Abstract
Following the first phase III study of edaravone for amyotrophic lateral sclerosis (ALS), this extension study was performed to evaluate longer-term efficacy and safety. Patients given edaravone in the first 24-week phase III study (Cycles 1–6) were randomised to edaravone (E-E) or placebo (E-P) in the subsequent 24-week double-blind period (Cycles 7–12). Patients given placebo in phase III were switched to edaravone (P-E). Subsequently, all patients received edaravone for 12 weeks (Cycles 13–15). Efficacy endpoints included revised ALS Functional Rating Scale (ALSFRS-R) score. Analysis populations were the full analysis set (FAS) and the efficacy-expected subpopulation (EESP) defined by post-hoc analysis of the first phase III study. The least-squares mean and standard error of the intergroup difference (E-E vs. E-P) of change in the ALSFRS-R score from Cycles 7–12 was 1.16 ± 0.93 (p = 0.2176) in the FAS, and 1.85 ± 1.14 (p = 0.1127) in the EESP. The ALSFRS-R score changed almost linearly in the E-E group throughout Cycles 1–15 (60 weeks). The incidence of serious adverse events associated with ALS progression was higher in E-E than in E-P. Edaravone might have potential efficacy for up to 15 cycles when used to treat patients in the EESP with careful safety monitoring.
Introduction
Amyotrophic lateral sclerosis (ALS) is a progressive disease with a fatal prognosis, for which no curative treatment is available. Oxidative stress is supposed to be involved in its progression (Citation1), and a drug such as edaravone (MCI-186), a free-radical scavenger, may be expected to ameliorate the disease progression of ALS.
The first phase III study (Citation2), in which patients received 24 weeks of treatment (Cycles 1–6), failed to demonstrate efficacy of edaravone in terms of change in the revised ALS Functional Rating Scale (ALSFRS-R) (Citation3,Citation4) score from baseline in the full analysis set (FAS). Post-hoc analysis, however, revealed a finding supporting improvement, compared with the placebo group, in a subgroup designated as the efficacy-expected sub-population (EESP) (Citation5). The least-squares mean and standard error of the intergroup difference (edaravone group vs. placebo group) of change in the ALSFRS-R score from Cycles 1–6 was 0.65 ± 0.78 (p = 0.4108) in the FAS, and 2.20 ± 1.03 (p = 0.0360) in the EESP.
In accordance with the original study plan, the first phase III study was followed by this extension study in order to investigate the long-term efficacy and safety of edaravone. We present the results of efficacy and safety during this extension study. We also compare the results in the FAS with those in the EESP, which had been defined in the statistical analysis plan before unblinding of the data from the first phase III study.
Materials and methods
Patients
Patients were enrolled if they completed treatment in the first phase III study without fulfilling any of the exclusion criteria and also gave consent to participate in this extension study. The exclusion criteria included renal dysfunction (creatinine clearance ≤50 ml/min) at the end of Cycle 6.
Patients who had already been treated with riluzole in the first phase III study could continue to receive it provided the regimen remained unchanged.
Study design
In the first randomised, double-blind, placebo-controlled phase III study, edaravone at 60 mg (E group) or placebo (P group) was administered for 24 weeks (Cycles 1–6; see the following section for details) after a 12-week pre-observation period. Patients who had received edaravone (E) were randomly assigned to receive either edaravone (E-E) or placebo (E-P) during the double-blind 24-week extension period (Cycles 7–12), while patients from the placebo (P) group were all assigned to receive edaravone (P-E). All of the patients were offered open-label edaravone for the following 12 weeks (Cycles 13–15) (). Primary efficacy analysis for the extension period (Cycles 7–12) was performed by comparing data for the E-E and E-P groups.
Figure 1. Study design.
This article reports this results of the extension study (Double blind period: Cycles 7–12, and Open label period: Cycles 13–15) indicated by the bold frame.
*As unblinding of the first phase III study was performed during this extension study, the blind was only maintained for the patients who were assigned to the E group in the first phase III study, but not for the patients who were assigned to the P group in the first phase III study.
