ABSTRACT
Immunocompromised children are at increased risk of severe illness from vaccine-preventable infections. However, inadequate vaccine coverage remains a concern. This scoping review sought to determine the current state of knowledge regarding vaccine coverage of immunocompromised children. Bibliographic databases were searched for primary research from any year. Data were analyzed quantitatively and narratively. Ninety-seven studies met inclusion criteria. The most commonly studied vaccines were pneumococcal (n = 46), influenza (n = 44), diphtheria/tetanus/pertussis/poliomyelitis/Haemophilus influenzae type B/hepatitis B-containing (n = 36), and measles- and/or mumps- and/or rubella-containing (n = 29). Immunocompromising conditions studied included cancer/stem cell transplants (n = 24), solid organ transplants (n = 23), sickle cell disease (n = 21), immunosuppressive therapy (n = 14), human immunodeficiency virus (n = 12), splenectomy (n = 4), and primary immunodeficiency (n = 2). As more children are treated with immunosuppressive therapies, it is critical to identify whether they are being appropriately vaccinated for age and condition. We identified gaps in the current state of knowledge for specific vaccine types in specific immunocompromised populations.
Introduction
Appropriate vaccination of immunocompromised children is critical, given that they have impaired immune systems due to conditions, illnesses, medical treatments, or medications that suppress their immune function.Citation1,Citation2 These children are more susceptible to infections and at higher risk of developing severe or complicated infections.Citation2 For example, among solid organ transplant recipients, vaccine-preventable infections have been shown to cause significant morbidity and mortality, resulting in hospitalization rates up to 87 times higher than the general population.Citation3 Optimizing vaccine coverage in this vulnerable population is of utmost importance as it is the best way to prevent severe and complicated infection and even death from vaccine-preventable diseases.Citation2
Although routinely used live and inactivated vaccines are both safe and effective for the vast majority of children, this is not always the case for immunocompromised children who have impaired immune systems due to conditions, illnesses, medical treatments, or medications that suppress their immune function.Citation1,Citation2 The goal of vaccination in this population is to maximize the benefits while minimizing harm, since some vaccines may not be as safe or effective.Citation4,Citation5 Thus, the individual’s underlying condition, disease progression, and timing of the vaccinations must be considered when health-care providers are weighing the risks versus the benefits of providing these children particular vaccines.Citation4 The reason why the child may be immunocompromised may affect vaccination requirements in different ways. Solid organ transplant recipients will remain on lifelong immunosuppression following transplant; therefore, vaccination should be optimized prior to transplantation and as early in the course of disease as possible when the maximum immune response would be expected and administration of live vaccines may be safe.Citation4,Citation6–8 In contrast, other immunocompromising conditions such as cancer or HIV may result in temporary immunosuppression, allowing for live vaccines to only be safely administered once the child in no longer considered to be immunocompromised.Citation4,Citation9
Despite known risks for vaccine-preventable infections, inadequate vaccine coverage has been identified as a concern for immunocompromised children.Citation6 In addition, immunocompromising conditions have become more prevalent as immunosuppressive therapies are being used for an increasing range of medical conditions and the life expectancy for patients with these conditions is increasing.Citation2 Thus, assessment of vaccine coverage among children with immunocompromising conditions is important.Citation4,Citation10 The purpose of this scoping review was to determine the current state of knowledge regarding vaccine coverage of children who are immunocompromised by mapping out the characteristics of the existing literature, in order to identify gaps in the existing research.
Methods
A scoping review was conducted in order to examine the extent, range, and nature of knowledge about vaccine coverage in this clinical population, as well as to identify research gaps. The review was guided by Arksey and O’Malley’s framework,Citation11 which includes five stages: (1) identifying the research question, (2) identifying relevant studies, (3) selecting relevant studies, (4) charting the data, and (5) collating, summarizing, and reporting results. The optional sixth stage of expert consultation was employed to identify any missing relevant literature. An unpublished review protocol is available upon request.
Study inclusion/exclusion criteria
The inclusion criteria were comprised of the following Population, Intervention, Comparison, and Outcome (PICO) criteria: (1) Population: all children from birth to 18 y of age who have an immunocompromising condition of any kind; (2) Intervention: vaccination with any active vaccine; (3) Comparison: studies with and without comparison groups were included; (4) Outcome: vaccine coverage, defined as the proportion of eligible children in the study population who received the vaccine(s) being investigated.Citation12 No limits were placed on study design or publication date. Primary research in the English language from any income country, and with extractable data were included. In order to identify potential publication bias, both published and unpublished research were included. Abstracts that met the study inclusion criteria were included. Reviews, case reports, editorials, letters and comments were excluded.
Search strategy
A research librarian searched the following databases for literature from any year: MEDLINE, Embase, Cochrane Library, CINAHL, ProQuest Dissertations and Theses Global, Scopus, and Web of Science Core Collection, which includes the Conference Proceedings Science Citation Index. Terms representing immunization/vaccination were combined with terms representing being immunocompromised or conditions or medications that might contribute to being immunocompromised. Reference lists of included studies and excluded review articles were also chain searched for relevant citations. The initial search was conducted May 15, 2019, with an updated search run on April 21, 2020.
Study selection
Two independent reviewers (AP, HT) conducted Level 1 (title/abstract) and Level 2 (full-text) screening based on the predetermined inclusion criteria using Covidence, a scoping review software platform.Citation13 Discrepancies were resolved by reaching a consensus through discussion and consulting a third reviewer (SM) when consensus could not be reached. We consulted with our clinical expert (CB) to verify that our included articles captured all key literature known to them.
Data collection/extraction
Two authors (AP and HT) developed, pilot tested, and revised the data extraction form in Google sheets. Data were extracted by one reviewer (AP) and verified by a second reviewer (HT). The following general data were extracted: record identification, title, author, year of publication, and country. Methodological elements were also extracted, including study design, aim, setting, sampling procedures, sample size, vaccine(s) and doses assessed, data collection methods, and comparison groups. Additional information about the population were extracted, including age range and the immunocompromising condition.
Quality appraisal
Two independent reviewers (AP and HT) appraised the quality of the evidence of all the included studies using the Mixed Methods Appraisal Tool (MMAT), version 2018.Citation14 The MMAT was chosen a priori, as the literature search allowed for inclusion of all research methodologies. In the case of discrepancies, a discussion occurred between the two reviewers until consensus was reached. A third reviewer was consulted when consensus was not reached. Studies were categorized into scores of 0.5–3.5 (low quality) and 4–5 (high quality).
Data analysis
A numerical analysis of the extracted variables was conducted to describe and quantify the following characteristics of the included articles: General characteristics such as geographic region, income of country, year of publication, and type of publication; study design, study aim; quality score; population characteristics such as immunocompromising condition, age, comparison group; sample size; and vaccine types, vaccine coverage, timepoints of measurement of coverage, and data source. A narrative synthesis and tabulation of these findings was prepared.
Results
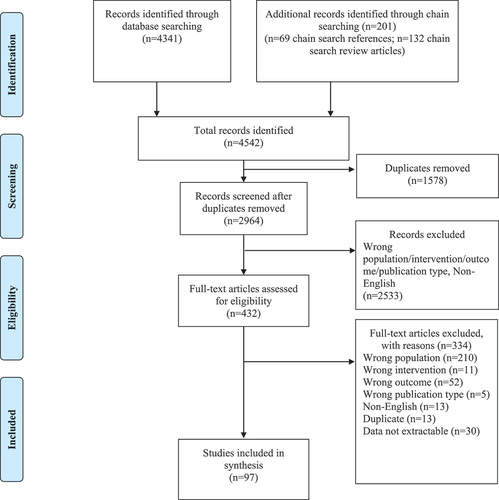
In total, 4542 records were retrieved through database searching and chain searching. After removal of duplicates and screening, 97 studies met our inclusion criteria (see ). A summary of the characteristics of included studies is presented in .
Table 1. Frequency of study characteristics of included studies (N = 97).a
Vaccine types
Vaccine coverage was studied for many different vaccines (see ). Several studies measured the vaccine coverage of all vaccines in the relevant vaccine guidelines or routine schedules (n = 24). Of these studies, 16 also reported coverage results for certain individual vaccines. Of the studies that examined specific vaccines, pneumococcal (n = 46) and influenza (n = 44) vaccines were the most commonly studied. Additional vaccines studied included measles- and/or mumps- and/or rubella (M/M/R)-containing (n = 29), varicella (n = 23), diphtheria- and/or tetanus- and/or pertussis- and/or poliomyelitis- and/or Haemophilus influenzae type B- and/or hepatitis B (D/T/P/Polio/Hib/Hep B)-containing (n = 36), meningococcal (n = 25), human papillomavirus (HPV) (n = 19), Bacillus Calmette-Guérin (BCG) (n = 10), hepatitis A (n = 11), rotavirus (n = 2), typhoid fever (n = 1), and yellow fever (n = 1) vaccines. One study did not state which vaccine types were studied.
