reported not having received any recommendation to vaccinate from HCWs in studies in Spain (2010–2011),Citation36 Italy (2012–2015),Citation29,Citation45,Citation75 Denmark (2010)Citation35 and France (2015),Citation29 an average of 26% of hesitant parents in studies from Spain (2010–2011)Citation36 and Italy (2012–2014)Citation27,Citation75 were advised not to vaccinate by their HCW. Additionally, 19% of general adolescents in a study from Germany in 2010 reported having been advised not to get the HPV vaccine by their physician.Citation95
Furthermore, an average of 47% of hesitant parents from studies in Romania (2010)Citation79 and Italy (2012)Citation27,Citation45 mentioned they had received contradictory advice and opinions from different healthcare professionals or specialists. A study published in 2015 in Italy, where both boys and girls have been vaccinated since 2017, showed 38% of general men who attended sexual health clinics reported receiving contradictory advice.Citation87
Some adolescents also reported being influenced by their parents, with 25% of hesitant adolescent girls from studies in the UK (2008–2013),Citation23,Citation68 39% of general adolescent girls from a study in Germany (2010)Citation95 and 1% of general adolescent girls from a study in Sweden (2008)Citation47 mentioning they could not get vaccinated because their parents had refused the vaccine.Citation25,Citation70 Additionally, 11% of hesitant adolescent girls from a 2015 study from Romania reported that their parents thought the vaccine was unsafe.Citation30
An average of 75% of hesitant parents from a 2010 study from RomaniaCitation79 and 39% of hesitant young women from a 2013 study from GermanyCitation37 reported being influenced by others who had refused HPV vaccination or who had recommended against it. Additionally, 49% of general adolescent girls from a 2007 study from the UK reported being influenced by rumours.Citation23
Qualitative results
Healthcare professionals, including school nurses and GPs, were commonly referred to in qualitative studies as having the most influence on HPV vaccination decisions.Citation52,Citation57,Citation68,Citation80,Citation99,Citation104,Citation117 Additionally, the influence of family members, friends, and parents of other children who did not vaccinate or who recommended against the vaccine were reported across some studies.Citation52,Citation57,Citation67,Citation100
Issues related to sexual behaviour
Quantitative results
The belief that HPV vaccination might encourage promiscuity or earlier sexual debut in young girls was observed in an average of 11% of hesitant study participants.Citation22,Citation23,Citation26,Citation32,Citation50,Citation71 This belief was also reported among 30% of general study participantsCitation51,Citation75,Citation85,Citation89,Citation90,Citation110,Citation118 and 6% of favourable study participants.Citation22,Citation71
An average of 20% of hesitant study participants,Citation22,Citation26,Citation27,Citation50,Citation74,Citation75,Citation107 20% of general study participants,Citation43,Citation90,Citation93,Citation97,Citation110 and 23% of favourable study participantsCitation22 also believed that the vaccine would lead to unsafe sexual behaviour and a decrease in the use of condoms and cervical cancer screening. This fear was reported by 19% of hesitant HCWs in studies in France (2007–2015)Citation22,Citation74,Citation107 and 23% of hesitant parents in studies in Italy (2007–2014).Citation27,Citation50,Citation75
Concerns about girls being too young were reported by an average of 13% of hesitant study participantsCitation23,Citation26,Citation27,Citation29,Citation30,Citation32,Citation35,Citation45,Citation75 and 30% of general study participants.Citation48,Citation110 It was most commonly reported by hesitant parents in a study in the Netherlands (2009, 52%)Citation26 and hesitant school nurses in a study in Sweden (2013, 48%).Citation110 The concern that it is too difficult to discuss HPV vaccination with adolescent girls was also discussed in studies by an average of 21% of hesitant parents and paediatricians,Citation35,Citation73 and 11% of general parents and HCWs.Citation22,Citation51,Citation82,Citation107,Citation109 An average of 27% of general GPs in a 2014 study in FranceCitation82 and 32% of general adolescents in a 2008 study in SwedenCitation47 also believed it was difficult to talk about the vaccine with parents, while 21% of general adolescents in a study in the UK (2007–2008) reported embarrassment around getting a vaccine against a sexually transmitted infection.Citation85
Cultural and religious barriers to vaccination were indicated by an average of 16% of hesitant participants,Citation26,Citation29,Citation31,Citation32 particularly in a study in the Netherlands (2009, 70%).Citation26
Qualitative results
Qualitative studies reported beliefs that HPV vaccination might encourage promiscuity or earlier sexual debut in young girlsCitation61,Citation65-Citation67,Citation100,Citation101,Citation106,Citation108,Citation119 and that the vaccine could lead to unsafe sexual behaviour and a decrease in the use of condoms and cervical cancer screening.Citation55,Citation57,Citation63,Citation64,Citation66,Citation67,Citation80,Citation106,Citation108,Citation109,Citation119
Additionally, some participants mentioned that the vaccine is being given to girls when they are too young because they have not started to menstruate, are not yet sexually active or married,Citation54,Citation57,Citation58,Citation61,Citation100,Citation108 or because they are too “naïve and immature” to give their consent.Citation98,Citation103 Some also reported that it is not easy to talk about HPV vaccination with girls that are so young.Citation20,Citation53,Citation54,Citation59,Citation61,Citation63,Citation79,Citation98,Citation99,Citation105,Citation108,Citation114
Finally, cultural and religious influences on vaccine acceptance reported in studies included perceived low risk of infection because of certain lifestyles (being virgins when marrying, having only one partner),Citation54,Citation57,Citation60,Citation61,Citation65,Citation99,Citation100,Citation105,Citation108,Citation109 and having a strong sense of fatality and/or belief that God will protect girls.Citation113
Against all or too many vaccines
Quantitative results
An average of 18% of hesitant study participantsCitation25,Citation26,Citation32,Citation36,Citation37,Citation77,Citation80 were against all vaccines in general. Additionally, 33% of general study participantsCitation90,Citation91 and 28% of hesitant study participantsCitation26,Citation29,Citation35,Citation50,Citation75,Citation80 believed children already receive too many vaccines, with 47% of hesitant parents in a 2015 study in GermanyCitation29 and 46% of hesitant parents in studies in Italy (2007–2015)Citation29,Citation50,Citation75 raising this concern.
Qualitative results
A few qualitative studies reported that some participants were against all vaccines.Citation54,Citation57,Citation59,Citation69,Citation99,Citation104
Access barriers
Quantitative results
In quantitative studies, cost was raised as an issue by an average of 32% of hesitant participants,Citation31,Citation38,Citation74,Citation75,Citation80 particularly in studies in Denmark (2009, 62%)Citation80 and Greece (2008–2014, 54%),Citation31 and was also raised as a concern for an average of 47% of general study participants in Germany, Greece, Hungary, Ireland, and Sweden.Citation46,Citation47,Citation94,Citation97,Citation120 The inconvenience of receiving multiple doses was reported by 56% of general participants in studies in Sweden (2007–2013),Citation96,Citation110 25% of hesitant participants in studies in France (2015)Citation74 and the UK (2007–2008),Citation71 and 10% of favourable participants in a study in the UK (2007–2008).Citation71
Increased workload created by administering the vaccine was raised as a barrier by general school nurses in a study in Sweden (2013, 40%),Citation110 while general adolescents in studies in Germany (2010, 12%)Citation95 and Greece (2008–2014, 1%)Citation31 reported being too busy to get vaccinated.
