ABSTRACT
Safety concerns about novel vaccines and necessity of COVID-19 vaccination for children, especially with underlying medical conditions, are the obstacle of COVID-19 vaccination program among pediatric population. The study was conducted to investigate the vaccine hesitancy reasons among the parents, and to monitor the adverse events of inactivated COVID-19 vaccines in children and teenagers with underlying medical conditions in China. Children with underlying medical conditions encountered to the Immunization Advisory Clinic for COVID-19 vaccine counseling were enrolled. They were given immunization recommendation and followed up at 72 h and 28 d after immunization to monitor the immunization compliance after consultation and adverse events. A total of 324 children aged 3–17 y were included. The top three primary medical conditions for counseling were allergy (33.6%), neurological diseases (31.2%) and rheumatic diseases (8.3%). COVID-19 vaccination was promptly recommended for 242 (74.7%) children. Seventy-one (65.7%) children who had allergy issues were recommend to take vaccination, which was significantly lower than that of other medical conditions (p < .05). The follow-up record showed that 180 children received 340 doses of inactivated COVID-19 vaccine after consultation. Overall, 39 (21.6%) children reported at least one adverse event within 28 d of either vaccination. No serious adverse reactions were observed. No difference of adverse effects between the first dose and the second dose of vaccination except fever. Parents’ hesitancy in COVID-19 vaccination for children with underling medical conditions are mainly due to the safety concerns. Specialist consultation is helpful to improve the vaccine uptake.
Introduction
The novel 2019 coronavirus disease (COVID-19) vaccines are critical to ending the pandemic. The vast majority of pediatric COVID-19 cases have milder symptoms than adult cases so that children play an important role in home, school and community transmission.Citation1,Citation2 COVID-19 vaccination for children is not only essential to protect susceptible pediatric population, but also a vital step to build-up herd immunity. Currently, there are five in nine World Health Organization (WHO) validated vaccines authorized for use in children and teenagers in different countries ().Citation3 BNT162b2 mRNA vaccine (by Pfizer-BioNTech) was the first COVID-19 vaccine approved for emergency use authorization (EUA) in teenagers older than 16 y by the US Food and Drug Administration (FDA) since 11 December 2020. The authorization was expanded to those 12–15 y of age on 10 May 2021 and 5–11 y of age on 29 October 2021.Citation4 Soon after, the China National Medical Products Administration (NMPA) granted a conditional authorization for inactivated whole-virus SARS-CoV-2 vaccine BBIBP-CorV (by Sinopharm) and CoronaVac (by Sinovac) to use in children and teenagers aged 3–17 y on early June 2021. COVID-19 vaccination program in pediatric population was initiated since late July 2021 across China. As for 16 December 2021, more than 140 million children aged 3–11 in China have received at least one dose of a COVID-19 vaccine.Citation5 Since then, the Immunization Advisory Clinic in our institute received increased parents’ counseling about the safety issues and necessity of COVID-19 vaccination for their children ≤17 y. Safety concern is the top reason for parental hesitancy about COVID-19 vaccination for children worldwide,Citation6–8 especially in those with underlying special medical conditions and previously confirmed or suspected adverse events following immunization (AEFI).Citation9–11 Since only previously healthy children were recruited in the pre-market clinical trials of the CoronaVac and BBIBP-CorV,Citation12,Citation13 the safety of COVID-19 vaccination in children with underlying medical conditions are not yet to be studied. The facts mentioned above contribute to the different description of contraindications from regulation agencies, vaccine manufacturers, pediatricians and primary care providers. Furthermore, there is little field evaluation and surveillance data of COVID-19 vaccines among these special pediatric population. Given the importance of addressing the concerns in this group and understanding the safety of COVID-19 vaccine, we carried out this study to investigate the vaccine hesitancy reasons among the counseled parents, and to actively monitor the adverse events of inactivated COVID-19 vaccines in children and teenagers with underlying medical conditions in China.
Table 1. Authorization for use in children and teens of COVID-19 vaccines validated for use by the WHO as of January 12, 2022.
