ABSTRACT
This study was designed to evaluate the emerging trends of research on mRNA vaccines. Altogether 3056 research articles related to mRNA vaccines published since 2010 were retrieved from the Web of Science database, based on which a co-citation analysis was conducted using CiteSpace. A total of 12 clusters were derived, all of which were classified into three periods according to the content and publication time of articles: (1) The preliminary exploratory period before early 2010s, when the potential of mRNA to induce immune response was evaluated; (2) the growing up period from early 2010s to 2019, when the stability and immunogenicity of mRNA vaccines were improved and the clinical development of products were pushed forward; (3) the rapid maturity period after the outbreak of COVID-19, when two products for COVID-19 were authorized for the first time. The approval of COVID-19 vaccines is an encouraging start, while the enormous potential of mRNA vaccines remains to be explored. Future research on mRNA-based infectious disease vaccines will focus on further optimizing mRNA modification and delivery, solving problems of the approved vaccines in real world, investigating mRNA vaccines for other infectious indications, and developing self-amplifying or thermostable vaccines. Future research on mRNA-based therapeutic cancer vaccines will focus on screening proper neoantigens, enhancing the delivery of mRNA into antigen-presenting cells and overcoming suppressive tumor microenvironment.
Background
The widespread use of conventional vaccines such as inactivated vaccines, live attenuated vaccines and subunit vaccines has saved numerous lives.Citation1 However, new types of vaccines are still in need for the reason that conventional ones cannot be developed or produced rapidly enough to face with the outbreak of emerging infectious diseases and may not be effective in treating cancers.Citation1,Citation2 mRNA vaccine is a new type of vaccine that introduces mRNA into the host body and uses the protein synthesis mechanism of the host cells to encode disease-specific antigens, thereby triggering the immune response of the host body to fight against diseases.Citation3 With the advantages of rapid to design, scalable production, favorable safety profiles and the potential to treat cancers, mRNA vaccine has been considered to be a promising direction in the future.Citation1,Citation3
The first protein expression post RNA and DNA expression vectors being injected into mouse skeletal muscle was reported in 1990, after when many in-vivo studies on mRNA vaccines were conducted.Citation4 In 1992, injection of vasopressin mRNA into rat brain was shown to be a potential protein replacement therapy for diabetes insipidus.Citation5 In 1993, the first liposome-mRNA vaccine was tested for preventing influenza in mice, and a specific antiviral immune response was elicited.Citation6 Even with these early promising results, the development of mRNA vaccines didn’t come to success in a long period of time thereafter, mainly owing to the instability and inefficient in vivo delivery of mRNA.Citation2,Citation3,Citation7 Over the past few decades, many scientists have devoted to overcome these problems and have made breakthroughs, especially on nucleoside modification and delivery systems.Citation8 In recent years, the pandemic of COVID-19 further accelerated the maturity of mRNA technology.Citation9,Citation10
Keeping up with the emerging trends of research on mRNA vaccines can provide references for scientists to adjust their research directions, for policy makers to determine the directions of funding and for enterprises to optimize their strategic layouts. In recent years, several reviews describing the advancement and progress of mRNA vaccines have been published, providing us a chance to quickly understand the knowledge evolution in this field.Citation1,Citation3,Citation9,Citation10 These reviews were mainly based on experts’ knowledge, which might be subjective and might be limited by the level of experts’ understanding of the industry. Therefore, a systematic and repeatable analysis is in need to provide more objective evidences.
Bibliometrics is an emerging discipline that measures science itself quantitatively. In the past few decades, theoretical and methodological contributions to bibliometrics have been made. Meanwhile, many science mapping tools have been developed, based on which the patterns in scientific literature can be analyzed to reflect the knowledge structures and emerging trends of research in a given field. Among all kinds of science mapping tools, CiteSpace is a commonly used one, the primary goal of which is to help detect the emerging trends of research based on the idea of co-citation analysis.Citation11–13 Two basic concepts, research front and intellectual base, were embedded in Citespace to conceptualize and visualize a field of knowledge.Citation11,Citation14 Research front refers to a group of emergent studies, and intellectual base refers to a group of references cited by publications forming the research front.Citation11 In addition, Kleinberg’s burst detection algorithm can be applied to detect sharp increase in citations and Freeman’s betweenness centrality metric can be used to detect potential pivotal nodes in a network.Citation11
This study aims to detect the emerging trends of research on mRNA vaccines, for which purpose, CiteSpace was used to visualize the knowledge structure of scientific literatures in this field, and in-depth interpretation of the knowledge structure was conducted to obtain objective evidences to facilitate the detection of emerging trends in addition to traditional systematic reviews.
Methods
Literature search
On 4 March 2022, publications related to mRNA vaccines were retrieved from the Web of Science (WOS) database. A relatively broad retrieval strategy was adopted. Specifically, publications with “RNA OR mRNA” and “vaccine*” appearing nearly in the titles, abstracts or keywords were included. In addition, publications with the product names of mRNA vaccines appeared in the above-mentioned fields were retrieved as well. The product names of mRNA vaccines were derived from the Cortellis database, which included “mRNA 1273,” “mRNA-1273,” “Moderna COVID 19 Vaccin*,” M-1273, M1273, Elasomeran, “COVID-19 Vaccine Moderna,” Spikevax, TAK-919, TAK919, “TAK 919,” BNT162*, “BNT-162*,” “Pfizer COVID 19 Vaccin*,” “Pfizer-BioNTech COVID 19 Vaccin*,” “BioNTech COVID 19 Vaccin*,” “ARCT 154,” Zorecimeran, CV -07,050,101, CVnCoV, Tozinameran, Comirnaty, “mRNA-1647,” Pidacmeran, Abdavomeran, Ganulameran, etc.
Considering that the purpose of this study is to explore the emerging trends of research, only research articles were included, while reviews, letters, editorial materials, meeting abstracts, news items, book chapters and proceeding papers were excluded. In addition, articles published before 2010 were not included in analysis because those published since 2010 are enough for detecting emerging trends.
Data analysis
The data analysis was conducted with CiteSpace 5.1.R6 from macro- to medium- and to micro-level. Based mainly on the objective evidences obtained from these analysis, and referring to the clinical progress of mRNA vaccines as well as perspectives from high-quality literatures and domain experts, emerging trends of research on mRNA can be detected.
At the macro-level, trend in the amount of publications was analyzed by displaying the amount of publications in each year, and WOS categories involved were displayed by generating a network of categories assigned to each article in the WOS database. The macro-level analysis provided a snapshot of the mRNA field.
At the medium level, evolution of research topics were detected by generating a co-citation network and by in-depth reading of each cluster in the network. Each article in the analytic set typically cites some references, and these cited references were represented as nodes constituting the co-citation network. It is assumed that two references often cited together are probably associated in some way, and therefore the co-citation network formed by these references based on how often they are cited together in the same articles can reflect the underling research topics of the scientific community.Citation11,Citation15 Based on the publication years of citing articles and cited references, the evolution of research topics can be detected.
Here we will describe the analysis process at the medium level in detail. First, with the top 50 references cited mostly in each year from 2010 to 2022, a co-citation network with different clusters was derived. In order to avoid including too many meaningless nodes, those cited for less than 3 times were excluded even if they were among the top 50 ones. For each cluster in the network, an ID was assigned by a descending order of the cluster size, with cluster #0 having the most references. Second, the Log-likelihood ratio test was conducted to extracted top terms for each cluster from the titles of citing articles. Based on these top terms and simultaneously based on in-depth reading of citing articles and cited references, each cluster was labeled with a research topic. Third, to detect the evolution of research topics, all clusters were classified into different development periods according to the publication years of citing articles and cited references. To show the development of each cluster, a time-line view was generated. Finally, the Sihouette value (S value) and the modularity value (Q value) were used to evaluate the quality of clustering, with the former one to measure the homogeneous of each cluster and the latter one to measure the significance of the whole network. An S value ≥0.7 and a Q value ≥0.3 means that the clustering results are convincing.Citation13,Citation15
At the micro-level, key nodes in the co-citation network were detected according to citations, betweeness centrality and citation bursts. References with the highest citations can be regarded as the landmarks with breakthrough contributions, those with the highest betweeness centrality may probably be important nodes located at key positions of the co-citation network linking different clusters, and those with a sharply increase in citations (citation bursts) may probably be the knowledge basis for studies conducted in recent years.Citation11,Citation12,Citation15
Results
Trends in the amount of publications and WOS categories involved
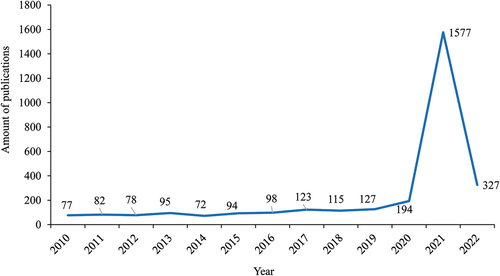
Altogether 6377 publications were obtained, among which 4217 were research articles and other 2160 were reviews, letters, meeting abstracts, editorial materials, etc. Among the 4217 research articles, 3056 (72.46%) published since 2010 were extracted for analysis in CiteSpace. As shown in , research articles related to mRNA vaccines increased steadily from 2010 to 2019 and sharply from 2020.
Figure 1. The amount of articles related to mRNA vaccines published between 2010 and 2022.
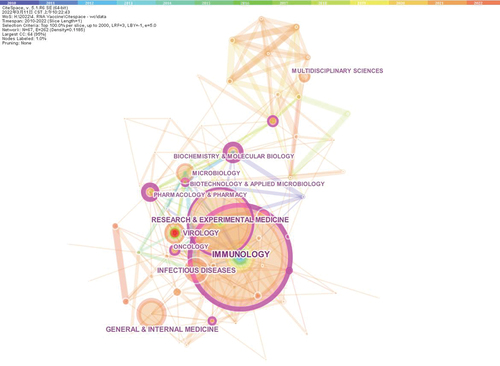
As shown in , Immunology was the largest WOS category, with 822 articles assigned, followed by Research & Experimental Medicine (557 articles), Virology (262 articles) and Infectious Diseases (258 articles). An obvious red node was shown in the category of Virology, indicating a strong citation burst detected.
