ABSTRACT
Soybean is one of the important oil crops and a major source of protein and lipids. Drought can cause severe soybean yields. Dehydrin protein (DHN) is a subfamily of LEA proteins that play an important role in plant responses to abiotic stresses. In this study, the soybean GmDHN9 gene was cloned and induced under a variety of abiotic stresses. Results showed that the GmDHN9 gene response was more pronounced under drought induction. Subcellular localization results indicated that the protein was localized in the cytoplasm. The role of transgenic Arabidopsis plants in drought stress response was further studied. Under drought stress, the germination rate, root length, chlorophyll, proline, relative water content, and antioxidant enzyme content of transgenic Arabidopsis thaliana transgenic genes were higher than those of wild-type plants, and transgenic plants contained less O2−, H2O2 and MDA contents. In short, the GmDHN9 gene can regulate the homeostasis of ROS and enhance the drought resistance of plants.
Introduction
Soybean (Glycine max L.) can be grown under a wide range of cultivation conditions and has a rich nutritional value. It can be utilized to produce different soy products, soybean oil, okara, and feed.Citation1 Abiotic stress caused by drought, a widespread meteorological hazard, is considered the most important limiting factor for soybean yields.Citation2 The aerobic metabolism of plants that maintain life activities produces reactive oxygen species (ROS), and under normal circumstances, their production and elimination are in a state of dynamic equilibrium.
However, when plants are subjected to drought stress, the balance of reactive oxygen species (ROS) in the plant is disrupted and the ROS scavenging capacity is reduced, leading to the accumulation of ROS, resulting in cell damage and death.Citation3–5
Elevated ROS levels lead to loss of organelle function, decreased metabolic function, lipid peroxidation, programmed cell death, and electrolyte leakage.Citation6 At the same time, the plant’s ability to scavenge reactive oxygen species reflects, to a certain extent, the plant’s tolerance to abiotic stresses. In order to balance the level of reactive oxygen species in plants, plants themselves reduce the level of reactive oxygen species through a variety of antioxidant enzyme reactions. Superoxide dismutase (SOD), catalase (CAT), and peroxidase (POD) can convert reactive oxygen radicals (O2−) and H2O2 into H2O and O2, which in turn functions to scavenge reactive oxygen species (ROS).Citation7 Chen et al. reported that the overexpression of ZmNAC2 in maize enhanced the scavenging ability of ROS and enhanced the osmotic stress tolerance of transgenic Arabidopsis.Citation8 Yan et al. found that GhCDPK60 positively regulated the drought resistance of transgenic Arabidopsis thaliana and cotton by regulating the content of proline (Pro) and ROS levels.Citation9 In another study, Wang et al. showed that OsLEA1a overexpression enhanced tolerance to different abiotic stresses in transgenic rice by inhibiting cell membrane damage and improving ROS clearance ability.Citation10
Late embryonic developmental abundant proteins (LEAs) are a widespread class of proteins in plants that protect plant cells from abiotic stress damage. LEA proteins are categorized into eight types based on their conserved motifs, amino acid sequences, and phylogenetic relationships, namely LEA-1, LEA-2, LEA-3, LEA-4, LEA-5, LEA-6, SMP, and DHN. Yu et al.Citation11 showed that overexpression of the rice OsEm1 gene increased the sensitivity of rice to ABA and improved the plant’s osmotic coordination. Huang et al.Citation12 found that the OsLEA5 gene is involved in ABA-mediated antioxidant defense and plays a vital role in rice’s drought and salt stress response. Sun et al.Citation13 suggested that GsPM30 overexpression enhances salt and drought tolerance in Arabidopsis seedlings and at the seedling stage. Dehydrin (DHN), a thermostable protein belonging to a subfamily of the LEA protein family, is expressed in large quantities in the later stages of embryonic development and has a highly hydrophilic sequence.Citation14 Dehydrogenases (DHNs) are proteins that are highly hydrophilic as well as stress-responsive to stress. Drought stress induced by drought causes dehydrogenase to accumulate during late embryonic development.Citation15 DHN proteins have a wide range of molecular weights, ranging from 9 to 200 kDa. The lysine-rich repeat structural domain (EKKGIMDKIKEKLPG) is the only conserved motif in DHN and may play a key role in protein-lipid interactions.Citation16–19 DHNs also contain multiple other conserved motifs, such as a tyrosine-rich Y fragment motif located at the N-terminal end of the protein-coding region (T/VDEYGNP) and an S segment containing a segment of 4–10 serine residues located between the Y and K segments.Citation20 Protein conformation and ion-binding activity can be regulated by phosphorylation of the modified serine residue composition.Citation21 There is a pair of hydrophobic Phe residues at the core of the F-segment sequence. The α segment is poorly conserved, mainly containing glycine and polar amino acids, which can interact with small molecular polar substances to improve the hydrophilicity of DHN and reduce the damage to proteins.Citation22 Depending on the presence, deletion, and number of repetitions of these fragments, different combinations of structural domains can be formed,Citation23 resulting in different taxa, classifying into six subclasses, namely: YnSKn, FnSKn, YnKn, SKn, Kn, and KnS.Citation24
