ABSTRACT
Trans -4-hydroxy-L-proline (Hyp) production by Escherichia coli (E. coli) in fermentation is a high-oxygen-demand process. E. coli secretes large amounts of soluble protein, especially in the anaphase of fermentation, which is an important factor leading to inadequate oxygen supply. And acetic acid that is the major by-product of Hyp production accumulates under low dissolved oxygen (DO). To increase DO and achieve high-level Hyp production, soluble protein was hydrolysed by adding protease in Hyp fermentation. The optimal protease, concentration, and addition time were trypsin, 0.2 g/L, and 18 h, respectively. With the addition of trypsin, the soluble protein in Hyp fermentation decreased by 43.5%. The DO could be maintained at 20–30% throughout fermentation. Hyp production and glucose conversion rate were 45.3 g/L and 18.1%, which were increases of 24.1% and 8.4%, respectively. The accumulation of acetic acid was decreased by 52.1%. The metabolic flux of Hyp was increased by 44.2% and the flux of acetate was decreased by 51.0%.
1. Introduction
Trans-4-hydroxy-L-proline (Hyp) is a valuable amino acid that is widely used in pharmaceutical, chemical, food and cosmetic industries[Citation1]. It is present in gelatin, collagen, and cell wall proteins and is also an important component of secondary metabolites of certain microorganisms[Citation2]. Hyp has conventionally been produced by biological extraction, a complex process which has many disadvantages such as high cost, low recovery rate, and serious pollution[Citation3]. With the increase of raw material price and people’s attention to environmental issues, the traditional method will be gradually eliminated, while microbial fermentation becomes a promising method due to its economic advantages and environmentally-benign features[Citation3].
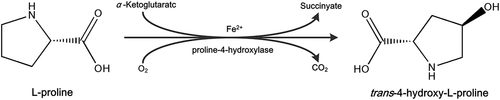
In a recombinant Escherichia coli (E. coli) strain, proline-4-hydroxylase (P4H), which is a member of dioxygenase family [Citation4–Citation6], catalyses the hydroxylation of L-proline to produce Hyp in the presence of α-ketoglutarate and Fe [Citation2]+ ()[Citation7]. In this hydroxylation reaction, an oxygen atom is introduced into L-proline to obtain Hyp, while another oxygen atom is introduced into α-ketoglutarate to obtain succinic acid and carbon dioxide [Citation8,Citation9]. Due to the catalytic properties of P4H, the production of Hyp is a high oxygen-demand process. However, the soluble protein secreted by E. coli during Hyp fermentation generates high-viscosity fermentation broths, hindering oxygen transfer, and reducing dissolved oxygen (DO) levels below that required to efficiently produce Hyp[Citation10]. In addition, acetic acid, which is the primary by-product of Hyp fermentation in E. coli, has inhibitory effects on cell growth and product synthesis[Citation11]. The accumulation of acetate is inversely correlated with DO levels, and acetate accumulation can be reduced by maintaining high DO levels at stationary growth phase [Citation12,Citation13]. Therefore, oxygen supply is generally considered to be a major limiting factor in Hyp production by recombinant strains[Citation14]. At present, there are two strategies for solving the problem of insufficient oxygen supply during high-density fermentation. One is the introduction of Vitreoscilla haemoglobin gene (vgb) into cells through metabolic engineering methods to enhance the cells’ respiration and energy metabolism under oxygen-limited conditions [Citation10,Citation15–Citation17]. The other strategy is to increase the stirring speed, aeration, and pressure of bioreactor in fermentation. However, these methods require a long production cycle or high energy consumption, which are not conducive to controlling production costs[Citation18].
To solve the problem of an insufficient oxygen supply in Hyp fermentation, a new method that adding protease to hydrolyse the soluble protein secreted by E. coli was proposed in this study. The added protease could degrade the proteins into small molecules, such as peptides and amino acids, which are used as nitrogen sources by microorganisms [Citation19–Citation21], resulting in the decrease of protein content and the increase of oxygen transmission efficiency in fermentation broth. In the present study, different proteases, concentrations, addition times, and the effect of adding protease on Hyp fermentation were examined. The results confirmed the feasibility of improving DO by hydrolysing the soluble protein to promote high-level Hyp production by this method.
2. Materials and methods
2.1. Microorganism
The Hyp-producing E. coli THLP strain used in this study was obtained from the Center of Culture Collection of Department of Life Science of Shanxi Datong University.