Figure 2. The network of Web of Science categories involved in articles related to mRNA vaccines published from 2010 to 2022.
Evolution of research topics revealed by the co-citation network
A co-citation network with 12 clusters was derived (). The Q value for the whole network was 0.7078, and the S values for each of the 12 clusters were close to 1, indicating that the network was of significance and most clusters were highly homogeneous ( and ). An ID from 0 to 11 was assigned to each cluster by a descending order of cluster size, with cluster #0 having 49 references and cluster #11 having 7 ones. The top terms extracted for each cluster were listed in , and the main citing articles and cited references in each cluster are listed in . The research topic of each cluster is labeled in the co-citation network in and in a time-line view in .
Figure 3. The co-citation network of references cited by articles related to mRNA vaccines published from 2010 to 2022.
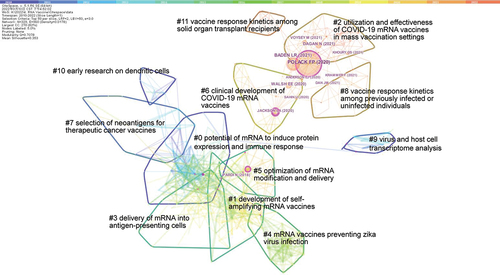
Figure 4. The timeline view of co-citation network.
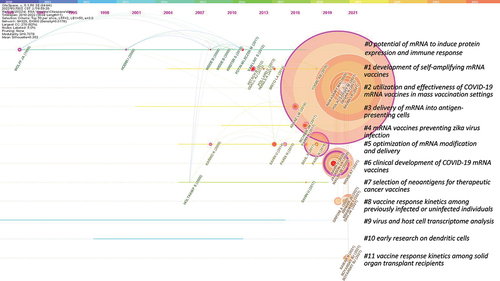
Table 1. Brief information of clusters in the co-citation network.
Table 2. Main citing articles and cited references in each cluster.
As shown in , Cluster #0, #1 and #5, located in the center of the network, were related to the improvement of generic technologies involved in mRNA vaccines, such as mRNA modification, delivery systems and novel vaccine designs. Other clusters, located at the surrounding positions, were related to vaccines for cancers or infectious diseases: cluster #2, #6, #8 and #11 were for COVID-19; cluster #3, #7 and #10 were for cancers. All the 12 clusters were classified into 3 periods, and the focus of research in each period were interpreted in detail in the following parts.
The preliminary exploratory period before early 2010s
Many studies were conducted to investigate the potential of mRNA to induce protein expression and immune response, and relatively less studies were on the functions of dendritic cells and the transcriptome features of virus and host cells.
Cluster #0 labeled as “potential of mRNA to induce protein expression and immune response” is the biggest cluster in the co-citation network. Top cited references in this cluster were mainly published before 2010. In 1990, the first protein expression post RNA and DNA expression vectors being injected into mouse skeletal muscle was reported.Citation4 Thereafter, many studies were conducted to test the immunogenicity of mRNA vaccines and to improve the delivery of mRNA. In 2000, a study shown that protected mRNA could stimulate similar immune response as naked mRNA, but could be stable for a longer time.Citation16 In 2008, a phase 1/2 trial conducted in 15 melanoma patients shown that direct injection of mRNA couldn’t bring enough clinical effectiveness even though antitumor humoral immune response was stimulated in some patients.Citation17 In 2009, another phase 1/2 trial conducted in 21 melanoma patients demonstrated the feasibility and safety of directly injected protamine-protected mRNA.Citation18 These studies supported the potential of mRNA to induce immune responses, especially with suitable delivery systems. With these basis, studies were conducted to detect the immune response through different delivery systems in early 2010s. In 2010, strong antigen-specific immunity elicited and cancer cure was achieved by intranodal vaccination with naked mRNA in mice.Citation19 In 2013, a study reported that good antigen-specific immune response was derived by lipid-based delivery of a mRNA vaccine,Citation20 another study proved the potential of cationic lipids and polymers to deliver mRNA to dendritic cells.Citation21 Publications in this cluster forms an important knowledge basis for all other clusters.
Besides publications forming cluster #0, there were also some studies forming two other clusters labeled as “virus and host cell transcriptome analysis” (cluster #9) and “early research on dendritic cells” (cluster #10). Although relatively less studies were included in these two clusters, they were important basis for further development of mRNA vaccines. Cluster #9 focused on the transcriptome analysis of virus and host cells,Citation22–26 which is a basis for nucleoside modification. Cluster #10 included some early studies investigating the functions of dendritic cells, the strategies of loading dendritic cells with cancer antigens, and the preliminary effect of dendritic cell vaccines on cancers,Citation27,Citation28 which were important basis for further development of therapeutic cancer vaccines.Citation29,Citation30
The growing up period from early 2010s to 2019
Many studies were conducted to improve mRNA modification and delivery and to accelerate the design of self-amplifying mRNA (SAM) vaccines, in addition to which, studies investigating mRNA vaccines for cancers or infectious diseases were pushed forward.
Cluster #5 labeled as “optimization of mRNA modification and delivery” is the cluster located at the middle of the co-citation network, which is a core cluster linked with many other ones. In 2008, scientists found that incorporation of pseudouridine into mRNA could increase the translational capacity and biological stability of mRNA, since when, optimizing the translation and stability of mRNA through nucleoside modification has been a promising direction for a long time,Citation8 and many researchers became to recognize the potential of in vitro transcribed mRNA as a new class of drug.Citation1,Citation3 Meanwhile, the delivery systems were also improved in 2010s, especially the lipid-based delivery system. Some studies proved the good kinetic characteristics of nucleoside-modified mRNA delivered in lipid nanoparticles.Citation31 With significant progress on nucleoside-modification and lipid nanoparticle, the problem of instable and inefficient in vivo delivery of mRNA had been overcome by late 2010s. Studies conducted in 2020 indicated that nucleoside-modified mRNA delivered in lipid nanoparticles could elicit strong immune responses.Citation32–34 These progress provided a firm foundation for the rapid development of mRNA vaccines for COVID-19.Citation32,Citation35,Citation36
Cluster #1 labeled as “development of self-amplifying mRNA vaccines” is a relatively large cluster in the network, which is a direction with great potential. With this platform, a large amount of antigens can be produced from a very low dose of vaccine, because the antigen-encoding mRNA can be replicated in vivo. In 2012, scientists developed a SAM vaccine encapsulated within an LNP, the immunogenicity of which was increased substantially as compared to conventional mRNA vaccines.Citation37 In 2013, a rapidly produced SAM vaccine for H7N9 influenza was proved to be immunogenic in mice.Citation38 In 2018, a SAM vaccine was proved to be equivalently protective against influenza at much lower doses as compared to conventional mRNA vaccines.Citation39 In order to push these technologies into clinical practice, the delivery systems need to be developed as well. Therefore, various cationic platforms for SAM vaccines, such as liposomes, solid lipid nanoparticles, polymeric nanoparticles and emulsions, have been examined in recent years.Citation40–42 With these development, SAM has been recognized as a promising platform.
Cluster #4 labeled as “mRNA vaccines preventing zika virus infection” is a cluster related to the development of mRNA vaccines for infectious diseases. It is known that conventional vaccines cannot adequately respond to the rapid spread of emerging infectious diseases for the reason of slow pace of design and production. Therefore, mRNA vaccine, which can be designed rapidly and produced in a large scale, has the potential to be an ideal choice for coping with emerging infectious diseases. After the epidemic emergence of zika virus in 2015–2016, scientists attempted to test the effect of mRNA vaccines in preventing zika virus infection in animals. Studies published in 2017 shown that LNP encapsulated nucleoside-modified mRNA vaccines could elicit rapid and durable protective immunity in mice and non-human primates.Citation43–45 As shown by these studies, mRNA vaccine represented a promising vaccine candidate for infectious diseases.
Cluster #7 labeled as “selection of neoantigens for therapeutic cancer vaccines” is the basis for developing mRNA vaccines for cancers. Actually, dendritic cell immunotherapy had shown the potential to treat cancers before 2010.Citation46 In 2013, oncological and immunological responses were observed in patients with uterine cancers who received wilms’ tumor gene 1 - loaded dendritic cell immunotherapy.Citation47 In 2017, individual mutations were exploited to develop vaccines for patient with melanoma, opening a path to personalized immunotherapy.Citation48 In 2020, studies shown that RNA-lipoplexes vaccines encoding non-mutant shared cancer antigens or CD4(+) T cell-recognized neoantigens might be potent immunotherapies for patients with melanoma.Citation49,Citation50 To improve immune responses, the selection of antigens is a key consideration. Therefore, further studies will be conducted to investigate mRNA vaccines based on different antigens.
Cluster #3 labeled as “delivery of mRNA into antigen-presenting cells” is another key factor that determines the success of mRNA vaccines for cancers. In 2015 2017, lipid nanoparticle, with the potential to induce potent cytotoxic T cell response, was shown to be a considerable promise for delivering mRNA to dendritic cells.Citation51–53 In recent years, other delivery systems were also tested. According to studies conducted in 2019, an mRNA polyplexes named PPx-GALA displayed the potential to enhance T cell response and DC maturation,Citation54 another delivery system using poly (lactic acid) nanoparticles and cell-penetrating peptides potentiated mRNA-based vaccine expression in dendritic cells,Citation55 and in addition, a neutral lipopolyplexes formulated with mRNA, cationic polymer and anionic liposomes delivered mRNA to dendritic cells successfully.Citation56 The delivery of mRNA into antigen-presenting cells, especially dendritic cells, is highly challenging. These preliminary exploration provided a good basis for the further development of mRNA vaccines for cancers.
