ABSTRACT
Infectious diseases represent a major cause of deaths worldwide. No vaccine or effective treatment exists nowadays, especially against intracellular pathogens. The increase in multiple drug and superbug antibiotic resistance strains, excessive medication, or misuse of drugs has prompted the search for other safe and effective alternatives. Consistent with this, adjuvants (Latin word “adjuvare”: “help or aid”) co-administered (Exo) in vaccines have emerged as a promising alternative to initiate and boost an innate, downstream signal that led to adaptative immune response. Nowadays, a promising model of strong immunogens and adjuvants at mucosal sites are the microbial bacterial toxins. Other adjuvants that are also used and might successfully replace aluminum salts in combination with nanotechnology are CpG-ODN, poly IC, type I IFNs, mRNA platforms. Therefore, in the present review, we focused to revisit the old to the new adjuvants compounds, the properties that make them friends in vaccine formulations against infectious diseases.
1. Introduction
Infectious diseases remain the most common cause of death in children less than 5 y of ageCitation1. Common infectious diseases such as diphtheria, tetanus, poliomyelitis, smallpox, and measles were able to prevent using relatively simple vaccines that stimulated robust antibody responses, except for malaria, tuberculosis, and human immunodeficiency virus (HIV) for whose there are not at present any vaccine or effective treatment and these pathogens are not effectively prevented by antibodies alone but long-lasting cellular responseCitation1. Furthermore, the emergence of new resistance mechanisms, the excessive and misuse of antibiotics in a clinic, the decrease in the development of antibiotics in the industry are urgent issues that represent challenges that lead us to consider adjuvants in vaccines candidates can enhance and boost innate and adaptative long memory response, precisely against intracellular pathogens.
The host–pathogen interaction could be visualized as a chemical reaction in which each element reacts by triggering molecular and genetic events that will transmit intracellular signals from the cell surface membrane to the nucleus, leading to changes in gene expression and inducing a robust effector and memory immune response, while also bringing about mechanisms that will change the course of the interaction toward a higher susceptibility or resistance (establishment of the infection) (). On one side, host response overcomes the mechanisms of evasion developed by pathogens, through the induction of innate and adaptive immune responses () while on other hand, the pathogens adjust and fitness physiological and genomic program for survival and adaptation (). What might be the potential role of the adjuvants compounds in the interaction host-pathogen? A key function of the adjuvants compounds is to initiate innate immune response (recognition receptor – ligand) that trigger the inflammatory response (cytokines) to the infection. In other words, adjuvants, enhance pathogen recognition and eliciting a response similar to the natural innate immune response. Thereby, adjuvants (from the Latin word “adjuvare” meaning “help or aid”), defined as compounds or molecules that can promote and enhance immune response – humoral or cellular,Citation2–5 either at the interface of the systemic or mucosal compartment.Citation2,Citation6–9
Figure 1. Most of the threatening infectious disease is targeted at the mucosal sites (around 400 mt2), therefore, the interaction at these sites constitutes the first line of the host defense. Upon host–pathogen interaction, a differential outcome for each other is the results of A + B, the product of this interaction, can be visualized as the outcome of the transcriptional and genomic program for the induction of protective adaptive immune response (HOST).; and for another side as an adjustment and fitness of the genomic program to develop evasion mechanism (PATHOGEN) for the successful establishment, survival, and adaptation into the host
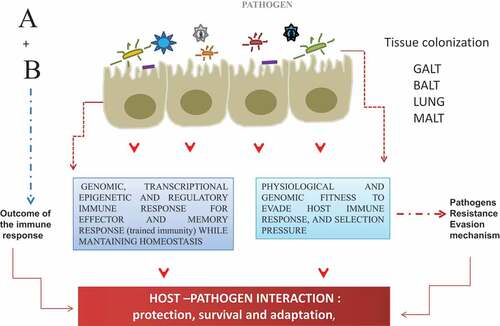
The repertoire of the adjuvants (endo-exo) in nature is broad from herbaceous secondary metabolites to antimicrobial peptides. The former is one of the effective mechanisms to kill bacteria – which could be induced upon natural infections (i.e. human defensins), but it could also be obtained from bacterial and marine sources.Citation10 In general, the world of adjuvant is not limited to aluminum salts (Alum salts mostly induce Th2 type immune responses and therefore, mostly enhance antibody responses) on the contrary, it has extended to other compounds derived from fruits, vegetables, and other types of plants like the Quillaga saponaria, QS-21 which a saponin of amphiphilic structure, formed by a structure of a carbón skeleton derived from squalene bonded to sugar residues (1, 2 o 3) with a relatively lipophilic a glycine moiety. The adjuvants compounds can be also of marine, or bacterial origin, such as recombinant subunits of bacterial toxins, such as heat-stable enterotoxins from Vibrio cholerae, or Escherichia coli (CTX/LTX), Bacillus thuringiensis Cry proteins, type I interferon (IFNs), Heparin-binding hemagglutinin adhesion (HBHA), Monophosphoryl Lipid A (MPLA), Unmethylated oligonucleotides (CpGODN) able to bias and balance the Th cellular immune response between the Th1 type and Th2 immune response. Poly(I: C) (Polyinosic: polycytidylic acid), compounds that can participate in dendritic cell maturation. (). In addition, calcium phosphate, chitosan have been replaced with aluminum salts because they bias Th cellular immune response toward Th1. Furthermore, the use of anti-inflammatory adjuvants in vaccine formulation to modulate the host response to pathogens toward T helper type (Th) Th2 or Th1 response, and the balance Th1/Th2 will dictate the outcome of humoral and effector cellular immune response. Thus, adjuvants in vaccine formulation as immune potentiators considering populations that are poor responders, elderly or vulnerable can influence positively in the induction of neutralizing antibody response and of CD8 + T lymphocytes (producers of granzymes, perforins) that are enough to limit virus or bacterial replication and establishment in the host cells.Citation2,Citation6–8,Citation11–15 Therefore, adjuvants are essential components of most clinically used vaccines. This is because the majority of non-living vaccines are relatively poor inducers of adaptive immunity unless effective adjuvants are co-administered. It is true not all vaccines need adjuvants, vaccines containing whole pathogens (live attenuated or inactivated), contain a heterogeneous mixture of diverse antigens and other pathogen components that act as intrinsic adjuvants, thereby these vaccines are capable of initiating innate immunity, which drives subsequent adaptive responses that lead to successful clearance of the pathogen. A problem is these vaccines are not suitable when natural infection itself does not confer long-standing immunity or when the pathogen is unable to be grown in culture. Thus, modern vaccines containing a limited number of purified antigens, which are also often less immunogenic due to the removal of pathogen features of the organism, and therefore, it is necessary to use the adjuvants because these compounds can improve immune responses in populations where responses to traditional vaccines are typically reduced such as infants, elderly, vulnerable and immunocompromised individuals, Despite some potential risks and safety considerations with the use of adjuvants, it is thought that the selection of the type the dose, and the route of administration, these compounds are key elements of current vaccines that are in development. In the present review, we aimed to revisit the old to the new adjuvant compounds, the properties that make them a friendly and promising strategy in vaccine formulations against infectious diseases (intracellular pathogens).
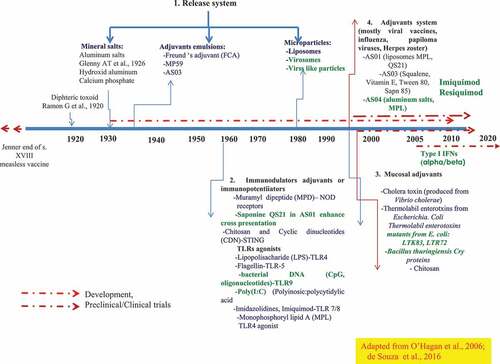
Figure 2. The repertoire of adjuvants compounds, categories, and development since 1920. A released system developed to target the vaccine subunits to the sites of the induction of the innate immune response (AS03, AS04, liposomes virosomes, viral-like particles). TLR agonist, immunostimulants (CpGODN, ISCOMS; Poly (I: C), MPL Flagellin; QS21, Imiquimod, Resiquimod). Of relevance is the availability and the potential of molecular mucosal adjuvants, -recombinant bacterial toxin, type I IFNs-that deserve further exploration and deep evaluation in phase I through phase III against intracellular pathogens
2. Types and classification of adjuvants compounds
Adjuvants can be categorized into two types: 1. Compounds/molecules that directly stimulate the host immune system called “Immunostimulants” and 2. Compounds that act indirectly included in vaccine formulations with live-attenuated pathogens (bacteria, viruses, fungi); purified antigens (recombinant proteins); subunits (toxoid, split viruses, fragments of pathogens)Citation16,Citation17 and schematized system that comprised of adjuvant plus vaccine or purified antigens plus adjuvant simple or in combination. Another adjuvant classification that has been well acceptedCitation16–18 as adjuvants consists of four groups: 1) Release system (e.g. mineral salts, aluminum salts, calcium phosphate).Citation19–21 2) Immunomodulators or immunopotentiators (e.g. MPLA, LPS (lipopolysaccharide), Flagellin, CpG, Poly IC, QS21; ISCOMs).Citation22–26 3). Mucosal adjuvants (bacterial toxins.Citation9,Citation15 4). Adjuvants system, such as (AS01, AS03, AS04Citation9,Citation22,Citation25; ). In more recent years, type I IFNs,Citation26–32 antimicrobial peptides;Citation33–42 chemokines;Citation43 and the use of nanoparticleCitation19–21 represent the new era of mucosal adjuvantsCitation2,Citation15–18 ().