DHN resists stress such as drought by protecting intracellular protein activity, binding metal ions to scavenge intracellular reactive oxygen radicals, binding DNA, stabilizing cell membranes with phospholipids, protecting enzyme activity, and scavenging ROS.Citation25 The CaDHN3 gene showed enhanced tolerance to salt and drought stress by reducing the accumulation of ROS.Citation26 Sorghum SbDHN1 gene improved the protective effect under high temperature and osmotic stress conditions.Citation27 Overexpression of polar DHN gene PtrDHN-3 enhanced A. thaliana‘s tolerance to salt stress by increasing antioxidant enzyme activity.Citation28 Tandem TaDHN genes responded strongly to stresses such as drought, cold, and high salt, whereas non-tandem genes responded poorly to all stress conditions. According to interaction network analysis, multiple DHN protein interactions are essential for plant resistance to abiotic stresses.Citation29 Plant DHNs are widely involved in physiological processes in response to abiotic stress and play an important role in improving plant stress tolerance. Many DHNs have been identified in previous studies, but the function of GmDHN9 has not been explored yet. In this study, we analyzed the response of the GmDHN9 gene to drought, and the results suggested that GmDHN9 might have a positive effect on the drought tolerance of plants by regulating ROS homeostasis.
Experiments and Methods
Plant Material
In this study, soybean “JN28” and Colombian wild-type Arabidopsis thaliana col were planted in soil and placed in an artificial culture chamber (25°C, 16 h light/8 h dark) for cultivation. When soybean grew to the three-leaf stage, it was subjected to drought (10% PEG6000), salt (100 mM NaCl), ABA (100 μM ABA), and low-temperature (4°C) stress treatments, respectively, and the leaves were harvested at treatments of 0, 3, 6, and 12 h. Harvested samples were frozen in a freezer at −80°C for use in the subsequent experiments.
Sequence Analysis
The GmDHN9 gene (Gene ID:547842) cDNA sequence was derived from NCBI (https://www.ncbi.nlm.nih.gov/). The upstream 2000bp promoter analysis of the GmDHN9 gene was performed using Plant CARE (http://bioinformatics.psb.ugent.be/webtools/plantcare/html/).
RNA Extraction and qRT-PCR Analysis
When soybeans grew to the three-leaf stage, they were treated with 100 μM ABA, 10% PEG6000, 100 mM NaCl, and a low temperature of 4°C for 0, 3, 6, and 12 h. Next, their leaves were plucked, respectively, and RNA was extracted using the TRIzol method. The extracted RNA was then reverse transcribed into cDNA for qRT-PCR (GeneCopeia Co., Ltd.) detection and analyzed using the 2−ΔΔCt method with three biological replicates.
Subcellular Localization
The gene cloning vector was detected by PCR using specific primers to amplify the CDS sequence of the GmDHN9 gene without an stop codon. Next, this was ligated into the pCAMBIA1302-GFP vector at the NcoI and SpeI sites to construct the pCAMBIA1302-GmDHN9-GFP recombinant vector. The recombinant vector was transferred into the Agrobacterium tumefaciens EHA105 strain, injected into tobacco epidermal cells, placed in a dark room at 25°C for 12 h, and then restored to light conditions for 48 h culturing, and observed using laser confocal microscopy.
Acquisition and Detection of Transgenic Arabidopsis
An overexpression pCAMBIA3301-GmDHN9 vector was constructed, and the recombinant plasmid was transferred into the Agrobacterium strain EHA105 and transformed into wild-type A. thaliana by flower immersion. After screening and PCR detection by Basta, T1 transgenic A. thaliana-positive plants were obtained and propagated to T3 generation A. thaliana plants. Quantitative PCR detection of transgenic A. thaliana with T3 generation was performed, and three transgenic lines with relatively high expression were selected for drought resistance identification.
Determination of Germination Rate and Root Length of Transgenic A. thaliana
The seeds of wild-type and T2 generation transgenic A. thaliana were sterilized with 75% alcohol for 1 min and 1% NaClO for 10 min, followed by rinsing with sterile water (3–4 times). Next, they were inoculated in 1/2 MS and 1/2 MS +100 mmol/L, 1/2 MS +200 mmol/L, and 1/2 MS +300 mmol/L mannitol, and maintained in dark conditions at 4°C for 3 days. They were then cultured in light conditions in the culture chamber, and the germination rate of A. thaliana was counted after 10 days. At the same time, A. thaliana was also cultured vertically to count their root length. Wild-type and T2 generation transgenic A. thaliana were planted in soil, and natural drought treatment was carried out in an artificial climate chamber, followed by rehydration process after 25 days of drought. Reference to Citation3,Citation30. The wilting and recovery of A. thaliana were statistically observed.