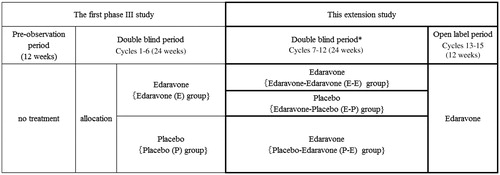
Patients were randomised to the E-E, E-P, and P-E group at a 1:1:2 ratio at the start of the first phase III study with the central randomisation system. Randomization was performed dynamically by the minimisation method using the following factors that were considered relevant to efficacy evaluation:
Change in the ALSFRS-R score during the 12-week pre-observation period (–1, –2/–3, –4)
Initial symptoms (bulbar or limb)
Use of riluzole (yes or no)
Study medication
Mitsubishi Tanabe Pharma Corporation provided the study drugs in ampoules. Only authorised personnel were permitted access to the key code until unblinding. Edaravone (60 mg) or placebo (saline) was administered once daily by intravenous infusion over 60 min.
As in the first phase III study, a single treatment cycle consisted of a 14-d study drug administration period followed by a 14-d observation period. Study drug was administered for 14 consecutive days in Cycle 1, and was administered for 10 out of 14-d in Cycles 2–15.
Efficacy evaluation
Efficacy endpoints included the ALSFRS-R score, the time to death or a specified state of disease progression (death, disability of independent ambulation, loss of upper-limb function, tracheotomy, use of respirator, and use of tube feeding), forced vital capacity (%FVC), Modified Norris Scale score (Citation6,Citation7), ALS Assessment Questionnaire (ALSAQ-40) score (Citation8,Citation9), hand grip strength (left/right mean), and pinch strength (left/right mean). The evaluations were carried out before the start of Cycle 7 and at the end of each treatment cycle.
Safety evaluation
Safety endpoints were adverse events (AEs), adverse drug reactions (ADRs), serious adverse events (SAEs), serious ADRs and laboratory tests throughout Cycles 7–15. SAEs were identified from the AEs according to the Good Clinical Practice (GCP) guideline.
Statistical analysis
Statistical sample size calculation to detect the difference of edaravone and placebo was performed for the first phase III study (Citation2).
The FAS (all patients with ALS and without significant GCP violations who received at least one dose of edaravone or placebo and had at least one efficacy data point after baseline in Cycle 7) was the main efficacy analysis set for this study. Efficacy evaluation was also similarly planned in the EESP (defined as patients with %FVC ≥80% and a score ≥2 for all items of ALSFRS-R at baseline in Cycle 1) before unblinding.
We thought the E-E group and the E-P group were comparable for baseline in Cycle 7 through the end of Cycle 12 because the baseline characteristics in Cycle 7 were considered to be similar between the E-E group and E-P group. Therefore, for efficacy and safety evaluation, we focussed on the comparison between E-E group and E-P group. We summarised the data after the end of Cycle 12 using descriptive statistics.
Analysis of variance (ANOVA) using treatment group and the change in the ALSFRS-R score during the pre-observation period (one of the factors employed for dynamic allocation) and treatment group as factors was performed for the ALSFRS-R score, %FVC, Modified Norris Scale score, ALSAQ-40 score, grip strength, and pinch strength. Each endpoint was compared between the E-E and the E-P group from the baseline (start of Cycle 7) to 24 weeks after the baseline (the end of Cycle 12). For the patients with missing data at 24 weeks after baseline, the last observation carried forward (LOCF) method was applied to impute missing data only for patients who completed at least Cycle 9.
To analyse data on the time to death or a specified state of disease progression, the stratified log-rank test and stratified generalised Wilcoxon test were performed using the change in the ALSFRS-R score during the pre-observation period as the stratification factor (other discontinuations were censored). Any of ‘death, disability of independent ambulation, loss of upper limbs function, tracheotomy, use of respirator, and use of tube feeding’ was defined as events, and for patients with more than one event, the onset date of the first event was defined as the date of event onset.
The level of significance was set at 5% (two-sided) for the FAS.