2.1. Immunological properties of the adjuvants
2.1.1. Release systems
Aluminum salts (alum) have been used as adjuvants with great success for almost a century and have been particularly effective at promoting protective humoral immunity. Aluminum salt/gel-based (alum) adjuvants remain the only standard versatile adjuvant licensed for human use in the United States. Alum can not induce a T helper type I (Th1) cell-mediated immune response that is important in fighting against certain viruses, bacteria, and parasites. Until now is the only approved adjuvants for human use, a Th2 type adjuvant that stimulates poor immunity to the elderly.Citation43 To note is that Aluminum-adjuvanted vaccines have not been successful in preventing infection due to intracellular pathogens. Another early adjuvant attempt was a mineral oil-in-water emulsion (Freund’s incomplete adjuvant) which was considered too reactogenic for continued use in humans Adjuvants have been used for more than 90 y and are currently of more than 30 licensed vaccines from different manufacturers.Citation17,Citation44 Alum exerts its immune-stimulatory activity by triggering the release of uric acid, a danger signal that amplifies the activation of DCs via the NALP3 inflammasome as shown by the increase in the co-stimulatory ligand CD86. This amplified DC activation leads to an immediate inflammatory response at the administration site, the generation of an adaptive cellular immune response, and a persistent Th2 immunity.Citation45,Citation46 Alum adjuvants induce the release of interleukin1-beta (IL-1β) from macrophages and dendritic cells and that this is abrogated in cells lacking various NALP3 inflammasome components.Citation47
The NALP3 inflammasome is also required in vivo for the innate immune responses to OVA in alum. The activation of the cellular immunity to OVA alum is initiated by monocytic dendritic cell precursors that induce the expansion of Ag-specific T cells in a NALP3 dependent way. It has been proposed that in addition to TLR stimulators, agonists of the NALP3 inflammasome, should also be considered as vaccine adjuvantsCitation48–54 ()).
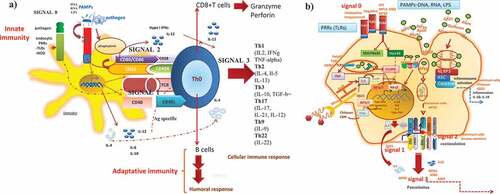
Figure 3. How adjuvant compounds in vaccine formulations initiate, boost and trigger the innate and adaptative immune response. The induction of the immune response occurs through the interaction of the mimic pathogen-associated molecular patterns (PAMPs) and the PRRS (e.g. TLRs, NLRs receptors) on antigen-presenting cells (APCs) (dendritic cells) that trigger an innate immune response leading to activation and maturation of APCs and initiation of downstream MyD88 signalization transduced to the nucleus, leading to pro-inflammatory response necessary to mount a specific durable and effective immune response by T CD4+ and T CD8+ lymphocytes (a). The mechanism of action of the adjuvants comprises a program of four signals which ended with the induction of the effector and memory cellular immune responses and thereby specific T and B cell responses. From the repertoire of adjuvants (TLR agonists, CpG-ODN, Poly(I: C, bacterial toxins), vehicles/carriers (ISCOMS, virosomes, liposomes), adjuvant system (AS03, AS04) as components of modern vaccines, which usually lack some of the components of the whole live microorganism, initiate the innate immune response by acting as PAMPs and thereby, participate by enhancing the interaction between the vaccine ((Ag) and the antigen-presenting cells (macrophages, dendritic cells, epithelial cells), by enhancing uptake, presentation, costimulation and activation, n of quality and magnitude of the activation of CD4 + T cells and CD8 + T cells and B cell differentiation to plasmacytoid B cells antibody producers, cytokines (TGF-β) and T cell homing molecules at MAL (mucosal-associated tissues) (b)
In a recent study, it was described that alum also induces high-level production of uric acid in vivo and this increased level of uric acid was required for infiltration of inflammatory cells. Although how this is done and which cells generate or release uric acid upon alum administration are open questions, it suggests that the increased level of uric acid leads to an amplification of the NALP3 inflammasome activation and, thus, IL1-β secretion. Interestingly, uric acid was identified not only as one of the most potent danger signals released from dying cells but also as an excellent adjuvant.Citation53–55
2.1.2. Immuno-stimulatory or immune-potentiators molecules
Adjuvants compounds that can enhance innate immunity. Adjuvants containing pathogen-associated molecular patterns act as ligands for TLRs. Thus, TLR9 was shown to be essential for the adjuvant effect of CpG oligonucleotides.Citation54 Alum adjuvants in contrast to bacteria-derived adjuvants do not activate TLRs. Alum adjuvants trigger activation of the NALP3 inflammasome. The potential of alum to trigger the NALP3 inflammasome leads to early activation of the innate cytokine IL-1β and an innate cellular immune response at the site of injection. Activation of the NALP3 inflammasome and the subsequent release of IL-1b leads to the recruitment of immature monocytes and DCsCitation47,Citation56 (). Production of IL-1β also leads to the activation of inflammatory monocytes and their migration to the lymph nodes draining the peritoneum. Interestingly, MPL® is the first non-alum vaccine adjuvant obtained from a Salmonella enterica endotoxin, which accounts for significant widespread and clinical market acceptance. The stimulatory dose–response curves revealed that most preparations of MPL are much more active in mice than in human cell systems, this is because in human cells correlated with human TLR4 inhibitory activity that resulted in a partial agonist profile.Citation57 While the biodegradable adjuvant, MCT®, was developed for application in the niche area of allergy immunotherapy (AIT), also in combination with a TLR-4 adjuvant-MPL®-producing the first adjuvant system approach for AIT in the clinic.Citation58
The Adjuvant System AS01 (a liposome-based vaccine adjuvant system containing two immunostimulants: 3-O-desacyl-4ʹ-monophosphoryl lipid A (MPL) and the saponin QS-21. AS01 is efficient at promoting CD4 + T cell-mediated immune responses and is an appropriate candidate adjuvant for inclusion in vaccines targeting viruses or intracellular pathogens. AS01 has been selected for the clinical development of several candidate vaccines including the RTS, malaria vaccine, and the subunit glycoprotein E varicella-zoster vaccine (both currently in phase III. AS01 to improve adaptive immune responses. Enhancement of the adaptive immunity by AS01 depends on activated dendritic cells and that depends on synergistic activities of QS-21 and MPL.Citation59,Citation60 MPL and aluminum salts are present in AS04, and both MPL and QS-21 are present in AS01 and AS02, which are liposome- and emulsion-based formulations, respectively. The licensing of two AS04-adjuvanted vaccines and the initiation of Phase III trials with an AS01-adjuvanted vaccine demonstrate the potential to develop new or improved human vaccines that contain MPL or MPL and QS-21.Citation61 In viral settings, it was compared AS01 versus other Adjuvant Systems in a candidate herpes zoster glycoprotein E subunit vaccine. It was evaluated formulated with AS01B, AS01E (50% less MPL and QS-21 than AS01B), AS03, or AS04 in C57BL6 mice primed with live-attenuated VZV. Four-weeks post-vaccination, the IgE-specific CD4 + T-cell response to gE/AS01B was 5.4, 2.8 and 2.2-fold greater than those to gE/AS03, gE/AS04 and gE/AS03, respectively (p < .001). Therefore in the VZV-primed mouse model, CD4 + T-cell responses to IgE were most enhanced by AS01.Citation62 In other vaccine formulations, liposomes containing monophosphoryl lipid A and QS-21 serve as an effective adjuvant for soluble circumsporozoite protein malaria vaccine FMP013 in a mouse model, C57BL6. FMP013 antigen in C57BL/6 mice formulated with two novel adjuvants of the Army Liposome Formulation (ALF) series and a commercially available adjuvant Montanide ISA 720 (Montanide) as a control. ALF is a liposomal adjuvant containing a synthetic monophosphoryl lipid A (3D-PHAD®). FMP013 was adjuvanted with ALF alone, ALF containing aluminum hydroxide (ALFA), or ALF containing QS-21 (ALFQ). Adjuvants ALF and ALFA induced similar antibody titers and protection against transgenic parasite challenges that were comparable to Montanide. FMP013+ ALFQ also augmented the numbers of splenic germinal center-derived activated B-cells and antibody-secreting cells compared to