Abstract
Our first phase III study failed to demonstrate efficacy of edaravone for amyotrophic lateral sclerosis (ALS) compared to placebo. Here, we performed post-hoc subgroup analysis to identify a subgroup in which edaravone might be expected to show efficacy. We focussed on two newly defined subgroups, EESP and dpEESP2y. The EESP was defined as the efficacy-expected subpopulation with % forced vital capacity of ≥80%, and ≥2 points for all item scores in the revised ALS functional rating scale (ALSFRS-R) score before treatment. The dpEESP2y was defined as the greater-efficacy-expected subpopulation within EESP having a diagnosis of ‘definite’ or ‘probable’ ALS according to the El Escorial revised Airlie House diagnostic criteria and onset of disease within two years. The primary endpoint of the post-hoc analysis was the change in the ALSFRS-R score during the 24-week treatment period. The intergroup differences of the least-squares mean change in the ALSFRS-R score ± standard error during treatment were 0.65 ± 0.78 (p = 0.4108) in the full analysis set, 2.20 ± 1.03 (p = 0.0360) in the EESP, and 3.01 ± 1.33 (p = 0.0270) in the dpEESP2y. Edaravone exhibited efficacy in the dpEESP2y subgroup. A further clinical study in patients meeting dpEESP2y criteria is warranted.
Introduction
Edaravone (MCI-186, Mitsubishi Tanabe Pharma Corporation, Tokyo, Japan), a free radical scavenger, was approved for the indication of ‘improvement of neurological symptoms, disorders in activities of daily living, and functional disorders with acute cerebral infarction’ in Japan in 2001 (Citation1).
We conducted the first phase III study (Citation2) to investigate the efficacy and superiority of edaravone versus placebo by using changes in the revised amyotrophic lateral sclerosis (ALS) functional rating scale (ALSFRS-R) (Citation3,Citation4) score as the primary endpoint. Changes in the ALSFRS-R scores in the placebo and edaravone groups during 24 weeks of treatment were compared by analysis of variance (ANOVA). However, this study failed to show any significant effect of edaravone at the primary endpoint. There are few treatment options for ALS other than riluzole. Therefore, we did not stop development of edaravone, but instead carried out a post-hoc analysis in order to investigate the reasons why the expected efficacy had not been found.
In this report, we present a post-hoc subgroup analysis of the first phase III study. The original study efficacy population (the full analysis set [FAS]) consisted of all patients who received at least one dose of edaravone or placebo and who had available efficacy data. Excluded from the FAS were patients with significant Good Clinical Practice (GCP) violations and patients subsequently determined not to have ALS. With the subgroup analysis we aimed at uncovering the reasons for the failure of the phase III study and identifying a subgroup of ALS patients in whom edaravone might be expected to show efficacy.
Materials and methods
The first phase III study has been reported in detail (Citation2). It was funded by Mitsubishi Tanabe Pharma Corporation and is registered with Clinical.Trial.gov, number NCT00330681.
The first phase III study design
The primary endpoint for the efficacy analysis was the change in the ALSFRS-R score, in which each item is clinically graded on a 5-point scale from 0 to 4 (12 items, maximum score 48) during the 24-week treatment period.
Patients were required to meet all of the following inclusion and exclusion criteria. Inclusion criteria were age 20–75 years; diagnosis of ‘definite’, ‘probable’ or ‘probable-laboratory-supported’ ALS according to the El Escorial revised Airlie House diagnostic criteria; % forced vital capacity (%FVC) of ≥70%; duration of disease within three years; change in the ALSFRS-R score during the 12-week pre-observation period of –1 to –4 points; and Japan ALS severity classification (Citation5) of 1 or 2 (1: able to work or perform housework; 2: independent living but unable to work). Exclusion criteria were described previously (Citation2).
Patients were randomised to the placebo group or the edaravone (60 mg) group. A single treatment cycle consisted of 14 days of study drug administration followed by a 14-day drug-free period. Study drugs were administered for six cycles.
