Abstract
Our objective was to explore the efficacy and safety of edaravone in amyotrophic lateral sclerosis (ALS) patients with a Japan ALS severity classification of Grade 3. In a 24-week, double-blind, randomized study, 25 patients who met all of the following criteria were enrolled: Japan ALS severity classification Grade 3; definite, probable, or probable-laboratory supported ALS (El Escorial/revised Airlie House); forced vital capacity (%FVC) ≥60%; duration of disease ≤3 years at consent; and change in the revised ALS functional rating scale (ALSFRS-R) score of –1 to –4 points during the 12-week pre-observation period. Patients received edaravone (n = 13) or placebo (n = 12) for six cycles. The efficacy outcome was change in the ALSFRS-R score. The least-squares mean change in the ALSFRS-R score ± standard error during the 24-week treatment was –6.52 ± 1.78 in the edaravone group and –6.00 ± 1.83 in the placebo group; the difference of –0.52 ± 2.46 was not statistically significant (p = 0.835). Incidence of adverse events was 92.3% (12/13) in the edaravone group and 100.0% (12/12) in the placebo group. There was no intergroup difference in the changes in the ALSFRS-R score. The incidences of adverse events were similar in the two groups.
Introduction
Amyotrophic lateral sclerosis (ALS) is a refractory, progressive disease that involves selective degeneration of upper and lower motor neurons (Citation1) leading to upper limbs dysfunction, gait disturbance, dysarthria, dysphagia, respiratory disorder, and death. Median survival is 20–48 months (Citation2).
The Japan ALS severity classification promulgated by the Specified Disease Treatment Research Program for ALS of the Ministry of Health, Labour and Welfare of Japan (Citation3) defines severity grades from 1 to 5, as follows: (1) Able to work or perform housework; (2) Independent living but unable to work; (3) Requiring assistance for eating, excretion or ambulation; (4) Presence of respiratory insufficiency, difficulty in coughing out sputum or dysphagia; (5) Using a tracheostomy tube, tube feeding or tracheostomy positive pressure ventilation. A 2005 survey assigned 6.2%, 18.8%, 23.7%, 16.0%, and 35.3% of ALS patients in Japan to Grades 1 through 5, respectively (Citation4).
Edaravone (MCI-186, Mitsubishi Tanabe Pharma Corporation, Tokyo, Japan) is a free radical scavenger approved in Japan in 2001 for improvement of neurological symptoms, disorder of activities of daily living, and functional disorder associated with acute ischemic stroke (Citation5). We conducted the first phase III confirmatory study in patients with Grade 1 or 2 ALS according to the Japan ALS severity classification (Citation6). However, we failed to demonstrate the efficacy in the primary endpoint of the ALSFRS-R score compared to placebo. We performed a post-hoc analysis of the first phase III study to identify the subgroup in which edaravone might be effective (Citation7). The second phase III study was conducted for ALS patients who met the subgroup criteria defined by the post-hoc analysis (ClinicalTrials.gov number: NCT01492686), and the results confirmed efficacy of edaravone in these patient subpopulations (Citation8).
Meanwhile, considering the possibility of administration of edaravone in patients with more advanced ALS patients than those in the first phase III study, an evaluation of edaravone in ALS patients at the Grade 3 stage of the Japan ALS Severity Classification was desirable. Therefore, we designed a clinical study to explore the efficacy and safety of edaravone in patients in this severity category. Here, we describe the results of this exploratory study.
Materials and methods
Patients
Patients were required to meet all of the following criteria: Grade 3 according to the Japan ALS severity classification; age 20–75 years; diagnosis of “definite”, “probable” or “probable-laboratory-supported” ALS according to the El Escorial revised Airlie House diagnostic criteria (Citation9,Citation10); forced vital capacity (%FVC) of at least 60%; duration of disease from the first symptom (any ALS symptom) within three years; and change in revised ALS functional rating scale (ALSFRS-R) (Citation11,Citation12) score during the 12-week pre-observation period before study drug administration of –1 to –4 points.