Montanide. Further, FMP013+ ALFQ induced antigen-specific IFN-γ ELISPOT activity, CD4 + T-cells, and a TH1-biased cytokine profile.Citation63 Moreover, immunization with Virus-Like Particles Encapsulated in Monophosphoryl Lipid A and Liposomes there is a promotion of Cellular and Humoral Immunity against Foot-and-Mouth Disease Virus.Citation64 In the study, it was described that MPL/DDA-VLPFMDV could induce strong cell-mediated immune responses by inducing not only VLP-specific IFN-γ+ CD4+ (Th1), IL-17A+CD4+ (Th17), and IFN-γ+ CD8+ (activated CD8 response) T cells, but also the development of VLP-specific multifunctional CD4+ and CD8+ memory T cells co-expressing IFN-γ, TNF-α, and IL-2 (). In addition, MPL/DDA-VLPFMDV vaccine markedly induced VLP-specific antibody titers; in particular, induce greater Th1-predominant IgG responses than VLPFMDV only and DDA-VLPFMDVCitation65. Furthermore, bacterial lipopolysaccharide (LPS) is toxic, and it has an excellent ability to mobilize innate immunity by Toll-like and other receptors and promote the maturation of dendritic cells.Citation66 However, its modified version of MPL is less toxic but a better adjuvant.Citation67,Citation68 Finally, a liposome-based delivery system incorporating CpG (is the most promising TLR Ligand that stimulates TLR9 in a pathway requiring the adaptor MyD88, leading to the activation of dendritic cells (DCs)Citation2,Citation23 which induces the rapid recruitment of neutrophils, enhances dendritic cell-associated Ag transport and influences the maturation of innate cells entering the afferent lymph, translated into an extended period of lymph node shutdown, the induction of IFN-γ-positive T cells, and enhanced production of Ag-specific Abs in a large animal model after vaccination of a dose comparable to that administered to humans.Citation65
2.1.3. Mucosal adjuvants
2.1.3.1. Bacterial toxins
Bacterial toxins are protein antigens, a condition that will result with time in the production of neutralizing antibodies that would abrogate their adjuvanticity and a loss of vaccine efficacy. This observation applies also to carriers containing proteins like keyhole lymphocyanin (KLH) and viral particles, where antibodies against the carrier’s protein(s) may inhibit immune response signal against the conjugated immunogens by a process known as carrier induced epitopes suppression (CIES).Citation69 A group of bacterial toxins, e.g. Vibrio cholerae, cholera toxin (CTx), and Escherichia coli heat-labile enterotoxins (LTx). ADP-ribosylating enterotoxins as vaccine adjuvantsCitation70–77 that are been considered for Alzheimer’s disease (AD) development. Since most of the pathogens enter the body by mucosal sites, therefore, it is key to develop mucosal vaccines that prevent local infection or invasion of pathogens, able to induce or to mount significant innate and adaptive immune responses in terms of sIgA antibodies, a subclass of IgG and tissue-resident memory CD4+ CD8 + T cells, Adenosine diphosphate (ADP)-ribosylating bacterial enterotoxins, such as cholera toxin (CT) and Escherichia coli heat-labile toxins (LTs) remain as the most strong mucosal immunogen and adjuvants. Cholera toxin (Ctx) and its close relative, Escherichia coli heat-labile enterotoxin (ETx) have long been established as potent mucosal and systemic adjuvants. Nontoxic-B-subunit of ETx (ETxB) is a highly potent mucosal adjuvant capable of potentiating protective immunity to viral infection by triggering specific signaling processes in lymphocyte populations, modulate differentially their activation differentiation and survival.Citation9,Citation70–77 Research on these toxins has been focused on their effects as mucosal adjuvants inducing Th2 type cellular immune response, their induced immunity depends on several factors, e.g administration route, and age of the animals. The wild types of toxins are toxic to human beings, however, the mutants or derivatives. The mechanism of LTB adjuvanticity of LTB was to enhance the turnover of dendritic cells (DCs) in the spleen and increase DC capacity to perform as antigen presentation cells (APCs) encountered with T cells. LTB also induces B and T cell clustering and delay/arrest in T–cell division following endocytosis or B cell receptor (BCR) uptake of antigen in a ganglioside (GM1)-mediated manner. A nontoxic mutant of CT that in young mice induced Th2 immunity, in aged mice induced both Th1 and Th2Citation74 Also it has been shown that other CT mutants induce Th17 type, a strong inflammatory response that may be damaging in AD vaccines. The enterotoxin LT is also being evaluated as an adjuvant for AD vaccines and because of the wild-type toxin’s toxicity, several nontoxic mutants have been developed. Like with CT mutants, the type of immunity induced by LT, either Th2 or Th1/Th2 type, would depend on the routes used for immunization.Citation75 Therefore, LT mutants depending on various factors may induce Th1 or Th17 inflammatory immunity not convenient for artherosclerosis (AS) vaccine but against intracellular pathogens.Citation76 Recent advancesCitation77 has pointed out that in the mechanism of adjuvanticity of thermolabile enterotoxin subunit B (LTB) is the immunogenicity and not the binding or the ADP-ribosylation activity that accounts for the observed adjuvanticity. Escherichia coli heat-labile enterotoxins B subunit is a more potent mucosal adjuvant than its closely related holotoxin, the B subunit of cholera toxin.Citation77 In a study, purified ETxB and CtxB were tested with hen egg lysozyme, and it was found that ETxB induced higher responses than CTxB, assessed by the induction of secretory antibody titers as well as by the stimulation of lymphocyte proliferation in the spleen and in draining lymph nodes, implying that both subunits should be considered independent in prospective vaccinesCitation78 (). Another toxin that has shown that act as adjuvants are the Cry proteins, obtained from Bacillus thuringiensis (Bt), a soil bacteria that have been used for several decades as bioinsecticides.Citation65,Citation66 However, in recent years, several pieces of evidence have indicated that they can induce adjuvant protective effects toward several parasites such as Naegleria fowleri,Citation78 metacestodes in ciscticercosis,Citation79 Brucella abortusCitation80, Plasmodium falciparum,Citation81 or enhance cellular immunity to Mycobacterium Bovis Bacillus Calmette Güerin (BCG).Citation14 The mechanism of action remains to be elucidated and defined. Despite this, it is tempting to propose this bacterial toxin as a safe alternative to induce robustness humoral and cellular immune responses at the systemic and mucosal levels.
3. How adjuvants initiate and boost innate and adaptive immune responses as components of vaccine formulations
The adjuvants, as immune potentiatiors can initiate and boost innate immune responseCitation16–18 through mimicking pathogen-associated molecular patterns (PAMPs), that interact with the pattern recognition receptors (PRRS) (e.g. TLRs, NLRs) on antigen-presenting cells (APCs) (dendritic cells, macrophages, epithelial cells ()), resembles a reaction receptor-ligand, that trigger an innate immune response leading to activation and maturation of APCs (e.g. dendritic cells) and initiation of downstream MyD88 signalization transduced to the nucleus, and leading to a pro-inflammatory response necessary to mount a specific durable, and long-term effective immune response upon the host–pathogen interaction () that will influence and impact onB and T lymphocyte population (CD4+ and CD8+) () and the Th subsets (Th1, Th2) ()). – How adjuvants compounds accomplished this task? The mechanism of action of adjuvants is wide and diverse.Citation2,Citation5–8,Citation24,Citation25,Citation27 But in general, it is well accepted that could be accomplished through a four-signal mechanism of actionCitation17–19()). The adjuvant molecule interacts with the receptors on innate immune cells, the antigen-presenting cells (macrophages, dendritic cells, epithelial cells, neutrophils)Citation6,Citation9,Citation11,Citation12,Citation15 or Signal 0: a first encounter that involves the initial interaction between the pattern of recognition receptors (PRRs) such as toll receptors (TLRs), other non-TLRs receptors; NOD-like receptors (NLRs); RIG-I-like-receptor (RLRS); dectin receptors or mannose lectin-like receptorsCitation11,Citation82–91 on the skin or mucosal innate cells (macrophages, dendritic cells, B cells, epithelial cells) and pattern associated molecular pathogens (PAMPs) (DNA, RNA, proteins, LPS) and/or the pathogen () and ).