The rapid maturity period after the outbreak of COVID-19
COVID-19 mRNA vaccines were developed rapidly and authorized for use for the first time in 2020, thereafter, the utilization and effectiveness of vaccines in mass vaccination settings and the response kinetics among special populations were studied to optimize vaccination strategies.
Cluster #6 labeled as “clinical development of COVID-19 mRNA vaccines” is mainly about the clinical studies of BNT162b2 and mRNA-1273, two products for COVID-19 developed in the fastest progression. In late 2020, BNT162b1 and BNT162b2 were studied and compared in phase 1 and phase 1/2 studies, the results of which supported further development of BNT162b2 in a pivotal phase 2/3 study.Citation35,Citation57,Citation58 Shortly afterward, a pivotal trial showing the 95% efficacy of two-dose regimen of BNT162b2 in preventing COVID-19 in persons aged ≥16 was published.Citation59 Nearly synchronously, in late 2020, a phase 1 study of mRNA-1273 supporting the further development of this vaccine was published.Citation60 In Feb 2021, a phase 3 key study showing the 94.1% efficacy of two-dose regimen of mRNA-1273 in preventing COVID-19 was published.Citation61 These data provided evidences and basis for the authorization of BNT162b2 and mRNA-1273 in late 2020 and early 2021. Thereafter, these two vaccines were investigated for efficacy and safety in special populations, such as patients with multiple sclerosis or those undergoing treatment for cancers, etc.Citation62
Cluster #2 labeled as “utilization and effectiveness of COVID-19 mRNA vaccines in mass vaccination settings” is mainly about studies conducted to solve problems faced by the two approved vaccines in real world. Since the widely utilization of BNT162b2 and mRNA-1273 in 2021, concerns regarding breakthrough infections, efficacy reduction over time, reduced efficacy against variants, efficacy in special populations, vaccination equity and vaccination accessibility have arisen.Citation10,Citation63 Regarding these concerns, studies were conducted to confirm the efficacy of mRNA vaccines in mass vaccination settings in different countries,Citation64–67 the efficacy against variants,Citation68,Citation69 the superiority of two doses over one doseCitation70 and the effect among special populations Citation71,Citation72 . The results of these studies are valuable for optimizing the vaccination strategy of the two vaccines in real world.
Cluster #8 labeled as “vaccine response kinetics among previously infected or uninfected individuals” is a cluster of studies detecting the levels of antibodies, memory B cells, memory CD8+ T cells or memory CD4+ T cells in SARS-CoV-2-naive persons or previously infected patients. According to these studies, SARS-CoV-2-naive persons might probably benefit from a second dose of vaccine, for the reason of rapid loss of antibodies from the first vaccination; while previously infected ones might get adequate protection from one dose of vaccination.Citation73–77 These results provided objective evidences for optimizing the vaccination strategy of these two populations.
Cluster #11 labeled as “vaccine response kinetics among solid organ transplant recipients” is a cluster of studies investigating the immune response elicited by mRNA vaccines in immunocompromised populations. In May, Jun and Sep in 2021, scientists in Johns Hopkins University published three papers on JAMA to illustrate the immunogenicity of 1-dose, 2-dose or 3-dose of mRNA vaccines in solid organ transplant recipients, reporting the inadequate immune response elicited by 1-dose or 2-dose of vaccines and indicating the necessity of 3-dose of vaccines in this population.Citation78–80 Thereafter, many studies were conducted to prove the necessity of a third-dose booster for solid-organ transplant recipients.Citation81–83
Key publications in the co-citation network
Citations
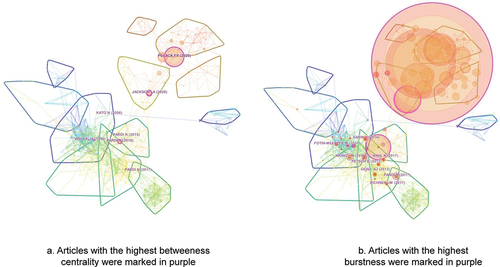
References with the highest citations can be regarded as the landmarks with breakthrough contributions. Among the top 10 most cited references, 5 were in cluster #2, 4 were in cluster #6, and 1 was in cluster #5 (). The most cited reference is Polack FP (2020) with 892 citations in our dataset,Citation59 followed by Baden LR (2021) with 531 citations,Citation61 both of which were in cluster #2 disclosing the promising results of key clinical trials for BNT162b2 and mRNA-1273, respectively.
Table 3. Cited references with the highest citations, bursts, centrality.
Betweenness centrality
References with the highest centrality are more likely to be located between different clusters, leading to insights into emerging trends.Citation15 Several structurally essential references in the network were shown in and . The reference with the highest centrality, located in the middle of the network, is Jackson LA (2020) reporting the preliminary results of a phase I study for mRNA-1273.Citation60 The one with the second highest centrality, located between cluster #6 and cluster #2, is Polack FP (2020) reporting the results of a key study for BNT162b2, which is also the most cited one.Citation59 The one with the third highest centrality, located in the middle of the network, is Pardi N (2018) reviewing the development and progression of mRNA vaccine technology.Citation1
Citation bursts
Citation bursts provide a means of value to track the development of research emphasis. References with the strongest citation bursts during 2010 and 2022 were shown in and . These references are mainly from cluster #1, #4 and #5, reflecting that these clusters, mainly about the modification and delivery of mRNA, the design of SAM vaccines and the effect of mRNA vaccines in preventing infectious diseases, are of the most concerns and may probably be the knowledge basis for studies conducted in recent years.
Discussion
mRNA vaccines, especially those for preventing infectious diseases, have been developed during the past decade, with COVID-19 vaccine being the most rapidly developed vaccine of any kind in history.Citation2,Citation61,Citation64 These vaccines have both advantages (such as the potential to treat chronic or recurrent infectious diseases, the potential to stimulate strong immune response, rapid to design and scalable production) and disadvantages (especially instability and inefficient in vivo delivery) relative to traditional vaccines.Citation1,Citation2,Citation61,Citation64 As early as 2010 -2015, there were individual studies investigating the effect of mRNA vaccines in preventing HIV-1 infection, influenza virus infection, etc.Citation84 In 2016 and 2017, the outbreak of zika virus infection in over 40 countries and its association with congenital malformations spawned a series of studies on mRNA vaccines, forming cluster #4 in our co-citation network.Citation43–45, Citation85 Thereafter, the outbreak of COVID-19 brought a breakthrough, and related studies have formed cluster #6 in our co-citation network.Citation35,Citation57 Two mRNA vaccines for COVID-19, BNT162b2 developed by Pfizer/BioNTech and mRNA-1273 developed by Moderna, have been approved, and the two articles disclosing the results of key clinical trials for these two vaccines have become the most cited articles in our co-citation network.Citation59,Citation61 At present, many other mRNA vaccines for infectious diseases are investigated in phase 3 clinical studies (refer to Table S1 for data searched from the Cortellis database). In addition, more than 50 studies investigating the effect of mRNA vaccines in preventing infectious diseases have been started in the first half year of 2022 (refer to Table S2 for data searched from the ClinicalTrials.gov database).
By contrast, the development of mRNA cancer vaccines has not gone smoothly, even though recent studies have formed two relevant clusters in our co-citation network, namely cluster #7 and cluster #3. People are full of expectations for using mRNA vaccines to treat cancers. However, there is still a long way to go before such products can be approved. At present, most therapeutic cancer vaccines are in early development stages, without any one being investigated in phase 3 studies (Table S1) and with only 3 studies related to therapeutic cancer mRNA vaccines started in the first half year of 2022 (Table S2).
Based mainly on the objective evidences obtained from this study, and referring to the clinical progress of mRNA vaccines as well as perspectives from high-quality literatures and domain experts, emerging trends of research on mRNA will be discussed in the following parts.
Emerging trends of research on mRNA-based infectious disease vaccines
The approval of COVID-19 mRNA vaccines brings hope for rapid response to other emerging infectious diseases in the future. However, this is just an encouraging start, while various problems encountered in real-world vaccination remains to be solved. For instance, how are the approved vaccines work in real world? Can we further enhance the efficacy and safety of mRNA vaccines? Whether mRNA vaccines are effective for other infectious diseases? To answer these questions, some emerging trends of research have appeared.
Further improving mRNA modification and delivery
It is well-known that the instability and inefficient in vivo delivery of mRNA is a main factor hindering the development of mRNA vaccines.Citation1,Citation3 To address this issue, extensive studies have been carried out and formed cluster #0 and #5 in our co-citation network. As shown in , cluster #6, namely clinical development of COVID-19 mRNA vaccines, is formed based on cluster #5, which can be traced back to cluster #0, indicating that important articles forming cluster #0 and #5 have laid a good foundation for the development of COVID-19 mRNA vaccines.Citation4,Citation8,Citation31
Nucleoside modification is a key factor determining the immunogenicity, efficacy and safety of mRNA vaccines.Citation86 In 2008, scientists found that incorporating pseudouridine into mRNA can increase the translational capacity and biological stability of mRNA.Citation8 Studies conducted in recent years further proved the feasibility of nucleoside-modified mRNA.Citation32–34 For both of the two approved mRNA vaccines, BNT162b2 and mRNA-1273, nucleosides were modified by substituting uridine with N1-methyl pseudouridine and efficacy have been proved in clinical trials.Citation59,Citation61 Some early studies show that unmodified nucleosides may lead to higher levels of protein expression in animals.Citation87,Citation88 However, the chemically unmodified mRNA vaccine, CVnCoV, only resulted an overall efficacy of 48.2% in preventing symptomatic COVID-19 as shown in a phase 2b/3 clinical trial and have been discontinued from development.Citation89 The divergences in different studies may be explained by different levels of mRNA purification, which need to be further investigated.Citation86 At present, many companies, including BioNTech, Moderna and CureVac, are advancing the development of mRNA vaccines using various-modified nucleosides, and further improvement is expected in the future (Table S1).
