ABSTRACT
Regulatory T cells (Tregs) play a crucial role in the control of the initiation and progression of type 1 diabetes (T1D). Various immunological interventions including those to ex vivo expansion Tregs transfer, in vivo induction of peripherally derived Treg (pTreg) have been considered as promising approaches for T1D therapy. In this study, we developed a novel tolerogenic vaccine using four autoantigenic peptides of islet-derived with cyclosporine A (CsA) as the pTreg inducer, designated as GAD-IN+CsA. This vaccine immunized into prediabetic NOD mice subcutaneously could induce IL-10 and TGF-β expressing pTregs and lead to suppressing autoreactive T cells responses, resulting in the prevention of T1D in these animals. Furthermore, we demonstrated that CsA with autoantigenic peptides modulates dendritic cells (DCs) to become immature IL-10hiCD40lo DCs. Such modulated DCs could foster naïve CD4+CD25− T cell into Tregs when presenting antigen peptides in vitro. This novel approach offers an alternative strategy to induce pTregs to treat T1D.
Introduction
The prevalence of Type 1 diabetes (T1D) is increasing globally among young children at a higher rate in developed countries over developing countries.Citation1 T1D is a severe chronic autoimmune disease characterized by progressive loss of pancreatic β cells resulting in deficient insulin production and hyperglycemia. T1D-related autoimmune activation has been highly suspected a malfunction of immunotolerance. Bold evidence has demonstrated that the balance of pathogenic and immune regulatory pathways underlies disease progression in T1D.Citation2,Citation3 Particularly, defective regulatory T cells (Tregs) caused uncontrolled autoimmune activation plays a pivotal role in the development of T1D.Citation4,Citation5 Re-enforcing the immune regulation by suppressing autoimmunity with various immunosuppressants has been tried in clinical settings only achieved short-term beneficial effects, suggesting temporary suppression on autoimmunity but not cure. Once withdraw of such drug, the disease progression develops as usual. Anti-CD3 or -CD4 antibody treatment in patients with T1D has also failed, which added another example to this category. Therefore, alternatives with more targeted approaches are urgently needed. One of such is to isolate polyclonal Tregs from patient with T1D, expanded into millions in vitro, and transferring back to the same patient.Citation6 However, this approach has been approved to have problems with short-lived and uncertainty of these Tregs after transfusion into a patient.Citation7
Over the past few years, there has been an increasing interest in the induction of Tregs for immunotherapeutic treatment against autoimmune diseases including T1D. For instance, anti-CD3 monoclonal antibody treatment has been demonstrated to induce adaptive Tregs and remission of the disease since this antibody directly interacts with T cells, interrupts its activation, and tips T effector cells (Teffs) toward more Tregs.Citation8,Citation9 Unfortunately, such a strategy fails to translate into clinical benefit for patients with T1D but raises more concerns on the consequence of biased systemic immunity. Other approaches aim to foster Tregs through inducing tolerogenic dendritic cells (tolDCs) in vitro or in vivo.Citation10-Citation12 Due to DCs have an essential function in initiating adaptive immune responses by recognizing, processing, and presenting specific antigens to T cells, it could highly activate Teffs and lead to the effective elimination of pathogens with full maturation status through up-regulating costimulatory molecules (e.g., CD40, CD86, CD80). Conversely, a DC encountering self-antigens could become a tolerogenic status resulting in an immune tolerance response against auto-reactive T cells via induction of pTregs. The latter mechanism has become a novel immunotherapeutic strategy to develop multiple laboratory methods against autoimmune diseases. The induction of pTregs fostered by tolDCs involves mechanisms largely unknown although some of the crucial factors having been demonstrated in an association during such induction, which includes the presence of anti-inflammatory cytokines (e.g., IL-10 or TGF-β) in local micro-environment as a tolerogenic environment and immature DCs when interacting with naïve T cells.Citation13,Citation14
To explore the induction of immune tolerance, particularly antigen-specific pTregs, influencing the micro-environment of DC-T interaction during an antigen processing and presentation with a minuscule amount of immunosuppressant could alter the outcome toward pTregs induction. This can be achieved through the administration of antigen with dexamethasone or cyclosporine A (CsA) subcutaneously as a “tolerogenic” vaccine as reported previously in our lab.Citation15,Citation16 Since T1D is an autoimmune disease with aberrant activation of Teffs and deficiency of Tregs, we hypothesized that autoantigens derived from the islet of pancreas formulated with CsA might be able to induce antigen-specific pTregs and potentiate immune tolerance against T1D in prediabetic NOD mice. In this study, we demonstrated that the formulated autoantigenic islet peptides (GAD65206-220, GAD65536-550, Insulin B9-23, and C17-A1) with CsA applied as a tolerogenic vaccine prevented the development of T1D in prediabetic NOD mice. This tolerogenic vaccine modulated DCs to acquire tolerogenic phenotypes and induced a higher level of pTregs, which could be a promising therapeutic approach for T1D.
Results
GAD-IN plus CsA vaccine induces the tolerogenic response in mice
Islet-derived peptides have been demonstrated as autoantigens associated with T cell activation in patients with T1D, suitable to develop tolerogenic vaccines. Here, we chose four islet-derived peptides (GAD65206-220, GAD65536-550, Insulin B9-23, and Insulin C17-A1), combined with CsA as GAD-IN+CsA vaccine. In detail, we mixed either 10, 20, or 40 μg of peptides together and formulated with 10 μg of CsA, whereas 40 μ of mixed multi-peptides alone, 10 μ of CsA alone or vehicle served as controls. The formulated GAD-IN+CsA vaccine was immunized subcutaneously into female BALB/c mice three immunizations on days 0, 6, and 12. The frequency of CD4+CD25+Foxp3+ pTregs was evaluated from the draining lymph node after vaccinations and showed an increase significantly in the groups immunized with 10, 20, and 40 μg of GAD-IN+CsA vaccine, while the 20 μg of GAD-IN+10 μ CsA regimen had achieved the highest level over other groups ().