Table 1. Approved TLR agonist as adjuvants
Signal 1: antigen (Ag) presentation (enhanced by aluminum salts, oils, emulsions) or antigen uptake and processing (MF59). The PRRS-Ag could be endocytosed or the pathogen itself could be endocytosed. All these processes would lead to the activation and maturation of the innate response and the activation of the key signalization pathways (Myd88, TRIF/TRAF, STING), from the cytosol to the nucleus (translocation of NF-kB), expression of IRF3/Citation7,Citation12,Citation29,Citation30 and induce an inflammatory response mediated by cytokines (IL-6, IL4, IL12), and chemokines as well ()).
Signal 2: costimulatory signal (enhanced by QS21, hydroxide aluminum, oil emulsions) or augmenting co-stimulatory molecules (CD80/CD86; CD40/CD40L) that make robust the antigen presentation to naive lymphocytes (CD4 + T cellsCitation2,Citation21–23; )).
Signal 3: the signal of potentiation for the differentiation of naive CD4 + T cells, toward Th1/Th2 (helper 1 T cells/helper 2 T cells) [MP2a, ASO4 potentiates for Th1-type cellular immune responses while MF-59, and hydroxide aluminum for Th2-type cellular immune response ()] and the different Th subsets [(Th17, Th9, Th22, Thf, follicular helper T cells, Tregs, regulatory T cells)] cytokine producers of IL-12, IL-17, IL-10, IL-22, IFN-gamma (IFN-γ) product will actívate macrophages (MØs), NK, NKT cells, CD8 + T cells (Cytotoxic T Lymphocyte); activation and differentiation of B cell to plasmatic B cells, antibody producers (neutralized or opsonized Abs)Citation2–8,Citation11,Citation15,Citation16 ()). Moreover, Tregs which express CTLA4Citation67,Citation88,Citation89 and FoxP3 produce IL-10 and TFG-beta (TGF-β), leading to a highly regulated immune response. In addition, direct antigen presentation of intracellular bacteria, viruses, protozoans o MHC-I-pathway to CD8 + T cell activation, differentiation to CD8 + T cell effectors (expression of FASL and perforin and granzyme production), which enabled the killing of infected macrophages ()). A similar action of the mucosal adjuvants, as well as those adjuvants of natural origin, marine or plant-derived (e.g. Quillaga saponaria) () can augment the immune responses to soluble antigens to contra rest the host’s self-tolerance.Citation12,Citation22,Citation86 It has been suggested that this type of adjuvants boosts the protective immunity and promotes specific humoral and cellular immune responses by following the three signal mechanism actions: signal 0, 1, and 2.Citation89 Thus, when they are co-administered with novel vaccines, such as those based on attenuated microorganism or recombinant bacteria, virus, or vaccines in proper vehicle (liposomes, virosomes, ISCOMS) formulations, like oil emulsions ()); adjuvants can exert a positive influence on antigen presentation, differentiation of naive CD4 + T cells, memory, and effector cellular immune responsesCitation2,Citation7,Citation9,Citation30,Citation43()). Moreover, adjuvants based on AS03, AS04, MF59 could enhance signal 0 and signal 1 (antigen uptake, processing), whilst bacterial toxins (e.g. cholera toxin of Vibrio cholera); thermolabile enterotoxins of Escherichia coli-their B subunits;Citation70–77 Bt Cry proteinsCitation14 and QuitosanCitation11,Citation16,Citation85 have a role in adaptive antigen-specific immune response (signal 2: antigen presentation, co-stimulatory molecules). Th1 or Th2 CD4 + T cells within the secondary lymphoid tissues follicle elicited cytokines, other than TGF-β during the inflammatory or pathogen-induced reactions produced by Th1 or Th2 CD4 + T cells, which may ensure that näive B cells are committed toward IgG2 (IFN-γ) or IgE (IL-4), maturing into gut-homing IgG2 or IgE producing plasma cells. IL-4 and TGF-β induce surface IgM-positive (sIgM+) B cells which switch to IgE and IgA. TGF-β1 could induce sIgM to sIgA B-cell. In humans, anti-CD40 stimulation of tonsillar B cells, together with TGF-β1 in the presence of IL-10, stimulates IgA synthesis. Differentiation of sIgA+ B cells into IgA-producing plasma cells is dependent on IL-5 and IL-6.Citation2,Citation37 (). In addition, the expression of T cell homing molecules plays a major role in the common mucosal immune system, which enables secretory IgA antibodies (SIgA) to be present in distal sites as in upper airwaysCitation5,Citation6,Citation11,Citation12,Citation82–86 (), and thereby be present in the frontline defense at mucosal sites, this represents a friendly action adjuvants compounds ().
4. Adjuvants in vaccine formulations
Adjuvants compounds as components of vaccine candidates can act or mimic pathogen-associated molecular patterns (PAMPs) in the vaccine formulation, such that vaccine components are identified as a threat and so, trigger an innate immune response through a variety of mechanisms with
a) activation and maturation of APCs and initiation of downstream adaptive immun e response ().Citation43
b) Adjuvants can increase the magnitude and durability of the response achievable using purified subunit antigen.
c) Adjuvants can reduce the number of antigens contained in individual vaccine doses. PAMPs that are typically associated with infections and facilitate target vaccines to their local action at the systemic and mucosal sites, and mostly at mucosal compartments since most of the pathogens enter via the mucosal routes.
The actual inclusion of adjuvants compounds in modern vaccine development is evident ( and ) since there are two challenges that the science of vaccination has to deal with it: One is one related to the characteristics of the pathogen itself, and the other related to the characteristics of the population (infants, young, elderly, vulnerable, immunocompromised individuals).Citation16–18,Citation43 First, related to the pathogen, among them, the evasion mechanism of the intracellular pathogens, the antigenic drift, the multiple serotypes, latent infection disease, and/or the short period of protection. The second one, related to the population features, like elderly adults with immunosenescence, populations with chronic disease, those immunosuppressed, and the infants with an immature immune system.Citation16,Citation17,Citation43 The adjuvants in vaccine formulations should also overcome several other important issues. The research and development, preclinical studies (animal studies) are very worthy to start the assessment of the safety of the adjuvant plus vaccine, to follow with phase I, II, III studies all of which constitute the pre-license stage before passing to the post-licensed stage that includes among others studies, phase IV safety studies, epidemiological studies. The benefit-risk profile should also be under constant reevaluation to warrant the safety and the feasibility of the final product. The potential safety concerns that have been described around the development of adjuvanted vaccines are the reactogenicity (swelling, pain, redness, general symptoms like fever, fatigue), especially it has been observed at the site of injection, the symptoms are from mild-to-moderate, despite this, all licensed adjuvanted vaccines have shown more a favorable benefit:risk ratio than adverse.Citation15–17,Citation43 This is the reason by which all the time this ratio has reevaluated to overcome any problem, especially in the elderly people. Another safety concern is the immune-mediated disease, due to exposure to vaccination in susceptible individuals, because of the adjuvant immune-stimulants properties that can lead to unwanted immune responses. The World Health Organization (WHO) encouraged animal and epidemiological studies, and prolonged follow-up studies after vaccination to evaluate adverse effects. Increasing efforts have been made to identify the risk of immune-mediated disease after vaccination with adjuvanted vaccines. Other key issues that are around the public confidence in vaccine safety profiles and efficacy are the trust in companies and agencies that manufacture it, and the science underlying vaccine research and development, because how to work the vaccines not always is known. To understand how the adjuvants can augment the innate immune response and therefore the adaptive immune response will assure and enable the development of new vaccines targeting especially toward intracellular pathogens (mycobacteria, virus, parasites) for which the old technologies are still ineffective nowadays.