2.2. Culture conditions
The off-line culture of fermentation broth was carried out in 500 mL shake flasks containing 30 mL of Hyp fermentation broth with various concentrations of alkaline protease, neutral protease, trypsin and complex protease, and cultured on a rotary shaker at 200 r/min, 36°C, and pH 7.0 for 6 h. The Hyp fermentation broth was prepared in a 5 L stirred fermenter (Shanghai Baoxing Bioengineering Equipment Co., Ltd) for 18 h.
The Hyp fermentation was conducted in a 5 L fermenter. The strains cultured on agar slant (10 g/L tryptone, 5 g/L yeast extract, 1 g/L KH2PO4, 0.5 g/L MgSO4 · 7H2O, 3 g/L NaCl and 20 g/L agar) at 37°C for about 12 h. Seed cultures were prepared by transferring an appropriate amount of agar slant cultured cells into 5 L seed fermenter containing 2 L seed medium (20 g/L glucose, 10 g/L yeast extract, 5 g/L (NH4)2SO4, 3 g/L KH2PO4, 1 g/L sodium citrate, and 1 g/L MgSO4 · 7H2O) at 36°C and pH 7.0 for about 10 h. The culture grown in the seed fermenter was transferred with 10% (V/V) inoculum into 5 L fermenter containing 3 L fermentation medium (20 g/L glucose, 5 g/L yeast extract, 3 g/L (NH4)2SO4, 4 g/L KH2PO4, 2 g/L sodium citrate, 1 g/L MgSO4 · 7H2O, and 0.1 g/L FeSO4 · 7H2O) in total. The fermentation temperature was maintained at 36°C, and the pH was adjusted to 7.0 by automated addition of 25% (V/V) ammonium hydroxide. The DO was maintained at 20–30% by adjusting the stirring speed and aeration. When the initial glucose was exhausted, 80% (m/V) glucose solution was fed into the fermenter at an appropriate rate to maintain the glucose concentration at 0–5 g/L.
2.3. Analysis of substrate and product
The biomass concentration was measured as the optical density at 600 nm (OD600) using a spectrophotomete[Citation22]. The glucose concentration was determined by a biological sensor (Shandong Academy of Sciences Institute of Biology). The concentrations of acetic acid and lactate were detected by high performance liquid chromatography (HPLC; Agilent 1200) using an Aminex HPX-87H column with H2SO4 (0.004 mol/L) as the mobile phase at a flow rate of 0.60 mL/min[Citation23]. The detection wavelength, temperature and injection volume were set as 210 nm, 35°C, and 20 μL, respectively. The Hyp concentration was measured according to the chloramine-T method[Citation24]. The protein concentration was determined with a kit (Nanjing Jiancheng Bioengineering Institute).
2.4. Analysis of metabolic flux
The distribution of metabolic flux with the control fermentation (CF) and the added-protease fermentation (APF) was calculated by MATLAB based on the analysis of metabolic flux balance and stoichiometry according to the method of Su et al. [Citation6,Citation25]
2.5. Statistical analysis
All experiments were conducted in triplicate and the dates represent mean values and standard deviation.
3. Results and discussion
3.1 Effect of different protease types and concentrations on hyp fermentation broth
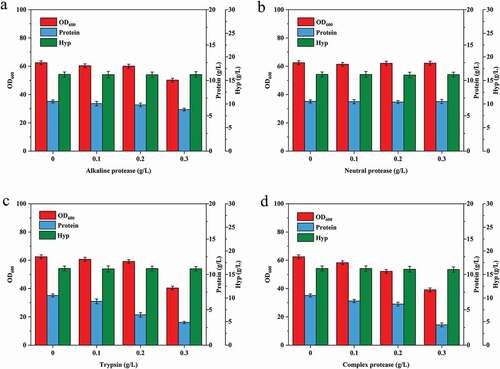
The soluble protein produced by E. coli during Hyp fermentation was hydrolysed by adding protease. To evaluate the effect of different protease types and concentrations on degrading soluble protein in Hyp fermentation broth without destroying Hyp and OD600 under the conditions of Hyp fermentation, the off-line culture was carried out. As shown in , there was no significant decrease in Hyp concentration following the addition of proteases, regardless of types or concentrations, indicating that Hyp was not destroyed by these proteases. When alkaline protease was added to Hyp fermentation broth at 0.1, 0.2, or 0.3 g/L (), the protein content was decreased by 4.41%, 6.97%, and 16.36%, respectively. shows that the neutral protease could not play a role on degrading protein under the conditions of Hyp fermentation. When 0.3 g/L trypsin was added to the fermentation broth (), the protein content was decreased by 54.34% from 7.03 g/L to 3.21 g/L, but OD600 decreased by 35.36%. In contrast, when trypsin was added at 0.2 g/L, the protein content was decreased by 39.12% to 4.28 g/L with a slight decrease in the OD600 of 5.28%. When the complex proteases were added (), OD600 decreased significantly as the protein content decreased. Therefore, trypsin was chosen as the optimal protease type, and the optimum amount was 0.2 g/L.