Figure 1. Islet autoantigenic multipeptides plus CsA induces Tregs and inhibits Teff immune response in prediabetic NOD mice. (a) The prediabetic NOD mice were injected with GAD-IN (20 μg each) and CsA (10 μg) or with controls on days 0, 6, and 12 s.c. The freshly isolated T cells from pancreatic LN on day 14 after the immunizations were in vitro stimulated with autoantigenic peptides (10 μg/ml each) for 8 h before performing antibody staining for gating strategy. The stimulated T cells were divided into two parts, the first part was gated on CD25+ and Foxp3+ to represent Treg cells, its percentage of Tregs (b) and levels of inhibitory cytokine expressions of IL-10 (c), TGF-β (d) and proliferative maker ki67 (e) were done by intracellular staining and measured by flow cytometry; the second part as autoreactive CD4+ T cells were also intracellularly analyzed their cytokine expressions for IFN-γ (f & g), TNF-α (F & H) and IL-2 (F & I). *, p value <.5, **, p value <.1, and ***, p value <.05.
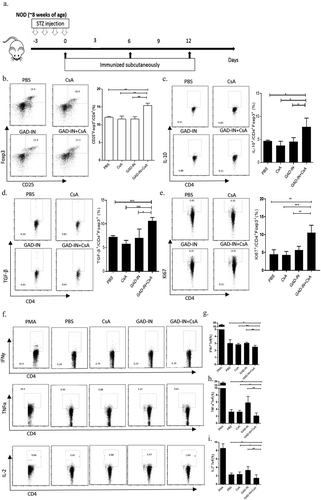
To demonstrate effectiveness to induce pTregs by the GAD-IN+CsA vaccine, 6–8 weeks old male NOD mice were administrated five times with 50 mg/ml STZ each day,Citation17-Citation19 and then given the 20 μg GAD-IN+CsA vaccine subcutaneously on days 0, 6, and 12 (). Twenty-four hours later after the last vaccination, a higher frequency of CD4+CD25+Foxp3+ pTregs was observed in the pancreatic lymph node of that 20 μg GAD-IN+CsA immunized NOD mice compared to that of the multiple-peptides mix without CsA, CsA alone or vehicle (). The expression levels of IL-10 and TGF-β in these induced Foxp3+ pTregs were elevated after GAD-IN+CsA vaccinations, indicating that these pTregs may bear suppressive function ( and ). In addition, the increased expression of ki67 in pTreg was detected after GAD-IN autoantigenic peptides stimulation in vitro, suggesting the antigen-specific pTreg were engendered after GAD-IN+CsA vaccination (). In contrast, expression levels of IFN-γ, TNF-α, and IL-2 of antigen-specific CD4+ Teff cells were significantly decreased in the GAD-IN+CsA immunized animals at the same period compared with the control treatments (–). The multipeptides without CsA induced higher levels of these inflammatory cytokines in the activated CD4+ Teff cells. The result indicated that these autoreactive pathogenic Teffs were suppressed in the group immunized with the GAD-IN+CsA vaccine by not in the other groups, and the peptides alone without CsA could induce more TNF-α and IL-2 production by CD4+ T cells.
GAD-IN plus CsA vaccine prevents T1D in diabetic NOD mice
To examine if GAD-IN+CsA-induced pTregs protect mice from T1D progression, we vaccinated the 8-wk-old NOD model mice with 20 μg GAD-IN+CsA for three immunizations with 6-day intervals. Control mice were treated with mixed peptides alone, CsA alone or vehicle. The GAD-IN+CsA vaccinations prevented the development of T1D with over 70% of NOD mice from diabetes and remained diabetes-free to the end of this study. By contrast, only approximately 25% of those with CsA alone, or untreated controls remained diabetes-free (). However, 95% of mice treated with mixed peptides without CsA had a more aggressive development of diabetes, indicating worsen autoimmunity by peptides alone. Consistently, fasting blood glucose levels were increased rapidly in the mice vaccinated with mixed peptides alone and vehicle, less aggressive with CsA alone, contrarily such level remained at normal range throughout the study in mice treated with the GAD-IN+CsA vaccine (). Further analysis by oral glucose tolerance test (OGTT) was revealed that the NOD mice after GAD-IN+CsA vaccinations exhibited well glucose tolerance over a period of 150 min throughout the study whereas other control groups had less resistance in the same period ().
Figure 2. Islet autoantigenic peptides plus CsA prevents T1D in prediabetic NOD mice. The blood glucose level of mice more than 15 mmol/L was identified as euglycemia mice. (a) The incidence of euglycemia from those immunized groups was monitored throughout the course of study. (b) The blood glucose levels were assessed after fasting 5 h in the GAD-IN+CsA vaccinated group (n = 12), compared with control groups including the GAD-IN alone (n = 12), the CsA alone (n = 12), or the vehicle (n = 12) every three days. (c) OGTT was performed to evaluate the islet function in these four groups at day 30 after the first vaccination. “*”, “#” and “Δ” represented the p values compared between the GAD-IN+CsA and GAD-IN alone, CsA alone or vehicle group, respectively. (d) Histopathological analysis of insulitis in H&E-stained sections of islets of isolated among the groups. (e) The degrees of insulitis were assessed in the four groups and percentages representing severity of insulitis was given accordingly. Each column represents a mean of 100 islets examined in five mice in each group. (f) Immunohistochemistry for infiltrating CD4+ T cells in pancreas sections was detected. Arrows indicate positive-stained cells.
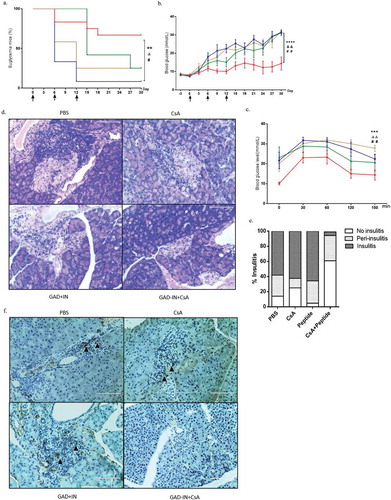
Islet lymphocytes infiltration causing insulitis hallmarks T1D. Thus, we next examined the islet tissues by section stained with hematoxylin and eosin (H&E) to reveal their structures and morphologies and rated the severity of destructions for insulitis by scoring the residual islets as insulitis, peri-insulitis, and no insulitis. As shown in and , severity scores ranging from mild peri-insulitis to no insulitis were evaluated in the GAD-IN+CsA-vaccinated mice, whereas most of the mice immunized with mixed peptides alone, CsA alone or the vehicle exhibited more severe insulitis and less peri-insulitis with the small percentage of no insulitis. Immunohistochemical analysis showed that large numbers of CD4+ T cells were eliminated in pancreas after GAD-IN+CsA vaccination in T1D model mice (), which may explain the diminished insulitis. Taken together, the combination of these islet-derived multipeptides formulated with CsA could suppress autoimmunity assault in mouse pancreas and prevent the onset of T1D effectively.
