Abstract
Objective: We recently reported that U.S. mortality rates for motor neuron disease (MND) at the level of the state are associated with well water use. However, data at the state level may not accurately reflect data at the individual level. We therefore examined the association between MND mortality and well water use utilizing data from smaller geographic units that may better reflect exposure and disease at the individual level. Methods: We used data on age-adjusted MND mortality rates at the level of the county, obtained from the CDC, and corresponding data on the prevalence of well water use, obtained from the U.S. Geological Survey. Data were analyzed by multivariate linear regression and by Getis-Ord Gi*, a measure of spatial clustering. Results: Age-adjusted mortality rates for MND in 923 U.S. counties were significantly correlated with the prevalence of well water (p < 0.0001). ‘Hot spots’ of MND mortality were significantly associated with ‘hot spots’ of well water use (p < 0.0005). Conclusions: These findings support the hypothesis that an agent present in well water plays an etiologic role in ALS. Further study of water use among individuals with ALS is warranted.
Introduction
Amyotrophic lateral sclerosis (ALS) is a degenerative disease of upper and lower motor neurons that commonly is fatal within three years from symptom onset. ALS occurs in familial (FALS) and sporadic (SALS) forms, which comprise about 10% and 90% of cases, respectively. Although large progress has been made in understanding the genetics of FALS, the cause(s) of SALS remains unknown (Citation1). Recently we reported that U.S. mortality rates for motor neuron disease (MND) at the level of the state are associated with the percentage of the state’s population that uses water from private wells (Citation2). If confirmed, that finding could greatly narrow the search for environmental causes of ALS. A limitation of that study is that measures of disease and exposure were obtained from relatively large geographic units, states, and therefore could be influenced by extraneous factors (i.e. confounding) and by misclassification. Because the effects of confounding and misclassification likely are greater in larger geographic units than in smaller ones (Citation3), we sought to confirm the association between MND mortality and well water employing data from smaller geographic units. We report that MND mortality rates in 923 U.S. counties are significantly correlated with the prevalence of well water. Moreover, spatial clusters of counties with high MND mortality rates (MND ‘hot spots’) are significantly associated with ‘hot spots’ of well water use.
Methods
Because most individuals with ALS die of their disease, mortality rates for MND (more than 90% of which is ALS) can serve as surrogates for ALS incidence rates (Citation4,Citation5). MND age-adjusted mortality rates for 1999–2014 for U.S. counties and county equivalents were obtained from the Centers for Disease Control (CDC) (Citation6). The CDC reported rates only for counties with at least 20 cases, as rates based on fewer cases were considered unstable.
We calculated the percent of each county’s population using domestic self-supplied water in 2010 from population-based water use data obtained from the U.S. Geological Survey (USGS) (Citation7). The USGS data were compiled from multiple sources, including government records for well permits, agencies that regulate utility rates, and bills from tax appraisers’ offices (Citation8). We used two methods of quality control. First, we generated scatter plots of the data for each state and queried USGS staff regarding apparent inconsistencies in the well water data. Secondly, we identified ‘outliers’ in the mortality and water data and excluded values more than three times the interquartile ranges of their respective distributions.
We calculated the correlation between age-adjusted MND mortality rates and the percent of the county’s population using self-supplied domestic water. Because higher MND mortality rates have been reported in northern states, we used linear regression to examine the correlation between MND mortality rates with well water use independently of latitude and other geographic factors (Citation9). Data on latitude and longitude were obtained from GIS data provided by Environmental Systems Research Institute (Redlands, CA). Data on mean annual temperature and precipitation were 30-year normals for 1981–2010 (Citation10). Finally, we used the Getis-Ord Gi* statistic, a measure of spatial clustering, with Delaunay Triangulation (‘natural neighbors’) weighting to identify significant ‘hot’ and ‘cold’ spots of MND rates and of well water use (ArcGIS 10.3, ESRI, Redlands, California) (Citation11). The similarity between the MND and well water ‘hot spots’ was assessed via Cohen’s kappa statistic (Citation12).