Determination of Physiological and Biochemical Indexes of Transgenic Arabidopsis
Wild-type and transgenic lines of A. thaliana were subjected to drought stress for 12 h (0 h as control), and the ROS content was determined in vivo by histochemical staining using 3,3’-diaminobenzidine (DAB) and p-nitroblue tetrachloride (NBT), which was based on the Soni ‘s method.Citation31 The H2O2, O2−, malondialdehyde (MDA), chlorophyll, and Pro contents were determined using the methods described by Jiao et al..Citation4 The determination of antioxidant enzyme activity, including SOD, POD, and CAT activities, was done according to Xiong’s methods.Citation32
Expression of Genes Associated with Drought Stress
Arabidopsis thaliana leaves with normal growth and under drought stress were selected. Total RNA was extracted, and the first strand of cDNA (Kangwei Reagent Technology Co., Ltd.) was synthesized for qRT-PCR (GeneCopeia Co., Ltd.) detection. The marker gene-specific primers DREB2A-F/R, RD17-F/R, RD26-F/R, and CBF3-F/R were used for qRT-PCR analysis, and AtEF1-F/R was used as the internal reference gene to calculate the expression of four stress-related marker genes.
Statistical Analysis
Differences between the data were determined by analysis of one-way variance using SPSS (SPSS Inc., Chicago, IL, USA) and considered statistically significant at p < .05 (*) or p < .01 (**).
Results
GmDhn9 Gene Was Significantly Upregulated Under Drought Stress
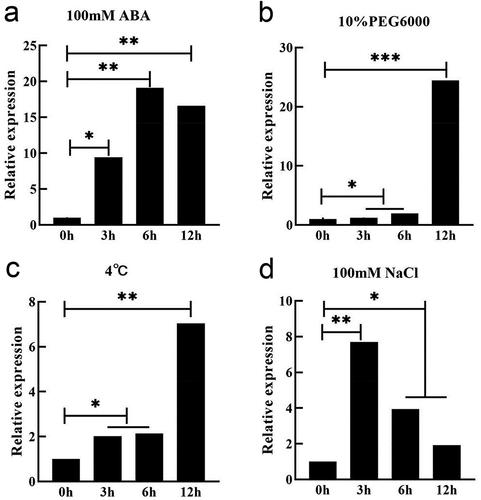
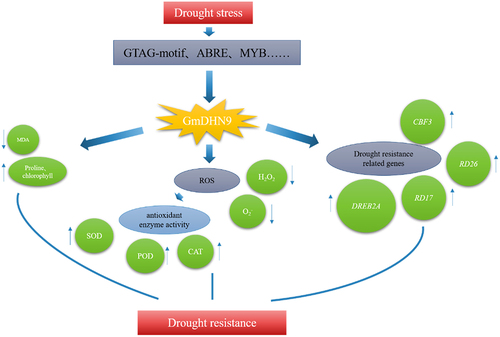
The conserved domain analysis of the gene (Fig. S1) showed that it contained the conserved domain of the DHN family. The gene encoded a protein of 226 amino acids, and the promoter sequence analysis of 2000bp upstream of the gene (Table S1) showed that it contained core promoter elements in response to drought, dehydration, abscisic acid, ABRE, and other related components, indicating that GmDHN9 gene might respond to drought stress. Subsequently, we analyzed the expression of GmDHN9 gene under drought (10% PEG6000), salt (100 mM NaCl), ABA (100 μM ABA), and low-temperature (4°C) stresses in soybean leaves, and the results showed () that the expression of the GmDHN9 gene was up-regulated under the different abiotic stresses, but was more pronounced in response to drought stress.
Subcellular Localization
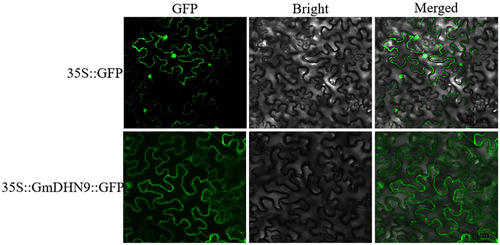
Knowing the location of gene expression products is important for functional analysis of genes. To determine subcellular localization of GmDHN9, the pCAMBIA1302-GmDHN9-GFP vector was constructed and expressed transiently in tobacco leaves. The results showed that GmDHN9 was localized in the cytoplasm ().
Acquisition and Detection of Transgenic Arabidopsis
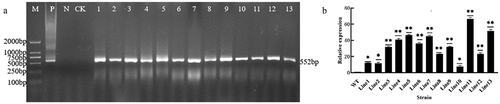
The recombinant plasmid pCAMBIA3301-GmDHN9 was transformed into wild-type Arabidopsis, and 13 positive lines of transgenic A. thaliana were obtained by Basta screening and PCR detection; the results are shown in , while those of real-time PCR of T3 generation transgenes are shown in . Three high-expression lines (Line5, Line11, Line13) were selected for follow-up experiments.