GAD-IN+CsA vaccine modulates DCs by upregulating Il-10hi and downregulating CD40lo in vitro
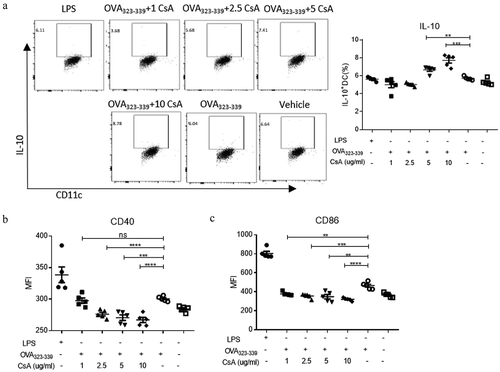
Having demonstrated tolerogenic responses induced by the immunizations of GAD-IN+CsA vaccine in mice, we next sought to examine whether the protection from T1D after vaccination was due to an induction of tolerogenic DCs that foster naïve T cells into Tregs. To test this notion, we switched the GAD-IN peptides to the OVA323-339 peptide since this OVA323-339 has been extensively studied the nature of class II MHC-peptide binding and T-cell activation as a model antigen.Citation20,Citation21 We isolated splenic CD11c+DCs from BALB/c mice using magnetic beads to achieve >90% purity and stimulated with antigen OVA323-339 with CsA at 1, 2.5, 5 or 10 μg/ml in vitro for 6 h, respectively, whereas the OVA323-339 alone, or the vehicle served as controls. Levels of expression of anti-inflammatory cytokine IL-10 and co-stimulatory molecules CD40 and CD86 were assessed after the stimulation. The results revealed that DCs after such stimulation significantly displayed a “tolerogenic” status with increased expression of IL-10 and decreased expressions of CD40 and CD86 on the surface of DCs in a dose-dependent fashion when compared with the controls (– and ). The optimal dose that induced tolerogenic DCs was at 10 μg/ml CsA and 20 μg/ml OVA323-339 peptide in vitro culture.
Figure 3. CsA modulates DCs as IL-10hiCD40lo phenotypic tolDC in vitro. CD11c+ DCs isolated from splenocytes of BALB/c or DO11.1 mouse were stimulated with 20 μg/ml of OVA323-339 peptide plus various concentrations of CsA, OVA323-339 alone, or vehicle for 6 h in vitro, respectively. LPS at 0.5 μg/ml was used to serve as a positive control. The stimulated DCs were performed a flow cytometry analysis on the level of intracellular cytokine IL-10 (a) and surface costimulatory markers CD40 (b) and CD86 (c). Data represent three independent experiments. *, p value <0.5, **, p value <0.1, and ***, p value <0.05.
Tolerogenic DCs could foster CD4+CD25− t cells into Tregs that prevents antigen-specific Teff cells response in vitro
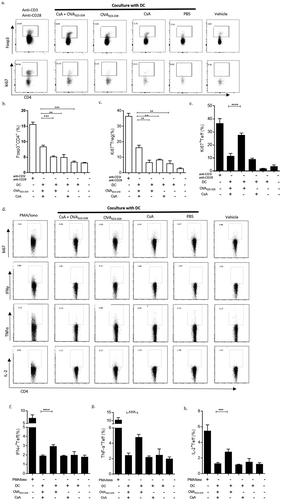
To further elucidate whether the tolerogenic DCs induced by in vitro stimulation can induce CD4+CD25− T cells into Tregs, we set up a co-culture system in which the DCs isolated from DO11.10 mice were in vitro stimulated with CsA and peptides for 4 h, followed by three times washes to remove any unbound substances. The treated DCs were then used to co-culture with CD4+CD25− T cells isolated from syngeneic DO11.10 mice for further 72 h. After this co-culture, we observed that co-cultured CD4+CD25− T cells were able to convert into more CD4+Foxp3+ Tregs when compared with the controls ( and ). In addition to the conversion, we also observed that the Ki67 expression of these Tregs was significantly higher when further stimulated with cognate antigen in vitro when compared with other groups ( and ). The data suggested that CsA plus antigen could induce tolerogenic DCs in vitro and lead to co-cultured naïve T cells switching into antigen recognized and expandable Tregs. More importantly, the induced Tregs, taken from the induction system described above, could suppress the proliferation of OVA primed CD4+CD25+Foxp3− Teff cells isolated from OVA immunized DO11.10 mice when co-cultured with the Treg cells (–). We further examined cytokine expressions from these Teff cells in such co-culture system to determine whether the cytokine profiles were also changed. The expression levels of IFN-γ, TNF-α, and IL-2 were significantly down-regulated when primed Teff cells were co-cultured with Tregs from the previous fostered with OVA323-339+ CsA treated DCs compared with other conditions (–). These results indicated that OVA323-339+ CsA-induced tolDCs could promote the expandable and functional CD4+Foxp3+ Tregs, resulting in the suppression of proliferation and functions of Teff cells in vitro.