Results
Age-adjusted MND mortality rates were available for 963 counties. Quality control identified 31 counties in North Carolina with well water data that were inconsistent with other counties in the state. Communication with USGS staff revealed that the data for those 31 counties were estimates; those data were censored (Doug Smith, USGS, personal communication, June 2, 2016). Data also were censored for counties in Alaska and Hawaii that were too distant for analysis by Getis-Ord Gi* (n = 6) and counties with outliers in mortality rates (n = 3). The final data set contained 923 counties (923/963, 96% of the total). The exclusions are shown in .
The average MND mortality rate was 2.25 per 100,000 (SD 0.54, range 0.67–4.23) and was highest in Waupaca County, WI, and lowest in Kings County, NY. The average percent of a county’s population using private well water was 21.2% (SD 18.4%, range 0–82%) and was highest in Putnam County, Florida, and was 0 in 42 counties.
MND mortality rates and well water use were significantly correlated, r = 0.278 (p < 0.0001; ). Regression analysis to predict MND mortality rates yielded two significant models: one containing annual temperature and one containing well water use. The best fit model, obtained by stepwise regression, included both temperature and well water use. Adding data on well water to the model that contained temperature significantly improved the model’s predictive power (adjusted r2 = 0.199; p < 0.0001, p for change in the F test, <0.0001).
Figure 2. Panel 2a. Scatterplot of MND mortality rates vs. well water prevalence for 923 U.S. counties. Panel 2b. “Hot” and “cold” spots for MND mortality rates. Counties at the 90th, 95th, and 99th percentile of the distribution of low and high MND mortality rates are shown in shades of blue and red, respectively. Panel 2c. “Hot” and “cold” spots for well water use. Counties at the 90th, 95th, and 99th percentile of the distribution of low and high well water use are shown in shades of blue and red, respectively.
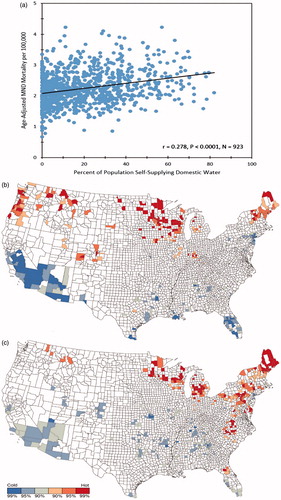
Getis-Ord Gi* maps for MND mortality and well water use showed substantial overall agreement in the co-occurrence of MND and well water use ‘hot’ and ‘cold’ spots that was highly significant, κ = 0.788 (95% CI 0.686–0.890), p < 0.0005 (Citation13). ().
Discussion
The key finding from this study is that mortality rates from MND in 923 U.S. counties are significantly correlated with the prevalence of well water use. Additionally, ‘hot spots’ of MND mortality are significantly associated with ‘hot spots’ of well water use.
Given that we recently reported an association between MND and well water at the state level, why re-investigate it at the county level? The reason is that associations present at the state level cannot validly be generalized to the county level or to the individual. (Citation14,Citation15). A striking illustration that associations do not generalize ‘downward’ is Zito’s example of an indecisive jury: the fact that a jury is indecisive does not imply that the individual jurors are indecisive; indeed, it is the resoluteness of the individual jurors that results in an indecisive jury (Citation16). A similar type of confounding could occur if state level data were generalized to the county or individual level. Consider a hypothetical situation in which the prevalence of wells was higher in eastern counties of states and MND mortality rates were higher in western counties. If the prevalence of wells in general was associated with a third factor that is causally related to MND, a positive association of MND and wells would result at the state level even though the association of MND with wells at the county level is negative. Thus, our finding that, in actuality, MND mortality rates are positively correlated with well water use at the county level is an important extension of our previous findings. This is underscored visually by the ‘hot spot’ analysis which illustrates that counties with high MND mortality rates are counties with high well water use.
We found a significant negative correlation between MND mortality rates and mean annual temperature. This is consistent with studies that reported higher rates of ALS in northern states (Citation17). The correlation between colder temperature and ALS could reflect a genuine relationship between ALS and temperature or may reflect regional differences in the accuracy of death certification (Citation3). Data on well water use significantly improved the model that included temperature, confirming an independent effect of well water.