Study characteristics
The majority of the studies were of cross-sectional design (n = 46). Others were of retrospective cohort (n = 19), prospective cohort interventional (n = 16), prospective cohort observational (n = 11), case–control (n = 4), or randomized controlled trial (n = 1) designs. Among most of the vaccine types, the cross-sectional design was most commonly used (). Retrospective cohort design (n = 14) was common among studies measuring pneumococcal vaccine coverage, and interventional prospective cohort design (n = 12) was common among studies measuring influenza vaccine coverage.
The most common method of determining the vaccine coverage of immunocompromised children was by reviewing the medical record or chart (n = 25). A variety of other data sources were used, including caregiver or patient questionnaire (n = 18), hospital unit questionnaire (n = 1), electronic medical records (n = 5), vaccination database or registry (n = 7), caregiver interview (n = 1), and physician obtained data (n = 2). There were 25 studies that used mixed data sources and 13 studies did not describe the data source used. Identified data sources were used broadly across vaccine types ().
There were studies with sample sizes under 25 (n = 9), between 25 and 50 (n = 14), between 51 and 100 (n = 24), and between 101 and 200 (n = 18). There were 28 studies with sample sizes greater than 200. The largest sample size consisted of 54,809 immunocompromised children. Five studies did not specify their sample size. Small sample sizes (<100 participants) were common among all vaccines studied, though the frequently studied vaccines (influenza and pneumococcal) had a greater number of studies with larger sample sizes ().
The distribution of age ranges studied varied across the studies. There were studies that included children from 0 to 4 y (n = 48), 5 to 11 y (n = 55), and 12 to 18 y (n = 50). However, several studies did not provide the age range of the population studied (n = 29).
Some studies compared the vaccine coverage of the immunocompromised population to a non-immunocompromised population (n = 24). Others compared to a population with the same immunocompromised condition with different characteristics, such as age, gender, or cohort year (n = 23). Three studies had a comparison group consisting of a population with a different immunocompromising condition. There were 50 studies that did not include a comparison group.
The studies included 67 peer-reviewed journal articles and 30 published abstracts. There were 34 studies that had a high-quality score (4–5 out of 5) and 63 studies that had a low-quality score (0.5–3.5 out of 5). Studies were commonly scored lower due to insufficient detail provided in the methods, such as when studies measured multiple variables besides vaccination coverage. Many of the studies were published between 2015 and 2020 (n = 50). Fewer studies were published from 2011 to 2014 (n = 27), and 2000 to 2010 (n = 18). Only two studies were published before the year 2000.
Country of origin
The 97 studies primarily came from the United States of America (n = 46) and Europe (n = 33). Six studies were from Africa and 6 were from Latin America/the Caribbean. Few studies were from Australia (n = 3), Canada (n = 2), or the Middle East (n = 2). The studies were also categorized according to country income level, as defined by The World Bank.Citation15 The majority of the studies were conducted in high-income countries (n = 84). Fewer studies were from upper-middle-income (n = 10), lower-middle-income (n = 2), and low-income (n = 4) countries.
The vaccine types studied in each geographic region are provided in . Many of the studies from North America and Europe measured the coverage of M/M/R-containing (n = 19), D/T/P/Polio/Hib/Hep B-containing (n = 27), meningococcal (n = 23), pneumococcal (n = 39), influenza (n = 41), and HPV (n = 19) vaccines. Fewer studies measured the coverage of the varicella vaccine (n = 17). Only two studies measured the coverage of the rotavirus vaccine, one in North America and one in Europe. BCG vaccine coverage was not studied in North America but was included in three studies from Europe. The three studies from Oceania (all from Australia) presented estimates for coverage of M/M/R-containing (n = 1), varicella (n = 3), D/T/P/Polio/Hib/Hep B-containing (n = 1), influenza (n = 1), and pneumococcal (n = 1) vaccines.
Table 2. Vaccine types and geographic regions.a
Table 3. Vaccine types and study designs.a
Table 4. Vaccine types and data sources.a
Table 5. Vaccine types and sample sizes.a
As with high-income countries, the vaccines most commonly studied in upper-middle, lower-middle, and low-income countries included M/M/R-containing (n = 9), D/T/P/Polio/Hib/Hep B-containing (n = 8), and pneumococcal (n = 6) vaccines. The BCG vaccine was more commonly studied in these countries (n = 7); typhoid fever (n = 1) and yellow fever (n = 1) vaccines were only studied in Africa. The HPV and rotavirus vaccines were not studied in these regions at all.
Vaccine coverage by immunocompromising condition
The included studies reported on a variety of immunocompromising conditions, including cancer/stem cell transplant, solid organ transplant, sickle cell disease, HIV, immunosuppressive therapy, splenectomy, primary immunodeficiency, and unspecified immunosuppression (disease/treatment not specified). The types of vaccines studied for each condition are summarized in . Vaccine coverage estimates for each condition are provided in ; although some studies reported either/both vaccine initiation (≥1 dose) and vaccine series completion, and/or pre- and post-intervention coverage, only series completion and pre-intervention results are included in the table.
Table 6. Immunocompromising conditions and vaccine Types.a
Table 7. Complete vaccine coverage, by immunocompromising condition.a
Cancer/stem cell transplant
Twenty-four articles (including six abstracts) reporting on vaccine coverage among children with cancer or stem cell recipients were retrieved. Study publication dates ranged from 2006 to 2020. Target populations included children who had completed treatment (n = 15) and those undergoing treatment (n = 6), with only one study reporting on pre-treatment vaccine coverage. One study did not specify the timepoint of coverage measurement. Most of the studies were completed in high-income countries (n = 23), including the USA (n = 14), Italy (n = 2), Australia (n = 2), Greece, Canada, Spain, the UK, and Germany (n = 1 per country). The remaining study was completed in Brazil (upper-middle-income country). No studies were completed in lower-middle or low-income countries.
The studies reported on influenza (n = 11), HPV (n = 9), M/M/R-containing (n = 5), varicella (n = 5), D/T/P/Polio/Hib/HepB-containing (n = 5), pneumococcal (n = 2), meningococcal (n = 2), and hepatitis A (n = 2) vaccine coverage. Eight studies reported on more than one vaccine type. For commonly studied vaccines, reported coverage estimates ranged broadly. For influenza, vaccine coverage estimates ranged from 3.4% to 87.1%; HPV coverage estimates ranged from 0%-27.3%, M/M/R-containing estimates ranged from 2.1%-90.9%. Coverage for all other vaccines studied was below 55%.
Solid Organ Transplant
Twenty-three studies (including nine abstracts) conducted among solid organ transplant recipients met the eligibility criteria. Studies were mainly published between 2007 and 2019, with one study from 1994. Studies most commonly reported pre-transplant vaccine status (n = 15), with four reporting post-transplant coverage, and three not specifying timepoint. A total of ten studies focused on liver transplant patients, six on kidney transplant and the remaining on multiple transplant types. Studies were conducted in the USA (n = 12), Iran (n = 2), Austria, Italy, the UK, Greece, Switzerland, Israel and Brazil (n = 1 per country). One study was conducted across four European countries (Germany, Italy, Turkey, and the UK).
Studies reported on pneumococcal (n = 14), D/T/P/Polio/Hib/Hep B-containing (n = 13), M/M/R-containing (n = 13), varicella (n = 12), influenza (n = 8), meningococcal (n = 7), hepatitis A (n = 6), HPV (n = 5), and BCG (n = 5) vaccines. For pre-transplant candidates, the reported coverage range for studied vaccines was >70%, except for HPV (reported coverage 27.3–90%) and BCG (reported coverage 97–100%).
Sickle Cell Disease
Twenty-one studies (including four abstracts) reported on vaccine coverage among children with sickle cell disease. Study publication dates ranged from 2008 to 2020, though most were published after 2015. The majority of studies were completed in high or upper-middle-income countries (n = 18), with two in low-income countries, and one mixed. Studies were completed in the USA (n = 11), Italy (n = 2), France, Brazil, Jamaica, the UK, Spain, Uganda, Burkina Faso (n = 1 for each). One study reported on participants in both the USA and Nigeria.
Studies reported on a wide range of vaccines, including pneumococcal (n = 16), influenza (n = 10), meningococcal (n = 7), D/T/P/Polio/Hib/HepB-containing (n = 5), hepatitis A (n = 3), M/M/R-containing (n = 2), varicella (n = 1), BCG (n = 1), typhoid fever (n = 1), and yellow fever (n = 1). The majority of studies (n = 13) reported on coverage for more than one type of vaccine. Reported pneumococcal vaccine coverage ranged from 0% to 97.5%; influenza and meningococcal coverage ranged from 0% to 90% and 25% to 90.2%, respectively.