Figure 4. TolDCs foster naïve T cells into Tregs and prevent antigen-specific Teff cells response in vitro. Fresh isolated splenic DCs were used to incubate with 20 μg/ml OVA323-339 plus 10 μg/ml CsA, OVA323-339 alone, CsA alone, or PBS for 4 h. After incubation, DCs were washed with PBS for three times, and seeded with co-cultured CD4+CD25− T cells obtained from OVA-immunized D011.10 mice in presence of IL-2. The ratio of DCs to CD4+CD25− T cells was the 1:10 for 72 h in vitro co-culture system. CD4+CD25− T cells were also set without DCs, but with coated anti-CD3 and anti-CD28, and soluble IL-2, TGF-β as differentiation control. Naive T cells were supplied with IL-2 as vehicle control. The percentage of CD4+Foxp3+ Tregs of total CD4+ T cells were detected after the co-culture (a & b). An ability to proliferate was assessed by measuring the level of ki67 expression on these CD4+Foxp3+ Tregs (a & c), or on Teffs (CD4+CD25+Foxp3−) (d & e). The functional cytokines IFN-γ (f), TNF-α (g) and IL-2 (h) of Teffs were further characterized by intracellularly staining after treated with Brefeldin A for 6 hr. Anti-CD3/anti-CD28 plus IL-2 activated T cells were stimulated with PMA and Ionomycin for last 6 h as positive control for these cytokines detection. Data represent three independent experiments. *, p value <0.5, **, p value <0.1, and ***, p value <0.05.
Discussion
Strategies of inducing antigen-specific tolerance to prevent T1D have been reported in the NOD animal model, but few translated into bedside successfully.Citation2,Citation3,Citation22 Although induction of antigen- specific pTregs has been considered as a safe and effective means to control T1D, it is a challenge to induce such antigen-specific pTregs by vaccination. In this study, we demonstrated that immunization of islet-derived multipeptides plus CsA (GAD-IN+CsA vaccine) in prediabetic NOD mice could induce a tolerogenic response against the development of T1D. The pTregs induced by the GAD-IN+CsA vaccine had higher expression of functional cytokines IL-10 and TGF-β and could expand in the presence of cognate antigen-loaded DCs. More importantly, such induced pTregs can effectively suppress the autoreactive T cell responses and prevent the onset of T1D.
Several recent approaches targeted to induce immunotolerance against T1D have been tried in clinical settings with limited success.Citation23,Citation24 Since Tregs are the most important immunotolerance player to regulate over inflammatory responses, which is potentially harmful to host, aiming to induce Tregs ex vivo or in vivo becomes an overt and favorable choice although it still has numerous technical challenges to be solved. Among them, ex vivo expansion of nTregs as an individualizing medicine can be given to patients with T1D by adoptive transfer of autologous expanded cells had advantages over other methods since it only required fewer manufacture processes and tests as a novel drug does.Citation25,Citation26 However, no significant improvements were observed for C-peptide as well as metabolic functions of these treated patients.Citation27 Despite the fact of earlier failures in several clinical trials, ex vivo for antigen-specific Tregs induction technical has been tried and tested in clinical trials.Citation22,Citation28 Those improved ex vivo strategies may further be proved the importance of Tregs in maintaining of immunotolerance for treating T1D.
Alternatively, the use of autoantigen(s)-based therapeutic vaccine has greater impacts on the treatment of patients with T1D. Once it has been proved to be effective, all patients with T1D will theoretically benefit from long-term administration of immunosuppressant. However, such promised strategies had not been efficacious in human since several clinical trials by using this type of approaches were not successfully reproduced in patients with T1D. For example, a vaccine based on the GAD65 formulated in alum adjuvant was given 219 patients with T1D in one clinical trial but ineffective with neither improvement of C-peptide nor controlling the blood glucose level over 15 months.Citation29 Another vaccine comprised of the insulin B-chain peptide formulated in Incomplete Freund’s adjuvant (IFA) given to patients with T1D has exhibited Treg cells responses but no C-peptide improvements.Citation30 Several similar antigen peptide or peptides based on insulin B chain or A chain without adjuvant have also been tried in clinical settings. No relief hypoglycemia was achieved, although some treated patients with T1D were responded to vaccinations with fluctuations of IL-10 levels, suggesting some degrees of induction of immunotolerance.Citation31,Citation32 Other approaches including the BCG immunizations intended to induce re-balanced immunity has also exhibited some degree of hyperglycemia improvement with elevating frequency of Tregs although its mechanism of action is still elusive.Citation33 Vaccines mentioned above had less efficient to induce desire high potential antigen-specific pTregs in patients despite facts these can be effective to treat T1D in animal models. Although the induction of potent antigen-specific pTregs had many approaches, auto-antigen itself is insufficient to do so since it often induces stronger autoimmune response or non-response. Adjuvants should be considered in designing a tolerogenic vaccine. Adjuvant containing Alum or IFA could help an antigen to induce Th2 biased response, but not favorable to promote the tolerogenic response. Other types of adjuvants facilitating to induce immunotolerance must be taken into consideration.
CsA is a widely used immunosuppressant against allograft rejections or treating various autoimmune diseases, such as rheumatoid arthritis, T1D, and psoriasis.Citation34-Citation37 CsA typically binds to the cyclophilin of immune cells and prevents the activation of T cells by inhibiting the transcription factor nuclear factor of activated-T cells (NFATc) signaling.Citation38 Previous studies have demonstrated that CsA also has a modulating effect on innate immune cells, including natural killer (NK) cell, macrophage, and DC.Citation39-Citation42 Those suggested that CsA could modulate DCs and affect outcomes of T cells. As our previous studies reported that the vaccine employing one immunosuppressant dexamethasone as adjuvant could induce a higher number of IL-10 producing immature DCs and in turn to induce expandable antigen-specific pTregs.Citation43 In this study, we showed that islet-derived multipeptides plus CsA could induce a higher level of antigen- specific pTregs control the blood glucose with less insulitis in NOD animal model. This type of pTregs induction could also be achieved using peptides plus CsA treated DCs in vitro. The immature IL-10hiCD40lo phenotype of DCs was generated through antigen plus CsA treatment in a dose-dependent manner in vitro. Although the mechanism of induction of pTreg cell by immature or tolerogenic DC is complicated, the molecular mechanisms have been documented previously. It may rely on the secretion of IL-10 or IDO, expression of programmed cell death ligands 1 (PD-L1) from tolerogenic DC to influence naïve T cell differentiation during DC-T antigen presentation.Citation13,Citation44,Citation45 Several independent studies have shown that a T cell with a regulatory phenotype can be induced through interacting with antigen-pulsed tolerogenic DCs treated with vitamin D3, rapamycin, aspirin, or simvastatin.Citation46-Citation49 In agreement of these pieces of evidence, the CsA plus antigen in vitro treatments could greatly modulate the process of DC maturation, and programs DCs to a tolerogenic state, which lead to pTregs induction.