Exclusion criteria included reduced respiratory function and complaints of dyspnea (ALSFRS-R score of 3 points or lower for any of the three items in ‘Dyspnea, Orthopnea and Respiratory Insufficiency in Respiration’); and renal dysfunction with creatinine clearance of 50 mL/min or below within 28 d before treatment.
Patients who had already been treated with riluzole could continue to receive it provided the regimen remained unchanged.
Study design
The design was the same as that of the double-blind, placebo-controlled first phase III study (Citation6).
The study period was 36 weeks, consisting of a 12-week pre-observation period before the start of the first cycle, followed by a 24-week treatment period ().
Figure 1. Trial profile. *4 discontinued treatment (2 patient’s request [Cycle 4], 1 adverse event [Cycle 5], 1 hard to visit the hospital due to worsening of ALS [Cycle 5]).
![Figure 1. Trial profile. *4 discontinued treatment (2 patient’s request [Cycle 4], 1 adverse event [Cycle 5], 1 hard to visit the hospital due to worsening of ALS [Cycle 5]).](/cms/asset/270fca6f-d776-4d70-b9cf-2b0aa6cf51bd/iafd_a_1361441_f0001_b.jpg)
After the 12-week pre-observation period, eligible patients were randomized at a ratio of 1:1 to the placebo or edaravone group, using dynamic allocation of minimization method with one prognostic factor of change in the ALSFRS-R score during the pre-observation period: the two categories –4, –3 or –2, –1.
Study medication
Saline (placebo) or edaravone (60 mg, diluted with approximately 100 mL saline) was administered once daily by intravenous infusion over 60 min. Mitsubishi Tanabe Pharma Corporation provided the study drugs in ampoules. Only authorized personnel, independent of the sponsor and investigators, had access to the key code until unblinding.
A single treatment cycle consisted of a 14-d study drug administration period followed by a 14-d observation period (28 d in total). Study drugs were administered every day for 14 d in the administration period of Cycle 1 and for 10 out of 14 d in the administration periods of Cycles 2–6.
Efficacy evaluation
Efficacy endpoints were changes from baseline to the end of Cycle 6 (or at discontinuation) in the ALSFRS-R score, %FVC, Modified Norris Scale score (Citation13,Citation14), ALS Assessment Questionnaire (ALSAQ-40) (Citation15,Citation16), grip strength (left/right mean), pinch strength (left/right mean), and time to death or a specified state of disease progression (disability of independent ambulation, loss of upper limbs function, tracheotomy, use of respirator, or use of tube feeding) occurring during the six cycles. The evaluations were carried out at the following times: ALSFRS-R, %FVC, grip strength and pinch strength before pre-observation, before the start of Cycle 1 and at the end of each cycle (after 14 d observation and before the first dosage of the next cycle); Modified Norris Scale and ALSAQ-40 before pre-observation, before the start of Cycle 1 and at the end of Cycle 6. As the study was exploratory, we did not classify primary or secondary endpoints.
Safety evaluation
The incidences of adverse events (AEs) and adverse drug reactions (ADRs) and the results of clinical laboratory tests (hematology, blood biochemistry, and urinalysis) were recorded. Serious adverse events (SAEs) were identified among the AEs according to the Good Clinical Practice (GCP) guideline.
Statistical analysis
Efficacy was examined using the full analysis set (FAS) defined as all patients with ALS, without significant GCP violations, who received at least one dose of edaravone or placebo and had at least one efficacy post-baseline data point. Testing of statistical significance was two sided, at a level of 5%. For the change in the ALSFRS-R score from the score before the start of Cycle 1 to the score at the end of Cycle 6, analysis of variance (ANOVA) was performed with treatment group and change in the ALSFRS-R score during the pre-observation period, which was used for dynamic allocation as factors. For the ALSFRS-R score from the end of Cycle 1 to the end of Cycle 6, repeated-measurement analysis of covariance was also performed using the treatment group, time, treatment-group-by-time interaction and change in the ALSFRS-R score during the pre-observation period as factors and the baseline value as a covariate.