Other accessibility issues such as not having a consent form, being absent from school when vaccination was being administered, recent migration or not being in the target age group were also reported by an average 10% of hesitant participants in studies in the Netherlands (2009),Citation26 the UK (2011–2013),Citation25,Citation39 Italy (2008–2009),Citation121 Denmark (2009),Citation80 and Portugal (2007–2008).Citation33
Qualitative results
The high cost of the HPV vaccine was mentioned in five qualitative studies, from 2009–2010 in Sweden,Citation64,Citation102 2010–2014 in Bulgaria,Citation98,Citation104 2014 in the UK,Citation98 2014 in Spain,Citation98 and 2012 in Ireland.Citation63 Another access barrier was the inconvenience of going to get multiple injections.Citation67,Citation115
The challenge of vaccinating in schools was discussed in studies in Sweden (2010–2014)Citation55-Citation57 and in the UK (2006–2015),Citation59,Citation61,Citation101,Citation116 with reasons including a lack of privacy, children needing a calmer environment, missing classes, and parental informed consent. The question of which healthcare professional should administer the vaccine was raised in studies in Sweden (2010)Citation55,Citation64 and the UK (2007–2008),Citation59 with feelings of competition and distrust between gynaecologists and paediatricians identified in a study in Greece (2016).Citation99
Perceived need for the vaccine and risk of disease
Quantitative results
A perceived lack of need for the HPV vaccine was reported by an average of 14% of hesitant study participants,Citation25,Citation31,Citation32,Citation36,Citation38,Citation70,Citation75,Citation121 5% of general study participants,Citation88,Citation95 and 2% of favourable study participants.Citation25
A perceived low risk of contracting HPV infection or developing cervical cancer was reported by an average of 22% of hesitant study participantsCitation23,Citation24,Citation26,Citation33,Citation35,Citation49,Citation76,Citation77,Citation79,Citation80,Citation114 and 26% of favourable study participants.Citation26 Among hesitant participants, the perceived low risk of HPV was particularly prevalent in studies in Italy (2007–2015, 44%)Citation49,Citation76,Citation114 and the Netherlands (2009, 38%).Citation26
Qualitative results
Some participants in qualitative studies expressed a perceived lack of need for the HPV vaccine due to a perceived low risk of contracting HPV and/or cervical cancer,Citation55,Citation59,Citation66,Citation80,Citation100,Citation109,Citation115 a perceived low severity of the disease,Citation63,Citation66,Citation81 and/or the availability of alternative prevention methods or abstinence.Citation54,Citation60,Citation66,Citation69,Citation101,Citation105,Citation108,Citation115
Fear of injections
Quantitative results
Fear of needles and injection pain was reported by 29% of general study participants,Citation47,Citation85,Citation89,Citation92 9% of hesitant study participants,Citation24,Citation25,Citation30,Citation31,Citation37,Citation39,Citation46,Citation72 and 5% of favourable study participants.Citation25
Qualitative results
Qualitative studies reported rumours among adolescents about vaccination pain,Citation102,Citation116 the size of the needles and the pain at injection increasing with each dose,Citation62,Citation116 the mistaken belief of the vaccine being administered in the cervix,Citation106,Citation116 concerns about needle cleanliness,Citation116 and the fear that the injection could lead to a loss of virginity.Citation68
Discussion
This systematic literature review identified 103 unique articles on determinants of HPV vaccine hesitancy in Europe. Across European studies, the most prevalent concerns were about: insufficient and inadequate information about HPV vaccination; potential side effects of the vaccine; issues around trust of health authorities, doctors, and new vaccines; and perceived low vaccine effectiveness. While issues about the sexual health aspects of the vaccine were reported in many qualitative studies, they were less prevalent in quantitative studies, which could be explained by the nature of qualitative studies that ask open-ended questions.
Some differences were observed between studies from different European countries, with studies from Italy reporting the highest average proportion of participants with concerns about vaccination in general, issues related to the sexual health aspects of the vaccine, and perceived low risk of HPV/cervical cancer and consequent doubts about the need for the HPV vaccine. Differences might be explained by different contexts and national immunisation programmes as well previous experiences with vaccination confidence crises but could also be due to differences in study designs and the methodology used for the systematic review.
Many concerns identified in this review were more frequently observed among study participants in general rather than in vaccine-hesitant participants, while some concerns were also reported by large proportions of favourable participants. This could be an effect of the methodology used to summarise proportions from different studies in this review: the differences could be explained by the fact that proportions from different years, countries, and population groups were averaged and compared. However, it could also point to other issues. More research should therefore be conducted to further explain these differences and explore perceptions about HPV vaccination across the continuum of vaccine hesitancy.
The most frequent concerns reported across studies and among most study participants were a perceived lack of information together with a fear of potential HPV vaccine side effects. This confirms results from previous studies about perceptions of vaccine safety being the most important determinant of vaccine hesitancy for vaccines in general in Europe.Citation4,Citation6 While some study participants raised specific concerns about infertility, most reported having general safety fears that they could not explain or specify. This could be a consequence of the vaccine still being perceived as too new and general uncertainty about the vaccine. A number of studies have shown that uncertainty can influence as much as empirical evidence in vaccination decision-making.Citation122,Citation123
Uncertainty is still often framed as something that can be overcome by filling information gaps with more facts; however, mistrust of health authorities and some doctors was commonly reported in this review, indicating that communication strategies need to include efforts to build and maintain public trust.Citation124 Communication strategies should engage communities, for instance by building alliances with civil society or disease associations, organising school information and discussion sessions with parents and peers, and online discussion groups overseen and managed by local health professionals. More research is needed to understand information-seeking behaviours as well as how individuals appraise and use information about HPV vaccination, and finally to evaluate which communication and engagement strategies are most effective with different population groups (e.g. parents, teenagers, ethnic/religious minorities, or HCWs).Citation4,Citation125,Citation126 Evaluations should focus on communication and engagement strategies developed by health authorities, but also on information that the public is exposed to every day, such as social media, online news, or television documentaries.