HIV
A total of 12 studies (including three abstracts) that met the inclusion criteria reported on vaccine coverage among children with HIV. Studies were mainly published between 2006 and 2018, with one study published in 2000. Seven studies were from high-income countries (Italy [n = 4], UK [n = 2], USA [n = 1]), two from upper-middle-income (mixed from Brazil, Mexico, Argentina, Peru, Jamaica [n = 2]), and three from low-income countries (Niger [n = 1], Zambia [n = 1], and Malawi [n = 1]).
Studies reported on vaccine coverage for D/T/P/Polio/Hib/HepB-containing (n = 7), influenza (n = 6), M/M/R-containing (n = 5), BCG (n = 4), meningococcal (n = 2), pneumococcal (n = 2), HPV (n = 2), and varicella (n = 1). Most of the studies (n = 9) reported on more than one vaccine. As with the other conditions, coverage estimates for the commonly studied vaccines ranged broadly. Reported vaccine coverage for D/T/P/Polio/Hib/HepB-containing ranged from 7.0% to 97.9%, influenza from 10% to 82%.
Immunosuppressive therapy
Fourteen studies (half of which were abstracts) described vaccine coverage in children undergoing immunosuppressive therapy. Studies were published between 2010 and 2020. Target populations included patients with inflammatory bowel disease (n = 7), juvenile idiopathic arthritis (n = 2), rheumatic disease (n = 2), autoimmune hepatitis (n = 1), chronic renal disease (n = 1), systemic lupus erythematosus (n = 1), or general use of immunosuppressive therapy (n = 2). All studies were completed in high- or upper-middle-income countries: USA (n = 5), Poland (n = 2), Canada, Australia, Germany, Greece, Portugal, Switzerland (n = 1 for each) and one study conducted across eight European countries.
Vaccine coverage for influenza (n = 9), pneumococcal (n = 7), meningococcal (n = 4), varicella (n = 3), D/T/P/Polio/Hib/HepB-containing (n = 3), HPV (n = 3), and M/M/R-containing (n = 3) vaccines were reported, with seven studies reporting on more than one vaccine type. Reported influenza vaccine coverage ranged from 1.7% to 84.3%, while coverage reported for pneumococcal and meningococcal vaccines was less than 45% in all included studies.
Splenectomy
A total of four studies examining vaccine coverage in children with splenectomy met the inclusion criteria, published between 1998 and 2019. Three studies addressed vaccine coverage post-splenectomy, while one presented coverage among children with idiopathic thrombocytopenia purpura (pre-splenectomy). Studies were completed in high-income countries, including the USA (n = 2), Switzerland (n = 1), and the Netherlands (n = 1).
All four studies examined vaccine coverage for pneumococcal vaccines, three also reported on D/T/P/Polio/Hib/HepB-containing and meningococcal vaccine coverage, and one additionally included influenza vaccine coverage. Pneumococcal vaccine coverage ranged from 44.4% to 100% in the included studies. Coverage for all other vaccines fell below 51%.
Primary Immunodeficiency
Only two studies examining vaccine coverage among children with primary immunodeficiency were retrieved, published in 2006 and 2019. Both were conducted in high-income countries (Australia and Italy). One study included a number of vaccines (M/M/R-containing, varicella, and pneumococcal vaccines), while the other focused on influenza vaccine coverage. Other than the M/M/R-containing vaccine (reported range 77–82%), all vaccine coverage levels were below 66%.
Unspecified
Three studies reported on vaccine coverage among children with unspecified immunosuppression, two were published in 2012 and one in 2016. Two studies were completed in the UK and one in the USA. All three studies focused exclusively on influenza vaccine coverage, with reported estimates ranging from 3.9% to 61.3%.
Discussion
We identified 97 studies that measured vaccine coverage in immunocompromised populations of children, 30 of which were abstracts. There was variability in the characteristics of the studies, such as the vaccine type, geographic location, immunocompromising condition, and vaccination data source.
The vaccine types studied varied by geographic region, with most studies occurring in high-income countries in North America and Europe. Variations in vaccine coverage considerations between these regions are likely related to differing vaccination priorities and schedules. For example, BCG vaccine coverage was not studied in North America, but was measured in areas in Europe in which there is a higher risk of tuberculosis and BCG is routinely recommended.Citation16 Although some studies reported on M/M/R-containing vaccine coverage, this should remain a focus for future studies, as measles is a reemerging concern in high-income countries.Citation17 The limited number of studies from Australia is surprising given the volume of research coming out of Australia related to vaccine coverage and determinants of uptake.Citation18 Therefore, clinicians and vaccine program administrators in Australia lack local evidence regarding which vaccines may have inadequate vaccine coverage in their immunocompromised populations. Given the difference in vaccine schedules and policies, additional country-specific coverage analysis is important.
Vaccines studied in lower-middle and low-income countries included typhoid fever, yellow fever, and BCG. These likely differed from those studied in North American and European countries due to the risk associated with these diseases in these geographic locations.Citation19–24 The HPV vaccine and rotavirus vaccines were not studied at all, which may be attributed to a lack of resources deeming this vaccine a lower priority for a resource-limited country. Future research on these two vaccines is important, given the high burden of HPV-associated cancersCitation25 and diarrhea-related morbidity and deathCitation26,Citation27 in low-resource settings.
Some vaccine types were studied extensively in certain immunocompromised populations, while others were studied minimally or not at all. Coverage for pneumococcal and influenza vaccines were the most commonly studied; these vaccine types were addressed for all studied conditions, with the exception of the unspecified immunosuppression group. The seasonal inactivated influenza vaccine is recommended for all immunocompromised populations, according to both Canadian and American immunization advisory committees.Citation4,Citation28,Citation29 Future research should expand focus on vaccines other than influenza and pneumococcal vaccines in order to provide broader understanding of vaccine coverage in these populations. Specific recommendations for each immunocompromised group are provided below.
Influenza vaccines were commonly studied in children with cancer and stem cell transplant recipients. People receiving cancer treatment are identified as a population at high-risk for influenza-related complications and hospitalization.Citation30 Additionally, a number of studies also included HPV vaccine in these populations. The HPV vaccine is especially important for childhood cancer survivors, as they have a higher risk than the general population for health complications, such as subsequent malignancies caused by HPV.Citation31,Citation32 However, certain vaccines have not been as extensively studied in this population, such as meningococcal and pneumococcal vaccines. Future research should focus on determining if children with cancer and/or stem cell transplants are being adequately vaccinated to protect them from these diseases.
Among solid organ transplant recipients, pneumococcal, D/T/P/Polio/Hib/HepB-containing, M/M/R-containing and varicella vaccines were commonly studied. As live vaccines are generally contraindicated after solid organ transplant, it is critical to optimize vaccination with live vaccines prior to transplant.Citation4,Citation33 Accordingly, most of the studies retrieved focused on pre-transplant vaccination status. While the use of live vaccines is generally not recommended after solid organ transplant, ongoing vaccination with inactivated vaccines can be given once a child is on baseline immunosuppression, which is usually about 6 months after transplant.Citation4,Citation6 With this in consideration, it is important to evaluate whether these children are adequately vaccinated according to recommended schedules both before and after transplantation. As guidelines on the use of live vaccines post-transplant continue to evolve,Citation34 it will be important to continue monitoring live vaccine coverage in these patients.
Children with sickle cell disease and those who have had a splenectomy are at increased risk of developing certain infections with encapsulated bacteria, such pneumococcus, meningococcus, and Haemophilus influenzae type B.Citation35,Citation36 Vaccination against these organisms is particularly important due to the higher risk of severe sepsis, meningitis, and pneumonia when infected with these organisms.Citation29,Citation37 Accordingly, coverage studies in these populations focused on these vaccines.
Pneumococcal vaccines were commonly studied in children with sickle cell disease. As pneumonia is a leading cause of death in infants and children with sickle cell disease,Citation36 studies of pneumococcal vaccines are particularly important in this population.Citation29,Citation38 However, there appears to be a lack of research in the vaccine coverage of M/M/R-containing, varicella, rotavirus, and HPV vaccines in children with sickle cell disease, so future research may be focused on these particular vaccines.
Studies from North America suggest that annual vaccination with the inactivated influenza vaccine is recommended among children who have received a splenectomy, to prevent severe and complicated influenza infection, as well as to reduce the risk of severe secondary bacterial infection.Citation6,Citation38 Despite this high importance, we identified only one study measuring influenza vaccine coverage in this population. Therefore, this is an area in which future research is warranted.
The vaccines that were studied in children with HIV varied. D/T/P/Polio/Hib/Hep B-containing and influenza vaccines were most common. This may be because immunization guidelines for individuals with HIV vary depending on the course of the illness and degree of immunosuppression. There is also variability in the vaccines studied in low- versus high-income countries, which reflect the vaccines routinely provided in those settings. Inactivated vaccines can be administered at any time, although are ideally administered at a time of low immune suppression when response will be improved. Routinely used live vaccines, such as M/M/R; varicella; and rotavirus, can be administered early in the disease course or after immune recovery with treatment.Citation4,Citation29 Other live vaccines, such as BCG, typhoid fever, and live influenza vaccines are not necessarily recommended.Citation4,Citation29 Thus, it is understandable why typhoid fever and yellow fever vaccines coverage were not studied, whereas it might be important for future research to focus on vaccine coverage for rotavirus vaccine.