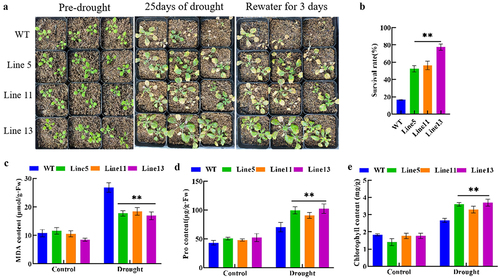
Overexpression of GmDhn9 Increased Germination Rate and Root Length in Transgenic Arabidopsis
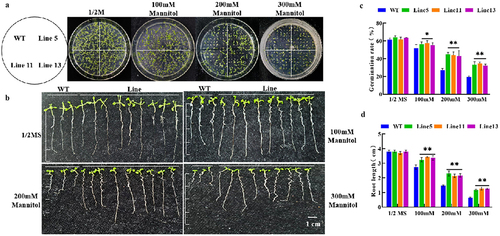
With the increase in mannitol concentration in the medium, the germination rate and root length of A. thaliana decreased. However, there were no significant differences in the germination rate and root length of A. thaliana between wild-type and transgenic lines on the media without mannitol. After adding mannitol, the germination rate of transgenic lines was significantly higher than that of wild-type A. thaliana (4-A, C) and root length, as shown in ). The germination rate of the three transgenic plants was 5%, 18%, and 16% higher than that of the wild-type plants at three concentration gradients of 100 mM, 200 mM, and 300 mM, respectively, which indicated that the transgenic Arabidopsis thaliana possessed more robust drought tolerance.
Overexpression of GmDhn9 Improves Drought Stress Tolerance in Transgenic Arabidopsis
To further validate the role of the GmDHN9 gene in drought stress, we subjected transgenic Arabidopsis to natural drought stress for 25 days, followed by rehydration for three days, and determined the survival rate of transgenic Arabidopsis. We found that the survival rates of the three transgenic Arabidopsis plants under natural drought stress were 36%, 39%, and 61% higher than those of the wild-type plants, respectively. In addition, we determined the contents of chlorophyll, MDA, and Pro in plants under drought stress. Compared with wild-type A. thaliana, the MDA content () in transgenic plants was significantly reduced, while the contents of Pro () and chlorophyll () were significantly increased. These results showed that overexpression of GmDHN9 improved the drought tolerance of transgenic A. thaliana.
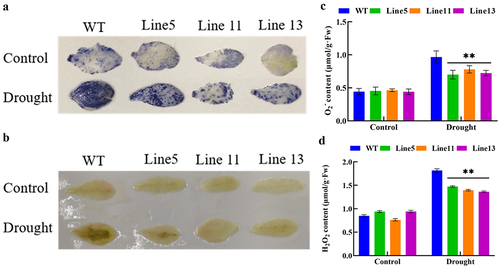
Overexpression of GmDhn9 Reduces the Accumulation of ROS in Transgenic Arabidopsis
To explore whether GmDHN9 can reduce ROS accumulation, we investigated ROS levels using NBT and DAB staining, and measured H2O2 and O2− contents. The results are shown in ). Under normal circumstances, there was no significant difference in H2O2 and O2− contents between wild-type and transgenic A. thaliana lines. After drought stress, transgenic A. thaliana showed lower H2O2 and O2− contents. The staining results using NBT and DAB (6-A, B) showed that the leaf color of wild-type A. thaliana was darker under drought stress, indicating that this plant accumulated more O2− and H2O2, which were consistent with the detection results of O2− and H2O2. This suggested that transgenic Arabidopsis lines had less ROS accumulation and cell membrane damage under drought stress. Therefore, it could be speculated that the expression of GmDHN9 might improve the drought tolerance of plants by reducing ROS accumulation.
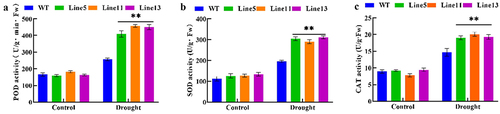
Overexpression of GmDhn9 Increases Antioxidant Enzyme Activity in Plants
To detect the ability of plants to remove ROS, we tested the activities of SOD, POD, and CAT antioxidant enzymes. Compared with wild-type A. thaliana under drought stress, the activities of SOD, POD, and CAT in transgenic plants were significantly improved. These results are shown in , indicating that transgenic A. thaliana had a stronger ability to clear ROS and an increased tolerance to drought stress. Therefore, it could be speculated that the expression of GmDHN9 might improve the drought resistance of plants by increasing the activity of antioxidant enzymes, thereby reducing ROS accumulation.