While influences from family members, friends, other parents or HCWs were commonly reported by parents in certain studies, adolescents in those studies also reported rumours circulating about HPV vaccination. The viral spread of negative rumours, particularly among adolescents through social media, could prove to be an important challenge should a confidence crisis arise. An example is the recent spread of unverified and subsequently disproved concerns in Denmark through YouTube videos and other social media that HPV vaccine might cause Postural Tachycardia Syndrome (PoTS) and Complex Regional Pain Syndrome (CRPS), as well as the occurrence of mass-psychogenic illnesses that have been observed and linked to HPV vaccination in Colombia and Australia.Citation127-Citation129 These examples illustrate the rapidity by which rumours can spread through social media and the importance of influencers and group dynamics in HPV vaccination confidence among adolescents as well as parents. Faster research is therefore needed, such as through media monitoring, to identify possible anxiety reactions, and to inform time-sensitive strategies – including crisis communication plans – on how to respond to them. The confidence crisis that occurred in Denmark provides a good example of how comprehensive strategies using a combination of social and online media interventions, engagement with mothers and adolescents, and risk communication strategies can successfully reinstate public trust in vaccination. Denmark’s success in reversing the decreasing vaccination coverage trend is in the process of being evaluated and will constitute a good case study for other countries facing similar issues.
HCWs, including GPs, paediatricians, and school nurses were identified in many studies as strong influencers for parents and adolescents but they were also found to have concerns about the safety and effectiveness of HPV vaccination, which could prevent them from recommending the vaccine.Citation109,Citation129 As HCWs are often considered the most trustworthy source of medical information,Citation130 it is important to address their own hesitancy, for instance by improving their training on the introduction of new vaccines. Additionally, as HCWs also reported facing difficulties talking about HPV vaccination in some studies, it is important to provide more support to HCWs and develop skills to manage difficult conversations. Some methods, such as motivational interviewingCitation131,Citation132 have been employed outside of Europe and could be adapted and evaluated in European countries.
Limitations
The results from this systematic literature review should be interpreted with some caution. While the screening of articles was conducted by two independent reviewers, data extraction and critical analysis of studies could only be conducted by a single reviewer. The results from the critical appraisal showed that many quantitative studies were methodologically weak, which can mainly be explained by the fact that the tool used to assess quantitative studies was designed for intervention studies while most studies in the field of vaccine hesitancy are observational, and often cross-sectional. This resulted in low scores for studies that did not use methods such as blinding, which are not applicable to observational studies. This limitation means that the results in this paper could not be discussed together with the critical appraisal results. While readers can refer to the results from the critical appraisal in the supplementary materials, they should therefore interpret these with caution. Information about each article’s reported conflicts of interests and funding sources was also extracted and is available at readers’ request. Additionally, two articles from Germany had to be omitted due to translations not being finalised in time for this report, which could have affected the findings for the country. The database searches were also only performed in English, which means some articles in other languages might have been omitted.
The heterogeneity of the data and the analysis conducted in the different studies, which did not allow for a meta-analysis, might have introduced some bias in the quantitative analysis. For instance, proportions of participants with specific concerns were compared across countries and population groups, although the denominators (i.e. entire population, vaccine refusers, or vaccine acceptors) were sometimes very different. This was partly managed by separating the analysis for different groups. Averages were used to quantify different concerns, although data from individual studies might have been misrepresented especially as very high ranges of proportions were observed. Finally, articles looking at socio-economic determinants of vaccine hesitancy were excluded as they were outside the scope of this review. Future reviews should be conducted looking specifically at the impact of those determinants on hesitancy.
Conclusion
Trust in HPV vaccination is currently being shaken in many European countries, the impact of which is indicated by low and/or decreasing coverage rates. Strategies developed with the goal of addressing HPV vaccine hesitancy should not only focus on providing more information about the safety and effectiveness of the vaccine, but also aim to rebuild and maintain trust in public health institutions, including HCWs and health authorities, in order to prevent or manage future potential confidence crises.
Disclosure of potential conflicts of interest
The authors report no conflict of interest. The LSHTM research group “The Vaccine Confidence Project” has received funding for other projects from the Bill & Melinda Gates Foundation, the Center for Strategic and International Studies, EU Innovative Medicines Initiative (IMI), GSK, National Institute for Health Research (UK), Novartis, and WHO. HL has done consulting on vaccine confidence with GSK and served on the Merck Vaccine Strategic Advisory Board.
Supplemental Material
Download MS Word (253.7 KB)Acknowledgments
The authors from this study would like to thank Pauline Paterson (LSHTM, UK) and Mark Petticrew (LSHTM, UK) for providing guidance on the methodology, as well as Franklin Apfel, Sabrina Cecconi, Jay Dowle, Camilla Hiul Suppli, Darina O’Flanagan, Jean Claude Desenclos, and Viorica Gheorghiu for reviewing this paper.
Supplementary material
Supplemental data for this article can be accessed on the publisher’s website.
Additional information
Funding
Notes
1 In this paper, the expression “determinant” refers to “influences of vaccine acceptance”, as understood by the WHO Sage working group on vaccine hesitancy, and does not always imply causation.
References
- Bruni L, Barrionuevo-Rosas L, Albero G, Serrano B, Mena M, Gómez D, Muñoz J, Bosch FX, de Sanjosé S. Human Papillomavirus and related diseases in Europe. Summary report. Barcelona, Spain: ICO Information Centre on HPV and Cancer (HPV Information Centre); 2017 Jul 27.
- Bruni L, Diaz M, Barrionuevo-Rosas L, Herrero R, Bray F, Bosch FX, de Sanjosé S, Castellsagué X. Global estimates of human papillomavirus vaccination coverage by region and income level: a pooled analysis. Lancet Glob Health. 2016;4(7):e453–e63. doi:10.1016/S2214-109X(16)30099-7.
- Karafillakis E, Larson HJ, Consortium A. The benefit of the doubt or doubts over benefits? A systematic literature review of perceived risks of vaccines in European populations. Vaccine. 2017;35(37):4840–50. Epub 2017/ 08/02. doi:10.1016/j.vaccine.2017.07.061.
- European Centre for Disease Prevention and Control. Rapid literature review on motivating hesitant population groups in Europe to vaccinate. Stockholm, Sweden: ECDC; 2015.
- Larson HJ, Jarrett C, Eckersberger E, Smith DM, Paterson P. Understanding vaccine hesitancy around vaccines and vaccination from a global perspective: a systematic review of published literature, 2007–2012. Vaccine. 2014;32(19):2150–59. doi:10.1016/j.vaccine.2014.01.081.
- Larson HJ, de Figueiredo A, Xiahong Z, Schulz WS, Verger P, Johnston IG, Cook AR, Jones NS. The state of vaccine confidence 2016: global insights through a 67-Country survey. EBioMedicine. 2016;12:295–301. doi:10.1016/j.ebiom.2016.08.042.
- Prue G, Shapiro G, Maybin R, Santin O, Lawler M. Knowledge and acceptance of human papillomavirus (HPV) and HPV vaccination in adolescent boys worldwide: A systematic review. J Cancer Policy. 2016;10:1–15. doi:10.1016/j.jcpo.2016.09.009.