Hypothesis for identifying the subpopulation
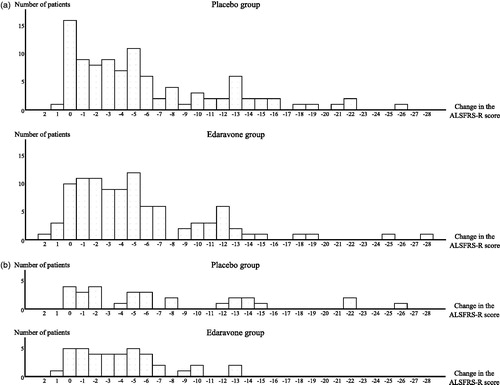
We considered that the study design of any new prospective phase III study should be basically the same as that of the first phase III study, in which change in the ALSFRS-R score had been the primary endpoint. With this background, we carefully considered that a possible reason why we had failed to demonstrate efficacy in the FAS population of the first phase III study was because there were large variations in the clinical course of the ALS patients enrolled, and consequently the range of changes in the ALSFRS-R score was too great, as illustrated in . Accordingly, in this post-hoc subgroup analysis, we set out to identify a patient subpopulation in which the changes in the ALSFRS-R score could be expected to be more consistent. In seeking criteria to define such a subgroup, we focussed on the following issues.
Figure 1. Histograms showing changes in the ALSFRS-R score during 24-week treatment by patient (LOCF for patients who had completed at least the third cycle). (a) Full analysis set (FAS). The greatest changes in score in the placebo group (n = 99) and in the edaravone group (n = 100), respectively, were -26 and -28, while the smallest changes were +1 and +2. The median change in both groups was -4. The proportion of the patients with a decrease in the ALSFRS-R score of ≥12 points was 20.2% in the placebo group and 14.0% in the edaravone group. (b) dpEESP2y. The greatest changes in score in the placebo group (n = 29) and in the edaravone group (n = 39), respectively, were -26 and -13, while the smallest changes were 0 and +1. The median changes in the placebo and edaravone groups were -5 and -4, respectively. The proportion of patients with a decrease in the ALSFRS-R score of ≥12 points was 31.0% in the placebo group and 5.1% in the edaravone group. EESP = efficacy-expected sub-population of ALS patients (% forced vital capacity of ≥ 80% before treatment and ≥ 2 points for all items. dpEESP2y = subgroup of the EESP, containing patients with a diagnosis of ‘definite’ or ‘probable’ ALS according to the El Escorial revised Airlie House diagnostic criteria and with disease duration of ≤2 years. ALSFRS-R = revised ALS functional rating scale. ALS = amyotrophic lateral sclerosis. LOCF = Last observation carried forward.
We defined two subgroups of patients with ALS within the FAS, using the following criteria at the start of treatment:
Efficacy-expected subpopulation with %FVC of ≥80% before treatment and ≥2 points for all item scores in the ALSFRS-R before treatment (designated as EESP).
Greater-efficacy-expected subpopulation within the EESP, with a diagnosis of ‘definite’ or ‘probable’ ALS according to the El Escorial revised Airlie House diagnostic criteria (Citation6,Citation7), and within two years of initial ALS symptom onset at the time of giving informed consent (designated as ‘definite’ or ‘probable’/two years/within the EESP, or dpEESP2y).
First, in patients with advanced disease, the ALSFRS-R score of items may already have decreased to 0 or 1, so that assessment of efficacy would be difficult in these patients. Accordingly, patients having no item score of <2 in the ALSFRS-R at the time of enrolment may be more appropriate for the present purpose. Such patients do not require complete support for all daily care. Patients with respiratory dysfunction were also considered inappropriate for efficacy assessment, since some patients with respiratory dysfunction may show rapid progression, which might mask any effect of edaravone. Therefore, patients with %FVC of ≥80% (almost normal respiratory function) were considered more appropriate for the present purpose. Thus, we selected the EESP subpopulation within the FAS as the patient population that satisfied the above two criteria.