3.2 Effect of protease addition time on hyp fermentation
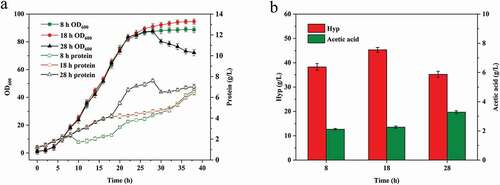
Based on the results of above experiments, Hyp fermentation experiments were carried out in 5 L fermenters. 0.2 g/L trypsin was added at 8, 18, and 28 h of Hyp fermentation respectively to determine the optimal time for addition. The OD600, protein, Hyp, and acetic acid contents are showed in . The addition of trypsin at 8 or 18 h had no adverse effect on bacterial growth. However, when adding trypsin at 28 h,OD600 decreased from 87.6 to 72.2 in the late stage of fermentation. From the perspective of protein content in fermentation broth, although the final protein content was roughly the same after adding trypsin at the three time points, the addition at 8 or 18 h could keep soluble protein at relatively low levels throughout the whole fermentation, and this effect was not observed when trypsin was added at 28 h. Besides, compared with adding trypsin at 8 or 28 h, Hyp production was the highest when trypsin was added at 18 h, and the acetate accumulation was also at a low level. Taken together, 18 h of fermentation was the optimal time for the addition of trypsin.
3.3 Effect of the added-protease fermentation on hyp production
Trypsin (0.2 g/L) was added to Hyp fermentation at 18 h, and the process was monitored. The stirring speed, aeration, DO, OD600, protein content, Hyp production, the rate of glucose conversion to Hyp, acetic acid content and production rate were evaluated(). In the prophase of fermentation, oxygen consumption gradually increased with the growth of bacteria, and the stirring speed and aeration volume were continuously adjusted to maintain DO at 20–30%. The stirring speed and aeration were adjusted to the maximum of 970 r/min and 8.0 L/min at 12 and 14 h, respectively ().With the effect of biomass increase and product formation on oxygen consumption, DO dropped to 10–20% at 18 h. After 18 h of fermentation, the DO in CF decreased significantly to <5%, while that in APF did not drop below 10%, and slowly recovered from 10–20% to 20–30% at 20 h. In the CF, DO was increased after 30 h, and aeration gradually decreased, falling to 3.0 L/min at 38 h. In contrast, the aeration decreased at 34 h in the APF, and the final amount was 6.4 L/min. It indicated that the activity of E. coli THLP in the middle and late stages of fermentation was improved in the APF. The final OD600 in the APF and CF were 94.6 and 88.1, respectively, representing an increase of 7.4% in the APF (). E. coli secretes soluble protein as they grow, resulting in a continuous increase of protein content in fermentation broth. However, the increase rate of protein content in APF was significantly lower than that in CF. Finally, protein content in CF and APF were 11.5 and 6.5 g/L, respectively, which was a decrease of 43.5%.
Figure 4. Effect of trypsin addition on trans-4-hydroxy-L-proline production. CF is control fermentation (no protease added); APF is added-protease fermentation (trypsin was added at 18 h). (a) Stirring speed, aeration and dissolved oxygen; (b) OD600 and protein content; (c) Hyp concentration and glucose conversion rate; (d) acetic acid concentration and production rate.