- Patel H, Jeve YB, Sherman SM, Moss EL. Knowledge of human papillomavirus and the human papillomavirus vaccine in European adolescents: A systematic review. Sex Transm Infect. 2016;92(6):474–79. doi:10.1136/sextrans-2015-052341.
- Hendry M, Lewis R, Clements A, Damery S, Wilkinson C. “HPV? never heard of it!”: A systematic review of girls’ and parents’ information needs, views and preferences about human papillomavirus vaccination. Vaccine. 2013;31(45):5152–67. doi:10.1016/j.vaccine.2013.08.091.
- Coles VAH, Patel AS, Allen FL, Keeping ST, Carroll SM. The association of human papillomavirus vaccination with sexual behaviours and human papillomavirus knowledge: a systematic review. Int J STD AIDS. 2015;26(11):777–88. doi:10.1177/0956462414554629.
- SAGE. Report of the SAGE working group on vaccine hesitancy. Geneva, Switzerland: WHO; 2014 Oct. Report No.
- Pope C, Mays N, Jennie P. Synthesising qualitative and quantitative health evidence: a guide to methods. Maidenhead, England: Open University Press; 2007.
- Wong G, Greenhalgh T, Westhorp G, Buckingham J, Pawson R. RAMESES publication standards: realist syntheses. BMC Med. 2013;11:21. Epub 2013/ 01/31. doi:10.1186/1741-7015-11-21.
- Wong G, Greenhalgh T, Westhorp G, Buckingham J, Pawson R. RAMESES publication standards: meta-narrative reviews. BMC Med. 2013;11:20. Epub 2013/ 01/31. doi:10.1186/1741-7015-11-20.
- Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, Moher D, Becker BJ, Sipe TA, Thacker SB. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis of observational studies in epidemiology (MOOSE) group. JAMA. 2000;283(15):2008–12. Epub 2000/ 05/02.
- Dixon-Woods M, Bonas S, Booth A, Jones DR, Miller T, Sutton AJ, Shaw RL, Smith JA, Young B. How can systematic reviews incorporate qualitative research? A critical perspective. Qual Res. 2006;6(1):27–44. doi:10.1177/1468794106058867.
- The Joanna Briggs Institute. The Joanna Briggs institute reviewers’ manual - methodology for JBI mixed methods systematic reviews. Adelaide, Australia: The University of Adelaide; 2014.
- Walsh D, Downe S. Meta-synthesis method for qualitative research: a literature review. J Adv Nurs. 2005;50(2):204–11. Epub 2005/ 03/25. doi:10.1111/j.1365-2648.2005.03380.x.
- Elaine B-P, Thomas J. Methods for the synthesis of qualitative research: a critical review. London: ESRC National Centre for Research Methods; 2009.
- Effective public health practice project. Quality assessment tool for quantitative studies; 2009. http://www.ephpp.ca/tools.html.
- Critical Appraisal Skills Programme. CASP qualitative checklist; 2017. http://www.casp-uk.net/casp-tools-checklists.
- Lasset C, Kalecinski J, Regnier V, Barone G, Leocmach Y, Vanhems P, Chauvin F, Lutringer-Magnin D. Practices and opinions regarding HPV vaccination among French general practitioners: evaluation through two cross-sectional studies in 2007 and 2010. Int J Public Health. 2014;59(3):519–28. doi:10.1007/s00038-014-0555-9.
- Brabin L, Roberts SA, Stretch R, Baxter D, Chambers G, Kitchener H, McCann R. Uptake of first two doses of human papillomavirus vaccine by adolescent schoolgirls in Manchester: prospective cohort study. BMJ. 2008;336(7652):1056–58. doi:10.1136/bmj.39541.534109.BE.
- Forster AS, Marlow LAV, Wardle J, Stephenson J, Waller J. Understanding adolescents’ intentions to have the HPV vaccine. Vaccine. 2010;28(7):1673–76. doi:10.1016/j.vaccine.2009.11.082.
- Forster AS, Waller J, Bowyer HL, Marlow LA. Girls’ explanations for being unvaccinated or under vaccinated against human papillomavirus: a content analysis of survey responses. BMC Public Health. 2015;15:1278. doi:10.1186/s12889-015-2657-6.
- Gefenaite G, Smit M, Nijman HW, Tami A, Drijfhout IH, Pascal A, Postma MJ, Wolters BA, van Delden JJ, Wilschut JC, et al. Comparatively low attendance during human Papillomavirus catch-up vaccination among teenage girls in the Netherlands: insights from a behavioral survey among parents. BMC Public Health. 2012;12:498. doi:10.1186/1471-2458-12-498.
- Giambi C, D’Ancona F, Del Manso M, De Mei B, Giovannelli I, Cattaneo C, Possenti V, Declich S. Exploring reasons for non-vaccination against human papillomavirus in Italy. BMC Infect Dis. 2014;14:545. doi:10.1186/s12879-014-0545-9.
- Hofman R, Van Empelen P, Richardus JH, Kok I, Koning H, Ballegooijen M, Korfage IJ. Predictors of HPV vaccination uptake: a longitudinal study among parents. Health Educ Res. 2014;29(1):83–96. doi:10.1093/her/cyt092.
- Lee Mortensen G, Adam M, Idtaleb L. Parental attitudes towards male human papillomavirus vaccination: a pan-European cross-sectional survey. BMC Public Health. 2015;15:624. doi:10.1186/s12889-015-1863-6.
- Maier C, Maier T, Neagu CE, Vledereanu R. Romanian adolescents’ knowledge and attitudes towards human papillomavirus infection and prophylactic vaccination. Eur J Obstet Gynecol Reprod Biol. 2015;195:77–82. doi:10.1016/j.ejogrb.2015.09.029.
- Mammas IN, Theodoridou M, Koutsaftiki C, Bertsias G, Sourvinos G, Spandidos DA. Vaccination against human Papillomavirus in relation to financial crisis: the “evaluation and education of Greek female adolescents on human Papillomaviruses’ prevention strategies” ELEFTHERIA study. J Pediatr Adolesc Gynecol. 2016;29(4):362–66. doi:10.1016/j.jpag.2015.12.007.
- Marlow LA, Wardle J, Forster AS, Waller J. Ethnic differences in human papillomavirus awareness and vaccine acceptability. J Epidemiol Community Health. 2009;63(12):1010–15. doi:10.1136/jech.2008.085886.
- Medeiros R, Ramada D. Knowledge differences between male and female university students about human papillomavirus (HPV) and cervical cancer: implications for health strategies and vaccination. Vaccine. 2010;29(2):153–60. doi:10.1016/j.vaccine.2010.10.068.
- Michail G, Smaili M, Vozikis A, Jelastopulu E, Adonakis G, Poulas K. Female students receiving post-secondary education in Greece: the results of a collaborative human papillomavirus knowledge survey. Public Health. 2014;128(12):1099–105. doi:10.1016/j.puhe.2014.09.005.