Table 2. Adjuvants in clinical phase studies of human infectious disease vaccines
Table 3. From old to new adjuvants compounds in preclinical testing (animal studies) for human vaccines
Reports of adjuvants that have been tested with promising results are (), e.g. the liposomal adjuvants systems, such as CAF01, a report by van Dissel et al.Citation92 as adjuvant of a vaccine formulation against M. tuberculosis, Ag85B-ESAT-6(H1) represents a first-in-man trial, that induced a Th1 response and antigen-specific T-cell responses of long-lasting as immunological memory after 150 weeks. In another study, the adjuvant IC31(®), plus Ag85B-ESAT6 boost individuals previously vaccinated with BCG and those latently infected with TB elicited strong antigen-specific T cell responses against Ag85B-ESAT-6 and both the Ag85B and ESAT-6 components that could be augmented by the second vaccination. The strong responses persisted through 32 weeks of follow-up, implied the induction of long-lasting immunological memory ().Citation92,Citation93 Molecular adjuvants such as type I IFNs, bacterial toxinsCitation10,Citation14,Citation31,Citation70–74,Citation78 () can reach the inductive sites is because they interact with receptors like molecules on the antigen-presenting cells (APC) and thus, triggering directly signalization to the nucleus for the production of the inflammatory response which links thus, with the adaptive immune response (humoral and cellular). The host response is fast, and it did not allow the expression of the bacterial resistance genes on the contrary it leads to an effect that can last in the activation of the surveillance mechanism of defense innate response. Work from us in the field of candidates adjuvants vaccines of BCG against Mycobacterium tuberculosis (MTb) have shown that immunodominant antigens such as HBHA () plus M. bovis BCG vaccine, represent a synergistic system, for one side a mycobacterial antigen that enhanced BCG vaccine immunity against M. tuberculosis, acted through a bacteriostatic effect, that is, limiting the growth and at the same time exert immunomodulation of the Th1 type cellular immune response.Citation94–96 Another in vivo study on this same concept is the systemic adjuvant effect of type I IFNs in a murine model of leprosyCitation31or tuberculosisCitation32 ().
On the other hand, adjuvants in SARS-Cov2 vaccine formulation. There are more than 100 COVID-19 vaccines under development and using a different platform as inactivated virus, recombinant proteins, viral vectors, ADN, RNA, and others and, some of them already authorized to use in humans (). Two vaccines based on inactivated SARS-Cov2, have more advanced results. Thus, the clinical trials ChiCTR2000031809/ChiCTR2000032459 performed by the Wuhan Institute of Biological Products/Sinopharm tested inactivated SARS-Cov2 adjuvanted with alum. In phase I/II clinical trial, this vaccine-induced high titers of antibodies in immunized individuals. The vaccine was safe, well-tolerated and, neutralizing antibody response was higher after two doses with 4ug than single doses with 8ug. It is approved in China for emergency use.Citation97,Citation98 The SINOVAC/CORONAVAC vaccine in phase I/II trial used two different doses 3ug or 6 ug antigen administered intramuscularly in a regimen including prime-boost at 2 and 4 weeks. Aluminum hydroxide was used as an adjuvant demonstrating safety and immunogenicity without adverse events. Overall, more than 90% of immunized individuals generated binding antibodies (NCT04352608 accessed 10 April 2021)Citation99.
The innovative application of nanomaterials as vaccine adjuvants have been increasingly investigated for immune protection and immunotherapy for infectious diseases. Thus, Novamax (USA) is testing Matrix-M1 which is composed of nanoparticles of saponin from the Quillaja saponaria, cholesterol and, phospholipids in perfusion-stabilized combined to full-length spike protein SARS-Cov2 in phase I /II. The results demonstrated that immunized individuals elicited a higher titer of neutralizing antibodies than the convalescents and the T cell response elicited was Th1 polarized. Phase II studies are still running (NCT04368988 accessed 10 April 2021).Citation100 Several ongoing clinical trials (phase I) are using a variety of adjuvants previously tested in other infectious diseases. AS03 (squalene based) and CpG 1018 (based on TLR9 agonist)/Alum combined with an innovate antigen recombinant SARS Cov2-trimeric spike protein produced in a mammalian cell culture-based expression system is applied via intramuscular route at 1 and 22 d (Clover Biopharmaceuticals Inc./ GSK/Dynavax, NCT04405908 accessed April 10, 2021). Similarly, KBP-Covid 19 (Kentucky Bioprocessing, Inc) plus CpG adjuvant is evaluated in Phase I in healthy seronegative individuals (NCT04473690 accessed 10 April 2021).
On the other hand, the vaccine developed by GSK/Dynavax is testing MF59 oil emulsion plus SARS CoV2 Spike protein stabilized with a molecular clamp (ACTRN1262000067 accessed 10 April 2021). Previous studies in mice demonstrated high neutralizing antibody titers and polyfunctional T cell CD4 and CD8 T cell response. Vaccine, use a novel adjuvant produced from microparticles of delta inulin (β-D-(2->1)-poly-fructo-furanosyl-D-glucose) named Advax-SM combined with a monovalent recombinant COVID-19 spike protein has been tested in recruiting individuals (NCT04453852 accessed 10 April 2021). Moreover, an mRNA vaccine platform with intrinsic adjuvanticity effect against prostate cancer and/or in SARS-Cov2, a TLR agonist of TLR-3, TLR-7, and TLR-8 have been also reported.Citation101–103
In COVID-19, still is challenging to develop a vaccine to prevent infection. All authorized vaccines for emergency uses only to prevent severe disease progression. It is essential to identify the suitable adjuvant in the SARS-Cov2 vaccine development to induce the appropriate immune response able to protect against the disease acquisition
5. Remarks and perspectives
The interaction host-pathogen is a potentially rich source of candidates and targets for the development of innovative, experimental, and bioinformatic technologies to provide a more rapid and accurate tool for the development of the most safety profile of adjuvants compounds an important component of modern vaccines. The validations of the aforementioned adjuvants enable the capacity to initiate, connect and boost host immune response to infectious diseases. These compounds can help to induce effective, prolonged, and specific immune responses (humoral and cellular) especially to a bystander and/or poor antigens, especially in elderly, vulnerable people that are a poor response to unadjuvanted vaccines. Optimization, and safety profiles, benefit:risk ratio, epidemiological survey and studies, and deep evaluation of the preclinical studies that include all experimental settings in the different animal models, should be followed and taken into account for further exploration and investigation. Nowadays, mineral salts (Freund’s adjuvant, alum salts), continue to be used in some formulations; however, it is more evidence that there are more alternatives such as that molecular adjuvants, type I IFNs, bacterial toxins, mRNA platform with intrinsic adjuvanticity that, in combination with nanotechnology, represent a friendly and promising strategy for clinical use (future scenery).
Disclosure of potential conflicts of interest
The authors declare not conflict of interest.
Acknowledgments
GGGM is grateful with SNI-CONACYT fellowship and COZCYT, Zacatecas, Zac. MEXICO.
Additional information
Funding
References
- World Health Organization. New Global Commitment to End Tuberculosis requires research support. 2018 Sept 18. http://www.who.int/tdr/news-room/fact-sheets/detail/tuberculosis.
- Lavelle EC, McLachlan JB. Editorial overview: immunomodulation: striking the right balance: using immunomodulators to target infectious diseases, cancer, and autoimmunity. Curr Opin Pharmacol. 2018:1–3. doi:10.1016/j.coph.2018.07.013.
- Mohan T, Zhu W, Wang Y, Wang BZ. Applications of chemokines as adjuvants for vaccine immunotherapy. Immunobiology 2018;223:477–85. doi:10.1016/j.imbio.2017.12.001.
- Dosler S, Karaaslan E, Gerceker AA. Antibacterial and anti-biofilm activities of melittin and colistin, alone and in combination with antibiotics against Gram-negative bacteria. J Chemother. 2016;28:95–103. doi:10.1179/1973947815Y.0000000004.
- Palucka K, Banchereau J, Mellman I. Designing vaccines based on biology of human dendritic cell subsets. Immunity 2010;33:464–71. doi:10.1016/j.immuni.2010.10.007.
- Longet S, Lundahl MLE, Lavelle EC. Targeted strategies for mucosal vaccination. Bioconjug Chem. 2018;29:613–23. doi:10.1021/acs.bioconjchem.7b00738.
- Mori A, Oleszycka E, Sharp FA, Coleman M, Osaka Y, Singh M, O’Hagan DT, Tajber I, Corngan OI, McNeela EA, et al. The vaccine adjuvant alum inhibits IL-12 by promoting PI3 kinase signaling while chitosan does not inhibit IL-12 and enhances Th1 and Th17 responses. Eur J Immunol. 2012;42:2709–19. doi:10.1002/eji.201242372.
- Carroll EC, Jin L, Mori A, Muñoz-Wolf N, Oleszycka E, Moran HBT, Mansouri S, McEntee CP, Lambe E, Agger EM, et al. The vaccine adjuvant Chitosan promotes cellular immunity via DNA sensor cGAS-STING-dependent induction of type I interferons. Immunity. 2016;44:597–608. doi:10.1016/j.immuni.2016.02.004.