For other populations that were on immunosuppressive therapy, influenza and pneumococcal vaccines were commonly studied. These vaccines are routinely recommended to individuals with chronic conditions who are on immunosuppressive therapy.Citation4,Citation6,Citation28 If possible, individuals should be vaccinated before commencing immunosuppressive therapy. Fortunately, chronic conditions such as inflammatory bowel disease are less common in children under 2 y of age, so often the majority of live vaccines will have been completed prior to the onset of disease and immunosuppressive therapy.Citation39 Once receiving immunosuppressive therapy, live vaccines are generally contraindicated.Citation4,Citation29 Aside from influenza and pneumococcal vaccines, all other commonly recommended vaccines were less frequently studied in this population, so these vaccines may be considered in further research.
Primary immunodeficiencies encompass a large variety of conditions, which include inherited disorders that result in defects in antibody production, complement deficiencies, or other aspects of cell-mediated immunity.Citation4,Citation29 For these individuals, other methods of protection against infection may be utilized, such as with replacement immune globulin or pathogen-specific immune globulin preparations. However, vaccination is still recommended whenever possible.Citation4,Citation6 While all inactivated vaccines should be given, specialist consultation is usually required to determine if or when administration of live vaccines is recommended.Citation4,Citation6,Citation8 As few vaccine types were studied in this population, this is clearly an area where more vaccine coverage research is needed to determine if these vulnerable children are being optimally protected against vaccine-preventable diseases.
Included studies were evaluated for quality using the MMAT. This review included both published and unpublished literature, as well as conference abstracts, in order to identify publication bias resulting from exclusion of unpublished works. This likely resulted in overall lower quality scores than may have been realized with only peer-reviewed articles. As many of the studies received a low-quality score, there is room for high-quality research to be conducted on this topic. To achieve a higher quality score, studies need to provide a more detailed description of the methods followed and gather vaccine coverage data from more accurate sources.
Variability in study design, data source, and sample size pose a challenge for drawing summary conclusions. Almost half the included studies used a cross-sectional design. Most intervention studies used a prospective cohort design; only one intervention study was designed as a randomized controlled trial, which is not surprising, given the ethical issues of using randomized controlled trials in this context. Vaccine coverage data sources ranged from more accurate sources, including charts, electronic medical records, and vaccination databases or registries, to less accurate sources, such parent/guardian self-report through questionnaires or interviews.Citation40 Parent recall typically overestimates vaccine coverage, erroneously identifying children as up-to-date.Citation40 The majority of studies had small sample sizes. The exception was influenza vaccine, in which most studies had larger samples. In order to provide evidence to vaccine program administrators and clinicians, more studies should be conducted using validated data sources and large sample sizes. The move toward use of administrative health data analysis will facilitate this, providing growing evidence to guide clinical practice in this area. While the results from each study may be useful for a particular setting and/or population, it was difficult to synthesize vaccine coverage results due to variations in methodologies and reporting.
An important area for future investigation is a review of barriers to vaccination in children with immunocompromising conditions and/or the effectiveness of interventions. Although these issues were not the focus of this review, a few barriers that were frequently stated by authors are noteworthy. These include concerns about the safety and effectiveness of vaccines and lack of information about the vaccines.Citation41–43 Strategies to help improve vaccination may include increased educationCitation44–49 and development of vaccination policies specific to immunocompromised populations.Citation50–52 Health-care providers should also consistently incorporate the review of vaccine records into their practice.Citation46,Citation52–54 Work should also be done to reduce barriers to vaccination, such as cost and accessibility.
Limitations
There were a few limitations to this review. Although we contacted authors of published abstracts to determine if a full-text publication was available, we did not contact authors when limited information was available in the full-text study report. Another limitation was the exclusion of articles that were not published in the English language (n = 13). Only primary research was included and relevant websites were not searched for additional citations. However, the search included world-wide publications, unpublished literature, and did not limit literature by methodology or year of publication.
Conclusion
To our knowledge, this is the first scoping review to comprehensively review the body of literature on vaccine coverage of immunocompromised children. This topic has gained more interest in recent years, as evidenced by the increasing amount of research conducted beyond the early 2000s. It is expected that this trend will continue as more children are being treated with immunosuppressive therapies and children with immunocompromising conditions are living longer, more functional lives. We have identified several substantial knowledge gaps with respect to vaccine coverage in immunocompromised children that should be used to guide future research in this important area. One specific area in which research should be focused is a systematic or scoping review of barriers to vaccination among immunocompromised populations, as this could facilitate development of meaningful interventions to improve appropriate vaccine coverage among this vulnerable population. These findings are critical to inform public health policy and practice surrounding the vaccination of immunocompromised children.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgments
Ms Palichuk received studentship funding from a University of Alberta Faculty of Nursing Undergraduate Student Summer Research Award; and with the generous support of the Stollery Children’s Hospital Foundation through the Women and Children’s Health Research Institute Student Summer Research Award. Dr MacDonald is the recipient of a Career Development Award from the Canadian Child Health Clinician Scientist Program. The team acknowledges the support of Megan Kennedy in conducting the updated literature search and Emmanuel Marfo’s contributions to data extraction.
References
- Esposito S, Prada E, Lelii M, Castellazzi L. Immunization of children with secondary immunodeficiency. Hum Vaccines Immunother. 2015;11(11):2564–70. doi:10.1080/21645515.2015.1039208.
- Weber DJ, Rutala WA. Immunization of immunocompromised persons. Immunol Allergy Clin North Am. 2003;23(4):605–34. doi:10.1016/S0889-8561(03)00100-0.
- Feldman AG, Beaty BL, Curtis D, Juarez-Colunga E, Kempe A. Incidence of hospitalization for vaccine-preventable infections in children following solid organ transplant and associated morbidity, mortality, and costs. JAMA Pediatrics. 2019;173(3):260–68. doi:10.1001/jamapediatrics.2018.4954.
- Government of Canada. Page 8: Canadian Immunization Guide: part 3 - Vaccination of Specific Populations. https://www.canada.ca/en/public-health/services/publications/healthy-living/canadian-immunization-guide-part-3-vaccination-specific-populations/page-8-immunization-immunocompromised-persons.html . Updated 2018. [accessed 2020 Dec 15]
- Moore DL. Immunization of the immunocompromised child: key principles. Paediatrics & Child Health. 2018;23(3):203–05. doi:10.1093/pch/pxx180.
- Rubin LG, Levin MJ, Ljungman P, Davies EG, Avery R, Tomblyn M, Bousvaros A, Dhanireddy S, Keyserling H, et al. Executive Summary: 2013 IDSA clinical practice guideline for vaccination of the immunocompromised host. Clin Infect Dis. 2014;58(3):309–18. 2014. doi:10.1093/cid/cit816.
- Danziger‐Isakov L, Kumar D. Vaccination of solid organ transplant candidates and recipients: guidelines from the American society of transplantation infectious diseases community of practice. Clin Transplant. 2019;33(9):1-10. doi:10.1111/ctr.13563.
- American Academy of Pediatrics. Immunization in special circumstances. In Kimberlin DW, Brady MT, Jackson MA, Long SS, editors. Red Book: 2015 Report of the Committee on Infectious Diseases. 30th ed. Elk Grove Village, IL: American Academy of Pediatrics. 2015: 68–101.
- Banerjee S, Dissanayake PV, Abeyagunawardena AS. Vaccinations in children on immunosuppressive medications for renal disease. Pediatr Nephrol. 2016;31(9):1437–48. doi:10.1007/s00467-015-3219-y.
- O’Donnell S, Dubé E, Tapiero B, Gagneur A, Doll MK, Quach C. Determinants of under-immunization and cumulative time spent under-immunized in a Quebec cohort. Vaccine. 2017;35(43):5924–31. doi:10.1016/j.vaccine.2017.08.072.
- Arksey H, O’Malley L. Scoping studies: towards a methodological framework. Int J Soc Res Methodol Theory Pract. 2005;8(1):19–32. doi:10.1080/1364557032000119616.
- MacDonald SE, Russell ML, Liu XC, Simmonds KA, Lorenzetti DL, Sharpe H, Svenson J, Svenson LW. Are we speaking the same language? An argument for the consistent use of terminology and definitions for childhood vaccination indicators. Hum Vaccines Immunother. 2019;15(3):740–47. doi:10.1080/21645515.2018.1546526.