For other endpoints, ANOVA and repeated-measures analysis of variance were performed. The time to death or a specified state of disease progression was defined as period from a first study drug administration date to a first specified event, and the other cases were defined as censored cases with the date of censoring, which was the last observation date. A stratified, generalized Wilcoxon test and log-rank test were performed using the change in the ALSFRS-R score during the pre-observation period, as a stratification factor.
For patients with missing data at the end of Cycle 6, the last observation carried forward (LOCF) method was applied to impute missing data only for the patients who completed Cycle 3.
Safety was examined using the safety analysis set, defined as the analysis set after exclusion of patients with significant GCP violations, patients who did not receive at least one dose of study drug, and patients for whom no safety data were available. The number of patients and the incidence of AEs, ADRs, SAEs and serious ADRs were calculated for each event, and the summary proportions were compared between the groups using Fisher’s exact test with a two-sided level of significance of 5%. The laboratory measurement changes were assessed by descriptive statistics.
Statistical analysis was performed using SAS software (version 9.1, SAS Institute, Cary, NC).
Ethics conduct
Five study sites in Japan participated in this study from December 2006 to July 2008. The study was conducted in compliance with GCP and in accordance with the ethical principals described in the Declaration of Helsinki. An institutional review board at each site approved the study protocol. All participants provided written informed consent before any study procedures. The study is registered in ClinicalTrials.gov with a registration number NCT00415519.
Results
Patients
Twenty-seven patients were registered. After the 12-week pre-observation period, two patients were excluded according to the inclusion and exclusion criteria (ineligible for this study, 1; patient’s request, 1), and the remaining 25 patients were randomized ().
The FAS and the safety analysis set both included all 25 patients. Treatment was discontinued for four patients in the edaravone group (patients’ request, 2 [Cycle 4]; AE, 1 [Cycle 5]; hard to visit the hospital due to worsening of ALS, 1 [Cycle 5]) and none in the placebo group. Thus, there was an imbalance between the groups regarding discontinuation.
All patients received 100% of the assigned dosages of study drug.
Patient characteristics are summarized in . There was an imbalance in the distribution of diagnostic criteria of ALS according to the revised Airlie House diagnostic criteria between the treatment groups.
Table 1. Subject demographic characteristics in the FAS.
Efficacy
The results of ANOVA for the change in the ALSFRS-R score during treatment and the results of repeated measures analysis of covariance are shown in . There were no statistically significant differences between the edaravone group and the placebo group in the ALSFRS-R score change (p = 0.835 and 0.945, respectively).
Table 2. Change in endpoints during treatment in the FAS.
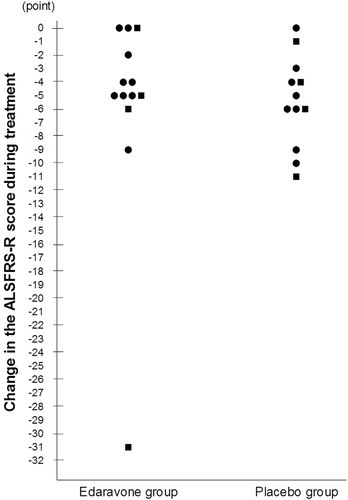
Mean ALSFRS-R scores through the study period are shown in , and the changes in the ALSFRS-R score in individual patients (LOCF) are shown in . One patient in the edaravone group showed a dramatic decline in the ALSFRS-R score (baseline, 32 points; at discontinuation, 1 point). The median values of the changes in the ALSFRS-R score were –5.0 points in the edaravone group and –5.5 points in the placebo group.