Secondly, to confirm the efficacy of edaravone with certainty, we focussed on the diagnosis of patients according to El Escorial revised Airlie House diagnostic criteria. The changes in the ALSFRS-R score in patients with a diagnosis of ‘probable-laboratory-supported’ were smaller than those of patients with a diagnosis of ‘definite’ or ‘probable’ (Citation2). Therefore, we decided to exclude patients with a diagnosis of ‘probable-laboratory-supported’ ALS and to focus on patients with a diagnosis of ‘definite’ or ‘probable’ ALS (abbreviated as ‘dp’), with the aim of increasing the ability of the ALSFRS-R score to distinguish between the edaravone group and placebo group over the 24-week timescale. In addition, we focussed on the rate of disease progression. According to ALS expert opinion, patients who have had ALS for more than two years are more likely to show less marked changes in symptoms and so can be expected to show smaller changes in the ALSFRS-R score. Therefore, we decided that only patients who were within two years of ALS symptom onset when they began 24 weeks of double-blind treatment should be included. Based on the above considerations, we selected a further subgroup within the EESP, designated dpEESP2y, which met all the following criteria: diagnosis of ‘definite’ or ‘probable’ ALS, within two years of initial ALS symptom onset, %FVC of ≥80%, and the ALSFRS-R score of ≥2 for all items.
Criteria for the FAS, EESP and dpEESP2y are summarised in .
Table 1. Comparison of analysis groups.
Post-hoc statistical analysis
For patients with missing data at 24 weeks after starting treatment, the last observation carried forward (LOCF) method was applied to impute missing data. Patients who completed the third cycle were eligible for LOCF. A two-sided level of significance was set at 5%.
ANOVA was performed on the changes in the ALSFRS-R scores during treatment, defined as the difference between the score before treatment and that 24 weeks after starting treatment for patients in the two subgroups after using the three factors ‘change in the ALSFRS-R score during pre-observation (–1, –2/–3, −4)’, ‘initial symptom (bulbar symptoms/limb symptoms)’ and ‘concomitant use of riluzole (yes/no)’ for dynamic allocation.
For supporting the result of the ALSFRS-R score, we also performed ANOVA analysis of %FVC, Modified Norris Scale score, and 40 items ALS assessment questionnaire (ALSAQ-40) score as secondary endpoints in the same manner as described for the ALSFRS-R scores.
Adverse events (AEs) and serious adverse events (SAEs) were assessed in the FAS, EESP and dpEESP2y. Proportions of AEs and SAEs were calculated and compared between placebo and edaravone groups using Fisher's exact test. SAEs were identified among AEs according to the GCP guideline.
Statistical analysis of efficacy and safety was performed using SAS software (version 9.1, SAS Institute, Cary, NC, USA).
Results
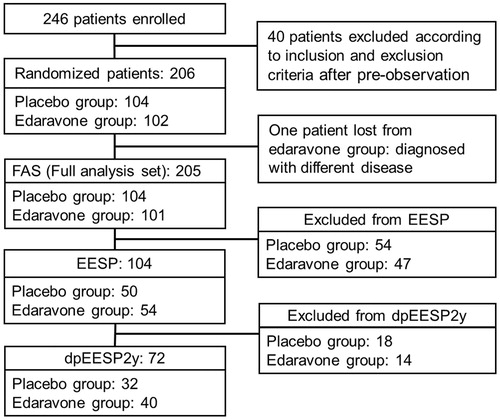
The FAS included 205 patients (104 patients in the placebo group and 101 patients in the edaravone group). The EESP included 104 patients (50 patients in the placebo group and 54 patients in the edaravone group). The dpEESP2y included 72 patients (32 patients in the placebo group and 40 patients in the edaravone group) ().