Patients and Methods
Subjects
Children aged ≤17 y old who visited the Immunization Advisory Clinic at our institute for COVID-19 vaccine counseling were enrolled from September 2021 to December 2021 during the rollout of COVID-19 vaccination in children in Shanghai. For each subject, vaccination concerns, medical conditions and immunization history were recorded by face-to-face interview. Recommendation for immunization of inactivated COVID-19 vaccine was made by the specialists of infectious diseases. To harmonize immunization recommendations across the team, the contraindications and precautions referred to the existing national and WHO guideline of COVID-19 vaccination, see .Citation3,Citation14 We conducted a phone call follow-up at 72 h and 28 d after to monitor the immunization compliance after consultation and AEFI, as prompted by and recorded in an electronic diary. All counseled parents were informed to report any unsolicited symptoms and signs within 28 d post-vaccination by telephone (). The children were informed to have further evaluation and management at the clinic if needed. Ethical approval was granted by the hospital's Institutional Review Board (IRB). Written consent was exempt because the information was collected anonymously and the follow-up were a sort of routine out-patient service. Since the China NMPA approved COVID-19 vaccination for use in children aged 3–17 y, those younger than 3 y old were excluded in this study. Also, to focus the safety assessment and surveillance on children with underlying medical conditions, those healthy children encountered for counseling were excluded.
Table 2. Contraindications and precautions to COVID-19 vaccination for children aged 3–17 y with underlying medical conditions.
Data collection
The following data was collected from the medical records in this study: (1) demographics: sex and age; (2) underlying medical conditions and current status; (3) previous immunization and AFEI history; (4) specialist recommendation on COVID-19 vaccination: starting or deferring vaccination. We recorded the follow-up data within 28 d after vaccination as follows: (1) COVID-19 immunization status and doses; (2) systemic adverse reactions; (3) unsolicited adverse events based on parents’ self-report.
Statistical analyses
Descriptive statistics were presented as mean ± standard deviation (SD) for the continuous variables and the number of cases for nominal variables. Nominal data, including sex, age and underlying medical conditions between the two groups were analyzed by Fisher’s Exact Test or Chi-squared test. In all cases, results with p < .05 were considered significant. All statistical analyses were performed by SPSS 21.0 (IBM, Armonk, NY, US)
Result
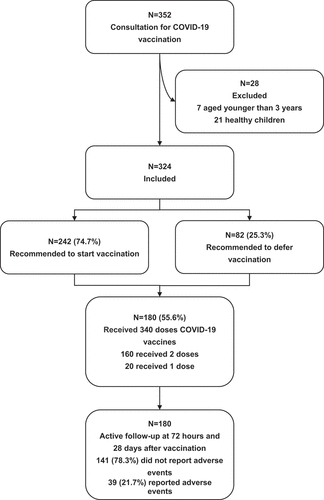
From September 2021 to December 2021, a total of 352 children visited our Immunization Advisory Clinic for COVID-19 vaccine counseling. Seven children aged younger than 3 y old and 21 previously healthy children were excluded from the final analysis. Finally, 324 children aged from 37 months to 215 months (mean ± SD: 118.7 ± 36.5 months) were included, among which 188 (58.0%) were boys and 136 (42.0%) were girls. The demographics and underlying medical conditions are summarized in . The top three primary medical conditions for counseling were allergy, neurological diseases and rheumatic diseases, accounting for 33.6%(n = 108), 31.2% (n = 101) and 8.3% (n = 27), respectively. Among the children with a history of AEFI, 6 was allergic to a prior dose of a COVID-19 vaccine, 2 to an unrelated vaccine, and 11 had non-allergic AEFI after unrelated vaccines.
Table 3. Demographics and medical conditions between the two groups recommended to start or defer CIVID-19 vaccination in the 324 subjects.
Overall, a prompt COVID-19 vaccination was recommended for 242 (74.7%) children (). No significant difference was found in sex or age group among children who were recommended to start or defer vaccination. Seventy-one (65.7%) children who had allergy issues were recommended to take COVID-19 vaccine, which was significantly lower than those with other medical conditions (p < .05). More than half children with allergy issues were diagnosed with a preexisting atopic disease, and followed by allergy to a non-medication such as food, pollen, pet and mite. Eighty (80%) children with neurological disease were recommended to take vaccination, among whom three-fourths had episodic diseases like epilepsy, febrile seizure and tic disorders. No child had immunocompromised conditions in the study children. All the children with solid and hematological cancer were cured cases. As per the national guideline of COVID-19 vaccination,Citation14 the reasons for deferring immunization were as follows: 52 (63.4%) had acute disease or acute exacerbation of chronic disease, 21 (25.6%) had uncontrolled epilepsy or other serious neurological diseases, 7 (8.5%) had severe systemic or allergic reactions to any previous vaccines, and 2 (2.4%) had allergy to any ingredient of COVID-19 vaccine.