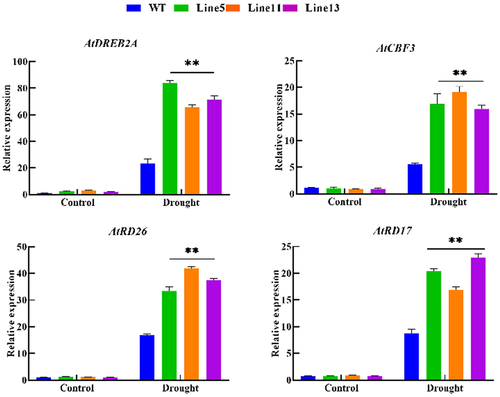
Transgenic A. thaliana Positively Regulates the Expression of Drought-Related Genes
The expression level of drought-resistant genes can be used to assess the response-ability of plants to drought stress, and related genes such as DREB2A, RD17, RD26, and CBF3 were selected since they significantly upregulate expression under drought stress. This experiment examined the relative expression levels of these four genes in wild-type and transgenic A. thaliana. Through the analysis of qRT-PCR results, the expression levels of genes related to DREB2A, RD17, RD26, and CBF3 were significantly higher than those of wild-type plants under drought stress, as shown in . Therefore, GmDHN9 may regulate the genes associated with plant drought stress, thereby positively regulating the response of plants to drought stress.
Discussion
Highly hydrophilic DHN protein acts as a protective agent against drying and accumulation in the embryo.Citation25 When bound to ligands (e.g., membranes), it can hinder the aggregation of other molecules, replacing inactivated chaperone proteins in the dry state to maintain the biological activity of cells.Citation33 Studies have reported that the expression of DHN genes plays an important role in coping with abiotic stresses such as plant drought, salinity, and low temperature.Citation34,Citation35 The results of this study were analyzed using the promoter sequence of the gene and it was seen that the GmDHN9 gene had related cis-acting elements in response to drought, dehydration, abscisic acid, and ABRE. Wild-type soybeans were treated with 10% PEG6000, 100 μM ABA, 100 mM NaCl, and low temperature (4°C) for 0, 3, 6, and 12 h. The qPCR results showed that GmDHN9 responded to drought, salt, ABA, and low temperature of 4°C, and the response to drought stress was stronger. Subsequently, subcellular localization of GmDHN9 was performed to verify its localization in the cytoplasm.
Under drought stress, many plants protect and stabilize subcellular structures by accumulating proline, buffering cellular redox potential, maintaining photosynthesis, scavenging free radicals, and regulating cell function.Citation36 The content of plant MDA can reflect the degree of damage to plant cell membranes. HbDHN1 and HbDHN2 enhanced the Pro content, which in turn improved the plant tolerance of bananas to drought and osmotic stress.Citation37 Maize ZmDHN13 enhanced the tolerance of transgenic tobacco to oxidative stress.Citation38 The EjDHN1 gene enhances cold tolerance in transgenic tobacco by mitigating oxidative responses and protecting against cell membrane damage.Citation39 In this study, compared with wild-type A. thaliana after drought stress treatment, the germination rate, root length, chlorophyll content, proline content, relative water content, and survival rate of transgenic ones were significantly increased, and the content of MDA was significantly reduced. It was suggested that overexpression of GmDHN9 enhanced the drought resistance of A. thaliana.
Plants have an antioxidant defense system that can effectively remove the accumulation of ROS in plants and can maintain ROS balance and intracellular redox homeostasis.Citation40 Drought peroxidizes cell membranes, and antioxidant enzymes eliminate superoxide radicals to mitigate membrane lipid peroxidation and membrane structure damage.Citation41,Citation42 It was seen that the drought stress tolerance of rice improved through excessive accumulation of antioxidant flavonoids.Citation43 In Arabidopsis, KS-type dehydrin AtHIRD11 inhibited the production of H2O2 and hydroxyl radicals in the copper ascorbate system to improve drought resistance.Citation44 In this study, the contents of H2O2 and O2− in transgenic A. thaliana were significantly reduced, and the activities of POD, SOD, and CAT were significantly increased after drought stress treatment, indicating that less ROS accumulated in them, and their ability to remove ROS was stronger, and it had greater tolerance to drought stress. We performed qRT-PCR on genes involved in drought stress (DREB2A, RD17, RD26, and CBF2). The results showed that the expression of all genes was up-regulated under drought stress, and the drought tolerance mechanism mediated by the GmDHN9 gene in Arabidopsis is shown in . In summary, GmDHN9 may be a positive regulator of drought tolerance in plants.