Figure 2. Subgroups for analysis. EESP = efficacy-expected sub-population of ALS patients (% forced vital capacity of ≥80% before treatment and ≥2 points for all item scores in the ALSFRS-R before treatment). dpEESP2y = subgroup of the EESP, containing patients with a diagnosis of ‘definite’ or ‘probable’ ALS according to the El Escorial revised Airlie House diagnostic criteria and with disease duration of ≤2 years. Last observation carried forward was applied to patients who completed the third cycle. ALS = amyotrophic lateral sclerosis. ALSFRS-R = revised ALS functional rating scale.
Demographic characteristics of patients in the FAS, EESP and dpEESP2y are summarised in .
Table 2. Subject demographic characteristics.
There were imbalances in ‘diagnosis’ in the FAS and EESP, and in ‘initial symptom’, ‘diagnosis’ and ‘use of riluzole’ in the dpEESP2y.
Efficacy results
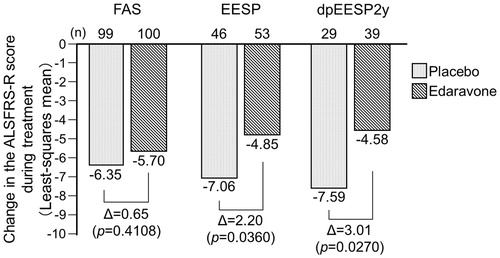
Histograms of changes in the ALSFRS-R score during treatment in the placebo and edaravone groups in the FAS and dpEESP2y are shown in ), respectively. The changes in the ALSFRS-R scores during treatment were compared using ANOVA; the differences between placebo and edaravone treatment in the FAS, EESP and dpEESP2y are shown in . The intergroup differences of the least-squares mean change in the ALSFRS-R score ± standard error during treatment were 0.65 ± 0.78 (p = 0.4108) in the FAS, 2.20 ± 1.03 (p = 0.0360) in the EESP, and 3.01 ± 1.33 (p = 0.0270) in the dpEESP2y. There was a significant intergroup difference in both the EESP and dpEESP2y. The inter-group differences were larger for the EESP and dpEESP2y than for the FAS, and the difference was larger for the dpEESP2y than for the EESP.
Figure 3. Changes in the ALSFRS-R score during treatment in the FAS, EESP and dpEESP2y (LOCF for patients who had completed at least the third cycle). Least-squares mean ± standard error of the inter-group differences in the ALSFRS-R score during treatment were 0.65 ± 0.78 (p = 0.4108) in the FAS, 2.20 ± 1.03 (p = 0.0360) in the EESP and 3.01 ± 1.33 (p = 0.0270) in the dpEESP2y. FAS = full analysis set. EESP = efficacy-expected subpopulation of ALS patients (% forced vital capacity of ≥80% before treatment and ≥2 points for all item scores in ALSFRS-R before treatment). dpEESP2y = subgroup of the EESP, containing patients with a diagnosis of ‘definite’ or ‘probable’ ALS according to the El Escorial revised Airlie House diagnostic criteria and with disease duration of ≤2 years. ALSFRS-R = revised ALS functional rating scale. ALS = amyotrophic lateral sclerosis. LOCF = last observation carried forward.
We also examined secondary endpoints, i.e. %FVC, Modified Norris Scale score and ALSAQ-40 score in the same manner used to evaluate the ALSFRS-R score, and the results are shown in . As in the case of the ALSFRS-R score, the intergroup differences of %FVC and those of Modified Norris Scale score were larger for the EESP and dpEESP2y than for the FAS, and the difference was larger for the dpEESP2y than for the EESP. In addition, the numbers of events (i.e. death or a specified state of disease progression, such as death, disability of independent ambulation, loss of upper limbs function, tracheotomy, use of respirator, and use of tube feeding) in the FAS, EESP and dpEESP2y are shown in .