- The Cochrane Community. About covidence. Published 2019. https://community.cochrane.org/help/tools-and-software/covidence/about-covidence . [Accessed 2019 Jun 18]
- Hong QN, Pluye P, Fàbregues S, Bartlett G, Boardman F, Cargo M, Dagenais P, Gagnon M, Griffiths F, Nicolau B, et al. Mixed Methods Appraisal Tool (MMAT), Version 2018 User Guide.; 2018. http://mixedmethodsappraisaltoolpublic.pbworks.com/ . [accessed 2019 June 18]
- The World Bank. World bank list of economies. Published. 2019. https://datahelpdesk.worldbank.org/knowledgebase/articles/906519-world-bank-country-and-lending-groups . [Accessed 2019 June 18]
- European Centre for Disease Prevention and Control. Tuberculosis surveillance and monitoring in Europe 2019. Published 2019. http://dx.publications.europa.eu/10.2900/512553. [Accessed 2020 Dec 15]
- Centers for Disease Prevention and Control. Measles cases and outbreaks. Published 2020. https://www.cdc.gov/measles/cases-outbreaks.html . [Accessed 2020 Dec 15]
- National Centre for Immunisation Research and Surveillance. Research to inform policy. Published 2019. http://www.ncirs.org.au/our-work/research-inform-policy . [Accessed 2020 Dec 15]
- Centers for Disease Control and Prevention. Typhoid vaccines: What you need to know. Published 2019. https://www.cdc.gov/vaccines/hcp/vis/vis-statements/typhoid.pdf . [Accessed 2020 Dec 15]
- Centers for Disease Control and Prevention. Yellow fever vaccine: What you need to know. Published 2019. https://www.cdc.gov/vaccines/hcp/vis/vis-statements/yf.pdf. [Accessed 2020 Dec 15]
- Government of Canada. Yellow fever. Published 2019. https://www.canada.ca/en/public-health/services/diseases/yellow-fever.html. [Accessed 2020 Dec 15]
- Government of Canada. Typhoid vaccine: Canadian immunization guide. Published 2019. https://www.canada.ca/en/public-health/services/publications/healthy-living/canadian-immunization-guide-part-4-active-vaccines/page-23-typhoid-vaccine.html . [Accessed 2020 Dec 15]
- Government of Canada. Bacille calmette-guérin (BCG) vaccine. Published 2014. https://www.canada.ca/en/public-health/services/publications/healthy-living/canadian-immunization-guide-part-4-active-vaccines/page-2-bacille-calmette-guerin-vaccine.html . [Accessed 2020 Dec 15]
- Nelson NP Chapter 4- travel-related infectious diseases - hepatitis A. Published 2020. https://wwwnc.cdc.gov/travel/yellowbook/2020/travel-related-infectious-diseases/hepatitis-a. [Accessed 2020 Dec 15]
- Wakeham K, Kavanagh K. The burden of HPV-associated anogenital cancers. Curr Oncol Rep. 2014;16(9):402. doi:10.1007/s11912-014-0402-4.
- Centers for Disease Control and Prevention. Diarrhea: Common illness, global killer. Published 2015. https://www.cdc.gov/healthywater/pdf/global/programs/Globaldiarrhea508c.pdf . [Accessed 2020 Dec 15]
- Troeger C, Khalil IA, Rao PC, Cao S, Blacker BF, Ahmed T, Armah G, Bines JE, Brewer TG, Colombara DV, et al. Rotavirus vaccination and the global burden of rotavirus diarrhea among children younger than 5 years. JAMA Pediatrics. 2018;172(10):958–65. doi:10.1001/jamapediatrics.2018.1960.
- Grohskopf LA, Alyanak E, Broder KR, Walter EB, Fry AM, Jernigan DB. Prevention and control of seasonal influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices — United States, 2019–20 influenza season. MMWR Recomm Reports. 2019;68:3. doi:10.15585/mmwr.rr6803a1.
- Centers for Disease Control and Prevention. Altered immunocompetence - General best practice guidelines for immunization: Best practices guidance of the advisory committee on immunization practices (ACIP). Published 2019. https://www.cdc.gov/vaccines/hcp/acip-recs/general-recs/immunocompetence.html . [Accessed 2020 Dec 15]
- Moore DL. Vaccine recommendations for children and youth for the 2018/2019 influenza season. Paediatrics & Child Health. 2018;23(8):565. doi:10.1093/pch/pxy150.
- Ojha RP, Tota JE, Offutt-Powell TN, Klosky JL, Minniear TD, Jackson BE, Gurney JG. Human papillomavirus-associated subsequent malignancies among long-term survivors of pediatric and young adult cancers. PLoS One. 2013;8(8):e70349. doi:10.1371/journal.pone.0070349.
- Temkin SM, Seibel NL. Are we missing an opportunity for cancer prevention? Human papillomavirus vaccination for survivors of pediatric and young adult cancers. Cancer. 2015;121(19):3395–402. doi:10.1002/cncr.29515.
- Centers for Disease Control and Prevention. Rotavirus vaccination: what everyone should know. Published 2018. https://www.cdc.gov/vaccines/vpd/rotavirus/public/index.html . [Accessed 2020 Dec 15]
- Suresh S, Upton J, Green M, Pham‐Huy A, Posfay‐Barbe KM, Michaels MG, Top KA, Avitzur Y, Burton C, Chong PP, et al. Live vaccines after pediatric solid organ transplant: proceedings of a consensus meeting, 2018. Pediatr Transplant. 2019;23(7):e13571. doi:10.1111/petr.13571.
- Booth C, Inusa B, Obaro SK. Infection in sickle cell disease: a review. Int J Infect Dis. 2010;14(1):e2-e12. doi:10.1016/j.ijid.2009.03.010.
- Centers for Disease Control and Prevention. Complications and treatments of sickle cell disease. Published 2019. https://www.cdc.gov/ncbddd/sicklecell/treatments.html#Infection . [Accessed 2020 Dec 15]
- Belli AK, Dönmez C, Özcan Ö, Dere Ö, Dirgen Çaylak S, Dinç Elibol F, Yazkan C, Yilmaz N, Nazli O. Adherence to vaccination recommendations after traumatic splenic injury. Ulus Travma Ve Acil Cerrahi Derg. 2018;24(4):337–42. doi:10.5505/tjtes.2017.84584.
- Government of Canada. Page 7: Canadian immunization guide: part 3 - vaccination of specific populations. Published 2016. https://www.canada.ca/en/public-health/services/publications/healthy-living/canadian-immunization-guide-part-3-vaccination-specific-populations/page-7-immunization-persons-with-chronic-diseases.html . [Accessed 2020 Dec 15]
- Bousvaros A, Lu Y. Immunizations in the child with inflammatory bowel disease. In Mamula P, Grossman A, Baldassano R, Kelsen J, Markowitz J, editors. Pediatric Inflammatory Bowel Diseaase. Cham, Switzerland: Springer; 2017. p. 663–68. doi:10.1007/978-3-319-49215-5_54.
- MacDonald SE, Schopflocher DP, Golonka RP. The pot calling the kettle black: the extent and type of errors in a computerized immunization registry and by parent report. BMC Pediatr. 2014;14(1):1. doi:10.1186/1471-2431-14-1.
- Doganis D, Tsolia M, Dana H, Bouhoutsou D, Pourtsidis A, Baka M, Varvoutsi M, Servitzoglou M, Kosmidis H. Compliance with immunization against H1N1 influenza virus among children with cancer. Pediatr Hematol Oncol. 2013;30(2):149–53. doi:10.3109/08880018.2012.753961.
- Giannattasio A, Squeglia V, Lo VA, Russo M, Barbarino A, Carlomagno R, Guarino A. Pneumococcal and influenza vaccination rates and their determinants in children with chronic medical conditions. Italian Journal of Pediatrics. 2010;36(1):28. doi:10.1186/1824-7288-36-28.
- Camerino M, Jackson S, Chinnakotla S, Verghese P. Effects of the influenza vaccine on pediatric kidney transplant outcomes. Pediatr Transplant. 2019;23(2):e13354. doi:10.1111/petr.13354.
- Fernández-Prada M, Rodríguez-Martínez M, García-García R, García-Corte MD, Martínez-Ortega C. Adapting immunisation schedules for children undergoing chemotherapy. Enferm Infecc Microbiol Clin. 2018;36(2):78–83. doi:10.1016/j.eimc.2016.09.003.
- Esposito S, Marchisio P, Droghetti R, Lambertini L, Faelli N, Bosis, Tosi S, Begliatti E, Principi N. Influenza vaccination coverage among children with high-risk medical conditions. Vaccine. 2006;24(24):5251–55. doi:10.1016/j.vaccine.2006.03.059.
- Carthon CE, Hall RC, Maxwell PR, Crowther BR. Impact of a pharmacist-led vaccine recommendation program for pediatric kidney transplant candidates. Pediatr Transplant. 2017;21(6):e12989. doi:10.1111/petr.12989.
- Wagner AL, Shrivastwa N, Potter RC, Lyon-callo SK, Boulton ML. Pneumococcal and meningococcal vaccination among Michigan children with sickle cell disease. J Pediatr. 2018;196:223–29. doi:10.1016/j.jpeds.2018.01.023.