Delivery system is another key factor determining the immunogenicity, efficacy and safety of mRNA vaccines. LNP has been proved to be a feasible delivery system, which is typically composed of an ionizable or cationic lipid, a helper phospholipid, a cholesterol analog and a polyethylene glycol (PEG)–lipid.Citation32–34, Citation86 To enhance tolerability and reduce safety risks and to reduce the potential of lipid accumulation, selecting a well-tolerated, safe and biodegradable lipid is critical. The accelerated elimination of lipid will help to enhance the immediate tolerability of prophylactic vaccines, and more importantly, will contribute to further development of cancer vaccines which probably require repeated dosing in a long period of time. Incorporating hydrolysable bonds to facilitate clearance has become a research trend in lipid development, however the formulation stability may be affected, which continues to be a challenge to be overcome in the future.Citation86 Polymeric nanoparticles (PNPs), although less advanced than LNPs, have also shown to be a promising delivery system, which typically composed of a biodegradable and amine-containing polymer and may be formulated with a helper phospholipid, a cholesterol analog and a PEG – lipid as needed in applications.Citation90 Future advancement in formulation science will promote the development of mRNA vaccines.Citation86,Citation91
Solving the problems of approved COVID-19 mRNA vaccines in real world vaccinations
Even though BNT162b2 and mRNA-1273 have been approved for use in clinical practice, various problems have been encountered in real-world vaccination. First, the preventive effect of mRNA vaccines against emerging mutant strains might be decreased.Citation63,Citation69 Second, the neutralizing antibodies induced by mRNA vaccines generally decreases over time.Citation73,Citation75 Third, some safety issues related to mRNA vaccines (such as local and systemic inflammation, increased risk of autoimmune reactions, edema, pathological thrombosis, etc.) are of concern.Citation1,Citation2,Citation86 Fourth, the shortage of vaccines makes it necessary to optimize vaccination strategies or to apply dose-sparing techniques, so as to allocate limited vaccines to those most in need.Citation10,Citation63 Fifth, some people with impaired or suppressed immunity may not obtain sufficient protection from ordinary doses of vaccination.Citation71,Citation78 In recent two years, many studies have been conducted to solving these problems, forming cluster #2, #8 and #11 in our co-citation network. The results of these studies have provided objective evidences for optimizing the vaccination strategy for various populations, such as less than ordinary doses for infected individuals, more than ordinary doses for immunosuppressed ones, a booster dose for variants, etc. Although studies in cluster #2, #8 and #11 have proposed some solutions, the unsolved problems or those arisen with the change of COVID-19 epidemic situation call for new studies. In the first half year of 2022, booster vaccination is the most important research focus, followed by the evaluation of mRNA vaccines in special populations and post-marketing surveillance. In addition to transplanted patients as indicated by cluster #11 in our network, attentions have also been paid to other populations such as older adults, children, those with a history of an adverse reaction to the first or second dose and those with a personal history of allergic reactions (Table S2).
Investigating mRNA vaccines for other infectious indications
The FDA approval of BNT162b2 and mRNA-1273 represents an epoch-making progress in the prevention of infectious diseases, however, the effect of mRNA vaccines needs to be validated in more diseases other than COVID-19. More ideally, an mRNA vaccine encoding multiple viral antigens and showing preventive effect for several diseases would be extremely encouraging and promising.Citation86 Encouraged by the success of COVID-19 vaccines, mRNA vaccines for other infectious indications are being pushed forward (Table S1). Currently, mRNA-1345 for preventing respiratory syncytial virus infection and mRNA-1647 for preventing cytomegalovirus infection are in phase 3 clinical studies. Some other mRNA vaccines protecting against HIV infection, influenza virus infection and zika virus infection are in phase 2 clinical studies (Table S1). Studies on mRNA vaccines protecting against zika virus infection have form cluster #4 in our co-citation network.Citation43,Citation45 It’s expected that publications related to other infectious diseases will form distinct clusters in the future, and such mRNA vaccines are anticipated to be available within several years.
Developing self-amplifying mRNA vaccines
SAM vaccines reserve intact genes encoding the RNA replication machinery, and therefore the antigen-encoding mRNA can be replicated in vivo. The bulk drug demand and the production burden of SAM vaccines are reduced significantly as compared non-replicating ones, because a very low dose of a SAM vaccine can stimulate enough immune response, which advantage makes it an ideal product in dealing with pandemics. Studies have demonstrated the potent immunogenicity of SAM vaccines in preventing infection of diverse viruses including influenza virus, human cytomegalovirus, rabies virus, hepatitis C virus and HIV.Citation38,Citation39,Citation86 Seeing from the results of our co-citation analysis, there has been a considerable amount of studies on SAM vaccines, promoting the rapid advancing of this technology. At present, there have been SAM vaccines for COVID-19 being investigated in phase 2 clinical studies, and several other ones for COVID-19, influenza, rabies virus infection or herpes simplex virus infection being investigated in phase 1 studies (Table S1). In 2022, Pfizer started a phase 1 clinical study to evaluate the effect of SAM vaccine in preventing influenza (NCT05227001, Table S2). It’s anticipated that more studies comparing SAM vaccines with non-replicating ones will be conducted, and clinical trials on SAM vaccines will be accelerated in the next few years.
Developing thermostable or lyophilized mRNA vaccines
From a global emergency perspective, millions of doses of vaccines must be delivered across a range of extreme temperature to countries with widely different levels of healthcare infrastructures. However, current approved mRNA vaccines must require low-temperature transportation and strict storage conditions, posing a challenge to vaccination in large-scale populations, especially in poor and tropical regions.Citation9,Citation10 At present, there have been some studies conducted to develop thermostable or lyophilized mRNA vaccines.Citation92 ARCoV originated by Suzhou Abogen Biosciences, manufactured in a liquid formulation, can be stored at room temperature for at least 1 week, and the long-term stability of this vaccine is currently under evaluation.Citation92 Pfizer has started a phase 3 study to compare the lyophilized formulation and frozen-liquid formulation of BNT162b2 in the aspects of safety, tolerability and immunogenicity in 2021 (NCT04816669). Wuhan Recogen Biotechnology has started a phase 1 study to evaluate the safety, reactogenicity, and immunogenicity of a lyophilized COVID-19 mRNA Vaccine in 2022 (NCT05366296, Table S2). Results of these clinical studies have not been disclosed, but an animal experiment has proved that a lyophilized influenza mRNA vaccine can be stable at room temperature for at least 12 weeks and at 4℃ for at least 24 weeks.Citation93 From the co-citation network in our study, only individual studies on thermostable or lyophilized mRNA vaccines have been published but have not form a distinct cluster.Citation92,Citation93 The storage and transportation of mRNA under room or refrigerating temperature is in need, but the development of thermostable or lyophilized mRNA vaccines is less than straightforward. In the next few years, the formulation science may bring new formulations of mRNA vaccines to a new stage.
Emerging trends of research on mRNA-based cancer vaccines
Unlike mRNA vaccines for infectious diseases, there are still many challenges to be addressed before therapeutic mRNA cancer vaccines can be used in clinical practice. Firstly, strong cytotoxic CD8+ T cell response must be induced to eradicate cancer cells; secondly, proper antigens being able to induce highly immune responses, which are highly variable across different individuals, must be selected; finally, the suppressive microenvironment preventing T cell infiltration into cancers and causing T cell exhaustion need to be improved.Citation86 To address these challenges, some emerging research trends have appeared.
Screening neoantigens for therapeutic cancer vaccines
The selection of antigens is a main difficulty in developing therapeutic cancer vaccines.Citation48 Currently, commonly used antigens are tumor-associated antigens (TAAs) that express at high levels on tumor cells but at low levels on normal cells.Citation50 The nonspecific expression of TAA makes mRNA vaccines prepared with TAA be prone to problems such as autoimmunity and resistance.Citation48,Citation50 Tumor-specific antigens (TSA) that express only on tumor cells, also known as neoantigens, can stimulate strong immune response and avoid the above-mentioned problems to a certain degree.Citation50 Therefore, many studies on neoantigens have been conducted in recent years, forming cluster #7 in our co-citation network. At present, several individualized neoantigen specific vaccines have been tested in clinical studies. BioNTech and Genentech are jointly promote the phase 2 clinical studies of BNT-122, an mRNA-based personalized cancer vaccine targeting neoantigens expressed on individual patient’s cancer cells (Table S1). Moderna is also promote the phase 2 clinical studies of neoantigen specific vaccines, such as mRNA-4157 and NCI-4650 (Table S1). With the rapid development of next generation sequencing and bioinformatics, selecting more ideal neoantigens have become possible, and the development of individualized mRNA cancer vaccines based on neoantigens will be a research trend in the future.
Enhancing the delivery of mRNA into antigen-presenting cells
To improve the effect of mRNA vaccines on cancers, efficient mRNA delivery to induce sufficient levels of T-cell immune response is a premise.Citation52,Citation53 Dendritic cells are the first choice of mRNA-based cancer vaccines, because they link innate and adaptive immune responses and are main regulators of cytotoxic and humoral adaptive responses.Citation55 Therefore, delivery of mRNA into antigen-presenting cells, mainly dendritic cells, has been the main focus of research in recent years, which forms Cluster #3 in our network. As mentioned above, a degradable or rapid-eliminated delivery system is extremely important for cancer vaccines for the reason that repeated dosing is always in need for cancer patients. Therefore, in addition to lipid nanoparticle, many other delivery systems such as mRNA polyplexes, neutral lipopolyplexes, cationic polymer, and anionic liposomes are in active research.Citation54–56 Formulations without relying on delivery vehicles are in development as well.Citation94 Continuous research on the delivery system of mRNA cancer vaccines should be conducted, to induce strong cytotoxic T-cell responses on one hand and to reduce the accumulation risk of delivery vehicles on the other hand.