However, the exact mechanism of tolerogenic DCs induced by CsA plus autoantigens still needs to be further explored. Both CD4+ and CD8+ T cell can play highly pathogenic roles in the development of T1D. The evaluation of inhibitory effect on CD8+ T cell after immunization with islet-derived multipeptides plus CsA (GAD-IN+CsA vaccine) should be conducted as well. Since islet autoantigenic peptides GAD65206-220, GAD65536-550, Insulin B9-23, and Insulin C17-A1 as applied in this study are epitopes recognized by CD4+ T cells both in NOD mice and patient with T1D.Citation50,Citation51 It could be readily adaptable for a clinical development once demonstrated its efficacy in NOD mice. Furthermore, the optimal effective dose for inducing tolerance and the safety in human are remained as ambiguous and challenging.
In summary, the experiments described in this paper showed that islet-derived multipeptides with CsA as a composition to induce tolDCs and in turn to foster expandable antigen-specific pTregs to suppress auto-reactive Teff cells effectively, in resulting a prevent T1D onset in NOD animals. This antigen-specific pTregs induction, in conjunction with our previous studies, ingeminates the role of tolerogenic adjuvant by using immunosuppressants in the modulation of the tolDCs-Tregs process. Due to such strategy adaptable for clinical development, this vaccine might promote clinical application to treat patients with T1D in the future.
Materials and methods
Animals and reagents
Adult female BALB/c mice (6–8 wks of age) were purchased from the Shanghai SLAC Laboratory Animal Co. Ltd. (Shanghai, China). Male NOD mice (6–8 wk old) were purchased from the Model Animal Research Center of Nanjing University (Nanjing, China). DO11.10 OVA-specific TCR-transgenic BALB/c mice were gifts from Dr. Shi Yan (Tsinghua University, Beijing, China). They were bred and housed under pathogen-free conditions, and all animal protocols were approved by the Animal Welfare Committee of Fudan University. Streptozocin (STZ), lipopolysaccharide (LPS) were all purchased from Sigma Aldrich (St Louis, MO, USA). Cyclosporin A (CsA) was purchased from Fujiang Kerui Parhmarceutic Co. LTD (Fujiang, China). Peptides were all synthesized by Shanghai Science Peptide Biological Technology Co. Ltd (Shanghai, China) with the purity higher than 95%.
Vaccine preparation, immunization, and diabetes monitoring
Four of CD4 epitopic peptides were selected based on previous reports as immunogens for this study, including GAD65206-220 (TYEIAPVFVLLEYVT), GAD65536-550 (RMMEYGTTMVSYQ-PL), Insulin B9-23 (SHLVEALYLVCGERG), and Insulin C17-A1 (GAGDLQTLALEVAQQKRG). Peptide mixture designated as GAD-IN and CsA was dissolved in propylene glycol/PBS (1:1) solution. The concentrations of each peptide and CsA were 200 and 100 μg/ml, respectively.
Before vaccination, male NOD mice aged 8 wk were injected i.p. with four doses of STZ (50 mg/kg) consecutively to be prediabetic. On the last STZ injection, the mice were immunized s.c. with GAD-IN (each 20 μg/mouse) and CsA 10 μg/mouse as one does, GAD-IN alone, CsA alone, or vehicle at days 0, 6, and 12. Mice were sacrificed 24 h after the last vaccination and their CD4+CD25+Foxp3+ pTreg or CD4+CD25+Foxp3− effector T cells were isolated from spleen and pancreatic lymph node and analyzed by Flow cytometry. Blood glucose level was assessed by Yicheng JPS-6 Glucometer with glucose test strips (Yicheng Bioelectronics Technology Company, Beijing, China) every 3 days. Mice with two consecutive blood glucose levels of ≥15 mmol/l were defined as the onset of diabetes.
Oral glucose tolerance test (OGTT)
All immunized or control NOD mice, which were taken from 30 days after the first vaccination, were orally administered glucose at a dose of 2 g/kg body weight following an overnight fast. Blood samples were obtained at time 0, 30, 60, 120, and 150 min after the glucose administration and used to measure glucose levels with glucose test strips.
Histopathology
On day 30 after the first immunization, the pancreas was fixed in formalin, embedded in paraffin, sectioned and stained with hematoxylin and eosin (H&E). Sections were analyzed under a light microscope to determine histological changes. Paraffin-embedded pancreas sections were used for CD4 immunostaining with purified anti-mouse CD4 antibody (Cell Signaling Technology, USA).
In vitro stimulation of purified DCs
DCs were purified from 6-wk-old female BALB/c and DO11.10 mice. In brief, single-cell suspensions were obtained from freshly isolated spleen tissues and used to incubate with anti-mouse CD11c-coated magnetic beads (Miltenyi Biotec, Bergisch Gladbach, Germany) at room temperature for 30 min followed with purification through MACS separation columns. The purity of DCs assessed over 90% by FACS. DCs were plated in 96-well plates at 1 × 105 cells/ml in R-10 medium (RPMI 1640/10% heat-inactivated fetal bovine serum) with either CsA (1, 2.5, 5 or 10 μg/ml) and 20 μg/ml OVA323-339 mixture, 20 μg/ml OVA323-339 peptide alone, 100 ng/ml LPS, or vehicle, respectively. After 6 h in vitro culture, the cells were washed in PBS three times to remove unbound substances and fraction of them were used for phenotype analysis, and the remained portions were further used for co-culture assay.
Phenotype analysis of DCs
After treatment with the different stimulus, isolated DCs were stained with specific mAb for phenotype analysis. Briefly, DCs (1 × 105) were incubated in FACS buffer (2% fetal bovine serum in PBS) at 4°C for 30 min along with surface costimulatory molecule mAbs and viability dye, measured by flow cytometry and analyzed as the median fluorescence intensity (MFI). For intracellular anti-inflammatory cytokine, cells were fixed in 1% paraformaldehyde (Sigma, St. Louis, MO, USA), permeabilized with 0.5% Tween 20 and intracellularly immunostained as described.Citation43 Anti-inflammatory cytokine-producing DCs were quantified relative to total CD11c+ DCs. Fixable viability dye eFlour780 (Biolegend, San Diego, CA, USA) was used to exclude dead cells following its staining protocol.