To assess safety endpoints, the incidence of AEs, SAEs, ADRs, and serious ADRs was calculated in the safety analysis set (defined as the analysis set after exclusion of patients with significant GCP violations, patients who did not receive at least one study drug, and patients for whom no safety data were available). Comparison of the incidence between the E-E and E-P group was performed using Fisher’s exact test.
Statistical analysis was performed using SAS software (version 9.1, SAS Institute, Cary, NC).
Ethics conduct
Twenty-nine study sites in Japan participated from May 2006 to May 2009. The study protocol was reviewed and approved by the respective institutional review boards. The study was conducted in compliance with GCP. Before any study procedures, each patient provided written informed consent. This study was sponsored by Mitsubishi Tanabe Pharma Corporation and was registered at ClinicalTrials.gov (NCT00424463).
Results
Patients
Among the 183 patients who completed the first phase III study, 181 were enrolled in this extension study. The FAS included 180 patients (48, 44 and 88 patients in the E-E, E-P and P-E groups, respectively). One subject met an exclusion criterion and was excluded from the FAS. The EESP included 96 patients (27, 25 and 44 patients in the E-E, E-P and P-E groups, respectively) ().
Figure 2. Disposition of patients.
FAS: Full analysis set, EESP: Efficacy-expected sub-population of ALS patients in the FAS (forced vital capacity of at least 80% before treatment and at least 2 points for all item scores in ALSFRS-R before treatment).
ALS: amyotrophic lateral sclerosis, ALSFRS-R: revised ALS functional rating scale.
Abbreviations of groups are as follows: E-E, edaravone group (the first phase III study) - edaravone group (this extension study); E-P, edaravone group (the first phase III study) - placebo group (this extension study); P-E, placebo group (the first phase III study) - edaravone group (this extension study).
Detailed reasons for discontinuation (numbers are numbers of patients):
*: patient's withdrawal request.
**: patient's request, E-E 2, E-P 4, P-E 5; adverse event, E-E 3, E-P 2, P-E 2; tracheotomy, E-E 7, E-P 1, P-E 6; patient's convenience, E-E 0, E-P 0, P-E 1; other reasons, E-E 2, E-P 0, P-E 2.
***: diagnosed as not having ALS. ****: patient's request, E-P 2, P-E 2; adverse event, E-E 1, tracheotomy, E-E 2, P-E 2; P-E 1; other reasons, P-E 2.
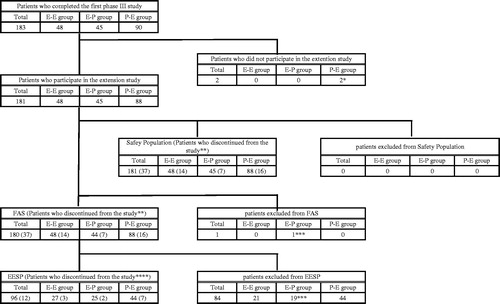
In both the FAS and the safety analysis set, treatment was prematurely discontinued by 14, seven and 16 patients in the E-E, E-P and P-E groups, respectively. In the EESP, treatment was prematurely discontinued by three, two and seven patients, respectively.
Demographic and other baseline characteristics of the FAS and the EESP are summarised in and , respectively. There were imbalances in sex, age and height at baseline in Cycle 1 in the FAS, and sex, height, body weight and initial symptom at baseline in Cycle 1 in the EESP.
Table 1. Subject demographic characteristics in the FAS.
Table 2. Subject demographic characteristics in the EESP.
Efficacy
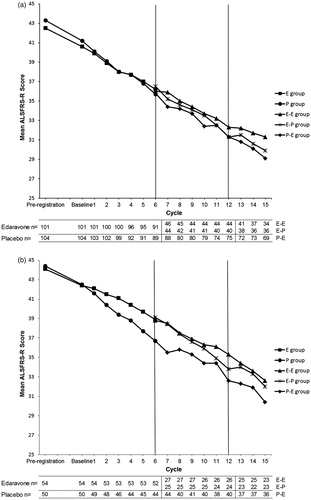
We examined changes in the ALSFRS-R score during the placebo-controlled double-blind extension period (Cycles 7–12). The intergroup difference (least-squares mean ± standard error, p-value) between E-E and E-P was not statistically significant in either the FAS or the EESP, although the difference was larger in the EESP (1.85 ± 1.14, p = 0.1127) than in the FAS (1.16 ± 0.93, p = 0.2176), as had also been found in the first phase III study. The changes in the ALSFRS-R score throughout the whole 60-week period (Cycles 1–15) in the FAS and the EESP are shown in , respectively.