Overcoming the suppressive tumor microenvironment by combining mRNA vaccines with other therapies
Even if strong immune response can be induced by a proper antigen and an effective delivery system, the suppressive microenvironment could lead to T cell exhaustion. There have been individual animal studies showing that the combination of an mRNA cancer vaccine with an anti-CTLA-4 monoclonal antibody or an immune checkpoint inhibitor could significantly improve the anti-cancer effect as compared to the vaccine or the monoclonal antibody given alone.Citation95–97 BioNTech started a phase 2 clinical trial in 2021 to evaluate the effect of BNT-111 with or without cemiplimab, a PD-1 monoclonal antibody, in patients with relapsed/refractory unresectable melanoma (NCT04526899). Some other exploratory studies have be started as well (NCT05192460, Table S2). As shown in our co-citation network, publications related to the combination of mRNA cancer vaccines with other therapies have not form a distinct cluster, more studies in this direction are anticipated in the future.
The applicability of CiteSpace-based co-citation analysis in detecting research trends
The research trends of mRNA vaccines revealed in this study are consistent with the objective facts mentioned in previously studies, reflecting the feasibility of CiteSpace-based co-citation analysis in revealing research trends. Specifically, an article published in Nature in 2021 described the history of mRNA vaccines in detail, based on which and some other important reviews, we can have a snapshot of the development history of mRNA vaccines.Citation1,Citation2,Citation9,Citation10 As early as 1984, mRNA was synthesized in laboratory for the first time, and in 1989 the mRNA translation post intramuscular injection in animal was reported for the first time, which is the breakthrough described in the landmark article in cluster #0.Citation2,Citation4 Between 1990 and 1999, researchers tried to develop mRNA products, especially those for cancers, but most attempts failed because of the instability of mRNA.Citation2 In late 1990s, lipid nanoparticle began to be used to deliver nucleic acid chains, which was a promising solution. Thereafter, during 2000 and 2010, a huge amount of funds was attracted into this field, many companies such as CureVac, BioNTech and Moderna were established, and pseudouridine theory was proved to be effective in enhancing the translational capacity and biological stability of mRNA, which is the breakthrough described in a landmark article in cluster #5.Citation8 From 2011 to 2019, the improvement of mRNA modification and delivery and the development of SAM vaccines solved the problems of easy degradation and insufficient immunogenicity of mRNA vaccines to a large extent, promoting some products into clinical trials.Citation1 Even so, the development of mRNA vaccines during this period was still not smooth, and not any mRNA vaccine had been investigated in phase 3 clinical trials by the end of 2019. It is the outbreak of COVID-19 pandemic that brings a fast breakthrough. Moderna made a prototype mRNA vaccine within several days of the sars-cov-2 genome sequence being made public, and Pfizer/BioNTech took only less than 8 months to go through the whole process from first-in-human trial to the emergency use approval.Citation2 These information support the reasonability of the three development periods revealed by our study.
Many applications have been available for co-citation analysis, and CiteSpace was used in this study because of its unique advantages: (1) The citing articles and cited references in each cluster can be viewed easily; (2) multiple information including total citations, citation burstness and centrality can be displayed simultaneously in a network, facilitating the detection of milestone articles; (3) The milestone articles in each cluster can be displayed in a time-line view; (4) two objective indicators, Q value and S value, can be used to evaluate the quality of clustering.
It should be pointed out that the Q value of our co-citation network is 0.7078, indicating that the overall quality of the network is satisfactory. The S values of most of the 12 clusters were above 0.8, indicating that the internal homogeneity of each cluster is convincing.Citation15 It might be unsatisfactory that the average S value is only 0.353. A reasonable explanation is that when selecting top 50 references in each time slice to form the co-citation network, some references cited by only several times were included and formed many small clusters that lowers the average S value. These small clusters were not displayed in because they were not connected with the largest component and were considered to be meaningless according to the algorithm of CiteSpace. The small amount of papers published in each year is the reason why some low-cited references being included in the network. Therefore, for other fields with more publications, an increased threshold can be used to avoid including low-cited documents, thereby reducing the number of meaningless clusters and significantly improving the average S value.
One more thing to mention is that a relatively broad search strategy was used instead of a precise search with only “RNA vaccin*” or “mRNA vaccine*” as search terms, for the reason that RNA vaccine or mRNA vaccine not always explicitly appear in the titles, abstracts or keywords of publications. As stated by Chen Chaomei, the developer of CiteSpace, when detecting trends of research on a specific technology, a broad search strategy is necessary, so as to avoid missing important publications.Citation15 The results of this study also support the feasibility of a broad search strategy in CiteSpace-based co-citation analysis.
Limitations
The main purpose of this study is to analyze the emerging trends of research on mRNA vaccines, for which purpose, this study has the following limitations. Firstly, the manual interpretations conducted based on the co-citation network to analyze the emerging trends of research were inevitably affected by the authors’ knowledge background. In order to ensure the accuracy and depth of interpretation and to avoid bias of individuals, a large amount of high-quality literatures as well as information on the progress of clinical translation drawn from the Cortellis and ClinicalTrials.gov databases were taken as references, in addition to which, experts in this field were consulted. Secondly, the data source of this article was limited to published scientific papers, therefore some studies conducted in recent months might probably be excluded because of the time lag of publication. As to this limitation, papers uploaded to the preprint platforms might serve as a supplementary source for readers to learn about the latest studies. Finally, only scientific papers were included in this study, however, many other data sources such as patent applications can also reflect emerging trends of research, thus analysis based on multi-dimensional data should be tried in the future to help obtain more comprehensive judgments.
Conclusions
Research on mRNA was active in the past few decades, especially after the outbreak of COVID-19. The development of mRNA vaccines has gone through three periods and significant progress has been made. In the preliminary exploratory period before early 2010s, studies were conducted to investigate the potential of mRNA to induce protein expression and immune response. In the growing up period from early 2010s to 2019, studies were conducted to improve the stability and immunogenicity of mRNA vaccines and to push forward the development of products for infectious diseases and cancers, while not any one was approved even with massive efforts. In the rapid maturity period after the outbreak of COVID-19, two mRNA vaccines, BNT162b2 and mRNA-1273, were authorized for the first time, and the studies afterward focused on solving problems arising in real-world vaccination.
The approval of COVID-19 vaccines is an encouraging start, while various problems encountered in real-world vaccination need to be solved and the enormous potential of mRNA vaccines remains to be explored in other conditions. Future research on mRNA-based infectious disease vaccines will focus on further optimizing mRNA modification and delivery, solving the problems of approved COVID-19 mRNA vaccines in real world vaccinations, testing the effect of mRNA vaccines in preventing other infectious indications, developing self-amplifying mRNA vaccines and developing thermostable or lyophilized mRNA vaccines. Future research on mRNA cancer vaccines will focus on screening proper neoantigens, enhancing the delivery of mRNA into antigen-presenting cells and overcoming the suppressive tumor microenvironment by combining mRNA vaccines with other therapies.
Authors’ contributions
The design of the study was put forwarded by Zhaolian Ouyang and Juan Chen. The collection, analysis and interpretation of data were conducted by Juan Chen, Ting Zhang and Xiaoyi Yang. The writing and revision of the manuscript were performed by Juan Chen and Yan Lu. All authors have read and approved the final manuscript.
Availability of data and materials
The datasets analyzed during the current study are available from the corresponding author on a reasonable request.
Ethics approval
The literature-based study was not conducted in animals or humans, therefore no ethical approval is required.
Supplemental Material
Download MS Word (2.7 MB)Disclosure statement
No potential conflict of interest was reported by the author(s).
Supplementary material
Supplemental data for this article can be accessed on the publisher’s website at https://doi.org/10.1080/21645515.2022.2110409.
Additional information
Funding
References
- Pardi N, Hogan MJ, Porter FW, Weissman D. mRNA vaccines - a new era in vaccinology. Nat Rev Drug Discov. 2018;17(4):1–17. doi:10.1038/nrd.2017.243.
- Dolgin E. The tangled history of mRNA vaccines. Nature. 2021;597(7876):318–324. doi:10.1038/d41586-021-02483-w.
- Sahin U, Kariko K, Tureci O. mRNA-Based therapeutics–developing a new class of drugs. Nat Rev Drug Discov. 2014;13(10):759–780. doi:10.1038/nrd4278.
- Wolff JA, Malone RW, Williams P, Chong W, Acsadi G, Jani A, Felgner PL. Direct gene transfer into mouse muscle in vivo. Science. 1990;247(4949 Pt 1):1465–1468. doi:10.1126/science.1690918.
- Jirikowski GF, Sanna PP, Maciejewski-Lenoir D, Bloom FE. Reversal of diabetes insipidus in Brattleboro rats: intrahypothalamic injection of vasopressin mRNA. Science. 1992;255(5047):996–998. doi:10.1126/science.1546298.
- Martinon F, Krishnan S, Lenzen G, Magné R, Gomard E, Guillet J-G, Lévy J-P, Meulien P. Induction of virus-specific cytotoxic T lymphocytes in vivo by liposome-entrapped mRNA. Eur J Immunol. 1993;23(7):1719–1722. doi:10.1002/eji.1830230749.
- Hou X, Zaks T, Langer R, Dong Y. Lipid nanoparticles for mRNA delivery. Nat Rev Mater. 2021;6(12):1078–1094. doi:10.1038/s41578-021-00358-0.