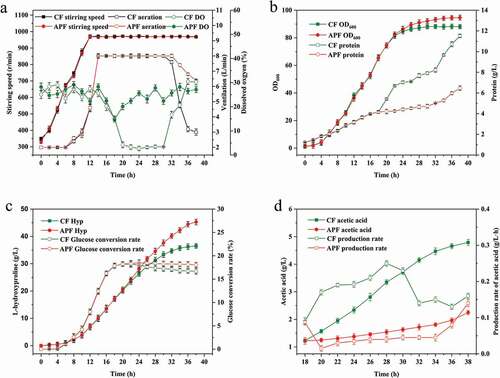
The previous study indicated that an insufficient supply of DO is an important issue in Hyp fermentation, as it leads to low Hyp yield and the accumulation of acetic acid [Citation11–Citation14]. Zhao et al [Citation10]. integrated the vgb gene into the E. coli chromosome to improve the oxygen transfer of Hyp fermentation, which led to Hyp concentration up to 14.4 g/L, and acetic acid concentration was decreased. In this study, the Hyp concentration in APF and CF were basically the same up to 18 h. After 18 h, DO in APF was maintained at the appropriate level (20–30%), while it was unable to reach this level in the CF. Thus, the Hyp production rate in APF was gradually higher than that in CF. At 38 h, Hyp production in APF and CF were 45.3 and 36.5 g/L, respectively, which was an increase of 24.1%. In addition, the rate of glucose conversion to Hyp in the APF was 18.1%, while that in CF was 16.7%, which was an increase of 8.4%. Acetic acid is the main metabolic by-product of Hyp production in E. coli, which is generated by metabolic overflow when the oxygen supply is limited[Citation14]. The acetic acid production rate in the APF was obviously lower than that in CF at 18–38 h, and the final concentrations in CF and APF were 4.8 g/L and 2.3 g/L, respectively, which was a decrease of 52.1%, suggesting improvement of oxygen transfer.
3.4 Effect of the added-protease fermentation on hyp production metabolic flux
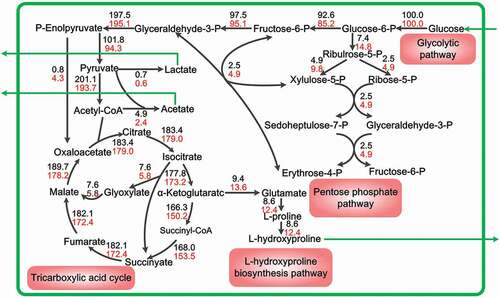
The metabolic flux distributions of Hyp biosynthesis in CF and APF during the later fermentation period (28–38 h) are presented in . The metabolic flux entering the Embden-Meyerhof-Parnas (EMP) pathway decreased by 8.0% in the APF compared to that in the CF, and the flux entering the pentose phosphate (PP) pathway increased from 7.4 to 14.8. The flux from isocitrate into the glyoxylic acid cycle in APF and CF were 6.8 and 7.6, respectively, showing a decrease of 10.5% in the APF. Compared with the CF, acetate flux in the APF decreased from 4.9 to 2.4, which was a decrease of 51.0%. The metabolic flux of Hyp increased from 8.6 to 12.4, which was an increase of 44.2% in the APF.
4. Conclusions
In this study, a strategy for improve the DO of Hyp fermentation by hydrolysing the soluble protein secreted by E. coli with the addition of protease was developed. The optimal protease, concentration, and addition time were trypsin, 0.2 g/L, and 18 h, respectively. The soluble protein in Hyp fermentation broth was decreased by 43.5%. DO in the APF was maintained at 20–30% throughout fermentation. Compared with the corresponding levels in the CF, Hyp titer and glucose conversion rate in the APF were 45.3 g/L and 18.1%, which were increases of 24.1% and 8.4%, respectively. The accumulation of acetic acid was decreased by 52.1%. Moreover, the metabolic flux of Hyp increased by 44.2% and the flux of acetate decreased by 51.0%. Thus the addition of trypsin appropriately during Hyp fermentation is an effectively approach to improving DO, reducing by-production acetate, and promoting Hyp production.
Disclosure statement
No potential conflict of interest was reported by the author.
Additional information
Funding
References
- Hara R, Kino K. Characterization of novel 2-oxoglutarate dependent dioxygenases converting L-proline to cis-4-hydroxy-L-proline. Biochem Biophys Res Commun. 2009;379(4):882–886.
- Hoa BT, Hibi T, Nasuno R, et al. Production of N-acetyl cis-4-hydroxy-L-proline by the yeast N-acetyltransferase Mpr1. J Biosci Bioeng. 2012;114(2):160–165.
- Shibasaki T, Mori H, Ozaki A. Enzymatic production of trans-4-hydroxy-L-proline by region and stereospecific hydroxylation of L-proline. J Agric Chem Soc Jpn. 2000;64(4):746–750.