- Mortensen GL. Parental attitudes towards vaccinating sons with human papillomavirus vaccine. Dan Med Bull. 2010;57(12): A4230.
- Navarro-Illana P, Caballero P, Tuells J, Puig-Barbera J, Diez-Domingo J. Acceptability of human papillomavirus vaccine in mothers from Valencia (Spain). Anales De Pediatria. 2015;83(5):318–27. doi:10.1016/j.anpedi.2014.11.018.
- Remschmidt C, Walter D, Schmich P, Wetzstein M, Delere Y, Wichmann O. Knowledge, attitude, and uptake related to human papillomavirus vaccination among young women in Germany recruited via a social media site. Hum Vaccin Immunother. 2014;10(9):2527–35. doi:10.4161/21645515.2014.970920.
- Sabiani L, Bremond A, Mortier I, Lecuyer M, Boubli L, Carcopino X. HPV prophylactic vaccine coverage in France: results of a survey among high school and university students in Marseilles’ area. Journal De Gynecologie, Obstetrique Et Biologie De La Reproduction. 2012;41(2):136–44. doi:10.1016/j.jgyn.2011.10.001.
- Sacks RJ, Copas AJ, Wilkinson DM, Robinson AJ. Uptake of the HPV vaccination programme in England: A cross-sectional survey of young women attending sexual health services. Sex Transm Infect. 2014;90(4):315–21. doi:10.1136/sextrans-2013-051179.
- Sotiriadis A, Dagklis T, Siamanta V, Chatzigeorgiou K, Agorastos T, Group LS. Increasing fear of adverse effects drops intention to vaccinate after the introduction of prophylactic HPV vaccine. Arch Gynecol Obstet. 2012;285(6):1719–24. doi:10.1007/s00404-011-2208-z.
- Tisi G, Salinaro F, Apostoli P, Bassani R, Bellicini A, Groppi L, Donarini P, Pecorelli S. HPV vaccination acceptability in young boys. Annali dell’Istituto Superiore Di Sanita. 2013;49(3):286–91. doi:10.4415/ANN_13_03_09.
- Papagiannis D, Rachiotis G, Symvoulakis EK, Daponte A, Grivea IN, Syrogiannopoulos GA, Hadjichristodoulou C. Vaccination against human papillomavirus among 865 female students from the health professions in central Greece: A questionnaire-based cross-sectional study. J Multidiscip Healthc. 2013;6:435–39. doi:10.2147/JMDH.S49558.
- Nadarzynski T, Smith HE, Richardson D, Ford E, Llewellyn CD. Sexual healthcare professionals’ views on HPV vaccination for men in the UK. Br J Cancer. 2015;113(11):1599–601. doi:10.1038/bjc.2015.403.
- van der Berg JD, Roorda J, Westerman MJ. Reasons not to have your daughter vaccinated against the human papilloma virus in Twente: a questionnaire study. [Dutch]. Ned Tijdschr Geneeskd. 2010;154:A1923.
- Baglioni A, Ceriale E, Bagnoli A, Mercone A, Nante N, Messina G. Parents’ awareness and acceptance of HPV vaccination in Italy. Igiene e Sanita Pubblica. 2014;70:489–98.
- Marek E, Dergez T, Rebek-Nagy G, Kricskovics A, Kovacs K, Bozsa S, Kiss I, Ember I, Gocze P. Adolescents’ awareness of HPV infections and attitudes towards HPV vaccination 3 years following the introduction of the HPV vaccine in Hungary. Vaccine. 2011;29(47):8591–98. doi:10.1016/j.vaccine.2011.09.018.
- Gottvall M, Larsson M, Hoglund AT, Tyden T. High HPV vaccine acceptance despite low awareness among Swedish upper secondary school students HPV and upper secondary school students Gottvall et al. Eur J Contracept Reprod Health Care. 2009;14(6):399–405. doi:10.3109/13625180903229605.
- Korfage IJ, Essink-Bot ML, Daamen R, Mols F, van Ballegooijen M. Women show mixed intentions regarding the uptake of HPV vaccinations in pre-adolescents: A questionnaire study. Eur J Cancer. 2008;44(9):1186–92. doi:10.1016/j.ejca.2008.03.018.
- Napolitano F, Napolitano P, Liguori G, Angelillo IF. Human papillomavirus infection and vaccination: knowledge and attitudes among young males in Italy. Hum Vaccin Immunother. 2016;12(6):1504–10. doi:10.1080/21645515.2016.1156271.
- Tozzi AE, Rava L, Stat D, Pandolfi E, Marino MG, Ugazio AG. Attitudes towards HPV immunization of Italian mothers of adolescent girls and potential role of health professionals in the immunization program. Vaccine. 2009;27(19):2625–29. doi:10.1016/j.vaccine.2009.02.050.
- Brabin L, Roberts SA, Farzaneh F, Kitchener HC. Future acceptance of adolescent human papillomavirus vaccination: a survey of parental attitudes. Vaccine. 2006;24(16):3087–94. doi:10.1016/j.vaccine.2006.01.048.
- Craciun C, Baban A. “Who will take the blame?”: understanding the reasons why Romanian mothers decline HPV vaccination for their daughters. Vaccine. 2012;30(48):6789–93. doi:10.1016/j.vaccine.2012.09.016.
- Fidalgo C, Mauron C, Nyffeler S, Pache B, Pereira PP. Vaccination against papillomavirus: words of adolescents. Rev Med Suisse. 2013;9:1438–39.
- Gordon D, Waller J, Marlow LAV. Attitudes to HPV vaccination among mothers in the British Jewish community: reasons for accepting or declining the vaccine. Vaccine. 2011;29(43):7350–56. doi:10.1016/j.vaccine.2011.07.083.
- Gottvall M, Tyden T, Larsson M, Stenhammar C, Hoglund AT. Challenges and opportunities of a new HPV immunization program. Perceptions among Swedish school nurses. Vaccine. 2011;29(28):4576–83. doi:10.1016/j.vaccine.2011.04.054.
- Gottvall M, Grandahl M, Hoglund AT, Larsson M, Stenhammar C, Andrae B, Tyden T. Trust versus concerns-how parents reason when they accept HPV vaccination for their young daughter. Ups J Med Sci. 2013;118(4):263–70. doi:10.3109/03009734.2013.809039.
- Grandahl M, Oscarsson M, Stenhammar C, Neveus T, Westerling R, Tyden T. Not the right time: why parents refuse to let their daughters have the human papillomavirus vaccination. Acta Pediatr, Int J Pediatr. 2014;103(4):436–41. doi:10.1111/apa.12545.
- Grandahl M, Tyden T, Gottvall M, Westerling R, Oscarsson M. Immigrant women’s experiences and views on the prevention of cervical cancer: a qualitative study. Health Expect. 2012;18(3):344–54. doi:10.1111/hex.12034.