Co-culture of tolerogenic DC with naïve CD4+CD25− T cell from DO11.10 mice
For the co-culture assay, splenic DCs were freshly isolated from DO11.10 (H-2d) mice, plated at 1 × 105 per well in a 96-well plate and further treated with 20 μg/ml OVA323-339 plus 10 μg/ml CsA, OVA323-339 alone, CsA alone, or vehicle in RPMI medium for 4 h in vitro, and followed by washing away free stimulants with fresh medium for three times. Naïve For Such pretreated DCs were co-cultured at 1:10 ratio with the CD4+CD25− T cells from DO11.10 transgenic mice previously immunized once with 10 μg of OVA protein in aluminum adjuvant and sorted by BD Aria II sorter (BD Bioscience, San Diego, CA, USA) supplied with 100 U/ml recombinant IL-2 (Biolegend). Anti-CD3 (2 μg/ml) and anti-CD28 (2 μg/ml) antibodies in the presence of 100 U/ml recombinant IL-2 and 0.5 ng/ml TGF-β (Biolegend) were used to induce Tregs from Naïve T cells served as quality control. After co-cultured for 72 h, CD4+ T cells were collected and washed in PBS. The percentage of Tregs and expression of Ki67 on these cells were analyzed by the flow cytometry, gated on live CD11c−CD4+Foxp3+ cells. The frequency and proliferation of activated primed Teff cells in the co-cultured system for 72 h were measured by quantified CD4+CD25+Foxp3− relative to total CD4+ T cells and intracellular staining of Ki67. Functional cytokines IFN-γ, IL-2 and TNF-α of Teff cell were also detected after adding protein transport inhibitor BD GolgiPlug™ (Brefeldin A) (BD Bioscience) at last 6 h. Naïve T cells were activated with coated anti-CD3 and anti-CD28 antibodies plus cytokines IL-2 for 3 days, and then stimulated with Phorbol 12-Myristate 13-Acetate (PMA) (Sigma, St. Louis, MO, USA) and a calcium ionophore (Ionomycin) (Sigma, St. Louis, MO, USA) for last 6 h as positive control for these cytokines detection.
Flow cytometry
The phenotypes of sorted DCs and T cells from pancreatic lymph nodes were analyzed by FACS FORTESSA with Diva software (BD Bioscience). Cells were stained with the following surface Abs: CD11c FITC (BD Bioscience), CD40 PerCP-Cy5.5 (eBioscience, USA), CD86 APC (eBioscience, USA), CD4 APC (eBioscience, USA) and CD25 (eBioscience, USA) Abs and incubated at 4°C for 30 min. For intracellular analysis of Foxp3 PE (eBioscience, USA), ki67 QD605 (Biolegend, San Diego, CA, USA), IL-10 BV421 (Biolegend, San Diego, CA, USA) and TGF-β PerCP-Cy5.5 (Biolegend, San Diego, CA, USA) expression, cells were stained with Foxp3 staining buffer according to the manufacturer’s protocol (eBioscience, USA). For intracellular cytokine IL-2 Brilliant violet 421 (Biolegend, San Diego, CA, USA), IFNγ PE-Cy7 (eBioscience, USA) and TNFα PE (eBioscience, USA) staining, T cells isolated from DO11.10 mice were treated with Brefeldin A for 6 h. After coculture, cells were washed in FACS buffer, fixed in 1% paraformaldehyde, permeabilized with 0.5% Tween 20, and stained with intracellular cytokine mAbs at 4°C for 40 min.
Statistical analysis
Data were analyzed using Prism 6 software (GraphPad Software, La Jolla, CA, USA) by either Student t-test (column analyses) or one-way ANOVA. The p values < 0.05 were considered significant.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Supplemental Material
Download MS Word (275 KB)Acknowledgments
This work was supported by grants from the National Natural Science Foundation of China Key Programs (30930068 and 31430027) and general Program (81672016) to Bin Wang. We also acknowledge Mr. Zhonghuai He and Ms. Yue Peng for their kind assistance in this work.
Supplemental data
Supplemental data for this paper can be accessed on the publisher’s website.
Additional information
Funding
References
- Atkinson MA, Eisenbarth GS, Michels AW. Type 1 diabetes. The Lancet. 2014;383:69–82. doi:10.1016/S0140-6736(13)60591-7.
- Santamaria P. The long and winding road to understanding and conquering Type 1 diabetes. Immunity. 2010;32:437–45. doi:10.1016/j.immuni.2010.04.003.
- Katsarou A, Gudbjornsdottir S, Rawshani A, Dabelea D, Bonifacio E, Anderson BJ, Jacobsen LM, Schatz DA, Lernmark Å. Type 1 diabetes mellitus. Nat Rev Dis Primers. 2017;3:17016. doi:10.1038/nrdp.2017.16.
- Ueda H, Howson JMM, Esposito L, Heward J, Snook H, Chamberlain G, Rainbow DB, Hunter KM, Smith AN, Di Genova G, et al. Association of the T-cell regulatory gene CTLA4 with susceptibility to autoimmune disease. Nature. 2003;423:506–11. doi:10.1038/nature01621.
- Long SA, Buckner JH. CD4(+)FOXP3(+) T regulatory cells in human autoimmunity: more than a numbers game. J Immunol. 2011;187:2061–66. doi:10.4049/jimmunol.1003224.
- Bayry J, Gautier JF. Regulatory T cell immunotherapy for Type 1 diabetes: a step closer to success? Cell Metab. 2016;23:231–33. doi:10.1016/j.cmet.2016.01.010.
- Roep BO, Tree TI. Immune modulation in humans: implications for type 1 diabetes mellitus. Nat Rev Endocrinol. 2014;10:229–42. doi:10.1038/nrendo.2014.2.