Figure 3. Changes in ALSFRS-R score in the first phase III study and this extension study in the FAS (a) and the EESP (b).
FAS: full analysis set
EESP: efficacy-expected sub-population in the FAS (forced vital capacity of at least 80% at baseline in Cycle 1 and at least 2 points for all item scores in ALSFRS-R).
ALS: amyotrophic lateral sclerosis.
ALSFRS-R: revised ALS functional rating scale.
In the first phase III study (from before pre-registration to the end of Cycle 6), the groups were as follows: E, edaravone group; P, placebo group.
In this extension study (baseline in Cycle 7 to the end of Cycle 15), the groups were as follows: E-E, edaravone group (the first phase III study) - edaravone group (this extension study); E-P, edaravone group (the first phase III study) - placebo group (this extension study); P-E, placebo group (the first phase III study) - edaravone group (this extension study).
The changes in other efficacy endpoints including %FVC, Modified Norris Scale score, ALSAQ-40 score, grip strength, and pinch strength, during the double-blind period in the FAS and the EESP are listed in .
Table 3. Changes in endpoints from baseline in Cycle 7 to the end of Cycle 12 in the FAS and the EESP.
summarises the numbers of patients in the FAS and the EESP who encountered death or a specified state of disease progression during the double-blind period. There were no statistically significant differences between the E-E and E-P group in the FAS (p = 0.0684, stratified generalised Wilcoxon test; p = 0.1540, stratified log-rank test), and in the EESP (p = 0.2402, stratified generalised Wilcoxon test; p = 0.3802, stratified log-rank test).
Table 4. Events of death or a specified state of disease progression in the FAS and the EESP.
Safety
All AEs reported in ≥5% of patients in either group are listed in . The most common AEs (≥10% of patients across all treatment groups) were nasopharyngitis, gait disturbance, constipation, dysphagia, and musculoskeletal disorder. There was no statistically significant difference in the incidence of AEs between the E-E and the E-P groups (p = 0.3625, Fisher’s exact test); the incidences were 91.7% (44/48 patients) in the E-E group and 97.8% (44/45 patients) in the E-P group. The largest differences were seen in gait disturbance (29.2% in E-E vs. 20.0% in E-P), and respiratory failure (12.5% in E-E vs. 4.4% in E-P).
Table 5. Adverse events in the Safety Analysis Set.
SAEs in each group are listed in . The most common SAEs (> 6% of patients across all treatment groups) were dysphagia and musculoskeletal disorder. There was statistical difference in the incidence of SAEs between the E-E and the E-P group (p = 0.0344, Fisher’s exact test); the incidences were 52.1% (25/48 patients) in the E-E group and 28.9% (13/45 patients) in the E-P group. The largest differences were in gait disturbance (8.3% in E-E vs. 0% in E-P) and respiratory failure (12.5% in E-E vs. 4.4% in E-P). The majority of SAEs were considered attributable to progression of ALS. There were no serious ADRs.
Table 6. All serious adverse events in the Safety Analysis Set.
No significant differences in laboratory measurements were observed between the E-E and E-P groups.
Discussion
Although there was no statistically significant difference in the change in the ALSFRS-R score between the E-E and E-P groups in the FAS or the EESP during the double-blind period of this extension study, the difference between the E-E and E-P groups was larger in the EESP than the FAS, as had also been found in the preceding first phase III study. It is noteworthy that the changes in ALSFRS-R score in the E-E group were almost linear over the whole period of Cycles 1–15 (60 weeks) with administration of edaravone 60 mg (). In the EESP, there seem to be different slopes in appearance between edaravone and the placebo administration period, in the E-P group or the P-E group, respectively, although statistical analysis was not carried out. These findings are meaningful, because the combination of the first phase III study and this extension study covers an administration time of 48 weeks. Including the subsequent open-label period, the total administration time has reached 60 weeks, which is the longest exposure in our series of clinical studies to date. The combined results of these studies suggest that edaravone might have potential efficacy for up to 15 cycles when used to treat ALS patients.