The follow-up record showed that 180 children received a total of 340 doses of either CoronaVac or BBIBP-CorV after consultation. Among the 180 vaccines, 160 children received 2 doses and 20 received 1 dose vaccine. Overall, 39 (21.6%) children reported at least one adverse reaction within 28 d of either vaccination (). Most systemic adverse events occurred within 3 d after vaccination and children recovered within 48 h. Fever (7.2%) was the most common systemic adverse reaction, followed by rash (2.8%) and fatigue (1.7%). The most common unsolicited adverse reaction was respiratory infections (15.6%), followed by flare of atopic dermatitis (2.2%) and flare of tics (1.1%). More cases of fever were observed after the first-dose vaccination than after the second one. Regarding other solicited adverse reactions than fever, no significant difference was observed between the first dose and the second dose of vaccination (). No difference was observed in the profile of adverse effects following vaccination according to sex or age group, but there were more cases of adverse effects reported in the children with allergy diseases (). No serious adverse reactions were observed, such as anaphylaxis, immune thrombocytopenia, Bell palsy, multisystem inflammatory syndrome in children, venous thromboembolism, thrombosis with thrombocytopenia syndrome, myocarditis and pericarditis. A 7-y-old girl complained about weaken eyesight and strabismus on day 2 after COVID-19 vaccination. However, ophthalmic examination was normal and she was recovered 1 week later. The case was finally considered to be psychogenic because she resented the reading pressure from her mother. A 12-y-old girl had recurrent fever pre- and post-vaccination. This girl was fully recovered from previously idiopathic thrombocytopenic purpura at the age of 6 y. She had low fever within 2 d after vaccination and recovered spontaneously. The persistent fever up to 39 Celsius degrees recurred on day 7 after vaccination and was accompanied with arthralgia. She was finally diagnosed as systemic lupus erythematosus.
Table 4. Adverse reactions reported within 28 d after vaccination in the 180 children receiving 340 doses of inactivated COVID-19 vaccines.
Table 5. Demographics and medical conditions between the two groups of reported adverse reactions and not reported adverse reactions within 28 d after vaccination.
Discussion
In this prospective hospital-based cross-sectional study, we encountered 354 children with or without underlying medical conditions to counsel COVID-19 vaccination at our Immunization Advisory Clinic in Shanghai. Massive COVID-19 vaccination roll-out commenced among children aged 15–17 y since 12 August 2021, 12–14 y since 1 September 2021, 6–11 y since 27 October 2021 and 3–5 y since 17 November locally. The counseling mainly came from the children with underlying medical conditions, but also from the healthy children. All parents expressed their safety concern on novel COVID-19 vaccines. So far, the phase 3 clinical trial data on CoronaVac and BBIBP-CorV conducted in pediatric subjects were not available, and the surveillance AEFI data after massive COVID-19 immunization among children and teenagers have not been reported in China. Children with underlying diseases may have higher risk of experience COVID-19 infection due to the frequent visits to the healthcare facility. Thus, vaccination for these special children is important to achieve immune protection. Addressing the parents’ safety concern will be helpful to improve COVID-19 vaccine uptakes. In this study, we observed that parents’ concerns include the potential allergic reactions of the vaccine, the relapse or worsening of the primary chronic disease, and unknown side-effects of the COVID-19 vaccine. The solicited systemic adverse events within 72 h after vaccination were almost the same as the data reported in the phase 1/2 clinical trials of CoronaVac of healthy children and adolescents.Citation12 The key strengths of this study are that it presents a full picture of what kind of underlying medical condition will cause vaccine hesitancy to COVID-19 vaccine among parents, and also it has a descriptive monitoring of AEFI within the 28 d after COVID-19 vaccination. Overall, our findings demonstrated the good safety of inactivated COVID-19 vaccines among children with underlying medical conditions.