Figure 4. Changes in the secondary endpoints during treatment in the FAS, EESP and dpEESP2y (LOCF for patients who had completed at least the third cycle). (a) % forced vital capacity: Least-squares mean ± standard error of the inter-group differences in % forced vital capacity during treatment were 2.92 ± 2.24 (p = 0.1928) in the FAS, 4.62 ± 2.31 (p = 0.0488) in the EESP and 6.30 ± 3.10 (p = 0.0467) in the dpEESP2y. (b) Modified Norris Scale score. Least-squares mean ± standard error of the inter-group differences in Modified Norris Scale score during treatment were 2.03 ± 1.89 (p = 0.2835) in the FAS, 6.86 ± 2.74 (p = 0.0141) in the EESP and 7.95 ± 3.63 (p = 0.0326) in the dpEESP2y. (c) ALSAQ-40 score: Least-squares mean ± standard error of the inter-group differences in ALSAQ-40 score during treatment were 0.48 ± 3.50 (p = 0.8921) in the FAS, -2.51 ± 5.11 (p = 0.6244) in the EESP and -3.14 ± 6.76 (p = 0.6442) in the dpEESP2y. ALSAQ-40 = ALS assessment questionnaire (40 items). ALS = amyotrophic lateral sclerosis. FAS = full analysis set. EESP = efficacy-expected sub-population of ALS patients (% forced vital capacity of ≥80% before treatment and ≥2 points for all item scores in the ALSFRS-R before treatment). dpEESP2y = subgroup of the EESP, containing patients with a diagnosis of ‘definite’ or ‘probable’ ALS according to the El Escorial revised Airlie House diagnostic criteria and with disease duration of ≤2 years. LOCF = last observation carried forward.
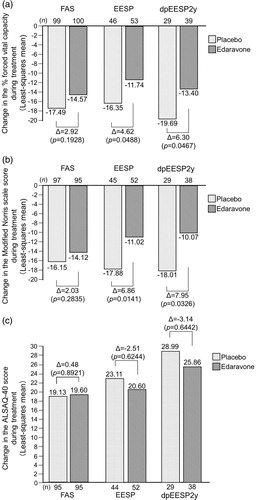
Table 3. Events of death or a specified state of disease progression in the FAS, EESP and dpEESP2y.
For reference, summary statistics of changes from baseline of the ALSFRS-R score in the FAS, EESP and dpEESP2y subgroups are shown in .
Table 4. Summary statistics of changes from baseline of the ALSFRS-R score in the FAS and subgroups (LOCF for patients who had completed at least the third cycle).
We also carried out post-hoc analyses employing ANOVA with LOCF using all available data for each patient and employing the mixed effects model for repeated measures (MMRM) in order to assess whether or not our predetermined criterion that LOCF imputation should be used only for patients who had completed at least the third treatment cycle had introduced any marked bias. The results of both analyses were consistent with the results of ANOVA with LOCF for patients who had completed at least the third cycle (data not shown).
Safety results
The proportions of AEs and SAEs in the all randomised patients in the FAS, EESP and dpEESP2y are shown in . The proportions of AEs in the EESP and dpEESP2y were similar to those in all randomised patients. There was a significant intergroup difference in the proportions of SAEs in both the EESP and dpEESP2y, with a higher proportion in the placebo group compared to the edaravone group (EESP: 24.0% vs. 1.9%, p = 0.0007 and dpEESP2y: 25.0% vs. 2.5%, p = 0.0085, respectively).
Table 5. Proportions of adverse events and serious adverse events in all randomized patients, the EESP and the dpEESP2y.
Discussion
ANOVA analysis showed significant differences regarding change in the ALSFRS-R score between placebo and edaravone groups in both the EESP and dpEESP2y, and the difference was larger in the dpEESP2y than the EESP (). These results suggest that edaravone inhibited the progression of functional disorder in patients with ALS in the EESP and dpEESP2y subpopulations, especially in the latter. Therefore, we concluded that it should be possible to demonstrate the efficacy of edaravone in a second prospective phase III study if the inclusion criteria were modified, i.e. if the dpEESP2y criteria were used to select an appropriate patient group.