- Kariko K, Muramatsu H, Welsh FA, Ludwig J, Kato H, Akira S, Weissman D. Incorporation of pseudouridine into mRNA yields superior nonimmunogenic vector with increased translational capacity and biological stability. Mol Ther. 2008;16(11):1833–1840. doi:10.1038/mt.2008.200.
- Flanagan KL, MacIntyre CR, McIntyre PB, Nelson MR. SARS-CoV-2 vaccines: where are we now? J Allergy Clin Immunol Pract. 2021;9(10):3535–3543. doi:10.1016/j.jaip.2021.07.016.
- Sharma K, Koirala A, Nicolopoulos K, Chiu C, Wood N, Britton PN. Vaccines for COVID-19: where do we stand in 2021? Paediatr Respir Rev. 2021;39:22–31. doi:10.1016/j.prrv.2021.07.001.
- Synnestvedt MB, Chen C, Holmes JH. CiteSpace II: visualization and knowledge discovery in bibliographic databases. AMIA Annu Symp Proc. 2005;724–728.
- Chen C. Searching for intellectual turning points: progressive knowledge domain visualization. Proc Natl Acad Sci USA. 2004;101 Suppl 1:5303–5310. doi:10.1073/pnas.0307513100.
- Chen CM. CiteSpace II: detecting and visualizing emerging trends and transient patterns in scientific literature. J Am Soc Inf Sci Tec. 2006;57(3):359–377. doi:10.1002/asi.20317.
- Price DJ. Networks of scientific papers. Science. 1965;149(3683):510–515. doi:10.1126/science.149.3683.510.
- Chen C, Hu Z, Liu S, Tseng H. Emerging trends in regenerative medicine: a scientometric analysis in CiteSpace. Expert Opin Biol Ther. 2012;12(5):593–608. doi:10.1517/14712598.2012.674507.
- Hoerr I, Obst R, Rammensee HG, Jung G. In vivo application of RNA leads to induction of specific cytotoxic T lymphocytes and antibodies. Eur J Immunol. 2000;30:1–7.
- Weide B, Carralot JP, Reese A, Scheel B, Eigentler TK, Hoerr I, Rammensee H-G, Garbe C, Pascolo S. Results of the first phase I/II clinical vaccination trial with direct injection of mRNA. J Immunother (1991). 2008;31(2):180–188. doi:10.1097/CJI.0b013e31815ce501.
- Weide B, Pascolo S, Scheel B, Derhovanessian E, Pflugfelder A, Eigentler TK, Pawelec G, Hoerr I, Rammensee H-G, Garbe C. Direct injection of protamine-protected mRNA: results of a phase 1/2 vaccination trial in metastatic melanoma patients. J Immunother (1991). 2009;32(5):498–507. doi:10.1097/CJI.0b013e3181a00068.
- Kreiter S, Selmi A, Diken M, Koslowski M, Britten CM, Huber C, Türeci Ö, Sahin U. Intranodal vaccination with naked antigen-encoding RNA elicits potent prophylactic and therapeutic antitumoral immunity. Cancer Res. 2010;70(22):9031–9040. doi:10.1158/0008-5472.CAN-10-0699.
- Pollard C, Rejman J, De Haes W, Verrier B, Van Gulck E, Naessens T, De Smedt S, Bogaert P, Grooten J, Vanham G. Type I IFN counteracts the induction of antigen-specific immune responses by lipid-based delivery of mRNA vaccines. Mol Ther. 2013;21(1):251–259. doi:10.1038/mt.2012.202.
- De Haes W, Rejman J, Pollard C, Merlin C, Vekemans M, Florence E, De Smedt SC, Grooten J, Vanham G, De Koker S. Lipoplexes carrying mRNA encoding Gag protein modulate dendritic cells to stimulate HIV-specific immune responses. Nanomedicine (Lond). 2013;8(1):77–87. doi:10.2217/nnm.12.97.
- Yang Z, Bruno DP, Martens CA, Porcella SF, Moss B. Simultaneous high-resolution analysis of vaccinia virus and host cell transcriptomes by deep RNA sequencing. Proc Natl Acad Sci USA. 2010;107(25):11513–11518. doi:10.1073/pnas.1006594107.
- Bullard JH, Purdom E, Hansen KD, Dudoit S. Evaluation of statistical methods for normalization and differential expression in mRNA-Seq experiments. BMC Bioinform. 2010;11:94. doi:10.1186/1471-2105-11-94.
- Schlee M, Roth A, Hornung V, Hagmann CA, Wimmenauer V, Barchet W, Coch C, Janke M, Mihailovic A, Wardle G. Recognition of 5′ triphosphate by RIG-I helicase requires short blunt double-stranded RNA as contained in panhandle of negative-strand virus. Immunity. 2009;31(1):25–34. doi:10.1016/j.immuni.2009.05.008.
- Pichlmair A, Schulz O, Tan CP, Rehwinkel J, Kato H, Takeuchi O, Akira S, Way M, Schiavo G, Reis E Sousa C. Activation of MDA5 requires higher-order RNA structures generated during virus infection. J Virol. 2009;83(20):10761–10769. doi:10.1128/JVI.00770-09.
- Myskiw C, Arsenio J, Booy EP, Hammett C, Deschambault Y, Gibson SB, Cao J. RNA species generated in vaccinia virus infected cells activate cell type-specific MDA5 or RIG-I dependent interferon gene transcription and PKR dependent apoptosis. Virology. 2011;413(2):183–193. doi:10.1016/j.virol.2011.01.034.
- Van Tendeloo VF, Ponsaerts P, Lardon F, Nijs G, Lenjou M, Van Broeckhoven C, Van Bockstaele DR, Berneman ZN. Highly efficient gene delivery by mRNA electroporation in human hematopoietic cells: superiority to lipofection and passive pulsing of mRNA and to electroporation of plasmid cDNA for tumor antigen loading of dendritic cells. Blood. 2001;98(1):49–56. doi:10.1182/blood.V98.1.49.
- Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392(6673):245–252. doi:10.1038/32588.
- Allard SD, De Keersmaecker B, de Goede AL, Verschuren EJ, Koetsveld J, Reedijk ML, Wylock C, De Bel AV, Vandeloo J, Pistoor F. A phase I/IIa immunotherapy trial of HIV-1-infected patients with Tat, Rev and Nef expressing dendritic cells followed by treatment interruption. Clin Immunol. 2012;142(3):252–268. doi:10.1016/j.clim.2011.10.010.
- Coosemans A, Wolfl M, Berneman ZN, Van Tendeloo V, Vergote I, Amant F, Van Gool SW. Immunological response after therapeutic vaccination with WT1 mRNA-loaded dendritic cells in end-stage endometrial carcinoma. Anticancer Res. 2010;30:3709–3714.
- Pardi N, Tuyishime S, Muramatsu H, Kariko K, Mui BL, Tam YK, Madden TD, Hope MJ, Weissman D. Expression kinetics of nucleoside-modified mRNA delivered in lipid nanoparticles to mice by various routes. J Control Release. 2015;217:345–351. doi:10.1016/j.jconrel.2015.08.007.
- Laczko D, Hogan MJ, Toulmin SA, Hicks P, Lederer K, Gaudette BT, Castaño D, Amanat F, Muramatsu H, Oguin TH, et al. A single immunization with nucleoside-modified mRNA vaccines elicits strong cellular and humoral immune responses against SARS-CoV-2 in mice. Immunity. 2020;53(4):724–732. doi:10.1016/j.immuni.2020.07.019.
- Freyn AW, Ramos DSJ, Rosado VC, Bliss CM, Pine M, Mui BL, Tam YK, Madden TD, de Souza Ferreira LC, Weissman D. A multi-targeting, nucleoside-modified mRNA influenza virus vaccine provides broad protection in mice. Mol Ther. 2020;28(7):1569–1584. doi:10.1016/j.ymthe.2020.04.018.
- Willis E, Pardi N, Parkhouse K, Mui BL, Tam YK, Weissman D, Hensley SE. Nucleoside-Modified mRNA vaccination partially overcomes maternal antibody inhibition of de novo immune responses in mice. Sci Transl Med. 2020;12(525). doi:10.1126/scitranslmed.aav5701.
- Sahin U, Muik A, Derhovanessian E, Vogler I, Kranz LM, Vormehr M, Baum A, Pascal K, Quandt J, Maurus D. COVID-19 vaccine BNT162b1 elicits human antibody and TH1 T cell responses. Nature. 2020;586(7830):594–599. doi:10.1038/s41586-020-2814-7.
- Ahammad I, Lira SS. Designing a novel mRNA vaccine against SARS-CoV-2: an immunoinformatics approach. Int J Biol Macromol. 2020;162:820–837. doi:10.1016/j.ijbiomac.2020.06.213.
- Geall AJ, Verma A, Otten GR, Shaw CA, Hekele A, Banerjee K, Cu Y, Beard CW, Brito LA, Krucker T. Nonviral delivery of self-amplifying RNA vaccines. Proc Natl Acad Sci USA. 2012;109(36):14604–14609. doi:10.1073/pnas.1209367109.
- Hekele A, Bertholet S, Archer J, Gibson DG, Palladino G, Brito LA, Otten GR, Brazzoli M, Buccato S, Bonci A. Rapidly produced SAM ® vaccine against H7N9 influenza is immunogenic in mice. Emerg Microbes Infect. 2013;2(1):e52. doi:10.1038/emi.2013.54.
- Vogel AB, Lambert L, Kinnear E, Busse D, Erbar S, Reuter KC, Wicke L, Perkovic M, Beissert T, Haas H. Self-Amplifying RNA vaccines give equivalent protection against influenza to mRNA vaccines but at much lower doses. Mol Ther. 2018;26(2):446–455. doi:10.1016/j.ymthe.2017.11.017.
- Anderluzzi G, Lou G, Gallorini S, Brazzoli M, Johnson R, O’Hagan DT, Baudner BC, Perrie Y. Investigating the impact of delivery system design on the efficacy of self-amplifying RNA vaccines. Vaccines (Basel). 2020;8(2). doi:10.3390/vaccines8020212.