Conclusion
The present study determined that GmDHN9 was a positive regulator of drought tolerance in Arabidopsis, and subcellular localization results showed its localization in the cytoplasm. Through phenotypic, physiological, biochemical, and molecular experiments on transgenic A. thaliana under drought stress, it was shown that overexpression of GmDHN9 increased their root length, germination rate, chlorophyll and Pro contents, reduced the contents of MDA, H2O2, and O2−, thereby improving the drought tolerance of plants. Furthermore, it enhanced the tolerance of these plants to drought stress by increasing antioxidant enzyme activity and removing accumulated ROS.
Author Contributions
SYL and XHF conceived the research plan and designed the experiment. JYF, YZZ, RJD and HJS conducted experiments. JYF wrote the draft. XYW, YS, YSJ, JYF, ZPL, ZYZ and HWY analyzed the data. SYL, SYG, PWW, XHF, PJ and JHY revised the manuscript. All authors read and approved the final manuscript.
Supplementary clean.docx
Download MS Word (28.9 KB)Acknowledgments
We thank SYL and XHF conceived research plans and designed experiments.
Disclosure Statement
No potential conflict of interest was reported by the author(s).
Supplementary Material
Supplemental data for this article can be accessed online at https://doi.org/10.1080/21645698.2024.2327116
Correction Statement
This article has been corrected with minor changes. These changes do not impact the academic content of the article.
Additional information
Funding
References
- Liu S, Liu J, Zhang Y, Jiang Y, Hu S, Shi A, Cong Q, Guan S, Qu J, Dan Y. et al. Cloning of the soybean sHSP26 gene and analysis of its drought resistance. Phyton-Int J Exp Bot. 2022;91(7):1465–82. doi:10.32604/phyton.2022.018836.
- Yu JQ, Ling W, Junhong X, Song W, Wu T, Han T, Wu C. Analysis of yield differences in different years and locations of soybean in Huanghuaihai summer. Crop Mag. 2022;4:221–226. doi:10.1002/agj2.20373.
- Jiang Y, Zhang Y, Duan R, Fan J, Jiao P, Sun H, Guan S, Liu S. Overexpression of maize glutathione S-Transferase ZmGST26 decreases drought resistance of arabidopsis. Agronomy. 2022;12(12):2948–2961. doi:10.3390/agronomy12122948.
- Jiao P, Wei X, Jiang Z, Liu S, Guan S, Ma Y. ZmLBD2 a maize (Zea mays L.) lateral organ boundaries domain (LBD) transcription factor enhances drought tolerance in transgenic Arabidopsis thaliana. Front Plant Sci. 2022;13:1000149–1000165. doi:10.3389/fpls.2022.1000149.
- Chen X, Su W, Zhang H, Zhan Y, Zeng F. Fraxinus mandshurica 4-coumarate-CoA ligase 2 enhances drought and osmotic stress tolerance of tobacco by increasing coniferyl alcohol content. Plant Physiol Bioch. 2020;155:697–708. doi:10.1016/j.plaphy.2020.08.031.
- Wang C, Gao B, Chen N, Jiao P, Jiang Z, Zhao C, Ma Y, Guan S, Liu S. A novel senescence-specific gene (ZmSAG39) negatively regulates darkness and drought responses in maize. Int J Mol Sci. 2022;23(24):15984–15997. doi:10.3390/ijms232415984.
- Zhang Y, Li G, Hu S, Liu J, Jiang Y, Liu S, Guan S, Qu J, Yao D, Shi A. et al. Cloning and drought resistance analysis of soybean GmHsps_p23-like gene. Phyton-Int J Exp Bot. 2022;91(6):1183–98. doi:10.32604/phyton.2022.018853.
- Chen Y, Li X, Xie X, Liu L, Fu J, Wang Q. Maize transcription factor ZmNAC2 enhances osmotic stress tolerance in transgenic Arabidopsis. Plant Physiol. 2023;82:153948~153952. doi:10.1016/j.jplph.2023.153948.
- Yan M, Yu X, Zhou G, Sun D, Hu Y, Huang C, Zheng Q, Sun N, Wu J, Fu Z. et al. GhCDPK60 positively regulates drought stress tolerance in both transgenic Arabidopsis and cotton by regulating proline content and ROS level. Front Plant Sci. 2022;13:1072584–1072596. doi:10.3389/fpls.2022.1072584.
- Wang Z, Zhang Q, Qin J, Xiao G, Zhu S, Hu T. OsLEA1a overexpression enhances tolerance to diverse abiotic stresses by inhibiting cell membrane damage and enhancing ROS scavenging capacity in transgenic rice. Funct Plant Biol. 2021;48(9):860–70. doi:10.1071/FP20231.
- Yu J, Lai Y, Wu X, Wu G, Guo C. Overexpression of OsEm1 encoding a group I LEA protein confers enhanced drought tolerance in rice. Biochem Bioph Res Co. 2016;478(2):703–09. doi:10.1016/j.bbrc.2016.08.010.