- Hilton S, Hunt K, Bedford H, Petticrew M. School nurses’ experiences of delivering the UK HPV vaccination programme in its first year. BMC Infect Dis. 2011;11:226. doi:10.1186/1471-2334-11-226.
- Hofman R, van Empelen P, Vogel I, Raat H, van Ballegooijen M, Korfage IJ. Parental decisional strategies regarding HPV vaccination before media debates: a focus group study. J Health Commun. 2013;18(7):866–80. doi:10.1080/10810730.2012.757390.
- Jackson C, Dyson L, Bedford H, Cheater FM, Condon L, Crocker A, Emslie C, Ireland L, Kemsley P, Kerr S, et al. UNderstanding uptake of immunisations in travelling aNd Gypsy communities (UNITING): a qualitative interview study. Health Technol Assess. 2016;20(72):1–176. doi:10.3310/hta20720.
- Kennedy C, Gray Brunton C, Hogg R. ‘Just that little bit of doubt’: scottish parents’, teenage girls’ and health professionals’ views of the MMR, H1N1 and HPV vaccines. Int J Behav Med. 2014;21(1):3–10. doi:10.1007/s12529-013-9356-4.
- McSherry LA, Dombrowski SU, Francis JJ, Murphy J, Martin CM, O’Leary JJ, Sharp L, Group A. ‘It’s a can of worms’: understanding primary care practitioners’ behaviours in relation to HPV using the theoretical domains framework. Implementation Sci. 2012;7:73. doi:10.1186/1748-5908-7-73.
- Oscarsson MG, Dahlberg A, Tyden T. Midwives at youth clinics attitude to HPV vaccination and their role in cervical cancer prevention. Sex Reprod Healthc. 2011;2(4):137–42. doi:10.1016/j.srhc.2011.09.001.
- Salad J, Verdonk P, De Boer F, Abma TA. “A Somali girl is Muslim and does not have premarital sex. Is vaccination really necessary?” A qualitative study into the perceptions of Somali women in the Netherlands about the prevention of cervical cancer. Int J Equity Health. 2015;14(1):68. doi:10.1186/s12939-015-0198-3.
- Waller J, Marlow LAV, Wardle J. Mothers’ attitudes towards preventing cervical cancer through human papillomavirus vaccination: a qualitative study. Cancer Epidemiol Biomarkers Prev. 2006;15(7):1257–61. doi:10.1158/1055-9965.EPI-06-0041.
- Williams K, Forster A, Marlow L, Waller J. Attitudes towards human papillomavirus vaccination: A qualitative study of vaccinated and unvaccinated girls aged 17–18 years. J Fam Plann Reprod Health Care. 2011;37(1):22–25. doi:10.1136/jfprhc.2010.0017.
- Camano-Puig R, Sanchis-Martinez MM. Human papilloma virus vaccination in teenage girls: a focus group evaluation. Revista de Salud Publica. 2014;16:646–57.
- Klotzler A, Kolip P. Decision for or against HPV vaccination–a qualitative study with adolescent girls. [German]. Gesundheitswesen (Bundesverband der Arzte des Offentlichen Gesundheitsdienstes (Germany)). 2012;74(11):716–21. doi:10.1055/s-0031-1286270.
- Paul-Ebhohimhen V, Huc S, Tissington H, Oates K, Stark C. HPV vaccination: vaccine acceptance, side effects and screening intentions. Community Pract. 2010;83:30–33.
- Stretch R, Roberts SA, McCann R, Baxter D, Chambers G, Kitchener H, Brabin L. Parental attitudes and information needs in an adolescent HPV vaccination programme. Br J Cancer. 2008;99(11):1908–11. doi:10.1038/sj.bjc.6604766.
- Marek E, Dergez T, Kricskovics A, Kovacs K, Rebek-Nagy G, Gocze K, Kiss I, Ember I, Gocze P. Difficulties in the prevention of cervical cancer: adults’ attitudes towards HPV vaccination 3 years after introducing the vaccine in hungary. Vaccine. 2011;29(32):5122–29. doi:10.1016/j.vaccine.2011.05.048.
- Esposito S, Bosis S, Pelucchi C, Begliatti E, Rognoni A, Bellasio M, Tel F, Consolo S, Principi N. Pediatrician knowledge and attitudes regarding human papillomavirus disease and its prevention. Vaccine. 2007;25(35):6437–46. doi:10.1016/j.vaccine.2007.06.053.
- Bouvret P, Mougin C, Pretet JL, Meurisse A, Bonnetain F, Fiteni F. Practices and attitudes regarding HPV vaccination among general practitioners from Besançon. Journal De Gynecologie Obstetrique Et Biologie De La Reproduction. 2015:08. doi:10.1016/j.jgyn.2015.12.002.
- Bianco A, Pileggi C, Iozzo F, Nobile CGA, Pavia M. Vaccination against human papilloma virus infection in male adolescents: knowledge, attitudes, and acceptability among parents in Italy. Hum Vaccin Immunother. 2014;10(9):2536–42. doi:10.4161/21645515.2014.969614.
- Di Giuseppe G, Abbate R, Liguori G, Albano L, Angelillo IF. Human papillomavirus and vaccination: knowledge, attitudes, and behavioural intention in adolescents and young women in Italy. Br J Cancer. 2008;99(2):225–29. doi:10.1038/sj.bjc.6604454.
- Blodt S, Holmberg C, Muller-Nordhorn J, Rieckmann N. Human Papillomavirus awareness, knowledge and vaccine acceptance: a survey among 18–25 year old male and female vocational school students in Berlin, Germany. Eur J Public Health. 2012;22(6):808–13. doi:10.1093/eurpub/ckr188.
- Bakogianni GD, Nikolakopoulos KM, Nikolakopoulou NM. HPV vaccine acceptance among female Greek students. Int J Adolesc Med Health. 2010;22:271–73.
- Abram Z, Tar G. Health education of mothers about their girls’ vaccination. 4th world conference on educational sciences. Procedia Soc Behav Sci. 2012;462012:5330–34.
- Mortensen GL. Drivers and barriers to acceptance of human-papillomavirus vaccination among young women: a qualitative and quantitative study. BMC Public Health. 2010;10:68. doi:10.1186/1471-2458-10-68.
- Haesebaert J, Lutringer-Magnin D, Kalecinski J, Barone G, Jacquard AC, Regnier V, Leocmach Y, Vanhems P, Chauvin F, Lasset C. French women’s knowledge of and attitudes towards cervical cancer prevention and the acceptability of HPV vaccination among those with 14–18 year old daughters: a quantitative-qualitative study. BMC Public Health. 2012;12:1034. doi:10.1186/1471-2458-12-1034.
- Collange F, Fressard L, Pulcini C, Sebbah R, Peretti-Watel P, Verger P. General practitioners’ attitudes and behaviors toward HPV vaccination: A French national survey. Vaccine. 2016;34(6):762–68. doi:10.1016/j.vaccine.2015.12.054.