Allergy concerns ranked the first position of children with underlying medical conditions counseling COVID-19 vaccines in our study. The clinical scenarios varied from allergy reactions (1) to a prior dose of a COVID-19 vaccine, (2) to an unrelated vaccine or medication, (3) to a non-medication, i.e., pollen, food, pet fur and mite etc., and (4) most frequently, having a preexisting atopic disease including asthma, atopic dermatitis and atopic rhinitis.Citation15 Parents not only worried that children with allergy conditions are more likely to have severe allergic reactions like life-threatening anaphylaxis, but also the vaccine may trigger the flare of stable atopic disease, particularly asthma. Parents are more willing to defer the vaccination in the allergy group as compared to other disease groups in our study. Our observational data show that more cases of adverse effects were reported in the children with allergy diseases. It may due to more attention paid to those children by the parents. Although there are some concern about the long-term allergic conditions possibly triggered by vaccination, the existing prospective studies have shown that childhood immunizations neither increased the risk of requiring asthma nor impacted the atopic disease activity.Citation16
For most vaccines, the reported rate of anaphylaxis ranges from 1 to 10 cases per million doses.Citation17 There were few cases of severe allergic reactions reported in the pre-marketing COVID-19 vaccine studies due to the low incidence of anaphylaxis. In the largest published interim analyses of post-approval surveillance mRNA COVID-19 vaccines in the US, the estimated incidence rate of confirmed anaphylaxis was 4.8–5.1 per million doses.Citation18 Meanwhile, this study also showed that 78% of individuals experiencing anaphylaxis had a history of allergies and 36% had a history of anaphylaxis.Citation16 Earlier reports also emphasized coexisting atopic disease, particularly asthma, as being clinical risk factors for anaphylaxis after vaccination.Citation19 However, the U.S. Centers for Disease Control and Prevention (CDC), the American Academy of Allergy, Asthma, and Immunology (AAAAI), and the European Academy of Allergy and Clinical Immunology (EAACI) all listed those had a history of any immediate allergic reaction to non-COVID-19 vaccines or injectable therapies as a precaution.Citation20–22 Some countries are more cautious. History of severe allergic reactions associated with any vaccine was listed as an exclusion criterion in almost all the clinical trials. So it is legally rational that some countries’ guidelines currently list the persons with any immediate allergic reaction to non-COVID-19 vaccines as contraindication, like in China and India.Citation14,Citation23
It has been a long-term issue for children with uncontrolled epilepsy and progressive neurological diseases may not be adequately vaccinated.Citation24 The main concern among this special group is that the vaccine may trigger the recurrence of seizure.Citation25 A multicentred healthy-control prospective study of adults in China found no evidence suggesting worsening seizures after inactivated COVID-19 vaccination in adults.Citation26 This is in consistence with a cross-sectional study in Kuwait, showing BNT162b2 and ChAdOx1nCoV-19 vaccines did not worsen epilepsy.Citation27 It is noteworthy that physicians also have the concerns on the safety and reliability of the vaccine.Citation28 Another barrier comes from the official guideline of vaccination, listing uncontrolled epilepsy and other serious neurological disorders, i.e., transverse myelitis, Guillain-Barré Syndrome, demyelinating disorders, and etc. as an contraindication.Citation14 Considering the prospective studies on episodic and progressive neurological diseases and COVID-19 vaccine for children remain lacking, it needs more surveillance data to entrust the safety of vaccines.
This study has some limitations. Our study is only hospital-based, small-scaled monitoring investigation, which cannot capture the general AEFI related to COVID-19 vaccine among all concerned children in the community. Some AEFI with the very low incidence, e.g., hypersensitivity and immune thrombocytopenia were not observed in this study due to the limited sample size. Second, the difference of adverse effects after COVID-19 vaccination between the two brands of inactivated vaccine or between different types of vaccines was not compared. Besides, immunocompromised children who are the key priority group for COVID-19 vaccination has not been included in this observational study, and the vaccine safety evidence is urgently needed for these special children. Prospective cohort studies on children with special conditions and massive population-based surveillance of AEFI are needed to provide high-quality evidence of the safety and efficacy of COVID-19 vaccines in the children with underlying medical conditions.
Conclusion
Both guidelines and healthcare providers remain cautious about recommending COVID-19 vaccines for children with underlying medical conditions due to the lack of published pre- and post-marketing safety surveillance data. Children with allergic diseases and neurological diseases will cause vaccine hesitancy to COVID-19 vaccine among parents. Our study demonstrated the good safety of inactivated COVID-19 vaccines among children with underlying medical conditions. No difference of adverse effects between the first dose and the second dose of vaccination except fever. Large population-based study will provide the robust evidence to entrust the safety of COVID-19 vaccination in children with underlying medical conditions.