- Huang L, Zhang M, Jia J, Zhao X, Huang X, Ji E, Ni L, Jiang M. An Atypical Late Embryogenesis Abundant Protein OsLEA5 Plays a Positive Role in ABA-Induced Antioxidant Defense in Oryza sativa L. Plant Cell Physiol. 2018;59(5):916–29. doi:10.1093/pcp/pcy035.
- Sun M, Shen Y, Yin K, Guo Y, Cai X, Yang J, Zhu Y, Jia B, Sun X. A late embryogenesis abundant protein GsPM30 interacts with a receptor like cytoplasmic kinase GsCBRLK and regulates environmental stress responses. Plant Sci. 2019;283:70–82. doi:10.1016/j.plantsci.2019.02.015.
- Liu J, Liu J, Deng L, Liu H, Liu H, Zhao W, Zhao Y, Sun X, Fan S, Wang H. et al. An intrinsically disordered region-containing protein mitigates the drought–growth trade-off to boost yields. Plant Physiol. 2023;7(1):274–92. doi:10.1093/plphys/kiad074.
- Darling AL, Zaslavsky BY, Uversky VN. Intrinsic disorder-based emergence in cellular biology: physiological and pathological liquid-liquid phase transitions in cells. Polym (Basel). 2019;11(6):990–1013. doi:10.3390/polym11060990.
- Abdul AM, Sabeem M, Mullath SK, Brini F, Masmoudi K. Plant group II LEA proteins: intrinsically disordered structure for multiple functions in response to environmental stresses. Biomolecules. 2021;11(11):1662~1689. doi:10.3390/biom11111662.
- Boddington KF, Graether SP. Binding of a Vitis riparia dehydrin to DNA. Plant Sci. 2019;287:110172–110182. doi:10.1016/j.plantsci.2019.110172.
- Gupta A, Marzinek JK, Jefferies D, Bond PJ, Harryson P, Wohland T. The disordered plant dehydrin Lti30 protects the membrane during water-related stress by cross-linking lipids. Journal Of Biological Chemistry. 2019;294(16):6468–82. doi:10.1074/jbc.RA118.007163.
- Murray MR, Graether SP. Physiological, structural, and functional insights into the cryoprotection of membranes by the dehydrins. Front Plant Sci. 2022;13:886525~886532. doi:10.3389/fpls.2022.886525.
- Luo D, Hou X, Zhang Y, Meng Y, Zhang H, Liu S, Wang X, Chen R. CaDHN5, a dehydrin gene from pepper, plays an important role in salt and osmotic stress responses. Int J Mol Sci. 2019;20(8):1989. IJMS 20:1989. doi:10.3390/ijms20081989.
- Malik AA, Veltri M, Boddington KF, Singh KK, Graether SP. Genome analysis of conserved dehydrin motifs in vascular plants. Front Plant Sci. 2017;8:709–721. doi:10.3389/fpls.2017.00709.
- Ghanmi S, Graether SP, Hanin M. The halophyte dehydrin sequence landscape. Biomolecules. 2022;12(2):330~342. doi:10.3390/biom12020330.
- Stival Sena J, Giguère I, Rigault P, Bousquet J, Mackay J. Expansion of the dehydrin gene family in the Pinaceae is associated with considerable structural diversity and drought-responsive expression. Tree Physiol. 2018;38(3):442–56. doi:10.1093/treephys/tpx125.
- Georgieva K, Mihailova G, Fernandez-Marin B, Bertazza G, Govoni A, Arzac MI, Laza JM, Vilas JL, García-Plazaola JI, Rapparini F. et al. Protective strategies of haberlea rhodopensis for acquisition of freezing tolerance: interaction between dehydration and Low Temperature. Int J Mol Sci. 2022;23(23):15050–15062. doi:10.3390/ijms232315050.
- Verma G, Dhar YV, Srivastava D, Kidwai M, Chauhan PS, Bag SK, Asif MH, Chakrabarty D. Genome-wide analysis of rice dehydrin gene family: its evolutionary conservedness and expression pattern in response to PEG induced dehydration stress. PLoS One. 2017;12(5):e176399. doi:10.1371/journal.pone.0176399.
- Meng YC, Zhang HF, Pan XX, Chen N, Hu, HF, Haq, SU, Khan A, Chen, RG. CaDHN3, a pepper (capsicum annuum L.) dehydrin gene enhances the tolerance against salt and drought stresses by reducing ROS accumulation. Int J Mol Sci. 2021;22(6):3205–3223. doi:10.3390/ijms22063205.
- Halder T, Upadhyaya G, Ray S. YSK2 type dehydrin (SbDhn1) from sorghum bicolor showed improved protection under high temperature and osmotic stress condition. Front Plant Sci. 2017;8(8):918–932. doi:10.3389/fpls.2017.00918.
- Zhou M, Peng N, Yang C, Wang C. The poplar (populus trichocarpa) dehydrin gene PtrDHN-3 enhances tolerance to salt stress in arabidopsis. Plants (Basel). 2022;11(20):2700–2714. doi:10.3390/plants11202700.