- Lee JB, Jang JE, Song MK, Chang J. Intranasal delivery of cholera toxin induces Th17 dominated T cell-response to bystander antigens. PLoS ONE. 2009;4:1–8. doi:10.1371/journal.pone.0005190.
- Pandiyan P, Lavelle EC. Immune cells in the mucosa. Ed. Frontiers Imunol. 2016;7:657–58. doi:10.3389/fimmu.2016.00657.
- Longhi MP, Trumpheller C, Idoyaga J, Caskey M, Matos I, Klager C, Salazar AM, Colonna M, Steinmman RM. Dendritic cells require a systemic type I interferon response to mature and induce CD4+Th1 immunity with poly IC adjuvants. JEM 2000;206:1589–602. doi:10.1084/jem.20090247.
- Sugai T, Mori M, Nakazawa M, Ichino M, Naruto T, Kobayashi N, Kobayashi Y, Minami M, Yokota S. A CpG-containing oligodeoxynucleotide as an efficient adjuvant counterbalancing the Th1/Th2 immune response in diphtheria-tetanus-pertussis vaccine. Vaccine. 2005;23:5450–56. doi:10.1016/j.vaccine.2004.09.041.
- Pirahmadi S, Zakeri S, Mehriz AA, Djadid ND, Raz-AA, Sani J. Combining Monophosphoryl Lipid A (MPL), CpG Oligodeoxynucleotide (ODN), and QS-21 Adjuvants Induces Strong and Persistent Functional Antibodies and T Cell Responses against Cell-Traversal Protein for Ookinetes and Sporozoites (CelTOS) of Plasmodium falciparum in BALB/c Mice. Infect Immun. 2019;87:e00911–18.
- Favela-Hernández JM, Balderas RI, Guerrero GG. The potential of a commercial product based on Bacillus thuringiensis Cry1A-Cry2A as a immunogen and adjuvant. Madridge J Immunol. 2018;2:58–64. doi:10.18689/mjim-1000114.
- O’Hagan DT, Valiante NM. Recent advances in the discovery and delivery of vaccine adjuvants. Nat Rev Drug Discov. 2003;2:727–35. doi:10.1038/nrd1176.
- de Souza AJ, Santos LVA, Coirada FC, Boscardin SB, Santoro RD. Adjuvants: classification, modus operandi, and licensing. J Immunol Res. 2016:1–16. ID. 1469394. doi:10.1155/2016/1459394.
- Di Pasquale A, Preiss S, Tavares Da Silva F, Garçon N. Vaccine adjuvants: from 1920 to 2015 and beyond. Vaccines. 2015;16:320–43. doi:10.3390/vaccines3020320.
- Reed SG, Bertholet S, Coler RN, Friede M. New horizons in adjuvants for vaccine development. Trend Immunol. 2009;30:23–30. doi:10.1016/j.it.2008.09.006.
- Lebre F, Bento D, Ribeiro J, Colaço M, Borchard G, Pedroso de Lima MC, Borges O. Association of Chitosan and aluminium as a new adjuvant strategy for improved vaccination. Int J Pharm. 2017;527:103–14. doi:10.1016/j.ijpharm.2017.05.028.
- Lebre F, Pedroso de Lima MC, Lavelle EC, Borges O. Mechanistic study of the adjuvant effect of Chitosan-aluminum nanoparticles. Int J Pharm. 2018;552:7–15. doi:10.1016/j.ijpharm.2018.09.044.
- Lin Y, Wang X, Huang X, Zhang J, Xia N, Zhao Q. Calcium phosphate nanoparticles as a new generation vaccine adjuvant. Expert Rev Vaccines. 2017;16:895–906. doi:10.1080/14760584.2017.1355733.
- Lacaille-Dubois MA, Wagner H. New perspectives for natural triterpene glycosides as potential adjuvants. Phytomedicine. 2017;37:49–57. doi:10.1016/j.phymed.2017.10.019.
- Fernandez-Tejada A, Chea EK, George C, Pillarsetty N, Gardner JR, Livingston PO, Ragupathi G, Lewis JS, Tan DS, Glin DY. Development of a minimal saponin vaccine adjuvant base don QS-21. Nat Chem. 2014;6:635–43. doi:10.1038/nchem.1963.
- Givena BE, Geary SM, Salem AK. Nanoparticle based CpG-oligonucleotides therapy for treating allergic asthma. Immunotherapy 2018;10:595–604. doi:10.2217/imt-2017-0142.
- Blaise C, Bioley G. Lipid-based particles: versatile delivery systems for mucosal vaccination against infection. Front Immunol. 2018;9:431–51. doi:10.3389/fimmu.2018.00431.
- Bracci L, Canini I, Puzelli S, Sestili P, Venditti M, Spleda M, Donatelli I, Belardelli F, Proietti E. Type I IFN is a powerful mucosal adjuvant for a selective intranasal vaccination against influenza virus in mice and affects antigen capture at mucosal level. Vaccine 2005;23:2994–3004. doi:10.1016/j.vaccine.2004.12.006.
- Prchal M, Pilz A, Simma O, Lingnau K, von Gabain A, Strobl B, Müller M, Decker T. Type I Interferon as mediators of immune adjuvants for T and B cell dependent acquired immunity. Vaccine 2009;275:G17–G20. doi:10.1016/j.vaccine.2009.10.016.
- Tough DF. Modulation of T-cell function by type I interferón. Immunol Cell Biol. 2012;90:493–97. doi:10.1038/icb.2012.7.
- Tovey MG, Lallemand CH, Thyphronitis G. Adjuvant activity of type I interferon. Biol Chem. 2008;389:541–45. doi:10.1515/bc.2008.051.
- Gonzalez-Navajas JM, Lee J, David M, Raz E. Immunomodulatory functions of type I IFNs. Nat Rev Immunol. 2012;12:125–35. doi:10.1038/nri3133.
- Guerrero GG, Rangel-Moreno J, Islas-Trujillo S, Rojas-Espinoza O. Successive Intramuscular Boosting with IFN-Alpha Protects Mycobacterium bovis BCG-Vaccinated Mice against M. lepraemurium Infection. BioMed Res Int. 2015;2015:1–9. ID. 414027. doi:10.1155/2015/414027.
- Rivas-Santiago, Guerrero GG. IFN-α boosting of Mycobacterium bovis BCG-vaccinated mice promoted Th1 type cytokines and protect against M. tuberculosis. Biomed Res Int. 2017; ID. 8796760. doi:10.1155/2017/8796760.
- Seo MD, Won HS, Kim JH, Mishig-Ochir T, Lee BJ. Antimicrobial peptides for therapeutic applications: a review. Molecules 2012;17:12276–86. doi:10.3390/molecules171012276.
- Mendez-Samperio P. Recent advances in the field of antimicrobial peptides in inflammatory diseases. Adv Biomed Res. 2013;2:50–55. doi:10.4103/2277-9175.114192.
- Lee J, Lee DG. Antimicrobial peptides (AMPs) with dual mechanisms: membrane disruption and apoptosis. J Microbiol Biotechnol. 2015;25:759–64. doi:10.4014/jmb.1411.11058.
- Ageitos JM, Sánchez-Pérez A, Calo-Mata P, Villa TG. Antimicrobial peptides (AMPs): ancient compounds that represent novel weapons in the fight against bacteria. Biochem Pharmacol. 2017;133:117–38. doi:10.1016/j.bcp.2016.09.018.
- Ladram A, Nicolas P. Antimicrobial peptides from frog skin: biodiversity and therapeutic promises. Front Biosci (Landmark Ed). 2016;21:341–1371. doi:10.2741/4461.
- Bechinger B, Lohner K. Detergent-like actions of linear amphipathic cationic antimicrobial peptides. Biochim Biophys Acta. 2006;1758:1529–39. doi:10.1016/j.bbamem.2006.07.001.
- Sumi CD, Yang BW, Yeo IC, Young TH. Antimicrobial peptides of the Genus Bacillus: a new era for antibiotics. Can J Microbiol. 2015;61:93–103. doi:10.1139/cjm-2014-0613.
- Rončević T, Puizina J, Tossi A. Antimicrobial Peptides as Anti-Infective Agents in Pre-Post-Antibiotic Era? Int J Mol Sci. 2019;14:1–22. doi:10.3390/ijms20225713.