- Herold KC, Gitelman SE, Ehlers MR, Gottlieb PA, Greenbaum CJ, Hagopian W, Boyle KD, Keyes-Elstein L, Aggarwal S, Phippard D, et al. Teplizumab (Anti-CD3 mAb) treatment preserves C-peptide responses in patients with new-onset Type 1 diabetes in a randomized controlled trial metabolic and immunologic features at baseline identify a subgroup of responders. Diabetes. 2013;62:3766–74. doi:10.2337/db13-0345.
- Belghith M, Bluestone JA, Barriot S, Megret J, Bach JF, Chatenoud L. TGF-beta-dependent mechanisms mediate restoration of self-tolerance induced by antibodies to CD3 in overt autoimmune diabetes. Nat Med. 2003;9:1202–08. doi:10.1038/nm924.
- Mahnke K, Johnson TS, Ring S, Enk AH. Tolerogenic dendritic cells and regulatory T cells: a two-way relationship. J Dermatol Sci. 2007;46:159–67. doi:10.1016/j.jdermsci.2007.03.002.
- Hammer GE, Ma A. Molecular control of steady-state dendritic cell maturation and immune homeostasis. Annu Rev Immunol. 2013;31:743–91. doi:10.1146/annurev-immunol-020711-074929.
- Terness P, Oelert T, Ehser S, Chuang JJ, Lahdou I, Kleist C, Velten F, Hämmerling GJ, Arnold B, Opelz G. Mitomycin C-treated dendritic cells inactivate autoreactive T cells: toward the development of a tolerogenic vaccine in autoimmune diseases. Proc Natl Acad Sci U S A. 2008;105:18442–47. doi:10.1073/pnas.0807185105.
- Boks MA, Kager-Groenland JR, Haasjes MS, Zwaginga JJ, van Ham SM, Ten Brinke A. IL-10-generated tolerogenic dendritic cells are optimal for functional regulatory T cell induction–a comparative study of human clinical-applicable DC. Clin Immunol. 2012;142:332–42. doi:10.1016/j.clim.2011.11.011.
- Martin S, Agarwal R, Murugaiyan G, Saha B. CD40 expression levels modulate regulatory T cells in Leishmania donovani infection. J Immunol. 2010;185:551–59. doi:10.4049/jimmunol.0902206.
- Geng S, Zhang H, Zhou X, He Y, Zhang X, Xie X, Li C, He Z, Yu Q, Zhong Y, et al. Diabetes tolerogenic vaccines targeting antigen-specific inflammation. Hum Vaccin Immunother. 2015;11:522–30. doi:10.1080/21645515.2014.1004024.
- Li C, Zhou X, Zhong Y, Li C, Dong A, He Z, Zhang S, Wang B. A recombinant G protein plus cyclosporine A-based respiratory syncytial virus vaccine elicits humoral and regulatory T cell responses against infection without vaccine-enhanced disease. J Immunol. 2016;196:1721–31. doi:10.4049/jimmunol.1502103.
- Reddy S, Chang M, Robinson E. Young NOD mice show increased diabetes sensitivity to low doses of streptozotocin. Ann N Y Acad Sci. 2006;1079:109–13. doi:10.1196/annals.1375.015.
- Baxter AGMT. Accelerated diabetes in non-obese diabetic (NOD) mice differing in incidence of spontaneous disease. Clin Exp Immunol. 1991;85:8.
- Dirice E, Kahraman S, Elpek GO, Aydin C, Balci MK, Omer A, Sanlioglu S, Sanlioglu AD. TRAIL and DcR1 expressions are differentially regulated in the pancreatic islets of STZ- versus CY-applied NOD mice. Exp Diabetes Res. 2011;2011:625813. doi:10.1155/2011/625813.
- Robertson JM, Jensen PE, Evavold BD. DO11.10 and OT-II T cells recognize a C-terminal ovalbumin 323–339 epitope. J Immunol. 2000;164:4706–12.
- McFarland BJ, Sant AJ, Lybrand TP, Beeson C. Ovalbumin(323-339) peptide binds to the major histocompatibility complex class II I-A(d) protein using two functionally distinct registers. Biochemistry. 1999;38:16663–70.
- Desreumaux P, Foussat A, Allez M, Beaugerie L, Hebuterne X, Bouhnik Y, Nachury M, Brun V, Bastian H, Belmonte N, et al. Safety and efficacy of antigen-specific regulatory T-cell therapy for patients with refractory Crohn’s disease. Gastroenterology. 2012;143:1207–17 e2. doi:10.1053/j.gastro.2012.07.116.
- Bluestone JA, Herold K, Eisenbarth G. Genetics, pathogenesis and clinical interventions in type 1 diabetes. Nature. 2010;464:1293–300.
- Yu H, Paiva R, Flavell RA. Harnessing the power of regulatory T-cells to control autoimmune diabetes: overview and perspective. Immunology. 2018;153:161–70. doi:10.1111/imm.12867.
- Putnam AL, Brusko TM, Lee MR, Liu W, Szot GL, Ghosh T, Atkinson MA, Bluestone JA. Expansion of human regulatory T-cells from patients with type 1 diabetes. Diabetes. 2009;58:652–62. doi:10.2337/db08-1168.
- Bluestone JA, Trotta E, Xu D. The therapeutic potential of regulatory T cells for the treatment of autoimmune disease. Expert Opin Ther Targets. 2015;19:1091–103. doi:10.1517/14728222.2015.1037282.
- Bluestone JA, Buckner JH, Fitch M, Gitelman SE, Gupta S, Hellerstein MK, Herold KC, Lares A, Lee MR, Li K, et al. Type 1 diabetes immunotherapy using polyclonal regulatory T cells. Sci Transl Med. 2015;7:315ra189. doi:10.1126/scitranslmed.aad3106.
- Veerapathran A, Pidala J, Beato F, Yu XZ, Anasetti C. Ex vivo expansion of human Tregs specific for alloantigens presented directly or indirectly. Blood. 2011;118:5671–80. doi:10.1182/blood-2011-02-337097.
- Ludvigsson J, Krisky D, Casas R, Battelino T, Castano L, Greening J, Kordonouri O, Otonkoski T, Pozzilli P, Robert -J-J, et al. GAD65 antigen therapy in recently diagnosed type 1 diabetes mellitus. N Engl J Med. 2012;366:433–42. doi:10.1056/NEJMoa1107096.