- Blakney AK, Zhu Y, McKay PF, Bouton CR, Yeow J, Tang J, Hu K, Samnuan K, Grigsby CL, Shattock RJ. Big is beautiful: enhanced saRNA delivery and immunogenicity by a higher molecular weight, bioreducible, cationic polymer. Acs Nano. 2020;14(5):5711–5727. doi:10.1021/acsnano.0c00326.
- Lou G, Anderluzzi G, Schmidt ST, Woods S, Gallorini S, Brazzoli M, Giusti F, Ferlenghi I, Johnson RN, Roberts CW. Delivery of self-amplifying mRNA vaccines by cationic lipid nanoparticles: the impact of cationic lipid selection. J Control Release. 2020;325:370–379. doi:10.1016/j.jconrel.2020.06.027.
- Pardi N, Hogan MJ, Pelc RS, Muramatsu H, Andersen H, DeMaso CR, Dowd KA, Sutherland LL, Scearce RM, Parks R. Zika virus protection by a single low-dose nucleoside-modified mRNA vaccination. Nature. 2017;543(7644):248–251. doi:10.1038/nature21428.
- Richner JM, Himansu S, Dowd KA, Butler SL, Salazar V, Fox JM, Julander JG, Tang WW, Shresta S, Pierson TC. Modified mRNA vaccines protect against zika virus infection. Cell. 2017;168(6):1114–1125. doi:10.1016/j.cell.2017.02.017.
- Richner JM, Jagger BW, Shan C, Fontes CR, Dowd KA, Cao B, Himansu S, Caine EA, Nunes BTD, Medeiros DBA. Vaccine mediated protection against zika virus-induced congenital disease. Cell. 2017;170(2):273–283. doi:10.1016/j.cell.2017.06.040.
- Holtkamp S, Kreiter S, Selmi A, Simon P, Koslowski M, Huber C, Türeci O, Sahin U. Modification of antigen-encoding RNA increases stability, translational efficacy, and T-cell stimulatory capacity of dendritic cells. Blood. 2006;108(13):4009–4017. doi:10.1182/blood-2006-04-015024.
- Coosemans A, Vanderstraeten A, Tuyaerts S, Verschuere T, Moerman P, Berneman ZN, Vergote I, Amant F, VAN Gool SW. Wilms’ tumor gene 1 (WT1)--loaded dendritic cell immunotherapy in patients with uterine tumors: a phase I/II clinical trial. Anticancer Res. 2013;33:5495–5500.
- Sahin U, Derhovanessian E, Miller M, Kloke B-P, Simon P, Löwer M, Bukur V, Tadmor AD, Luxemburger U, Schrörs B, et al. Personalized RNA mutanome vaccines mobilize poly-specific therapeutic immunity against cancer. Nature. 2017;547(7662):222–226. doi:10.1038/nature23003.
- Sahin U, Oehm P, Derhovanessian E, Jabulowsky RA, Vormehr M, Gold M, Maurus D, Schwarck-Kokarakis D, Kuhn AN, Omokoko T. An RNA vaccine drives immunity in checkpoint-inhibitor-treated melanoma. Nature. 2020;585(7823):107–112. doi:10.1038/s41586-020-2537-9.
- Salomon N, Vascotto F, Selmi A, Vormehr M, Quinkhardt J, Bukur T, Schrörs B, Löewer M, Diken M, Türeci Ö, et al. A liposomal RNA vaccine inducing neoantigen-specific CD4 + T cells augments the antitumor activity of local radiotherapy in mice. Oncoimmunology. 2020;9(1):1771925. doi:10.1080/2162402X.2020.1771925.
- Oberli MA, Reichmuth AM, Dorkin JR, Mitchell MJ, Fenton OS, Jaklenec A, Anderson DG, Langer R, Blankschtein D. Lipid nanoparticle assisted mRNA delivery for potent cancer immunotherapy. Nano Lett. 2017;17(3):1326–1335. doi:10.1021/acs.nanolett.6b03329.
- Kranz LM, Diken M, Haas H, Kreiter S, Loquai C, Reuter KC, Meng M, Fritz D, Vascotto F, Hefesha H. Systemic RNA delivery to dendritic cells exploits antiviral defence for cancer immunotherapy. Nature. 2016;534(7607):396–401. doi:10.1038/nature18300.
- Kauffman KJ, Dorkin JR, Yang JH, Heartlein MW, DeRosa F, Mir FF, Fenton OS, Anderson DG. Optimization of lipid nanoparticle formulations for mRNA delivery in vivo with fractional factorial and definitive screening designs. Nano Lett. 2015;15(11):7300–7306. doi:10.1021/acs.nanolett.5b02497.
- Lou B, De Koker S, Lau C, Hennink WE, Mastrobattista E. mRNA polyplexes with post-conjugated GALA peptides efficiently target, transfect, and activate antigen presenting cells. Bioconjug Chem. 2019;30(2):461–475. doi:10.1021/acs.bioconjchem.8b00524.
- Coolen AL, Lacroix C, Mercier-Gouy P, Delaune E, Monge C, Exposito J-Y, Verrier B. Poly(lactic acid) nanoparticles and cell-penetrating peptide potentiate mRNA-based vaccine expression in dendritic cells triggering their activation. Biomaterials. 2019;195:23–37. doi:10.1016/j.biomaterials.2018.12.019.
- Perche F, Clemencon R, Schulze K, Ebensen T, Guzman CA, Pichon C. Neutral lipopolyplexes for in vivo delivery of conventional and replicative RNA vaccine. Mol Ther Nucleic Acids. 2019;17:767–775. doi:10.1016/j.omtn.2019.07.014.
- Walsh EE, Frenck RJ, Falsey AR, Kitchin N, Absalon J, Gurtman A, Lockhart S, Neuzil K, Mulligan MJ, Bailey R. Safety and immunogenicity of two RNA-based Covid-19 vaccine candidates. N Engl J Med. 2020;383(25):2439–2450. doi:10.1056/NEJMoa2027906.
- Mulligan MJ, Lyke KE, Kitchin N, Absalon J, Gurtman A, Lockhart S, Neuzil K, Raabe V, Bailey R, Swanson KA. Phase I/II study of COVID-19 RNA vaccine BNT162b1 in adults. Nature. 2020;586(7830):589–593. doi:10.1038/s41586-020-2639-4.
- Polack FP, Thomas SJ, Kitchin N, Absalon J, Gurtman A, Lockhart S, Perez JL, Pérez Marc G, Moreira ED, Zerbini C, et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med. 2020;383(27):2603–2615. doi:10.1056/NEJMoa2034577.
- Jackson LA, Anderson EJ, Rouphael NG, Roberts PC, Makhene M, Coler RN, McCullough MP, Chappell JD, Denison MR, Stevens LJ. An mRNA vaccine against SARS-CoV-2 — Preliminary report. N Engl J Med. 2020;383(20):1920–1931. doi:10.1056/NEJMoa2022483.
- Baden LR, El SH, Essink B, Kotloff K, Frey S, Novak R, Diemert D, Spector SA, Rouphael N, Creech CB. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med. 2021;384(5):403–416. doi:10.1056/NEJMoa2035389.
- Goshen-Lago T, Waldhorn I, Holland R, Szwarcwort-Cohen M, Reiner-Benaim A, Shachor-Meyouhas Y, Hussein K, Fahoum L, Baruch M, Peer A. Serologic status and toxic effects of the SARS-CoV-2 BNT162b2 vaccine in patients undergoing treatment for cancer. JAMA Oncol. 2021;7(10):1507–1513. doi:10.1001/jamaoncol.2021.2675.
- Tregoning JS, Flight KE, Higham SL, Wang Z, Pierce BF. Progress of the COVID-19 vaccine effort: viruses, vaccines and variants versus efficacy, effectiveness and escape. Nat Rev Immunol. 2021;21(10):626–636. doi:10.1038/s41577-021-00592-1.
- Dagan N, Barda N, Kepten E, Miron O, Perchik S, Katz MA, Hernán MA, Lipsitch M, Reis B, Balicer RD, et al. Bnt162b2 mRNA Covid-19 vaccine in a nationwide mass vaccination setting. N Engl J Med. 2021;384(15):1412–1423. doi:10.1056/NEJMoa2101765.
- Haas EJ, Angulo FJ, McLaughlin JM, Anis E, Singer SR, Khan F, Brooks N, Smaja M, Mircus G, Pan K. Impact and effectiveness of mRNA BNT162b2 vaccine against SARS-CoV-2 infections and COVID-19 cases, hospitalisations, and deaths following a nationwide vaccination campaign in Israel: an observational study using national surveillance data. Lancet. 2021;397(10287):1819–1829. doi:10.1016/S0140-6736(21)00947-8.
- Flacco ME, Soldato G, Acuti MC, Carota R, Di Luzio R, Caponetti A, Manzoli L. Interim estimates of COVID-19 vaccine effectiveness in a mass vaccination setting: data from an Italian province. Vaccines (Basel). 2021;9(6). doi:10.3390/vaccines9060628.
- Tartof SY, Slezak JM, Fischer H, Hong V, Ackerson BK, Ranasinghe ON, Frankland TB, Ogun OA, Zamparo JM, Gray S. Effectiveness of mRNA BNT162b2 COVID-19 vaccine up to 6 months in a large integrated health system in the USA: a retrospective cohort study. Lancet. 2021;398(10309):1407–1416. doi:10.1016/S0140-6736(21)02183-8.
- Lopez BJ, Andrews N, Gower C, Gallagher E, Simmons R, Thelwall S, Stowe J, Tessier E, Groves N, Dabrera G. Effectiveness of Covid-19 vaccines against the B.1.617.2 (Delta) variant. N Engl J Med. 2021;385(7):585–594. doi:10.1056/NEJMoa2108891.