Author’s contributions
Drs Hailing Chang and Tianxing Feng are joint first authors and contributed equally to the study. Drs Mei Zeng and Xiaowen Zhai are joint corresponding authors. Drs Mei Zeng and Xiaowen Zhai initiated and conceived this study. Dr Mei Zeng supervised this study. Dr Tianxing Feng wrote the first and subsequent drafts of the paper. Dr Hailing Chang and Ms Wenjie Ma conducted the statistical analyses and takes responsibility for the integrity of the data and accuracy of the data analyses. Drs Yanling Ge, Yanfeng Zhu and Xiangshi Wang reviewed all children included on case level with Dr Mei Zeng. Dr Hailing Chang and Ms Wenjie Ma conducted follow-up work. Ms Wenjie Ma provided administrative and material support. Drs Mei Zeng and Xiaowen Zhai are guarantors. All authors have approved the final version of this manuscript submitted for publication. The corresponding author attests that all listed authors meet authorship criteria and that no others meeting the criteria have been omitted.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Imamura T, Saito M, Ko YK, Imamura T, Otani K, Akaba H, Ninomiya K, Furuse Y, Miyahara R, Sando E, et al. Roles of children and adolescents in COVID-19 transmission in the community: a retrospective analysis of nationwide data in Japan. Front Pediatr. 2021;9:1. doi:10.3389/fped.2021.705882.
- Pediatrics AAo. Children and COVID-19: state-level data report 2022 [accessed 2022 Jan 24]. https://www.aap.org/en/pages/2019-novel-coronavirus-covid-19-infections/children-and-covid-19-state-level-data-report/.
- Organization WH. Coronavirus disease (COVID-19): vaccines 2021 [accessed 2021 Nov 29]. https://www.who.int/news-room/questions-and-answers/item/coronavirus-disease-(covid-19)-vaccines.
- Administration USFaD. FDA approves first COVID-19 vaccine 2021 [accessed 2021 Aug 23]. https://www.fda.gov/news-events/press-announcements/fda-approves-first-covid-19-vaccine.
- China NHCo. State Council Joint Prevention and Control Mechanism Press Conference on December 20, 2021; 2021. http://www.nhc.gov.cn/xcs/yqfkdt/202112/bb163e5e345546669130c0a894a91ca1.shtml.
- Alfieri NL, Kusma JD, Heard-Garris N, Davis MM, Golbeck E, Barrera L, Macy ML. Parental COVID-19 vaccine hesitancy for children: vulnerability in an urban hotspot. BMC Public Health. 2021 Sep 13;21(1):1662. doi:10.1186/s12889-021-11725-5.
- Bagateli LE, Saeki EY, Fadda M, Agostoni C, Marchisio P, Milani GP. COVID-19 vaccine hesitancy among parents of children and adolescents living in Brazil. Vaccines (Basel). 2021 Sep 30;9(10). doi:10.3390/vaccines9101115.
- Yigit M, Ozkaya-Parlakay A, Senel E. Evaluation of COVID-19 vaccine refusal in parents. Pediatr Infect Dis J. 2021 Apr 1;40(4):e134–8. doi:10.1097/INF.0000000000003042.
- Skeens M, Guttoo P, Stanek JR, Taylor K, Stratz E, Ardura MI, Rangarajan HG. An exploration of COVID-19 impact and vaccine hesitancy in parents of pediatric Hematopoietic Stem Cell Transplant (HCT) recipients. Bone Marrow Transplant. 2022 Jan 24;57(4):547–553. doi:10.1038/s41409-022-01587-9.
- Tsai R, Hervey J, Hoffman K, Wood J, Johnson J, Deighton D, Clermont D, Loew B, Goldberg SL. COVID-19 vaccine hesitancy and acceptance among individuals with cancer, autoimmune diseases, or other serious comorbid conditions: cross-sectional, internet-based survey. JMIR Public Health Surveill. 2022 Jan 5;8(1):e29872. doi:10.2196/29872.
- Waters AR, Kepka D, Ramsay JM, Mann K, Vaca Lopez PL, Anderson JS, Ou JY, Kaddas HK, Palmer A, Ray N, et al. COVID-19 vaccine hesitancy among adolescent and young adult cancer survivors. JNCI Cancer Spectr. 2021;5(3):kab049. doi:10.1093/jncics/pkab049.
- Han B, Song Y, Li C, Yang W, Ma Q, Jiang Z, Li M, Lian X, Jiao W, Wang L, et al. Safety, tolerability, and immunogenicity of an inactivated SARS-CoV-2 vaccine (CoronaVac) in healthy children and adolescents: a double-blind, randomised, controlled, phase 1/2 clinical trial. Lancet Infect Dis. 2021;21(12):1645–1653. doi:10.1016/S1473-3099(21)00319-4.