Hot and cold spots of MND mortality were significantly associated with hot and cold spots of well water use (p < 0.0005). Agreement in the location of hot spots was especially marked in Maine, New Hampshire, Wisconsin and Minnesota. Conversely, agreement was not apparent in Virginia and North Carolina, which showed hot spots for well water but not MND, and in Colorado, Oregon, and Washington, which showed hot spots for MND but not well water. MND hot spots unaccompanied by well water hot spots suggest that there are causes of ALS other than well water. One cause may be heredity, as clustering could result if individuals with an increased genetic risk for ALS lived near one another (Citation18). The observations that many individuals with SALS harbour genetic mutations characteristic of FALS suggests that gene-environment interactions may contribute to the geographic distribution of ALS (Citation19). Lastly, well water hot spots unaccompanied by MND hotspots suggest that well water per se may not be the etiologic factor; e.g. the factor may be a contaminant present in some, but not all wells.
Limitations of this study include biases caused by selection, misclassification, and migration. Mortality rates were available only for counties with at least 20 cases. Less populous, predominantly rural counties, may have been disproportionately excluded. The use of MND mortality rates may have introduced disease misclassification. However, death certificates can reliably identify patients with ALS, as the reported positive predictive value of MND death certificates ranges from 65 to 82% (Citation20,Citation21). Some individuals with ALS may have been exposed to an etiologic factor in one county but moved and died in another. Migration would bias an association between MND and well water toward the null, as it is unlikely that individuals with MND migrate selectively to high well water use counties. Importantly, because the units of analysis in this study are counties, not individuals, an association between well water use and ALS in individuals cannot validly be deduced from it. However, our results are consistent with the results of several studies that report an association between ALS and exposure to well water in individuals (Citation2,Citation22,Citation23).
How could well water increase the risk of ALS? One possibility is via infection with Legionella, a bacterium that is a frequent contaminant of wells (Citation2,Citation24). Legionella are the agent of Legionnaires’ Disease, a multi-system disease associated with a potentially fatal pneumonia (Citation25,Citation26). Approximately 50% of patients infected with Legionella develop neurological disease, which may persist long after resolution of the lung infection (Citation27,Citation28). It is noteworthy that patients with legionellosis have been reported with lesions of the corpus callosum and cortical-spinal tracts that, on magnetic resonance imaging, have been described as ‘consistent with ALS’ (Citation29,Citation30).
Conclusion
MND mortality rates and well water use in the U.S. are significantly positively associated at the level of the county. These findings extend our previous findings at the level of the state and implicate a factor present in well water as a possible environmental cause of ALS. Further research on the water supply of individuals with ALS is warranted.
Declaration of interest
The authors declare that they have no conflict of interest regarding the ideas or data presented in this paper.
References
- Al-Chalabi A, Hardiman O. The epidemiology of ALS: a conspiracy of genes, environment and time. Nat Rev Neurol. 2013;9:617–28.
- Schwartz GG, Klug MG. Motor neuron disease mortality rates in U.S. states are associated with well water use. Amyotroph Lateral Scler Frontotemp Degen. 2016;17:528–34.
- Elliot P, Savitz DA. Design issues in small-area studies of environment and health. Environ Health Perspect. 2008;116:1098–2204.
- Armon C. Amyotrophic lateral sclerosis. In: Nelson SM, Tanner CM, van den Eeden SK, McGuire VM, eds. Neuroepidemiology: from principles to practice. Oxford, UK Oxford University Press; 2004:162–87.
- Martin B, Couratier P, Preux P-M, Logroscino G. Can mortality data be used to estimate amyotrophic lateral sclerosis incidence? Neuroepidemiology. 2011;36:29–38.
- Centers for Disease Control and Prevention. http://wonder.cdc.gov/ucd-icd10.html. Accessed May 1, 2016.
- U.S Department of the Interior. 2016. United States Geological Survey, Water Use for the Nation. Available at: http://waterdata.usgs.gov/nwis. Accessed June 2, 2016.
- Hutson SS. Guidelines for preparation of state water use estimates for 2005. U.S. Department of the Interior, U.S. Geological Survey.