As regards safety, there appeared to be no notable safety issues in the EESP and dpEESP2y subpopulations in items of the incidence of AEs and SAEs compared with the placebo group, although the number of subjects was limited.
Based on the above conclusion, the second phase III study in ALS patients who met the dpEESP2y criteria was subsequently carried out in Japan (ClinicalTrials.gov number: NCT01492686), and the results confirmed efficacy of edaravone in this patient subpopulation (Citation8).
We believe the results of this post-hoc analysis of the first phase III study will be of interest to clinicians and researchers studying various diseases where efficacy evaluation is problematic for various reasons. The possibility that a drug may benefit a well-defined subgroup of patients deserves careful attention.
The Edaravone (MCI-186) ALS 16 Study Group: Site investigators
The Edaravone (MCI-186) ALS 16 Study Group investigators are as follows.
Hidenao Sasaki, Hokkaido University Hospital; Asako Takei and Isao Yamashita, Hokuyukai Neurological Hospital; Takashi Imai, National Hospital Organisation Miyagi National Hospital; Imaharu Nakano††, Jichi Medical School Hospital; Koichi Okamoto, Gunma University Hospital; Yuichi Maruki, Saitama Center of Neurology and Psychiatry; Shuichi Mishima and Jin Nishimiya, Kohnodai Hospital, National Center for Global Health and Medicine; Yasuo Iwasaki, Toho University Omori Medical Center; Mineo Yamazaki, Nippon Medical School Hospital; Yuji Takahashi, The University of Tokyo Hospital; Mieko Ogino and Yutaka Ogino, Kitasato University East Hospital; Masafumi Ogawa, National Center of Neurology and Psychiatry (NCNP); Tetsumasa Kamei, Shonan Fujisawa Tokushukai Hospital; Tsuyoshi Uchiyama, Seirei Hamamatsu General Hospital; Hirohisa Watanabe, Nagoya University Hospital; Yasumasa Kokubo, Mie University Hospital; Hideyuki Sawada, National Hospital Organisation Utano Hospital; Takanori Hazama, Osaka General Medical Center; Fumiharu Kimura, Osaka Medical College Hospital; Harutoshi Fujimura, National Hospital Organisation Toneyama National Hospital; Hirofumi Kusaka, Kansai Medical University Takii Hospital; Tsukasa Hashimoto, National Hospital Organisation Ehime National Hospital; Takeshi Yamada, Yuji Kanamori and Kenji Yamasaki, Saiseikai Fukuoka General Hospital; Shizuma Kaku, Fukuoka Tokushukai Medical Center; Hitoshi Kikuchi, Murakami Karindoh Hospital; Shigehiro Imamura, National Hospital Organisation Kumamoto Saishunso National Hospital; Seiichiro Sugimoto and Masahiko Kishi, National Hospital Organisation Miyazaki Higashi Hospital. ††Deceased.