- Xia S, Zhang Y, Wang Y, Wang H, Yang Y, Gao GF, Tan W, Wu G, Xu M, Lou Z, et al. Safety and immunogenicity of an inactivated COVID-19 vaccine, BBIBP-CorV, in people younger than 18 years: a randomised, double-blind, controlled, phase 1/2 trial. Lancet Infect Dis. 2022;22(2):196–208. doi:10.1016/S1473-3099(21)00462-X.
- China NHCo. Practical guideline of COVID-19 vaccination (First Edition) 2021 [accessed 2021 Mar 29]. http://www.nhc.gov.cn/jkj/s3582/202103/c2febfd04fc5498f916b1be080905771.shtml.
- Moreno MA. Atopic diseases in children. JAMA Pediatr. 2016;170(1):96. doi:10.1001/jamapediatrics.2015.3886.
- Nilsson L, Brockow K, Alm J, Cardona V, Caubet J-C, Gomes E, Jenmalm MC, Lau S, Netterlid E, Schwarze J, et al. Vaccination and allergy: EAACI position paper, practical aspects. Pediatr Allergy Immunol. 2017;28(7):628–640. doi:10.1111/pai.12762.
- Dreskin SC, Halsey NA, Kelso JM, Wood RA, Hummell DS, Edwards KM, Caubet J-C, Engler RJM, Gold MS, Ponvert C, et al. International Consensus (ICON): allergic reactions to vaccines. World Allergy Organ J. 2016;9(1):32. doi:10.1186/s40413-016-0120-5.
- Klein NP, Lewis N, Goddard K, Fireman B, Zerbo O, Hanson KE, Donahue JG, Kharbanda EO, Naleway A, Nelson JC, et al. Surveillance for adverse events after COVID-19 mRNA vaccination. Jama. 2021;326(14):1390–1399. doi:10.1001/jama.2021.15072.
- McNeil MM, Weintraub ES, Duffy J, Sukumaran L, Jacobsen SJ, Klein NP, Hambidge SJ, Lee GM, Jackson LA, Irving SA, et al. Risk of anaphylaxis after vaccination in children and adults. J Allergy Clin Immunol. 2016;137(3):868–878. doi:10.1016/j.jaci.2015.07.048.
- American Academy of Allergy A, and Immunology. Vaccine reactions. 2022.
- Prevention USCfDCa. Interim clinical considerations for use of COVID-19 vaccines currently approved or authorized in the United States 2021 [accessed 2021 Nov 3]. https://www.cdc.gov/vaccines/covid-19/info-by-product/clinical-considerations.html.
- Sokolowska M, Eiwegger T, Ollert M, Torres MJ, Barber D, Del Giacco S, Jutel M, Nadeau KC, Palomares O, Rabin RL, et al. EAACI statement on the diagnosis, management and prevention of severe allergic reactions to COVID-19 vaccines. Allergy. 2021;76(6):1629–1639. doi:10.1111/all.14739.
- Welfare IMoHaF. Who will get the vaccine? 2021 [accessed 25 Mar 2021]. https://www.mohfw.gov.in/covid_vaccination/vaccination/faqs.html#who-will-get-the-vaccine.
- Richter D, Anca I, André FE, Bakir M, Chlibek R, Čižman M, Mangarov A, Mészner Z, Pokorn M, Prymula R, et al. Immunization of high-risk paediatric populations: Central European Vaccination Awareness Group recommendations. Expert Rev Vaccines. 2014;13(6):801–815. doi:10.1586/14760584.2014.897615.
- Li N, Chu C, Lin W. A survey of hesitancy and response to the COVID-19 vaccine among patients with epilepsy in Northeast China. Front Neurol. 2021;12:778618. doi:10.3389/fneur.2021.778618.
- Lu L, Zhang Q, Xiao J, Zhang Y, Peng W, Han X, Chen S, Yang D, Sander JW, Zhou D, et al. COVID-19 vaccine take-up rate and safety in adults with epilepsy: Data from a multicenter study in China. Epilepsia. 2022;63(1):244–251. doi:10.1111/epi.17138.
- Massoud F, Ahmad SF, Hassan AM, Alexander KJ, Al–hashel J, Arabi M. Safety and tolerability of the novel 2019 coronavirus disease (COVID-19) vaccines among people with epilepsy (PwE): a cross-sectional study. Seizure. 2021;92:2–9. doi:10.1016/j.seizure.2021.08.001.
- Asadi-Pooya AA, Sahraian A, Badv RS, Sahraian MA. Physicians’ opinions on the necessity of COVID-19 vaccination in patients with epilepsy. Epileptic Disord. 2021 Jun 1;23(3):485–489. doi:10.1684/epd.2021.1282.