With regard to safety, the incidence of ALS-related SAEs recorded in case report forms as ‘worsening of ALS’ or ‘progression of ALS’ (such as ‘dysphagia’, ‘musculoskeletal disorder’ and ‘respiratory failure’ etc.) was noticeably higher in the E-E group (45.8%) than in the E-P group (24.4%). In addition, the incidence of SAEs of the system organ class of ‘respiratory, thoracic and mediastinal disorders’ was 25% (12/48) and 13.3% (6/45) in the E-E and E-P groups, respectively. Moreover, the efficacy measure of change in %FVC from baseline in Cycle 7 to Cycle 12 in the FAS, adjusted mean change (the LS Mean ± SE) was −13.33 ± 2.29 and −10.15 ± 2.44 in the E-E and E-P groups, respectively. We have not been able to identify the detailed causes for the above safety and efficacy finding in the E-E group. However, the demographic characteristics also revealed a higher proportion of elderly patients in the E-E group than in the E-P group. Patients with elderly-onset ALS may present with respiratory failure in the early phase of the disease (Citation10,Citation11). Imbalance in age may have been one of the factors in these deteriorations in respiratory function in the E-E group due to the following results. In the E-E group, 12 patients were judged to have SAEs classified as ‘respiratory, thoracic and mediastinal disorders’, and a majority of patients (7/12) were aged over 65 years. The proportion of elderly (over 65 years) patients with these events was higher in the E-E group than in the E-P group (2/6 patients). It is considered necessary to observe carefully serious respiratory AEs that may directly lead to death. When edaravone is administered to a patient with ALS for a long period of time, the patient's condition should be monitored carefully to ensure the safety of treatment.
Because long-term intravenous administration of placebo is ethically unrealistic, we limited placebo administration to six cycles (24 weeks) for all patients. Consequently, we did not establish a P-P group to receive placebo throughout the whole of the first phase III study and this extension study. This means that controlled comparison is not possible, and so interpretation of the results is difficult. Evaluation of long-term treatment for efficacy and safety, with an important focus on determining whether the observed differences in long-term efficacy and SAEs of long-term treatment with edaravone between the E-E group and the E-P group were due to the underlying characteristics of the two patient populations or whether they highlight real risks of long-term use of edaravone, remains a future issue.
Declaration of interests
Mr. Abe received travel funds and speaker honoraria from Mitsubishi Tanabe Pharma Corporation. Mr. Itoyama received speaker honoraria from Mitsubishi Tanabe Pharma Corporation. Mr. Tsuji received travel funds and speaker honoraria from Mitsubishi Tanabe Pharma Corporation. Mr. Sobue received travel funds and speaker honoraria from Mitsubishi Tanabe Pharma Corporation, and serves on the scientific advisory board for the Kanae Science Foundation for the Promotion of Medical Science, Naito Science Foundation, and as an advisory board member of Brain, an editorial board member of Degenerative Neurological and Neuromuscular Disease, the Journal of Neurology, and Amyotrophic Lateral Sclerosis and Frontotemporal Degeneration, and has received funding from several Japanese government agencies. Mr. Aoki received travel funds, speaker honoraria, and fees for conducting and consulting on pharmacological testing of edaravone in a rat ALS model, from Mitsubishi Tanabe Pharma Corporation, and has received research grants from several Japanese government agencies, including an Intramural Research Grant for Neurological Psychiatric Disorders from the National Center of Neurology and Psychiatry (NCNP). Mr. Doyu received travel funds and speaker honoraria from Mitsubishi Tanabe Pharma Corporation. Mr. Hamada is a consultant for Chugai Pharmaceutical Co., Ltd., Taiho Pharmaceutical Co., Ltd., Kowa Company. Ltd., Sanwa Kagaku Kenkyusho Co., Ltd., Maruho Co., Ltd., Daiichi Sankyo Co., Ltd., Eisai Co., Ltd., Mochida Pharmaceutical Co., Ltd., Otsuka Pharmaceutical Co., Ltd., Nippon Shinyaku Pharmaceutical Co., Ltd. and Mitsubishi Tanabe Pharma Corporation. Mr. Tanaka, Mr. Akimoto, Ms. Nakamura, Mr. Naito, Ms. Murakami, Mr. Takahashi and Mr. Kondo are employees of Mitsubishi Tanabe Pharma Corporation. Mr. Yoshino received travel funds and speaker honoraria from, had co-owned a patent with, and is a consultant for Mitsubishi Tanabe Pharma Corporation. This study was funded by Mitsubishi Tanabe Pharma Corporation.