- Nero AC, Akuete K, Reeves SL, Dombkowski KJ. Pneumococcal vaccination rates in children with sickle cell disease. J Public Heal Manag Pract. 2014;20(6):587–90. doi:10.1097/PHH.0000000000000034.
- Setse RW, Siberry GK, Moss WJ, Wheeling J, Bohannon BA, Dominguez KL. Meningococcal conjugate and tetanus toxoid, reduced diphtheria toxoid and acellular pertussis vaccination among HIV-infected youth. Pediatr Infect Dis J. 2016;35(5):e152–e157S. doi:10.1097/INF.0000000000001078.
- de de la Fuente Garcia I, Coïc L, Leclerc J-M, Laverdière C, Rousseau C, Ovetchkine, Tapiéro B. Protection against vaccine preventable diseases in children treated for acute lymphoblastic leukemia. Pediatr Blood Cancer. 2017;64(2):315–20. doi:10.1002/pbc.26187.
- Cecinati V, Esposito S, Schicchitano B, Delvecchio GC, Amato D, Pelucchi C, Jankovic M, De Mattia, Principi N. Effectiveness of recall systems for improving influenza vaccination coverage in children with oncohematological malignancies. Hum Vaccin. 2010;6(2):194–97. doi:10.4161/hv.6.2.10253.
- Chen C-J, Bakeera-Kitaka S, Mupere E, Kasirye P, Munube D, Idro R, Hume H, Pfeffer B, LaRussa P, Green NS, et al. Paediatric immunisation and chemoprophylaxis in a Ugandan sickle cell disease clinic. J Paediatr Child Health. 2019;55(7):795–801. doi:10.1111/jpc.14291.
- Wong CI, Billett AL, Weng S, Eng K, Thakrar U, Davies KJ. A quality improvement initiative to increase and sustain influenza vaccination rates in pediatric oncology and stem cell transplant patients. Pediatr Qual Saf. 2018;3(1):e052. doi:10.1097/pq9.0000000000000052.
- Cortina G, Ojinaga V, Zlamy M, Goner T, Riedl M, Rauchenzauner M, Entenmann A, Müller T. Vaccination status in pediatric solid-organ transplant recipients and their household members. Exp Clin Transplant. 2019;1(1):1–6. doi:10.6002/ect.2018.0184.
- Khemani K, Steele MK, Bakshi N, Krishnamurti L, Yildirim I. Vaccination adherence in pediatric patients post-hematopoietic stem cell transplant. Blood. 2018;132(Supplement1):3406. doi:10.1182/blood-2018-99-119283.
- Keeley NE, Cherven BO, Weinzierl E, Olson E, Thompson A, Mertens A, Haight AE. Re-Immunization Practices Among Survivors of Pediatric Hematopoietic Stem Cell Transplant. Biol Blood Marrow Transplant. 2011;17(2):S276. doi:10.1016/j.bbmt.2010.12.370.
- Mason KK, Becton D, Mack JM. Got polio? A QI project improving vaccinations rates in off-therapy cancer patients at Arkansas Children’s Hospital. J Investig Med. 2020;68:2. doi:10.1136/jim-2020-SRM.622.
- Crawford NW, Heath JA, Ashley D, Downie P, Buttery JP. Survivors of childhood cancer: an Australian audit of vaccination status after treatment. Pediatr Blood Cancer. 2010;54(1):128–33. doi:10.1002/pbc.22256.
- Patel SR, Bate J, Mathews R, Chisholm J, Heath PT. Vaccination status of children with cancer after completion of standard-dose chemotherapy and after haematopoietic stem cell transplant. Pediatr Blood Cancer. 2012;59(6):1116. doi:10.1002/pbc.
- Volc SM, Almeida MTA, Abadi MD, Cornacchioni AL, Filho VO, Cristofani LM. Measles and rubella antibody status in children after treatment for acute lymphoblastic leukemia. J Pediatr (Rio J). 2006;82(6):481–84. doi:10.2223/JPED.1532.
- Marshall HS, McIntyref P, Richmond P, Buttery JP, Royle JA, Gold MS, Wood N, Elliott EJ, Zurynski Y, Toi CS, et al. Changes in patterns of hospitalized children with varicella and of associated varicella genotypes after introduction of varicella vaccine in Australia. Pediatr Infect Dis J. 2013;32(5):530–37. doi:10.1097/INF.0b013e31827e92b7.
- Pettke A, Jocham S, Wiener A, Löcken A, Groenefeld J, Ahlmann M, Groll AH. Vaccination against influenza at a European pediatric cancer center: immunization rates and attitudes among staff, patients, and their families. Support Care Cancer. 2017;25(12):3815–22. doi:10.1007/s00520-017-3813-6.
- Tishler D, Escobedo E, Alhushki W, Margol AS, Mehta B, Rushing T. Influenza vaccine immunization in a pediatric oncology ambulatory practice. J Clin Oncol. 2013;31(31_suppl):139. doi:10.1200/jco.2013.31.31_suppl.139.
- Freedman JL, Reilly AF, Powell SC, Bailey LC. Quality improvement initiative to increase influenza vaccination in pediatric cancer patients. Pediatrics. 2015;135(2):e540–e546. doi:10.1542/peds.2014-0943.
- Olshefski RS, Bibart M, Frost R, Wood E, Hampl J, Mangum R, Ardura M, Guinipero T, Cripe TP. A multiyear quality improvement project to increase influenza vaccination in a pediatric oncology population undergoing active therapy. Pediatr Blood Cancer. 2018;65(9):e27268. doi:10.1002/pbc.27268.
- Castellino SM, Allen KE, Pleasant K, Keyes G, Poehling KA, Tooze JA. Suboptimal uptake of human papillomavirus (HPV) vaccine in survivors of childhood and adolescent and young adult (AYA) cancer. J Cancer Surviv. 2019;13(5):730–38. doi:10.1007/s11764-019-00791-9.
- Klosky JL, Hudson MM, Chen Y, Connelly JA, Wasilewski-Masker K, Sun C-L, Francisco L, Gustafson L, Russell KM, Sabbatini G, et al. Human papillomavirus vaccination rates in young cancer survivors. J Clin Oncol. 2017;35(31):3582–90. doi:10.1200/JCO.2017.74.1843.
- Klosky JL, Russell KM, Canavera KE, Gammel HL, Foster RH, Parra GR, Simmons JL, Green DM, Hudson MM, et al. Risk factors for non-initiation of the human papillomavirus vaccine among adolescent survivors of childhood cancer. Cancer Prev Res. 2013;6(10):1101–10. doi:10.1158/1940-6207.CAPR-13-0127.
- Klosky JL, Russell KM, Simmons JL, Foster RH, Peck K, Green DM, Hudson MM. Medical and sociodemographic factors associated with human papillomavirus (HPV) vaccination adherence among female survivors of childhood cancer. Pediatr Blood Cancer. 2015;62(9):1630–36. doi:10.1002/pbc.25539.
- Höcker B, Aguilar M, SchniHodgestzler P, Pape L, Webb NJA, Bald M, Genc G, Billing H, König J, et al. Incomplete vaccination coverage in European children with end-stage kidney disease prior to renal transplantation. Pediatr Nephrol. 2018;33(2):341–50. doi:10.1007/s00467-017-3776-3.
- McGhee W, McHugh C, Mazariegos G, Squires R, Remaley L, Zetler K, Brown-Bakewell R, Green M, Michaels M. Improving immunization compliance in pediatric transplant candidates. Pediatr Transplant. 2017;21(Supplement 1):92–93. doi:10.1111/petr.12978.
- Thall TV, Rosh JR, Schwersenz AH, Eickmeyer, Fernandez M, Benkov KJ, LeLeiko NS. Primary immunization status in infants referred for liver transplantation. Transplant Proc. 1994;26:191.
- Island E, Pearson A, Kaufman S, Fishbein T. Vaccination status in pediatric patients referred for liver transplantation. Am J Transplant. 2013; 13(Supplement 5): 249. http://ovidsp.ovid.com/ovidweb.cgi?T=JS&PAGE=reference&D=emed11&NEWS=N&AN=71057295 .
- Murray J, Wollersheim S, Yazigi N, Khan K, Kaufman S, Island E. Vaccination status of pediatric liver transplant candidates. Pediatr Transplant. 2015;19:120–21.
- Gonzalez B, Saracusa C. Barriers to vaccination of pediatric solid organ transplant candidates at a pediatric transplant center. Pediatr Transplant. 2017;21(Supplement 1):81–82. doi:10.1111/petr.12976.
- Urschel S, Cremer S, Birnbaum J, DallaPozza R, Fuchs A, Jäger G, Schmitz C, Belohradsky BH, Netz H. Lack of serologic immunity against vaccine-preventable diseases in children after thoracic transplantation. Transpl Int. 2009;23(6):619–27. doi:10.1111/j.1432-2277.2009.01030.x.