- D’Hauwers KWM, Gadet PFE, Donders ART, Tjalma WAA. Impact of medical education on knowledge and attitudes regarding the human papilloma virus and vaccination: comparison before and 6 years after the introduction of the vaccines. Vaccine. 2013;31(49):5843–47. doi:10.1016/j.vaccine.2013.09.068.
- Dahlstrom LA, Tran TN, Lundholm C, Young C, Sundstrom K, Sparen P. Attitudes to HPV vaccination among parents of children aged 12–15 years - A population-based survey in Sweden. Int J Cancer. 2010;126(2):500–07. doi:10.1002/ijc.24712.
- Brabin L, Roberts SA, Stretch R, Baxter D, Elton P, Kitchener H, McCann R. A survey of adolescent experiences of human papillomavirus vaccination in the Manchester study. Br J Cancer. 2009;101(9):1502–04. doi:10.1038/sj.bjc.6605362.
- Hofman R, de Bekker-Grob EW, Raat H, Helmerhorst TJ, van Ballegooijen M, Korfage IJ. Parents’ preferences for vaccinating daughters against human papillomavirus in the Netherlands: a discrete choice experiment. BMC Public Health. 2014;14:454. doi:10.1186/1471-2458-14-454.
- Giuliani M, Vescio MF, Dona MG, Latini A, Frasca M, Colafigli M, Farinella M, Rezza G, Cristaudo A. Perceptions of human Papillomavirus (HPV) infection and acceptability of HPV vaccine among men attending a sexual health clinic differ according to sexual orientation. Hum Vaccin Immunother. 2016;12(6):1542–50. doi:10.1080/21645515.2015.1115935.
- Donders GGG, Gabrovska M, Bellen G, Van Keirsbilck J, Van Den Bosch T, Riphagen I, Verjans M. Knowledge of cervix cancer, human papilloma virus (HPV) and HPV vaccination at the moment of introduction of the vaccine in women in Belgium. Arch Gynecol Obstet. 2008;277(4):291–98. doi:10.1007/s00404-007-0487-1.
- Marlow LAV, Waller J, Evans REC, Wardle J. Predictors of interest in HPV vaccination: A study of British adolescents. Vaccine. 2009;27(18):2483–88. doi:10.1016/j.vaccine.2009.02.057.
- Marlow LA, Waller J, Wardle J. Parental attitudes to pre-pubertal HPV vaccination. Vaccine. 2007;25(11):1945–52. doi:10.1016/j.vaccine.2007.01.059.
- Marlow LAV, Waller J, Wardle J. Trust and experience as predictors of HPV vaccine acceptance. Hum Vaccin. 2007;3:171–75.
- Navarro-Illana P, Diez-Domingo J, Navarro-Illana E, Tuells J, Aleman S, Puig-Barbera J. Knowledge and attitudes of Spanish adolescent girls towards human papillomavirus infection: where to intervene to improve vaccination coverage. BMC Public Health. 2014;14:490. doi:10.1186/1471-2458-14-490.
- Piana L, Noel G, Uters M, Laporte R, Minodier P. Standpoint and practice concerning the human Papillomavirus vaccine among French family physicians. Med Mal Infect. 2009;39(10):789–97. doi:10.1016/j.medmal.2009.08.007.
- Sadlier C, Lynam A, O’Dea S, Delamere S, Quinlan M, Clarke S, Sheils O, Bergin C. HPV vaccine acceptability in HIV-infected and HIV negative men who have sex with men (MSM) in Ireland. Hum Vaccin Immunother. 2016;12(6):1536–41. doi:10.1080/21645515.2016.1151588.
- Stocker P, Dehnert M, Schuster M, Wichmann O, Delere Y. Human papillomavirus vaccine uptake, knowledge and attitude among 10th grade students in Berlin, Germany, 2010. Hum Vaccin Immunother. 2013;9(1):74–82. doi:10.4161/hv.22192.
- Sundstrom K, Tran TN, Lundholm C, Young C, Sparen P, Dahlstrom LA. Acceptability of HPV vaccination among young adults aged 18–30 years–a population based survey in Sweden. Vaccine. 2010;28(47):7492–500. doi:10.1016/j.vaccine.2010.09.007.
- Kuitto K, Pickel S, Jahn D. Perspectives on and experiences with early detection and preventive measures against cervical cancer. Results of an expert survey among physicians in Mecklenburg-Western Pomerania. (Konzertierter Einsatz von Niedergelassenen, Betriebsarzten und Patienten. Impfstrategien und Gesundheitsforderung.) [German]. Pravention und Gesundheitsforderung. 2010;5(Suppl. 1):38–45. doi:10.1007/s11553-010-0235-4.
- Gray Brunton C, Farver I, Jager M, Lenneis A, Parve K, Patarcic D, Petrova D, Hogg R, Kennedy C, Garcia-Retamero R, et al. Young women’s constructions of the HPV vaccine: a cross-cultural, qualitative study in Scotland, Spain, Serbia and Bulgaria. Int J Behav Med. 2014;21(1):11–19. doi:10.1007/s12529-013-9357-3.
- Karamanidou C, Dimopoulos K. Greek health professionals’ perceptions of the HPV vaccine, state policy recommendations and their own role with regards to communication of relevant health information. BMC Public Health. 2016;16:467. doi:10.1186/s12889-016-2831-5.
- Mupandawana ET, Cross R. Attitudes towards human papillomavirus vaccination among African parents in a city in the north of England: A qualitative study. Reprod Health. 2016;13(1):97. doi:10.1186/s12978-016-0209-x.
- Noakes K, Yarwood J, Salisbury D. Parental response to the introduction of a vaccine against human papilloma virus. Hum Vaccin. 2006;2:243–48.
- Oscarsson MG, Hannerfors AK, Tyden T. Young women’s decision-making process for HPV vaccination. Sex Reprod Healthc. 2012;3(4):141–46. doi:10.1016/j.srhc.2012.10.002.
- Stretch R, McCann R, Roberts SA, Elton P, Baxter D, Brabin L. A qualitative study to assess school nurses’ views on vaccinating 12–13 year old school girls against human papillomavirus without parental consent. BMC Public Health. 2009;9:254. doi:10.1186/1471-2458-9-254.
- Todorova I, Alexandrova-Karamanova A, Panayotova Y, Dimitrova E, Kotzeva T. Managing uncertainty: healthcare professionals’ meanings regarding the HPV vaccine. Int J Behav Med. 2014;21(1):29–36. doi:10.1007/s12529-013-9343-9.
- Hutton S, Finlay F. Allaying parental concerns about the human papillomavirus vaccine. Paediatr Nurs. 2009;21(9):20–23. doi:10.7748/paed2009.11.21.9.20.c7356.
- Morison LA, Cozzolino PJ, Orbell S. Temporal perspective and parental intention to accept the human papillomavirus vaccination for their daughter. Br J Health Psychol. 2010;15(Pt 1):151–65. doi:10.1348/135910709X437092.