Figure 2. Mean ALSFRS-R scores through the study period. For patients with missing values at the end of Cycle 6, data were imputed by the LOCF method, provided that they had completed at least Cycle 3. ALS: amyotrophic lateral sclerosis. ALSFRS-R: Revised ALS Functional Rating Scale. LOCF: last observation carried forward; SD: standard deviation.
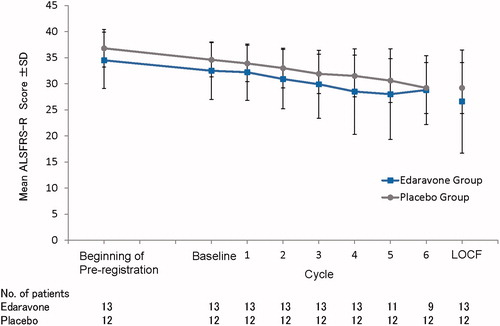
Figure 3. Changes of the ALSFRS-R score during treatment in individual patients. ALSFRS-R: revised amyotrophic lateral sclerosis functional rating scale. Change of the ALSFRS-R score defined as the change from baseline to the end of Cycle 6 (or at discontinuation). For patients with missing values at the end of Cycle 6, data were input by the last observation carried forward (LOCF) method, provided that they had completed at least Cycle 3. •: change in the ALSFRS-R score during pre-observation period: −1, −2. ▪: change in the ALSFRS-R score during pre-observation period: −3, −4.
The results of other endpoints are also presented in . There was no statistically significant difference between the edaravone group and the placebo group in %FVC, Modified Norris Scale, ALSAQ-40, grip strength, or pinch grip strength.
Numbers of events defined as death or a specified state of disease progression are summarized in . Death or a specified state of disease progression occurred in four patients in the edaravone group and three patients in the placebo group. There was no statistically significant difference between the edaravone group and the placebo group (p = 0.0782, stratified generalized Wilcoxon text; p = 0.1058, stratified log-rank test).
Table 3. Events of death or a specified state of disease progression in the FAS.
Safety
All AEs reported in more than two patients in either group are listed in . The most commonly reported AEs (≥3 patients in either group) were diarrhoea, dyschezia, gait disturbance, headache, stomatitis, and upper respiratory tract inflammation. The incidence of reported ADRs was 23.1% in the edaravone group (three of 13 patients experienced three events: muscular weakness, feeling cold and rash) and 8.3% in the placebo group (one of 12 patients experienced one event: hemorrhage subcutaneous). There were no significant intergroup differences in the incidence of AEs (p = 1.000) or ADRs (p = 0.593).
Table 4. Adverse events that occurred in more than two patients in either group (safety analysis set).
All SAEs are listed in . The most commonly reported SAE was dysphagia (two patients in the edaravone group and none in the placebo group). There was no significant inter-group difference in the incidence of SAE (p = 1.000).
Table 5. All serious adverse events in the safety analysis set.
There was one death in the edaravone group and none in the placebo group. The patient in the edaravone group experienced several SAEs (dysphagia, gait disturbance, musculoskeletal disorder and respiratory failure) associated with progression of ALS leading to death due to respiratory failure. There were no serious ADRs in either group. No notable differences in laboratory measurement changes were observed between the groups.
Discussion
In this small-scale study to explore the efficacy and safety of edaravone in ALS patients at Japan ALS severity classification Grade 3 who require assistance for eating, excretion or ambulation, we found no statistically significant intergroup difference changes in the ALSFRS-R score and other endpoints between the edaravone group and the placebo group.