- Patel S, Akhtar N. Antimicrobial peptides (AMPs): the quintessential ‘Offense and Defense’ molecules are more than antimicrobials. Biomed Pharmacother. 2017;95:1276–83. doi:10.1016/j.biopha.2017.09.042.
- Wilmes M, Hans-Georg Sahl HG. Defensin-based anti-infective strategies. Int J Med Microbiol. 2014;304:93–99. doi:10.1016/j.ijmm.2013.08.007.
- Sambhara S, McEIhaney JE. Immunosenescence and influenza vaccine efficacy. Curr Top Microbiol Immunol. 2009;333:413–29. doi:10.1007/978-3-540-92165-3_20.
- Luchner M, Reinke S, Milicic A. TLR agonists as vaccine adjuvants targeting cancer and infectious disease. Pharmaceutics 2021;13:142–58. doi:10.3390/pharmaceutics13020142.
- Kool M, Petrilli V, De Smedf T, Rolaz A, Hammad H, van NInwegen M, Bergen IM, Castillo R, Lambrecht BN, Tschopp J. Cutting edge: alum adjuvant stimulates inflammatory dendritic cells through activation of the NALP3 inflammasome. J Immunol. 2008;181:3755–59. doi:10.4049/jimmunol.181.6.3755.
- Marrack P, McKee AS, Munks MW. Towards an understating of the adjuvant action of aluminium. Nat Rev Immunol. 2009;9:287–93. doi:10.1038/nri2510.
- Kool M, Soullie T, van Nimwegen M, Willart MA, Musken F, Jung S, Hoogsteden HC, Hammad H, Lambrecht BN. Alum adjuvant boosts adaptive immunity by inducing uric acid and activating inflammatory dendritic cells. J Exp Med. 2008;205:869–82. doi:10.1084/jem.20071087.
- Gavin AI, Hoebe K, Duong B, Ota T, Martin C, Beutler B, Nemazee D. Adjuvant-enhanced antibody response in the absence of Toll-like receptor signaling. Science 2006;314:1936–38. doi:10.1126/science.1135299.
- Fritz JH, Ferrero RL, Philpott DJ, Girardin SE. Nod-like protein in immunity, inflammation and disease. Nat Immunol. 2006;7:1250–57. doi:10.1038/ni1412.
- Franchi I, Park JH, Shaw MH, Marina-Garcia N, Chen G, Kim GY, Nuñez G. Intracellular NOD-like receptors in innate immunity, infection and disease. Cell Microbio. 2008;10:1–8. doi:10.1111/j.1462-5822.2007.01059.x.
- Martinon F, Tschopp J. Inflammatory caspases and inflammasomes master switches of inflammation. Cell Death Differ. 2007;14:10–22. doi:10.1038/sj.cdd.4402038.
- Petrilli VC, Dostert DA, Muruve DA, Tschopp J. The inflammasome a danger sensing complex triggering innate immunity. Curr Opi Immunol. 2007;19:615–22. doi:10.1016/j.coi.2007.09.002.
- Eisenbarth SC, Colegio OR, O’Connor W, Sutterwala FS, Flavell RA. Crucial role for the Nalp3 inflammasome in the immunostimulatory properties of aluminum adjuvants. Nature 2008;453:1122–26. doi:10.1038/nature06939.
- Shi Y, Evans JE, Rock KI. Molecular identification of a danger signal that alerts the immune system to dying cells. Nature. 2003;452:516–21. doi:10.1038/nature01991.
- Akira S, Takeda K. Toll-like receptor signalling. Nat Rev Immunol. 2004;4:499–511. doi:10.1038/nri1391.
- Daubenberger CA. TLR9 agonists as adjuvants for prophylactic and therapeutic vaccines. Curr Opin Mol Ther. 2007;9:45–52.
- Wang Y-Q, Bazin-Lee H, Evams JT, Casella CR, Mitchell TC. MPL adjuvant contains competitive antagonists of human TLR4. Front Immunol. 2020;11:577823–35. doi:10.3389/fimmu.2020.577823.
- Tripathi RP, Tewari N, Dwivedi N, Trwan VK. Fighting tuberculosis: an old disease with new challenges. Med Res Rev. 2005;25:93–131. doi:10.1002/med.20017.
- Didierlaurent AM, Laupieze B, Di Pasquale A, Hergli N, Collignon C, Garcon N. Adjuvant system AS01: helping to overcome the challenges of modern vaccines. Expert Rev Vaccines. 2017;16:55–63. doi:10.1080/14760584.2016.1213632.
- Didierlaureant AM, Collignon C, Bourguignon P, Wouters S, Fierens K, Fochesato M, Dendouga N, Langlet CH, Melissen B, Lambrecht BN, et al. Enhancement of adaptive immunity by the human vaccine adjuvant AS01 depends on activated dendritic cells. J Immunol. 2014;193:1920–30. doi:10.4049/jimmunol.1400948.
- Garçon N, Van Mechelen M. Recent clinical experience with vaccines using MPL- and QS-21-containing adjuvant systems. Expert Rev Vaccines. 2011;10:471–86. doi:10.1586/erv.11.29.
- Fochesato M, Dendouga N, Boxus M. Comparative preclinical evaluation of AS01 versus other adjuvant systems in a candidate herpes zoster glycoprotein E subunit vaccine. Hum Vaccin Immunother. 2016;12:2092–95. doi:10.1080/21645515.2016.1154247.
- Heath MD, Mohsen MO, de Kam P-J, Carreno VTL, Hewings SJ, Kramer MF, Kunidng TM, Bachmann MF, Skinner MA. Shaping modern vaccines: adjuvant systems using microcrystalline tyrosine (MCT ®). Front Immunol. 2020;11:59491–594923. doi:10.3389/fimmu.2020.594911.
- Kim WS, Zhi Y, Guo H, Byun E-B, Lim JH, Seo HS. Promotion of cellular and humoral immunity against foot-and-mouth disease virus by immunization with virus-like particles encapsulated in monophosphoryl lipid a and liposomes. Vaccines 2020;8:633.647. doi:10.3390/vaccines8040633.
- De Maagd RA, Bravo A, Berry N, Crickmore N, Schnepf HE. Structure, diversity, and evolution of proteins toxins from spore-forming entomopathogenic bacteria. Annu Rev Genet. 2003;37:409–33. doi:10.1146/annurev.genet.37.110801.143042.
- De Maagd RA, Bravo A, Crickmore N. How Bacillus thuringiensis has evolved specific toxins to colonize the insect world. Trends Genet. 2001;17:193–99. doi:10.1016/s0168-9525(01)02237-5.
- Salkowski CA, Detore GR, Vogel SN. Lipopolysaccharide and monophosphoryl lipid A differentially regulate interleukin-12, gamma interferon, and interleukin-10 mRNA production in mRNA production in macrophages. Infect Immun. 1997;65:3239. doi:10.1128/IAI.65.8.3239-3247.1997.
- Thompson BS, Chilton PM, Ward JR, Eans JT, Mitchell TC. The low-toxicity versions of LPS, MPL, adjuvant and RC529, are efficient adjuvants for CD4+ T cells. J Leukoc Biol. 2005;78:1273–80. doi:10.1189/jlb.0305172.
- Jegerlehner A, Wiesel M, Dietmeier K, Zabel F, Gatto D, Saudan P, Bachmann MF. Carrier induced epitopic suppression of antibody responses induced by virus-like particles is a dynamic phenomenon caused by carrier-specific antibodies. Vaccine 2010;28:5503–12. doi:10.1016/j.vaccine.2010.02.103.
- Lycke N, Lebrero-Fernández C. ADP-ribosylating enterotoxins as vaccine adjuvants. Curr Opin Pharmacol. 2018;41:42–51. doi:10.1016/j.coph.2018.03.015.
- Williams NA. Immune modulation by the cholera-like enterotoxin B-subunits: from adjuvant to immunotherapeutic. Int J Med Microbiol. 2000;290:447–53. doi:10.1016/S1438-4221(00)80062-4.
- Datta SK, Sabet M, Nguyen KPI, Valdez PA, Gonzalez-Navajas JM, Islam S, Mihajlov I, Fierer J, Insel PA, Webster NJ, et al. Mucosal adjuvant activity of cholera toxin requires Th17 cells and protects against inhalation anthrax. PNAs 2010;107:10638–43. doi:10.1073/pnas.1002348107.
- Cai CY, Kurita-Ochiai T, Kobayashi R, Hashizume T, Yamamoto M. Nasal immunization with the 40 KDa outer membrane protein of Porphyromonas gingivalis plus cholera toxin induces protective immunity in aged mice. J Oral Sci. 2013;55:107–14. doi:10.2334/josnusd.55.107.