- Orban T, Farkas K, Jalahej H, Kis J, Treszl A, Falk B, Reijonen H, Wolfsdorf J, Ricker A, Matthews JB, et al. Autoantigen-specific regulatory T cells induced in patients with type 1 diabetes mellitus by insulin B-chain immunotherapy. J Autoimmun. 2010;34:408–15. doi:10.1016/j.jaut.2009.10.005.
- Thrower SL, James L, Hall W, Green KM, Arif S, Allen JS, Van-Krinks C, Lozanoska-Ochser B, Marquesini L, Brown S, et al. Proinsulin peptide immunotherapy in type 1 diabetes: report of a first-in-man Phase I safety study. Clin Exp Immunol. 2009;155:156–65. doi:10.1111/j.1365-2249.2008.03814.x.
- Alhadj Ali M, Liu YF, Arif S, Tatovic D, Shariff H, Gibson VB, Yusuf N, Baptista R, Eichmann M, Petrov N, et al. Metabolic and immune effects of immunotherapy with proinsulin peptide in human new-onset type 1 diabetes. Sci Transl Med. 2017. doi:doi:10.1126/scitranslmed.aaf7779.
- Kuhtreiber WM, Tran L, Kim T, Dybala M, Nguyen B, Plager S, Huang D, Janes S, Defusco A, Baum D, et al. Long-term reduction in hyperglycemia in advanced type 1 diabetes: the value of induced aerobic glycolysis with BCG vaccinations. NPJ Vaccines. 2018;3:23. doi:10.1038/s41541-018-0062-8.
- European FK506 Multicenter Liver Study Group. Randomised trial comparing tacrolimus (FK506) and cyclosporin in prevention of liver allograft rejection.Lancet. 1994;344:423–28.
- Dougados M, Awada H, Amor B. Cyclosporin in rheumatoid arthritis: a double blind, placebo controlled study in 52 patients. Ann Rheum Dis. 1988;47:127–33. doi:10.1136/ard.47.2.127.
- Assan R, Debraysachs M, Laborie C, Chatenoud L, Feutren G, Quinioudebrie MC, Chatenoud L, Bach JF. Metabolic and immunological effects of cyclosporin in recently diagnosed Type-1 diabetes-mellitus. Lancet. 1985;1:67–71.
- Laburte C, Grossman R, Abi-Rached J, Abeywickrama KH, Dubertret L. Efficacy and safety of oral cyclosporin A (CyA; Sandimmun) for long-term treatment of chronic severe plaque psoriasis. Br J Dermatol. 1994;130:366–75.
- Mccaffrey PG, Luo C, Kerppola TK, Jain J, Badalian TM, Ho AM, Burgeon E, Lane WS, Lambert JN, Curran T. Isolation of the cyclosporine-sensitive T-cell transcription factor Nfatp. Science. 1993;262:750–54.
- Fric J, Zelante T, Wong AYW, Mertes A, Yu HB, Ricciardi-Castagnoli P. NFAT control of innate immunity. Blood. 2012;120:1380–89. doi:10.1182/blood-2012-02-404475.
- Manome H, Aiba S. Dexamethasone and cyclosporin A affect the maturation of monocyte-derived dendritic cells differently. Int Arch Allergy Immunol. 2000;122:76–84. doi:10.1159/000024406.
- Tajima K, Amakawa R, Ito T, Miyaji M, Takebayashi M, Fukuhara S. Immunomodulatory effects of cyclosporin A on human peripheral blood dendritic cell subsets. Immunology. 2003;108:321–28.
- Palay DA, Cluff CW, Wentworth PA, Ziegler HK. Cyclosporine inhibits macrophage-mediated antigen presentation. J Immunol. 1986;136:4348–53.
- Kang Y, Xu L, Wang B, Chen A, Zheng G. Cutting edge: immunosuppressant as adjuvant for tolerogenic immunization. J Immunol. 2008;180:5172–76.
- Baban B, Chandler PR, Sharma MD, Pihkala J, Koni PA, Munn DH, Mellor AL. IDO activates regulatory T cells and blocks their conversion into Th17-like T cells. J Immunol. 2009;183:2475–83. doi:10.4049/jimmunol.0900986.
- Francisco LM, Salinas VH, Brown KE, Vanguri VK, Freeman GJ, Kuchroo VK, Sharpe AH. PD-L1 regulates the development, maintenance, and function of induced regulatory T cells. J Exp Med. 2009;206:3015–29. doi:10.1084/jem.20090847.
- Unger WWJ, Laban S, Kleijwegt FS, van der Slik AR, Roep BO. Induction of Treg by monocyte-derived DC modulated by vitamin D-3 or dexamethasone: differential role for PD-L1. Eur J Immunol. 2009;39:3147–59. doi:10.1002/eji.200839103.
- Turnquist HR, Raimondi G, Zahorchak AF, Fischer RT, Wang ZL, Thomson AW. Rapamycin-conditioned dendritic cells are poor stimulators of allogeneic CD4(+) T cells, but enrich for antigen-specific Foxp3(+) T regulatory cells and promote organ transplant tolerance. J Immunol. 2007;178:7018–31. doi:10.4049/jimmunol.178.11.7018.
- Buckland M, Jago C, Fazekesova H, George A, Lechler R, Lombardi G. Aspirin modified dendritic cells are potent inducers of allo-specific regulatory T-cells. Int Immunopharmacol. 2006;6:1895–901.
- Zhang X, Tao YZ, Wang JZ, Garcia-Mata R, Markovic-Plese S. Simvastatin inhibits secretion of Th17-polarizing cytokines and antigen presentation by DCs in patients with relapsing remitting multiple sclerosis. Eur J Immunol. 2013;43:281–89. doi:10.1002/eji.201242566.
- Mallone R, Brezar V, Boitard C. T cell recognition of autoantigens in human type 1 diabetes: clinical perspectives. Clin Dev Immunol. 2011;2011:513210. doi:10.1155/2011/513210.
- Di Lorenzo TP. MPaBOR. translational mini-review series on Type 1 diabetes, systematic analysis of T cell epitopes in autoimmune diabetes. Clin Exp Immunol. 2007;148:16. doi:10.1111/j.1365-2249.2007.03362.x.