- Sejvar JJ, Holman RC, Bresse JS. Amyotrophic lateral sclerosis mortality in the United States, 1979-2001. Neuroepidemiology. 2005;25:144–52.
- PRISM Climate Group. 2016. http://prism.oregonstate.edu/accessed. Accessed May 15, 2016.
- Smith TB, Smith N, Weleber RG. Comparison of nonparametric methods for static visual field interpolation. Med Biol Eng Comput. 2016;. [Epub ahead of print]. DOI:10.1007/S11517-016-1485-x.
- Cohen J. Weighted kappa: nominal scale agreement with provision for scaled disagreement or partial credit. Psychol Bull. 1968;70:213–20.
- Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977;33:159–74.
- Piantodosi S, Byar DP, Green SB. The ecological fallacy. Am J Epidemiol. 1988;127:893–904.
- Greenland S. Divergent biases in ecologic and individual-level studies. Stat Med. 1992;11:1209–23.
- Zito G. Methodology and meanings: varieties of sociological inquiry. New York: Praeger Press; 1975.
- Bharucha NE, Schoenberg BS, Raven RH, Pickle LW, Byar DP, Mason TJ. Geographic distribution of motor neuron disease and correlation with possible etiologic factors. Neurology. 1983;36:281–302.
- Ishiura H, Takahashi Y, Mitsui J, Yoshida S, Kihura T, Kokubo Y, et al. C9ORF72 repeat expansion in amyotrophic lateral sclerosis in the Kii peninsula of Japan. Arch Neurol. 2012;69:1154–8.
- Van Damme P, Robberecht W. Clinical implications of recent breakthroughs in amyotrophic lateral sclerosis. Curr Opin Neurol. 2013;26:466–72.
- Atickler SE, Royer JA, Hardin JW. Accuracy and usefulness of ICD-10 death certificate coding for the identification of patients with ALS: results from the South Carolina ALS Surveillance Pilot Project. Amyotroph Lateral Scler. 2012;13:69–73.
- Kioumourtzoglou MA, Seals RM, Himmerslev I, Heiman-Patterson T, Lacomis D, Rana S, et al. Comparison of diagnoses of amyotrophic lateral sclerosis by use of death certificates and hospital discharge data in the Danish population. Amyotroph Lateral Scler Frontotemporal Degener. 2015;16:224–9.
- Malek AM, Burchowsky A, Bowser R, Gredal O, Hansen J, Weisskopf MG. Environmental and occupational risk factors for amyotrophic lateral sclerosis: a case-control study. Neurodegener Dis. 2012;14:31–8.
- Das K, Nag C, Ghosh M. Familial, environmental, and occupational risk factors in development of amyotrophic lateral sclerosis. N Am J Med Sci. 2012;4:350–5.
- Strauss WI, Plouffe JF, File JM, Lipman HB, Hackman BH, Salstrom SJ, et al. Risk factors for domestic acquisition of legionnaires disease. Ohio legionnaires Disease Group. Arch Intern Med. 1996;156:1685–92.
- Steinert M, Hentschel U, Hacker J. Legionella pneumophila: an aquatic microbe goes astray. FEMS Microbiol Rev. 2002;26:149–62.
- Yoder J, Roberts V, Craun FG, Hill V, Hicks LA, Alexander NT, et al. Surveillance for waterborne disease and outbreaks associated with drinking water and water not intended for drinking: United States 2005-2006. MMWR Surveill Summ. 2008;57:39–62.
- Weir AI, Bone I, Kennedy DH. Neurological involvement in legionellosis. J Neurol Neurosurg Psychiatry. 1982;45:603–8.
- Harris LF. Legionnaires' disease associated with acute encephalomyelitis. Arch Neurol. 1981;38:462–3.
- Malhotra P, Wurth DM, Parimon T, Albert TJ. A fatal case of Legionella pneumonia with acute neurological complications involving cortico-spinal tracts and corpus callosum. Am J Resp Crit Care Med. 2010;181:A6878.
- Yoshida S, Endo K, Konno F, Higuchi J. Reversible diffusion hyperintensity of bilateral pyramidal tracts and the corpus callosum in Legionnaires’ disease. Neurol Clin Neuroscience. 2014;2:96.