- Hausinger RP. Fe(II)/α-ketoglutarate-dependent hydroxylases and related enzymes. CRC Crit Rev Biochem. 2004;39(1):21–68.
- Purpero V, Moran GR. The diverse and pervasive chemistries of the α-keto acid dependent enzymes. J Biol Inorg Chem. 2007;12(5):587–601.
- Theodosiou E, Frick O, Bühler B, et al. Metabolic network capacity of Escherichia coli for Krebs cycle-dependent proline hydroxylation. Microb Cell Fact. 2015;14(1):108.
- Falcioni F, L M B, Frick O, et al. Proline availability regulates proline-4-hydroxylase synthesis and substrate uptake in proline-hydroxylating recombinant Escherichia coli. Appl Environ Microbiol. 2013;79(9):3091–3100.
- Carolis ED, Luca VD. 2-Oxoglutarate-dependent dioxygenase and related enzymes: biochemical characterization. Phytochemistry. 1994;36(5):1093–1107.
- Shibasaki T, Mori H, Chiba S, et al. Microbial proline 4-hydroxylase screening and gene cloning. Appl Environ Microbiol. 1999;65(9):4028–4031.
- Zhao TX, Li M, Zheng X, et al. Improved production of trans-4-hydroxy-L-proline by chromosomal integration of the Vitreoscilla hemoglobin gene into recombinant Escherichia coli with expression of proline-4-hydroxylase. J Biosci Bioeng. 2016;123(1):109.
- Castañocerezo S, Pastor JM, Renilla S, et al. An insight into the role of phosphotransacetylase (pta) and the acetate/acetyl-CoA node in Escherichia coli. Microb Cell Fact. 2009;8(1):54.
- Zhao C, Cheng L, Xu Q, et al. Improvement of the production of L-tryptophan in Escherichia coli, by application of a dissolved oxygen stage control strategy. Ann Microbiol. 2016;66(2):843–854.
- Pablos TE, Sigala JC, Le BS, et al. Aerobic expression of Vitreoscilla hemoglobin efficiently reduces overflow metabolism in Escherichia coli. Biotechnol J. 2014;9(6):791–799.
- Falcioni F, Bühler B, Schmid A. Efficient hydroxyproline production from glucose in minimal media by Corynebacterium glutamicum. Biotechnol Bioeng. 2015;112(2):322–330.
- Chi PY, Webster DA, Stark BC. Vitreoscilla hemoglobin aids respiration under hypoxic conditions in its native host. Microbiol Res. 2009;164(3):267–275.
- Sumer F, Stark BC, Akbas MY. Efficient ethanol production from potato and corn processing industry waste using E. coli engineered to express Vitreoscilla hemoglobin. Environ Technol. 2015;36(18):2319–2327.
- KKaira GS, Kr D, Pandey A. A psychrotolerant strain of Serratia marcescens (MTCC 4822) produces laccase at wide temperature and pH range. Amb Express. 2015;5(1):43.
- Smith JJ, Lilly MD, Fox RI. The effect of agitation on the morphology and penicillin production of Penicillium chrysogenum. Biotechnol Bioeng. 1990;35(10):1011–1023.
- Yamamoto N, Ejiri M, Mizuno S. Biogenic peptides and their potential use. Curr Pharm Des. 2003;9(16):1345–1355.
- Surowka K, Zmudzinski D. Functional properties modification of extruded soy protein concentrate using neutrase. Czech J Food Sci. 2004;22(5):163–174.
- Yamamoto N, Akino A, Takano T. Purification and specificity of a cell-wall-associated proteinase from Lactobacillus helveticus CP790. J Biochem. 1993;114(5):740–745.
- Tian Y, Fan Y, Liu J, et al. Effect of nitrogen, carbon sources and agitation speed on acetoin production of Bacillus subtilis SF4-3. J Biotechnol. 2016;19(1):41–49.
- Xu Q, Bai F, Chen N, et al. Removing the by-products acetic acid and NH4+ from the L-tryptophan broth by vacuum thin film evaporation during L-tryptophan production. Electron J Biotechnol. 2018;33:46–51.
- Reddy GK, Enwemeka CS. A simplified method for the analysis of hydroxyproline in biological tissues. Clin Biochem. 1996;29(3):225–229.
- Su YW, Guo QQ, Wang S, et al. Effects of betaine supplementation on L-threonine fed-batch fermentation by Escherichia coli. Bioprocess Biosyst Eng. 2008;41:1509–1518.