- Hao Y, Hao M, Cui Y, Kong L, Wang H. Genome-wide survey of the dehydrin genes in bread wheat (Triticum aestivum L.) and its relatives: identification, evolution and expression profiling under various abiotic stresses. BMC Genomics. 2022;23(1):73. doi:10.1186/s12864-022-08317-x.
- Jiao P, Jiang Z, Wei X, Liu S, Qu J, Guan S, Ma Y. Overexpression of the homeobox-leucine zipper protein ATHB-6 improves the drought tolerance of maize (Zea mays L.). Plant Sci. 2022;316:111159–111176. doi:10.1016/j.plantsci.2021.111159.
- Soni S, Kumar A, Sehrawat N, Kumar A, Kumar N, Lata C, Mann A. Effect of saline irrigation on plant water traits, photosynthesis and ionic balance in durum wheat genotypes. Saudi J Biol Sci. 2021;28(4):2510–17. doi:10.1016/j.sjbs.2021.01.052.
- Xiong J, Zhang W, Zheng D, Xiong H, Feng X, Zhang X, Wang Q, Wu F, Xu J. et al. ZmLBD5 increases drought sensitivity by suppressing ROS accumulation in arabidopsis. Plants. 2022;11(10):1382–1394. doi:10.3390/plants11101382.
- Eriksson S, Eremina N, Barth A, Danielsson J, Harryson P. Membrane-induced folding of the plant-stress protein Lti30. Plant Physiol. 2016;171(2):932–943. doi:10.1104/pp.15.01531.
- Alqurashi M, Thomas L, Gehring C, Marondedze C. A microsomal proteomics view of H₂O₂ and ABA-Dependent responses. Proteomes. 2017;5(4):22~37. doi:10.3390/proteomes5030022.
- Gouveia C, Gananca J, Slaski JJ, Lebot V, Pinheiro de Carvalho MÂA. Abscisic acid phytohormone estimation in tubers and shoots of ipomoea batatas subjected to long drought stress using competitive immunological assay. Physiol Plant. 2021;172(2):419–30. doi:10.1111/ppl.13192.
- Zhang Y, Zhang H, Fu J, Du Y-Y, Qu J, Song Y, Wang P-W. The GmXTH1 gene improves drought stress resistance of soybean seedlings. Mol Breed. 2022;42(1):3. doi:10.1007/s11032-021-01258-5.
- Cao Y, Xiang X, Geng M, You Q, Huang X. Effect of HbDHN1 and HbDHN2 genes on abiotic stress responses in Arabidopsis. Front Plant Sci. 2017;8(8):470–486. doi:10.3389/fpls.2017.00470.
- Liu Y, Wang L, Zhang T, Yang X, Li D. Functional characterization of KS-type dehydrin ZmDHN13 and its related conserved domains under oxidative stress. Sci Rep 4. 2017;7(1):7361–7375. doi:10.1038/s41598-017-07852-y.
- Xu HX, Li XY, Xu CJ, Chen JW. Overexpression of loquat dehydrin gene EjDHN1 promotes cold tolerance in transgenic tobacco. Russ J Plant Physiol. 2018;65(1):69–77. doi:10.1134/S102144371801020X.
- Chen N, Fan X, Wang C, Jiao P, Jiang Z, Ma Y, Guan S, Liu S. Overexpression of ZmDHN15 enhances cold tolerance in yeast and Arabidopsis. Int J Mol Sci. 2022;24(1):480–495. doi:10.3390/ijms24010480.
- Liu J, Zhang Y, Jiang Y, Sun H, Duan R, Qu J, Yao D, Liu S, Guan S. Formation mechanism and occurrence law of pod shattering in soybean: a review. Phyton-Int J Exp Bot. 2022;91(7):1327–40. doi:10.32604/phyton.2022.019870.
- Wei X, Fan X, Zhang H, Jiao P, Jiang Z, Lu X, Liu S, Guan S, Ma Y. Overexpression of ZmSRG7 improves drought and salt tolerance in maize (Zea mays L.). Int J Mol Sci. 2022;23(21):13349–13365. doi:10.3390/ijms232113349.
- Jan R, Khan MA, Asaf SL, Waqas M, Park J-R, Asif S, Kim N, Lee I-J, Kim K-M. et al. Drought and UV radiation stress tolerance in rice is improved by overaccumulation of non-enzymatic antioxidant flavonoids. Antioxid (Basel). 2022;11(5):917–931. doi:10.3390/antiox11050917.
- Punia H, Tokas J, Malik A, Bajguz A, El-Sheikh MA, Ahmad P. Ascorbate–glutathione oxidant scavengers, metabolome analysis and adaptation mechanisms of ion exclusion in sorghum under salt stress. Int J Mol Sci. 2021;22(24):13249–13262. doi:10.3390/ijms222413249.