- Lutringer-Magnin D, Kalecinski J, Barone G, Leocmach Y, Regnier V, Jacquard AC, Soubeyrand B, Vanhems P, Chauvin F, Lasset C. Human papillomavirus (HPV) vaccination: perception and practice among French general practitioners in the year since licensing. Vaccine. 2011;29(32):5322–28. doi:10.1016/j.vaccine.2011.05.006.
- Marlow LAV, Wardle J, Waller J. Attitudes to HPV vaccination among ethnic minority mothers in the UK: an exploratory qualitative study. Hum Vaccin. 2009;5(2):105–10. doi:10.4161/hv.5.2.7368.
- Lutringer-Magnin D, Kalecinski J, Barone G, Borne H, Regnier V, Vanhems P, Chauvin F, Lasset C. Gynaecologists’ attitudes and practices towards HPV vaccination: A quantitative-qualitative study in Rhone-Alpes. [French]. Gynecologie Obstetrique Fertilite. 2011;39(12):687–93. doi:10.1016/j.gyobfe.2011.07.015.
- Grandahl M, Tyden T, Rosenblad A, Oscarsson M, Neveus T, Stenhammar C. School nurses’ attitudes and experiences regarding the human papillomavirus vaccination programme in Sweden: a population-based survey. BMC Public Health. 2014;14:540. doi:10.1186/1471-2458-14-540.
- Firenze A, Marsala MGL, Bonanno V, Maranto M, Ferrara C, Giovannelli L, Restivo V. Facilitators and barriers HPV unvaccinated girls after 5 years of program implementation. Hum Vaccin Immunother. 2015;11(1):240–44. doi:10.4161/hv.36158.
- Perez MR, Violeta VB, Del Campo AV, Ruiz C, Castano SY, Conde LP, Lopez JS. Cross-sectional study about primary health care professionals views on the inclusion of the vaccine against human papillomavirus in the vaccine schedules. Infect Agent Cancer. 2015;10:41. doi:10.1186/s13027-015-0034-9.
- Pop CA. Cervical cancer narratives: invoking ‘God’s will’ to re-appropriate reproductive rights in present-day Romania. Cult Health Sex. 2015;17(1):48–62. doi:10.1080/13691058.2014.948491.
- Pelullo CP, Di Giuseppe G, Angelillo IF. Human papillomavirus infection: knowledge, attitudes, and behaviors among lesbian, gay men, and bisexual in Italy. PLoS One. 2012;7(8). doi:10.1371/journal.pone.0042856.
- Martinez L, Tugaut B, Raineri F, Arnould B, Seyler D, Arnould P, Benmedjahed K, Coindard G, Denis F, Gallais JL, et al. The commitment of French general practitioners to vaccination: the DIVA study (Determinants of vaccination intentions). Sante Publique. 2016;28(1):19–32.
- Hilton S, Smith E. “I thought cancer was one of those random things. I didn’t know cancer could be caught…”: adolescent girls’ understandings and experiences of the HPV programme in the UK. Vaccine. 2011;29(26):4409–15. doi:10.1016/j.vaccine.2011.03.101.
- Brabin L, Roberts SA, Kitchener HC. A semi-qualitative study of attitudes to vaccinating adolescents against human papillomavirus without parental consent. BMC Public Health. 2007;7:20. doi:10.1186/1471-2458-7-20.
- Woodhall SC, Lehtinen M, Verho T, Huhtala H, Hokkanen M, Kosunen E. Anticipated acceptance of HPV vaccination at the baseline of implementation: a survey of parental and adolescent knowledge and attitudes in Finland. J Adolesc Health. 2007;40(5):466–69. doi:10.1016/j.jadohealth.2007.01.005.
- Martin E, Senior N, Abdullah A, Brown J, Collings S, Racktoo S, Walpole S, Zeiton M, Heffernan C. Perceptions of HPV vaccine amongst UK university students. Health Educ. 2011;111(6):498–513. doi:10.1108/09654281111180481.
- Gesouli-Voltyraki E, Tsetsekou E, Marneras C, Krapis K, Yfantis A, Noula M. Hpv vaccination acceptance among women in Greek provincial areas. Arch Hellenic Med. 2010;27:522–28.
- Giambi C, Donati S, Declich S, Salmaso S, Degli Atti MLC, Alibrandi MP, Brezzi S, Carozzi F, Collina N, Franchi D, et al. Estimated acceptance of HPV vaccination among Italian women aged 18–26 years. Vaccine. 2011;29(46):8373–80. doi:10.1016/j.vaccine.2011.08.079.
- Dubé E, Laberge C, Guay M, Bramadat P, Roy R, Bettinger JA. Vaccine hesitancy: an overview. Hum Vaccin Immunother. 2013;9(8):1763–73. doi:10.4161/hv.24657.
- Hobson-West P. Understanding vaccination resistance: moving beyond risk. Health Risk Soc. 2003;5(3):273–83. doi:10.1080/13698570310001606978.
- Calman KC. Communication of risk: choice, consent, and trust. The Lancet. 2002;360(9327):166–68. doi:10.1016/S0140-6736(02)09421-7.
- Jarrett C, Wilson R, O’Leary M, Eckersberger E, Larson HJ. Strategies for addressing vaccine hesitancy - A systematic review. Vaccine. 2015;33(34):4180–90. Epub 2015/ 04/22. doi:10.1016/j.vaccine.2015.04.040.
- Dubé E, Gagnon D, MacDonald NE. Strategies intended to address vaccine hesitancy: review of published reviews. Vaccine. 2015;33(34):4191–203. doi:10.1016/j.vaccine.2015.04.041.
- Larson HJ. The world must accept that the HPV vaccine is safe. Nature. 2015;528:9. doi:10.1038/528009a.
- Buttery JP, Madin S, Crawford NW, Elia S, La Vincente S, Hanieh S, Smith L, Bolam B. Mass psychogenic response to human papillomavirus vaccination. Med J Aust. 2008;189:261–62.
- Simas C, Munoz N, Arregoces L, Larson HJ. HPV vaccine confidence and cases of mass psychogenic illness following immunization in Carmen de Bolivar, Colombia. Hum Vaccin Immunother. 2018:1–4. doi:10.1080/21645515.2018.1511667.
- Bouder F, Way D, Lofstedt R, Evensen D. Transparency in Europe: a quantitative study. Risk Anal. 2015;35(7):1210–29. Epub 2015/ 05/02. doi:10.1111/risa.12386.
- Leask J, Kinnersley P, Jackson C, Cheater F, Bedford H, Rowles G. Communicating with parents about vaccination: a framework for health professionals. BMC Pediatr. 2012;12(1):154. doi:10.1186/1471-2431-12-34.
- MacDonald NE, Finlay JC. Working with vaccine-hesitant parents. Paediatr Child Health. 2013;18(5):265–67. doi:10.1093/pch/18.5.265.