- L’Huillier AG, Wildhaber BE, Belli DC, Rodriguez M, Siegrist CA, Posfay-Barbe KM. Successful serology-based intervention to increase protection against vaccine-preventable diseases in liver-transplanted children: a 19-yr review of the Swiss national reference center. Pediatr Transplant. 2012;16(1):50–57. doi:10.1111/j.1399-3046.2011.01600.x.
- Dehghani SM, Shakiba MA, Ziaeyan M, Imanieh MH, Sedaghat M, Alborzi A, Malek-Hosseini SA. Evaluation of immunity status to routine vaccination in pediatric liver transplant candidates. The Turkish Journal of Gastroenterology. 2015;25(1):26–31. doi:10.5152/tjg.2014.5139.
- Daniels H, Saracusa C, Worley S, Gonzalez B. Measles and varicella vaccination status and evidence of immunity during solid organ pre-transplantation evaluation. Am J Transplant. 2018;18(Supplement 4):739. doi:10.17159/2078-516x/2016/v28i5a1627.
- Dehghani SM, Shakiba MA, Ziaeyan M, Imanieh MH, Haghighat M, Bahador A, Nikeghbalian S, Salahi H, Alborzi A, Malek-Hosseini SA, et al. Vaccination status in pediatric liver transplant candidates. Pediatr Transplant. 2009;13(7):820–22. doi:10.1111/j.1399-3046.2009.01177.x.
- Chaves TSS, Pereira LM, Santos SDS, David-Neto E, Lopes MH. Evaluation of the vaccination status in pediatric renal transplant recipients. Pediatr Transplant. 2008;12(4):432–35. doi:10.1111/j.1399-3046.2007.00820.x.
- Feldman AG, Sundaram SS, Beaty BL, Torres R, Curtis D, Juarez-Colunga E, Kempe A. Immunization status at the time of liver transplant in children and adolescents. JAMA. 2019;322(18):1822–24. doi:10.1001/jama.2019.14386.
- Fela E, Rivard K, Pallotta A, Spinner M, Lepak M, Gonzalez BE. Pre-transplant vaccination adherence in pediatric solid organ transplant patients at a large academic medical center. Open Forum Infect Dis. 2018;5(suppl_1):S745–undefined. doi:10.1093/ofid/ofy210.2137.
- Vo HD, Florescu DF, Brown CR, Chambers HE, Mercer DF, Vargas LM, Grant WJ, Langnas AN, Quiros-Tejeira RE. Invasive pneumococcal infections in pediatric liver-small bowel-pancreas transplant recipients. Pediatr Transplant. 2018;22(3):e13165. doi:10.1111/petr.13165.
- Printza N, Farmaki E, Bosdou J, Gkogka C, Papachristou F. Pandemic influenza A 2009 (H1N1) vaccination in high risk children with chronic renal diseases: acceptance and perceptions. Hum Vaccin. 2010;6(10):819–22. doi:10.4161/hv.6.10.12846.
- Bhagavatula M, Hawkins A, Inward C, Van Der, Van Der Voort J. Immunisation in renal transplantation –a joint audit. Arch Dis Child. 2012;97(Suppl 1):A159–A160. doi:10.1136/archdischild-2012-301885.374.
- Peleg N, Zevit N, Shamir R, Chodick G, Levy I. Seasonal influenza vaccination rates and reasons for non-vaccination in children with gastrointestinal disorders. Vaccine. 2015;33(1):182–86. doi:10.1016/j.vaccine.2014.10.086.
- Britto MT, Schoettker PJ, Pandzik GM, Weiland J, Mandel KE. Improving influenza immunisation for high-risk children and adolescents. Quality and Safety in Health Care. 2007;16(5):363–68. doi:10.1136/qshc.2006.019380.
- Freiberg AS, Singleton AC. Adequacy of vaccine documentation against encapsulated bacteria in a population of children with sickle cell disease. Blood. 2011;118(21):4853. doi:10.1182/blood.V118.21.4853.4853.
- Gomes LMX, Reis TC, Vieira MM, de Andrade-Barbosa TL, Caldeira AP. Quality of assistance provided to children with sickle cell disease by primary healthcare services. Rev Bras Hematol Hemoter. 2011;33(4):277–82. doi:10.5581/1516-8484.20110077.
- Rodríguez-Moldes B, Carbajo AJ, Sánchez B, Fernández M, Garí M, Fernández MC, Álvarez J, García A, Cela E. Seguimiento en Atención Primaria de los recién nacidos con enfermedad falciforme detectados en el cribado neonatal de la Comunidad de Madrid. Anales De Pediatría. 2015;82(4):222–27. doi:10.1016/j.anpedi.2014.04.007.
- Yé D, Kouéta F, Dao L, Kaboret S, Sawadogo A. [Pediatric management of sickle cell disease: experience at the Charles de Gaulle University Children’s Hospital in Ouagadougou (Burkina Faso)]. Sante. 2008;18(2):71–75. doi:10.1684/san.2008.0113.
- Howard-Jones M, Randall L, Bailey-Squire B, Clayton J, Jackson N. An audit of immunisation status of sickle cell patients in Coventry, UK. J Clin Pathol. 2009;62(1):42–45. doi:10.1136/jcp.2008.058982.
- Infanti LM, Elder JJ, Franco K, Simms S, Statler VA, Raj A. Immunization adherence in children with sickle cell disease: a single-institution experience. J Pediatr Pharmacol Ther. 2020;25(1):39–46. doi:10.5863/1551-6776-25.1.39.
- Nofal R, Lorenzana A. Immunization rates in patients with sickle cell disease: a single institution experience. Pediatr Blood Cancer. 2019;66(Supplement 2):263. doi:10.1002/pbc.27713.
- Rhodes MM, Adkins L, Anderews T, Thornton J, Jaggi P. Meningococcal vaccination in children with sickle cell disease: who is responsible? Am J Hematol. 2012;87:E32–E33.
- Akingbola TS, Tayo BO, Salako B, Layden JE, Hsu LL, Cooper RS, Gordeuk VR, Saraf SL. Comparison of patients from Nigeria and the USA highlights modifiable risk factors for sickle cell anemia complications. Hemoglobin. 2014;38(4):236–43. doi:10.3109/03630269.2014.927363.
- Meier ER, Brown L, Hampton K, Bloom E, Lawson D, Hall SA. A novel sickle cell outreach program improves access to TCD screening, vaccines and hydroxyurea in a medically underserved area. Blood. 2019;134(Supplement_1):4696. doi:10.1182/blood-2019-130209.
- Brousse V, Arnaud C, Lesprit E, Quinet B, Odièvre M-H, Etienne-Julan M, Guillaumat C, Elana G, Belloy M, Garnier N, et al. Evaluation of outcomes and quality of care in children with sickle cell disease diagnosed by newborn screening: a real-world nation-wide study in France. J Clin Med. 2019;8(10):1594. doi:10.3390/jcm8101594.
- Reeves SL, Jary HK, Gondhi JP, Kleyn M, Wagner AL, Dombkowski KJ. Pneumococcal vaccination coverage among children with sickle cell anemia, sickle cell trait, and normal hemoglobin. Pediatr Blood Cancer. 2018;65(10):e27282. doi:10.1002/pbc.27282.
- Colombatti R, Montanaro M, Guasti F, Rampazzo P, Meneghetti G, Giordan M, Basso G, Sainati L. Comprehensive care for sickle cell disease immigrant patients: a reproducible model achieving high adherence to minimum standards of care. Pediatr Blood Cancer. 2012;59(7):1275–79. doi:10.1002/pbc.24110.
- Hardie R, King L, Fraser R, Reid M. Prevalence of pneumococcal polysaccharide vaccine administration and incidence of invasive pneumococcal disease in children in Jamaica aged over 4 years with sickle cell disease diagnosed by newborn screening. Ann Trop Paediatr. 2009;29(3):197–202. doi:10.1179/027249309X12467994693851.
- Meier ER, Janson IA, Hampton K, Bloom E, Duncan N, Roberson C, Rampersad A. Adherence to quality of care indicators and location of sickle cell care within Indiana. J Community Health. 2020;45(1):81–87. doi:10.1007/s10900-019-00721-x.
- Sobota AE, Kavanagh PL, Adams WG, McClure E, Farrell D, Sprinz PG. Improvement in influenza vaccination rates in a pediatric sickle cell disease clinic. Pediatr Blood Cancer. 2015;62(4):654–57. doi:10.1002/pbc.25390.
- Colombatti R, Perrotta S, Masera N, Palazzi G, Notarangelo LD, Pusiol A, Bonetto E, De Zen L, Nocerino A, Samperi P, et al. Lessons learned from the H1N1 pandemic: the need to improve systematic vaccination in sickle cell disease children. A multi center survey in Italy. Vaccine. 2011;29(6):1126–28. doi:10.1016/j.vaccine.2010.11.089.
- Bailey A, Bandi S. G136(P) Immunisation of HIV positive children. Arch Dis Child. 2013;98(Suppl 1):A63–A63. doi:10.1136/archdischild-2013-304107.148.