- Da Hora VP, Conceicao FR, Dellagostin OA, Doolan DL. Non-toxic derivatives of LT as potent adjuvants. Vaccine 2011;29:1538–44. doi:10.1016/j.vaccine.2010.11.091.
- Thiam F, Chernilienne A, Poncet D, Kohh E, Basset C. B subunits of cholera toxin and thermolabile enterotoxin of Escherichia coli have similar adjuvant effect as whole molecules on rotavirus 2/6-VLP specific antibody responses and induce a Th17-like response after intrarectal immunization. Microb Pathog. 2015;89:27–34. doi:10.1016/j.micpath.2015.08.013.
- Yongping M. Recent advances in nontoxic Escherichia coli heat-labile toxin and its derivative adjuvants. Expert Rev Vaccines. 2016;15:1361–71. doi:10.1080/14760584.2016.1182868.
- Millar DG, Hirst TR, Snider DP. Escherichia coli heat-labile enterotoxin B subunit is a more potent mucosal adjuvant than its closely related homologue, the B subunit of cholera toxin. Infect Immun. 2001;69:3476–82. doi:10.1128/IAI.69.5.3476-3482.2001.
- Rojas-Hernández S, Rodriguez-Monroy MA, López-Revilla R, Reséndiz-Albor AA, Moreno-Fierros L. Intranasal coadministration of the Cry1Ac protoxin with amoebal lysates increases protection against Naegleria fowleri meningoencephalitis. Infect Immun. 2004;72:4368–75. doi:10.1128/IAI.72.8.4368-4375.2004.
- Ibarra-Moreno S, García-Hernández AL, Moreno-Fierros L. Coadministration of protoxin Cry1Ac from Bacillus thuringiensis with metacestode extract confers protective immunity to murine cysticercosis. Parasite Immunol. 2014;36:266–70. doi:10.1111/pim.12103.
- González-González E, García-Hernández AL, Flores-Mejía R, López-Santiago R, Moreno-Fierros L. The protoxin Cry1Ac of Bacillus thuringiensis improves the protection conferred by intranasal immunization with Brucella abortus RB51 in a mouse model. Vet Microbiol. 2016;175:382–88. doi:10.1016/j.vetmic.2014.11.021.
- Legorreta-Herrera M, Oviedo MR, Moreno-Fierros L. Pretreatment with Cry1Ac protoxin modulates the immune response,and increases the survival of Plasmodium-infected CBA/Ca mice. J Biomed Biotech. 2010;2010:1–11. ID 198921. doi:10.1155/2010/198921.
- Tundup S, Srivastava I, Harn D Jr. Polarization of the host immune responses by helminth-expressed glycans. Ann NY Acad Sci. 2012;1253:EI–EI3. doi:10.1111/j.1749-6632.2012.06618.x.
- Demet CJ, Ishii KJ. Nucleic acid sensing at the interface between innate and adaptive immunity in vaccination. Nat Rev Immunol. 2012;12:479–91. doi:10.1038/nri3247.
- Kubelkova K, Macela A. Innate immune recognition: an issue more complex than expected. Front Cell Infect Microbiol. 2019;9:1–19. doi:10.3389/fcimb.2019.00241.
- Kumar S, Ingle H, Prasad DVR, Kumar H. Recognition of bacterial infection by innate immune sensors. Crit Rev Microbiol. 2013;39:229–46. doi:10.3109/1040841X.2012.706249.
- Pulendra B, Ahmed R. Immunological mechanism of vaccination. Nat Immunol. 2011;12:509–17. doi:10.1038/ni.2039.
- Fujkuyama Y, Tokuhara D, Kataoka K, Gilbert RS, McGhee JR, Yuki Y, Kiyono H. Fujihashi K novel vaccine development strategies for inducing mucosal immunity. Expert Rev Vaccines. 2012;11:367–79. doi:10.1586/erv.11.196.
- Walker LS, Sansom DM. The emerging role of CTLA4 as a cell-extrinsic regulator of T cell responses. Nat Rev Immunology. 2011;11:852–63. doi:10.1038/nri3108.
- Walker LSK, Lecture EFIS. Understanding the CTLA-4 checkpoint in the maintenance of immune homeostasis. Immunol Letter. 2017;184:43–50. doi:10.1016/j.imlet.2017.02.007.
- Schijns VE. Immunological concepts of vaccine adjuvant activity. Curr Opin Immunol. 2000;12:456–63. doi:10.1016/s0952-7915(00)00120-5.
- Yoshino S, Sasatomi E, Ohsawa M. Bacterial lipopolysaccharide acts as adjuvant to induce autoimmune arthritis in mice. Immunology 2000;4:607–14. doi:10.1046/j.1365-2567.2000.00015.x.
- van Dissel JT, Arend SM, Prims C, Bang P, Tugkov PN, Lingnau K, Nouta J, Klein MR, Rocenkrands I, Ottenhoff THM. Ag85B-ESAT-6 adjuvanted with K31 promotes strong and long-lived Mycobacterium tuberculosis specific T cell responses in naive human volunteers. Vaccine 2010;28:3571–81. doi:10.1016/j.vaccine.2010.02.094.
- Fraser CK, Diener KR, Brown MP, Huyball JD. Improving vaccine by incorporating immunological adjuvants. Expert Rev Vaccines. 2007;6:559–78. doi:10.1586/14760584.6.4.559.
- Locht C, Houghardy JM, Rouanet C, Place S, Mascart F. Heparin-binding haemagglutinin, from an extrapulmonary dissemination factor to a powerful diagnostic and protective antigen against tuberculosis. Tuberculosis 2006;86:303–09. doi:10.1016/j.tube.2006.01.016.
- Guerrero GG, Debrie AS, Locht C. Boosting with mycobacterial heparin-binding haemagglutinin enhanced protection of Mycobacterium bovis BCG vaccinated newborns mice against M. tuberculosis. Vaccine 2010;28:4340–47. doi:10.1016/j.vaccine.2010.04.062.
- Guerrero GG, Locht C. Recombinant HBHA boosting effect on BCG-induced immunity against Mycobacterium tuberculosis infection. Clin Dev Immunol. 2011;2011:730702. doi:10.1155/2011/730702.
- Xia S, Duan K, Zhang Y, Zhao D, Zhang H, Xie Z, Li X, Peng C, Zhang Y, Zhang W, et al. Effect of an inactivated vaccine against SARS-CoV-2 on safety and immunogenicity outcomes: interim analysis of 2 randomized clinical trials. JAMA 2020;324:951–60. doi:10.1001/jama.2020.15543.
- Xia S, Zhang Y, Wang Y, Wang H, Yang Y, Gao FG, Tan W, Wu G, Xu M, Lou Z, et al. Safety and immunogenicity of an inactivated SARS-CoV-2 vaccine, BBIBP-CorV: a randomised, double-blind, placebo-controlled, phase 1/2 trial. Lancet Infect Dis. 2021;21:39–51. doi:10.1016/S1473-3099(20)30831-8.
- Zhang Y, Zeng G, Pan H, Li C, Hu Y, Chu K, Han W, Chen Z, Tang R, Yin W, et al. Safety, tolerability, and immunogenicity of an inactivated SARS-CoV-2 vaccine in healthy adults aged 18-59 years: a randomised, double-blind, placebo-controlled, phase 1/2 clinical trial. Lancet Infect Dis. 2021;21:181–92. doi:10.1016/S1473-3099(20)30843-4.
- Keech C, Albert G, Cho I, Robertson A, Reed P, Neal S, Plested JS, Zhu M, Cloney-Clark S, Zhou H, et al. Phase 1–2 trial of a SARS-CoV-2 recombinant spike protein nanoparticle vaccine. N Engl J Med. 2020;383:2320–32. doi:10.1056/NEJMoa2026920.
- Topol EJ. Messenger RNA vaccines against SARS-CoV2. Cell 2021;184:1401–02. doi:10.1016/j.cell.2020.12.039ç.
- Polack FP, Thomas SJ, Kitchin N, Absalon J, Gurtman A, Lockhart S, John L, Perez GM, Moreira ED, Zerbini C, et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. Clinical Trial N Engl J Med. 2020;383:2603–15. doi:10.1056/NEJMoa2034577.
- Pulendran B, Arunachalam PS, O’Hagan DT. Emerging concepts in the science of vaccine adjuvants. Nat Rev Drug Discov. 2021;6:1–22. doi:10.1038/s41573-021-00163-y.