The efficacy of edaravone could not have been statistically evaluated in this study because of very small sample size. A post-hoc analysis for the first confirmatory study (Citation6) has been performed to define the ‘well-defined’ subgroup in which edaravone might be effective (Citation7). Targeted to this well-defined subgroup, the efficacy of edaravone has been confirmed for patients at Japan ALS severity classification Grade 1 or 2 in the second confirmatory study (Citation8). On the other hand, in this study, it would be hard to define such a subgroup because of the sample size limitation. Even if it was successfully defined, we would still have limited information out of the entire group of patients at Grade 3 Japan ALS severity classification. Moreover, a sample size would need to be larger than the first confirmatory study (i.e. more than 200 patients) since the difference between treatment groups in change in ALSFRS-R score for patients at Grade 3 is estimated to be smaller than for patients at Grade 1 or 2. Hence, it would be difficult to conduct the further study on patients at Grade 3 with the sample size larger than the first confirmatory study.
With regard to the incidence rates of AE, ADR, SAE and laboratory tests, there were no significant differences between the edaravone group and the placebo group. Thus, no particular concern over the safety of edaravone was found in this exploratory study.
In conclusion, this exploratory study of edaravone in ALS patients with a Japan ALS severity classification at Grade 3 did not show a statistically significant difference of changes in the ALSFRS-R score compared with the placebo group. From the findings of this study, the effect of edaravone in ALS patients at Grade 3 was not conclusive, and remains as a future issue.
Declaration of interest
Mr. Abe received travel funds and speaker honoraria from Mitsubishi Tanabe Pharma Corporation. Mr. Itoyama received speaker honoraria from Mitsubishi Tanabe Pharma Corporation. Mr. Tsuji received travel funds and speaker honoraria from Mitsubishi Tanabe Pharma Corporation. Mr. Sobue received travel funds and speaker honoraria from Mitsubishi Tanabe Pharma Corporation and serves on the scientific advisory board for the Kanae Science Foundation for the Promotion of Medical Science, Naito Science Foundation, and as an advisory board member of Brain, an editorial board member of Degenerative Neurological and Neuromuscular Disease, the Journal of Neurology, and Amyotrophic Lateral Sclerosis and Frontotemporal Degeneration, and has received funding from several Japanese government agencies. Mr. Aoki received travel funds, speaker honoraria, and fees for conducting and consulting on pharmacological testing of edaravone in a rat ALS model from Mitsubishi Tanabe Pharma Corporation and has received research grants from several Japanese government agencies, including an Intramural Research Grant for Neurological Psychiatric Disorders from the National Center of Neurology and Psychiatry (NCNP). Mr. Doyu received travel funds and speaker honoraria from Mitsubishi Tanabe Pharma Corporation. Mr. Hamada is a consultant for Chugai Pharmaceutical Co., Ltd., Taiho Pharmaceutical Co., Ltd., Kowa Company, Ltd., Sanwa Kagaku Kenkyusho Co., Ltd., Maruho Co., Ltd., Daiichi Sankyo Co., Ltd., Eisai Co., Ltd., Mochida Pharmaceutical Co., Ltd., Otsuka Pharmaceutical Co., Ltd., Nippon Shinyaku Pharmaceutical Co., Ltd., and Mitsubishi Tanabe Pharma Corporation. Mr. Tanaka, Mr. Akimoto, Ms. Nakamura, Ms. Sumii, Mr. Takahashi and Mr. Kondo are employees of Mitsubishi Tanabe Pharma Corporation. Mr. Yoshino received travel funds and speaker honoraria from, had co-owned a patent with, and is a consultant for Mitsubishi Tanabe Pharma Corporation.
This study was funded by Mitsubishi Tanabe Pharma Corporation.
Open access publication of this article and editorial support were funded by Mitsubishi Tanabe Pharma America, Inc.
Acknowledgements
We thank all participating patients and their family members and participating staff at all study sites; Teresa Oblak of Covance Market Access, Inc., for editorial assistance; Richard Steele of WRS Steele Scientific and Technical Editing for proofreading the manuscript; Koji Takei and Kikumi Tsuda of Mitsubishi Tanabe Pharma Development America for critical review of the manuscript.
References
- Rowland LP, Shneider NA. Amyotrophic lateral sclerosis. N Engl J Med. 2001;344:1688–700.