- Rovida F, Cassaniti I, Paolucci S, Percivalle E, Sarasini A, Piralla A, Giardina F, Sammartino JC, Ferrari A, Bergami F. SARS-CoV-2 vaccine breakthrough infections with the alpha variant are asymptomatic or mildly symptomatic among health care workers. Nat Commun. 2021;12(1):6032. doi:10.1038/s41467-021-26154-6.
- Sadarangani M, Marchant A, Kollmann TR. Immunological mechanisms of vaccine-induced protection against COVID-19 in humans. Nat Rev Immunol. 2021;21(8):475–484. doi:10.1038/s41577-021-00578-z.
- Weinberger B. Vaccines and vaccination against SARS-CoV-2: considerations for the older population. Vaccines (Basel). 2021;9(12). doi:10.3390/vaccines9121435.
- Nordstrom P, Ballin M, Nordstrom A. Association between risk of COVID-19 infection in nonimmune individuals and COVID-19 immunity in their family members. JAMA Intern Med. 2021;181(12):1589–1595. doi:10.1001/jamainternmed.2021.5814.
- Krammer F, Srivastava K, Alshammary H, Amoako AA, Awawda MH, Beach KF, Bermúdez-González MC, Bielak DA, Carreño JM, Chernet RL, et al. Antibody responses in seropositive persons after a single dose of SARS-CoV-2 mRNA vaccine. N Engl J Med. 2021;384(14):1372–1374. doi:10.1056/NEJMc2101667.
- Dan JM, Mateus J, Kato Y, Hastie KM, Yu ED, Faliti CE, Grifoni A, Ramirez SI, Haupt S, Frazier A. Immunological memory to SARS-CoV-2 assessed for up to 8 months after infection. Science. 2021;371(6529):6529. doi:10.1126/science.abf4063.
- Ebinger JE, Fert-Bober J, Printsev I, Wu M, Sun N, Prostko JC, Frias EC, Stewart JL, Van Eyk JE, Braun JG. Antibody responses to the BNT162b2 mRNA vaccine in individuals previously infected with SARS-CoV-2. Nat Med. 2021;27(6):981–984. doi:10.1038/s41591-021-01325-6.
- Manisty C, Otter AD, Treibel TA, McKnight Á, Altmann DM, Brooks T, Noursadeghi M, Boyton RJ, Semper A, Moon JC, et al. Antibody response to first BNT162b2 dose in previously SARS-CoV-2-infected individuals. Lancet. 2021;397(10279):1057–1058. doi:10.1016/S0140-6736(21)00501-8.
- Grifoni A, Weiskopf D, Ramirez SI, Mateus J, Dan JM, Moderbacher CR, Rawlings SA, Sutherland A, Premkumar L, Jadi RS. Targets of T cell responses to SARS-CoV-2 coronavirus in humans with COVID-19 disease and unexposed individuals. Cell. 2020;181(7):1489–1501. doi:10.1016/j.cell.2020.05.015.
- Boyarsky BJ, Werbel WA, Avery RK, Tobian AAR, Massie AB, Segev DL, Garonzik-Wang JM. Immunogenicity of a single dose of SARS-CoV-2 messenger RNA vaccine in solid organ transplant recipients. JAMA. 2021;325(17):1784–1786. doi:10.1001/jama.2021.4385.
- Boyarsky BJ, Werbel WA, Avery RK, Tobian AAR, Massie AB, Segev DL, Garonzik-Wang JM. Antibody response to 2-dose SARS-CoV-2 mRNA vaccine series in solid organ transplant recipients. JAMA. 2021;325(21):2204–2206. doi:10.1001/jama.2021.7489.
- Werbel WA, Boyarsky BJ, Ou MT, Massie AB, Tobian AAR, Garonzik-Wang JM, Segev DL. Safety and immunogenicity of a third dose of SARS-CoV-2 vaccine in solid organ transplant recipients: a case series. Ann Intern Med. 2021;174(9):1330–1332. doi:10.7326/L21-0282.
- Reindl-Schwaighofer R, Heinzel A, Mayrdorfer M, Jabbour R, Hofbauer TM, Merrelaar A, Eder M, Regele F, Doberer K, Spechtl P. Comparison of SARS-CoV-2 antibody response 4 weeks after homologous vs heterologous third vaccine dose in kidney transplant recipients: a randomized clinical trial. JAMA Intern Med. 2022;182(2):165–171. doi:10.1001/jamainternmed.2021.7372.
- Karaba AH, Zhu X, Liang T, Wang KH, Rittenhouse A, Akinde O, Eby Y, Ruff JE, Blankson J, Abedon AT. A third dose of SARS-CoV-2 vaccine increases neutralizing antibodies against variants of concern in solid organ transplant recipients. Am J Transplant. 2022;22(4):1253–1260. doi:10.1111/ajt.16933.
- Peled Y, Ram E, Lavee J, Segev A, Matezki S, Wieder-Finesod A, Halperin R, Mandelboim M, Indenbaum V, Levy I. Third dose of the BNT162b2 vaccine in heart transplant recipients: immunogenicity and clinical experience. J Heart Lung Transplant. 2022;41(2):148–157. doi:10.1016/j.healun.2021.08.010.
- Petsch B, Schnee M, Vogel AB, Lange E, Hoffmann B, Voss D, Schlake T, Thess A, Kallen K-J, Stitz L. Protective efficacy of in vitro synthesized, specific mRNA vaccines against influenza a virus infection. Nat Biotechnol. 2012;30(12):1210–1216. doi:10.1038/nbt.2436.
- Fernandez E, Diamond MS. Vaccination strategies against zika virus. Curr Opin Virol. 2017;23:59–67. doi:10.1016/j.coviro.2017.03.006.
- Barbier AJ, Jiang AY, Zhang P, Wooster R, Anderson DG. The clinical progress of mRNA vaccines and immunotherapies. Nat Biotechnol. 2022;40(6):840–854. doi:10.1038/s41587-022-01294-2.
- Kauffman KJ, Mir FF, Jhunjhunwala S, Kaczmarek JC, Hurtado JE, Yang JH, Webber MJ, Kowalski PS, Heartlein MW, DeRosa F. Efficacy and immunogenicity of unmodified and pseudouridine-modified mRNA delivered systemically with lipid nanoparticles in vivo. Biomaterials. 2016;109:78–87. doi:10.1016/j.biomaterials.2016.09.006.
- Thess A, Grund S, Mui BL, Hope MJ, Baumhof P, Fotin-Mleczek M, Schlake T. Sequence-Engineered mRNA without chemical nucleoside modifications enables an effective protein therapy in large animals. Mol Ther. 2015;23(9):1456–1464. doi:10.1038/mt.2015.103.
- Kremsner PG, Ahuad GR, Arana-Arri E, Aroca Martinez GJ, Bonten M, Chandler R, Corral G, De Block EJL, Ecker L, Gabor JJ. Efficacy and safety of the CVnCov SARS-CoV-2 mRNA vaccine candidate in ten countries in Europe and Latin America (HERALD): a randomised, observer-blinded, placebo-controlled, phase 2b/3 trial. Lancet Infect Dis. 2022;22(3):329–340. doi:10.1016/S1473-3099(21)00677-0.
- Begines B, Ortiz T, Perez-Aranda M, Martínez G, Merinero M, Argüelles-Arias F, Alcudia A. Polymeric nanoparticles for drug delivery: recent developments and future prospects. Nanomaterials (Basel). 2020;10(7):1403. doi:10.3390/nano10071403.
- Ibba ML, Ciccone G, Esposito CL, Catuogno S, Giangrande PH. Advances in mRNA non-viral delivery approaches. Adv Drug Deliv Rev. 2021;177:113930. doi:10.1016/j.addr.2021.113930.
- Zhang NN, Li XF, Deng YQ, Zhao H, Huang Y-J, Yang G, Huang W-J, Gao P, Zhou C, Zhang R-R. A thermostable mRNA vaccine against COVID-19. Cell. 2020;182(5):1271–1283. doi:10.1016/j.cell.2020.07.024.
- Muramatsu H, Lam K, Bajusz C, Laczkó D, Karikó K, Schreiner P, Martin A, Lutwyche P, Heyes J, Pardi N. Lyophilization provides long-term stability for a lipid nanoparticle-formulated, nucleoside-modified mRNA vaccine. Mol Ther. 2022;30(5):1941–1951. doi:10.1016/j.ymthe.2022.02.001.
- Loomis KH, Lindsay KE, Zurla C, Bhosle SM, Vanover DA, Blanchard EL, Kirschman JL, Bellamkonda RV, Santangelo PJ. In vitro transcribed mRNA vaccines with programmable stimulation of innate immunity. Bioconjug Chem. 2018;29(9):3072–3083. doi:10.1021/acs.bioconjchem.8b00443.
- Liu L, Wang Y, Miao L, Liu Q, Musetti S, Li J, Huang L. Combination immunotherapy of MUC1 mRNA nano-vaccine and CTLA-4 blockade effectively inhibits growth of triple negative breast cancer. Mol Ther. 2018;26(1):45–55. doi:10.1016/j.ymthe.2017.10.020.
- Wang Y, Zhang L, Xu Z, Miao L, Huang L. mRNA vaccine with antigen-specific checkpoint blockade induces an enhanced immune response against established melanoma. Mol Ther. 2018;26(2):420–434. doi:10.1016/j.ymthe.2017.11.009.
- Bialkowski L, Van der Jeught K, Bevers S, Tjok JP, Renmans D, Heirman C, Aerts JL, Thielemans K. Immune checkpoint blockade combined with IL-6 and TGF-beta inhibition improves the therapeutic outcome of mRNA-based immunotherapy. Int J Cancer. 2018;143(3):686–698. doi:10.1002/ijc.31331