Declaration of interest
Mr. Abe received travel funds and speaker honoraria from Mitsubishi Tanabe Pharma Corporation. Mr. Itoyama received speaker honoraria from Mitsubishi Tanabe Pharma Corporation. Mr. Tsuji received travel funds and speaker honoraria from Mitsubishi Tanabe Pharma Corporation. Mr. Sobue received travel funds and speaker honoraria from Mitsubishi Tanabe Pharma Corporation, and serves on the scientific advisory board for the Kanae Science Foundation for the Promotion of Medical Science, Naito Science Foundation, and as an advisory board member of Brain, and editorial board member of Degenerative Neurological and Neuromuscular Disease, the Journal of Neurology, and Amyotrophic Lateral Sclerosis and Frontotemporal Degeneration, and has received funding from several Japanese government agencies. Mr. Aoki received travel funds, speaker honoraria, and fees for conducting and consulting on pharmacological testing of edaravone in a rat ALS model from Mitsubishi Tanabe Pharma Corporation, and has received research grants from several Japanese government agencies, including an Intramural Research Grant for Neurological Psychiatric Disorders from the National Center of Neurology and Psychiatry (NCNP). Mr. Doyu received travel funds and speaker honoraria from Mitsubishi Tanabe Pharma Corporation. Dr. Hamada is a consultant for Chugai Pharmaceutical Co. Ltd., Taiho Pharmaceutical Co. Ltd., Kowa Company Ltd., Sanwa Kagaku Kenkyusho Co. Ltd., Maruho Co. Ltd., Daiichi Sankyo Co. Ltd., Eisai Co. Ltd., Mochida Pharmaceutical Co. Ltd., Otsuka Pharmaceutical Co. Ltd., Nippon Shinyaku Pharmaceutical Co. Ltd., and Mitsubishi Tanabe Pharma Corporation. Mr. Togo, Mr. Tanaka, Mr. Akimoto, Ms. Nakamura, Mr. Takahashi and Mr. Kondo are employees of Mitsubishi Tanabe Pharma Corporation. Mr. Yoneoka is an employee of and had co-owned a patent with Mitsubishi Tanabe Pharma Corporation. Mr. Yoshino received travel funds and speaker honoraria from, had co-owned a patent with, and is a consultant for Mitsubishi Tanabe Pharma Corporation.
This study was funded by Mitsubishi Tanabe Pharma Corporation.
Open access publication of this article and editorial support were funded by Mitsubishi Tanabe Pharma America, Inc.
Acknowledgements
We thank all participating patients, their family members, and participating staff at all study sites; Teresa Oblak of Covance Market Access, Inc., for editorial assistance; Aya Tokaji and Marika Ogasawara of MDS-CMG Inc., for publication assistance; Richard Steele of WRS Steele Scientific and Technical Editing for proofreading the manuscript; Koji Takei and Kikumi Tsuda of Mitsubishi Tanabe Pharma Development America for critical review of the manuscript.
Additional information
Funding
References
- The Edaravone Acute Brain Infarction Study Group. Effect of a novel free radical scavenger, edaravone (MCI-186), on acute brain infarction. Randomized, placebo-controlled, double-blind study at multicenters. Cerebrovasc Dis. 2003; 15:222–9.
- Abe K, Itoyama Y, Sobue G, Tsuji S, Aoki M, Doyu M, et al. Confirmatory double-blind, parallel-group, placebo-controlled study of efficacy and safety of edaravone (MCI-186) in amyotrophic lateral sclerosis patients. Amyotroph Lateral Scler Frontotemporal Degener. 2014; 15:610–7.
- Cedarbaum JM, Stambler N, Malta E, Fuller C, Hilt D, Thurmond B, et al. The ALSFRS-R: a revised ALS functional rating scale that incorporates assessments of respiratory function. J Neurol Sci. 1999; 169:13–21.
- Ohashi Y, Tashiro K, Itoyama Y, Nakano I, Sobue G, Nakamura S, et al. Study of functional rating scale for amyotrophic lateral sclerosis: revised ALSFRS (ALSFRS-R) Japanese version. Brain Nerve. 2001;53:346–55. [Japanese]
- Japan Intractable Diseases Information Center [online]. Last updated March 2, 2015. Available at: http://www.nanbyou.or.jp/entry/52. Accessed August 14, 2017. [Japanese]
- Brooks BR. Defining optimal management in ALS: from first symptoms to announcement. Neurology. 1999; 53:S1–S3.
- Brooks BR, Miller RG, Swash M, Munsat TL. El Escorial revisited: revised criteria for the diagnosis of amyotrophic lateral sclerosis. Amyotroph Lateral Scler Other Motor Neuron Disord. 2000; 1:293–9.
- The Writing Group on behalf of the Edaravone (MCI-186) ALS 19 Study Group. Safety and efficacy of edaravone in well defined patients with amyotrophic lateral sclerosis: a randomised, double-blind, placebo-controlled trial. Lancet Neurol. 2017; 16:505–12.