- Chio A, Logroscino G, Hardiman O, Swingler R, Mitchell D, Beghi E, et al. Prognostic factors in ALS: a critical review. Amyotroph Lateral Scler. 2009;10:310–23.
- Japan intractable diseases information center. Available at: http://www.mhlw.go.jp/file/06-Seisakujouhou-10900000-Kenkoukyoku/0000089881.pdf. Accessed February 2, 2017.
- Atsuta N, Watanabe T, Ito M, Senda J, Sobue G. Problems of the current clinical personal clinical survey sheet in amyotrophic lateral sclerosis. Workshop of the Specified Disease Treatment Research Program. 2005. Japanese.
- The Edaravone Acute Brain Infarction Study Group. Effect of a novel free radical scavenger, Edaravone (MCI-186), on acute brain infarction. Cerebravascuar Dis. 2003; 15:222–9.
- Abe K, Itoyama Y, Sobue G, Tsuji S, Aoki M, Doyu M, et al. Comfirmatory double-blind, parallel-group, placebo-controlled study of efficacy and safety of edaravone (MCI-186) in amyotrophic lateral sclerosis patients. Amyotroph Lateral Scler Frontotemporal Degener. 2014;15:610–17.
- The Edaravone (MCI-186) ALS 16 Study Group. A post-hoc subgroup analysis of outcomes in the first phase III clinical study of edaravone (MCI-186) in amyotrophic lateral sclerosis. Amyotroph Lateral Scler Frontotemporal Degener. 2017;18(Suppl). [Epub ahead of print]. doi: 10.1080/21678421.2017.1363780.
- The Writing Group on behalf of the Edaravone (MCI-186) ALS 19 Study Group. Safety and efficacy of edaravone in well defined patients with amyotrophic lateral sclerosis: a randomised, double-blind, placebo-controlled trial. Lancet Neurol. 2017;16:505–12.
- Brooks BR. Introduction defining optimal management in ALS: from first symptoms to announcement. Neurology. 1999;53:S1–S3.
- Brooks BR, Miller RG, Swash M, Munsat TL. El Escorial revisited: revised criteria for the diagnosis of amyotrophic lateral sclerosis. Amyotroph Lateral Scler Other Motor Neuron Disord. 2000;1:293–9.
- Cedarbaum JM, Stambler N, Malta E, Fuller C, Hilt D, Thurmond B, et al. The ALSFRS-R: a revised ALS functional rating scale that incorporates assessments of respiratory function. J Neurol Sci. 1999;169:13–21.
- Ohashi Y, Tashiro K, Itoyama Y, Nakano I, Sobue G, Nakamura S, et al. Study of functional rating scale for amyotrophic lateral sclerosis: Revised ALSFRS (ALSFRS-R) Japanese Version. No To Shinkei. 2001;53:346–55. Japanese.
- Norris FHJr., Calanchini PR, Fallat RJ, Panchari S, Jewett B. The administration of guanidine in amyotrophic lateral sclerosis. Neurology. 1974;24:721–8.
- Oda E, Ohashi Y, Tashiro K, Mizuno Y, Kowa H, Yanagisawa N. Reliability and factorial structure of a rating scale for amyotrophic lateral sclerosis. No To Shinkei. 1996;48:999–1007. Japanese.
- Jenkinson C, Fitzpatrick R, Brennen C, Swash M. Evidence for the validity and reliability of the ALS assessment questionnaire: the ALSAQ-40. Amyotroph Lateral Scler Other Motor Neuron Disord. 1999;1:33–40.
- Yamaguchi T, Ohbu S, Ito Y, Moriwaka F, Tashiro K, Ohashi Y, et al. Validity and clinical applicability of the Japanese version of amyotrophic lateral sclerosis: Assessment questionnaire 40 (ALSAQ-40). No To Shinkei. 2004; 56:483–94. Japanese.