- Setse R, Cutts F, Monze M, Ryon J, Quinn T, Griffen D, Moss W. HIV-1 infection as a risk factor for incomplete childhood immunization in Zambia. J Trop Pediatr. 2006;52(5):324–28. doi:10.1093/tropej/fmk002.
- Dhesi A, Bandi S, Blake K, Welch S, Thompson M. Immunisation of children with HIV. Arch Dis Child. 2012;97(Suppl 1):A32. doi:10.1136/archdischild-2012-301885.82.
- Tchidjou HK, Vescio MF, Sanou Sobze M, Souleyman A, Stefanelli P, Mbabia A, Moussa I, Gentile B, Colizzi V, Rezza G, et al. Low vaccine coverage among children born to HIV infected women in Niamey, Niger. Hum Vaccines Immunother. 2016;12(2):540–44. doi:10.1080/21645515.2015.1069451.
- Succi RCM, Krauss MR, Harris DR, Machado DM, de Moraes-Pinto MI, Mussi-Pinhata MM, Ruz NP, Pierre RB, Kolevic L, Joao E, et al. Undervaccination of perinatally HIV-Infected and HIV-Exposed uninfected children in Latin America and the Caribbean. Pediatr Infect Dis J. 2013;32(8):845–50. doi:10.1097/INF.0b013e31828bbe68.
- Succi RCM, Krauss MR, Harris DR, Machado DM, de Moraes-Pinto MI, Mussi-Pinhata MM, Pavia Ruz N, Pierre RB, Kolevic Roca LA, Joao E, et al. Immunity after childhood vaccinations in perinatally HIV-exposed children with and without HIV infection in Latin America. Pediatr Infect Dis J. 2018;37(4):304–09. doi:10.1097/INF.0000000000001831.
- Taha TE, Graham SM, Kumwenda NI, Broadhead RL, Hoover DR, Markakis D, van der Hoeven L, Liomba GN, Chiphangwi JD, Miotti PG, et al. Morbidity among Human Immunodeficiency Virus-1-Infected and -Uninfected African children. Pediatrics. 2000;106(6):e77–e77. doi:10.1542/peds.106.6.e77.
- Pandolfi E, Carloni E, Marino MG, Ciofi degli Atti ML, Gesualdo F, Romano M, Giannattasio A, Guarino A, Carloni R, Borgia P, et al. Immunization coverage and timeliness of vaccination in Italian children with chronic diseases. Vaccine. 2012;30(34):5172–78. doi:10.1016/j.vaccine.2011.02.099.
- Giagnorio MG, Minicucci V, Mariano M, Romano R, Napolitano C, Manchisi M, Macchiaroli A, Vecchio AL, Raia V, Giannattasio A, et al. 177 Strategies to improve influenza vaccination coverage in at-risk children: the experience of patients with cystic fibrosis. J Cyst Fibros. 2013;12(Supplement 1):S93. doi:10.1016/s1569-1993(13)60318-5.
- Welzel T, Zumbrunn T, Bonhoeffer J, Cannizzaro Schneider E, Hofer M, Kaiser D, Hentgen V, Woerner A. High vaccine coverage rates are not enough: vaccination delay and risk for vaccinepreventable diseases in pediatric patients with rheumatic diseases with and without immunosuppressive therapy. Swiss Med Wkly. 2018;184(Supplement 228):6S.
- Nicol S, Lawrence S, Jacobson K. Inadequate vaccination uptake in children receiving anti-TNF therapy for inflammatory bowel disease. J Pediatr Gastroenterol Nutr. 2017;64(Supplement 1):501. doi:10.1097/01.mpg.0000516381.25680.b4.
- Franczak-Chmura P, Tomaszek MB, Sobiesiak A, Opoka-Winiarska V. The vaccination status of patients with juvenile idiopathic arthritis. Pediatr Rheumatol. 2018;16(Supplement 2):128. doi:10.1186/s12969-018-0265-6.
- Sousa H, Carvalho S, Rodrigues M, Brito I, Reis P, Rocha T, Ramos M, Santos M, Ramos F, Cabral M, Guedes M. Vaccination coverage in Portuguese children with rheumatic diseases undergoing immunosuppressive therapy. Pediatr Rheumatol. 2018;16(Supplement 2):196–97. doi:10.1002/art.40466.
- Martinelli M, Giugliano FP, Miele E, Strisciuglio C, Urbonas V, Serban DE, Banaszkiewicz A, Assa A, Hojsak I, Lerchova T, et al. Vaccinations and immunization status in pediatric inflammatory bowel disease: a multicenter study from the pediatric IBD porto group of the ESPGHAN. Inflamm Bowel Dis. 2020;26(9):1407–14. doi:10.1093/ibd/izz264.
- Cagol L, Seitel T, Ehrenberg S, Frivolt K, Krahl A, Lainka E, Gerner P, Lenhartz H, Vermehren J, Radke M, et al. Vaccination rate and immunity of children and adolescents with inflammatory bowel disease or autoimmune hepatitis in Germany. Vaccine. 2020;38(7):1810–17. doi:10.1016/j.vaccine.2019.12.024.
- Feldon M, Furnier A, Lehmann C, Fletcher B, Kues J, Speer B, Kramer S, Morgan P, Siegle L, Brady R, et al. Improving pneumococcal immunization rates among immunocompromised adolescent patients at a tertiary care children’s hospital. Arthritis Rheumatol. 67(Supplement10):2015. http://ovidsp.ovid.com/ovidweb.cgi?T=JS&PAGE=reference&D=emed13&NEWS=N&AN=72094916 .
- Temtem T, Whitworth J, Bagga B. Pneumococcal polysaccharide vaccination in pediatric inflammatory bowel disease. Glob Pediatr Heal. 2019;6(1):1–4. doi:10.1177/2333794x19849754.
- Ayers M, Mahajan L, Anani A, Collyer E. Immunization rates for PPSV23 (pneumovax) in immunocompromised pediatric patients with inflammatory bowel disease: room for improvement. J Pediatr Gastroenterol Nutr. 2016;63:S76.
- Banaszkiewicz A, Klincewicz B, Lazowska-Przeorek I, Grzybowska-Chlebowczyk U, Kąkol P, Mytyk A, Kofla A, Radzikowski A. Influenza vaccination coverage in children with inflammatory bowel disease. Influenza Other Respi Viruses. 2014;8(4):431–35. doi:10.1111/irv.12236.
- Taylor J, Haddix EF, Badinghaus K, Burns M, Moore T, Ranz J, Anneken A, Fiorini P, Spiegel E, Morgan-Dewitt E, et al. Participation in hospital influenza collaborative is influential in improving vaccination rates in patients with juvenile idiopathic arthritis and systemic lupus erythematosus. Arthritis Rheum. 2011;63(Supplement 10):S616.
- Spijkerman R, Teuben MPJ, Hietbrink F, Kramer WLM, Leenen LPH. A cohort study to evaluate infection prevention protocol in pediatric trauma patients with blunt splenic injury in a Dutch level 1 trauma center. Patient Prefer Adherence. 2018;12:1607–17. doi:10.2147/PPA.S169072.
- Khasawneh MA, Contreras-Peraza N, Hernandez MC, Lohse C, Jenkins DH, Zielinski MD. Outcomes after splenectomy in children: a 48-year population-based study. Pediatr Surg Int. 2019;35(5):575–82. doi:10.1007/s00383-019-04439-8.
- Kind EA, Craft C, Fowles JB, McCoy CE. Pneumococcal vaccine administration associated with splenectomy: missed opportunities. Am J Infect Control. 1998;26(4):418–22. doi:10.1016/S0196-6553(98)70038-0.
- Berkhout A, Varghese V, Prasad V, Heussler H, Preece K, Clark J, Wen S. Optimising immunisations in children with 22Q11 microdeletion. J Paediatr Child Health. 2019;55(S2):15–15. doi:10.1111/jpc.14468.
- Tennis P, Toback SL, Andrews EB, McQuay LJ, Ambrose CS. A US postmarketing evaluation of the frequency and safety of live attenuated influenza vaccine use in nonrecommended children younger than 5 years: 2009–2010 season. Vaccine. 2012;30(42):6099–102. doi:10.1016/j.vaccine.2012.07.031.
- Rajaram S, Steffey A, Blak B, Hickman M, Christensen H, Caspard H. Uptake of childhood influenza vaccine from 2012–2013 to 2014–2015 in the UK and the implications for high-risk children: a retrospective observational cohort study. BMJ Open. 2016;6(8):e010625. doi:10.1136/bmjopen-2015-010625.
- Sammon CJ, McGrogan A, Snowball J, De Vries CS. Factors associated with uptake of seasonal and pandemic influenza vaccine among clinical risk groups in the UK: an analysis using the general practice research database. Vaccine. 2012;30(14):2483–89. doi:10.1016/j.vaccine.2011.11.077.