Open access publication of this article and editorial support were funded by Mitsubishi Tanabe Pharma America, Inc.
Acknowledgements
We thank all participating patients and their family members, and participating staff at all study sites; Contract Clinical Research Associates; Teresa Oblak of Covance Market Access, Inc., for editorial assistance; Richard Steele of WRS Steele Scientific and Technical Editing for proofreading the manuscript; Koji Takei and Kikumi Tsuda of Mitsubishi Tanabe Pharma Development America for critical review of the manuscript.
Additional information
Funding
References
- Beckman JS, Carson M, Smith CD, Koppenol W. ALS, SOD and peroxynitrite. Nature. 1993;364:584.
- Abe K, Itoyama Y, Sobue G, Tsuji S, Aoki M, Doyu M, et al. Confirmatory double-blind, parallel-group, placebo-controlled study of efficacy and safety of edaravone (MCI-186) in amyotrophic lateral sclerosis patients. Amyotroph Lateral Scler Frontotemporal Degener. 2014;15:610–17.
- Cedarbaum JM, Stambler N, Malta E, Fuller C, Hilt D, Thurmond B, et al. The ALSFRS-R: a revised ALS functional rating scale that incorporates assessments of respiratory function. J Neurol Sci. 1999;169:13–21.
- Ohashi Y, Tashiro K, Itoyama Y, Nakano I, Sobue G, Nakamura S, et al. Study of functional rating scale for amyotrophic lateral sclerosis: revised ALSFRS (ALSFRS-R) Japanese version. No to Shinkei. 2001;53:346–55. Japanese.
- The Edaravone (MCI-186) ALS 16 Study Group. A post-hoc subgroup analysis of outcomes in the first phase III clinical study of edaravone (MCI-186) in amyotrophic lateral sclerosis. Amyotroph Lateral Scler and Frontotemporal Degener. 2017;18 (Suppl). Epub ahead of print. doi. 10.1080/21678421.2017.1363780.
- Norris FH Jr, Calanchini PR, Fallat RJ, Panchari S, Jewett B. The administration of guanidine in amyotrophic lateral sclerosis. Neurology. 1974;24:721–8.
- Oda E, Ohashi Y, Tashiro K, Mizuno Y, Kowa H, Yanagisawa N. Reliability and factorial structure of a rating scale for amyotrophic lateral sclerosis. No to Shinkei. 1996;48:999–1007. Japanese.
- Jenkinson C, Fitzpatrick R, Brennan C, Swash M. Evidence for the validity and reliability of the ALS assessment questionnaire: The ALSAQ-40. Amyotroph Lateral Scler Other Motor Neuron Disord. 1999;1:33–40.
- Yamaguchi T, Ohbu S, Saito M, Ito Y, Moriwaka F, Tashiro K, et al. Validity and clinical applicability of the Japanese version of amyotrophic lateral sclerosis –assessment questionnaire 40 (ALSAQ-40). No to Shinkei. 2004;56:483–94. Japanese.
- Terao S, Miura N, Osano Y, Adachi K, Sobue G. Clinical characteristics of elderly Japanese patients with amyotrophic lateral sclerosis; with special reference to the development of respiratory failure. Clin Neurol. 2006;46:381–9. Japanese.
- Magnus T, Beck M, Giess R, Puls I, Naumann M, Toyka KV. Disease progression in amyotrophic lateral sclerosis: predictors of survival. Muscle Nerve. 2002;25:709–14.
