Abstract
We aimed to explore the longer-term efficacy and safety of edaravone in an active-treatment extension period following the double-blind period of the second phase III study. Patients who met all the following criteria (scores ≥2 points on all 12 items of the revised amyotrophic lateral sclerosis functional rating scale [ALSFRS-R], forced vital capacity ≥80%, definite or probable ALS, and disease duration ≤2 years) were randomised to 60 mg intravenous edaravone or placebo for six cycles in the double-blind period, and then offered the opportunity to proceed to this 24-week open-label extension period. One hundred and twenty-three of 137 patients continued to the extension period: 65 edaravone-edaravone (E-E group) and 58 placebo-edaravone (P-E group). Change (mean ± standard deviation; SD) in the ALSFRS-R score from baseline in the double-blind period was –4.1 ± 3.4 and –6.9 ± 5.1 in the E-E group and P-E group, respectively, while it was –8.0 ± 5.6 in the E-E group and –10.9 ± 6.9 in the P-E group over the whole 48-week period. The ALSFRS-R score changed almost linearly throughout Cycles 1–12 in the E-E group. The most commonly reported adverse events were constipation, dysphagia, and contusion. There was no sudden deterioration in the ALSFRS-R score of the E-E group. No safety concerns related to edaravone were detected.
Introduction
Edaravone (MCI-186) is a free radical scavenger (Citation1–3) that has been used in 1.7 million patients since its approval in Japan in 2001 for improvement of neurological symptoms, disorder of activities of daily living, and functional disorders associated with acute ischaemic stroke (Citation4). Potential benefits of edaravone in ALS include the ability to scavenge radical species (e.g. hydroxyl radicals), ameliorate oxidative stress, and suppress degeneration of spinal motor neurons (Citation1,Citation5,Citation6). Previous studies have demonstrated potential benefits of edaravone in the treatment of ALS (Citation7–10).
We designed the second phase III study (Citation11) in a prospectively defined group of ALS patients meeting the criteria identified in the post-hoc analysis of the first phase III study. This study consisted of a 12-week pre-observation period, a 24-week, randomised, placebo-controlled, double-blind period, and a 24-week, open-label, extension active-treatment period. In the double-blind period, edaravone showed a statistically significant difference from placebo in the least-squares mean (LS mean) change in the revised ALS functional rating scale (ALSFRS-R) with standard error (SE) (difference: 2.49 ± 0.76, p = 0.0013). The results at secondary endpoints (forced vital capacity [%FVC], Modified Norris Scale Score, ALS Assessment Questionnaire (ALSAQ-40) and time to death or a specified state of disease progression) supported the primary analysis result of the ALSFRS-R score. The findings in the double-blind period of the second phase III study have been reported in detail (Citation11).
The open-label, active-treatment extension period was included for exploratory evaluation of efficacy and safety for ethical reasons, so that all patients who had participated in the double-blind period would have the opportunity to receive edaravone if they so wished. Here we describe the results of the extension period of the second phase III study, and we discuss their implications for the longer term – up to 48 weeks – efficacy of edaravone.
Materials and methods
Patients
Patients were required to meet all the following inclusion criteria:
1) Scores ≥2 points, which correspond to a condition not requiring assistance, on all items of the ALSFRS-R (note that a score of 4 was required for each of the three items in ‘Dyspnoea, Orthopnoea and Respiratory Insufficiency in Respiration’; see exclusion criteria below)
2) %FVC ≥80%, as the criterion for normal respiratory function
3) Definite or probable ALS (El Escorial/revised Airlie House criteria (Citation12))
4) Duration of disease from the first symptom (any ALS symptom) ≤ 2 years.
In addition, eligible patients were Grade 1 or 2 (Citation11) ALS severity according to the Japan ALS Severity Classification, aged 20 to 75 years and had to show a change in the ALSFRS-R score during the 12-week pre-observation period before study drug administration of –1 to –4 points. Exclusion criteria included reduced respiratory function and complaints of dyspnoea (ALSFRS-R score of 3 points or lower for any of the three items in ‘Dyspnoea, Orthopnoea and Respiratory Insufficiency in Respiration’), and renal dysfunction with creatinine clearance of 50 ml/min or below within 28 d before treatment. Patients who had already been treated with riluzole could continue to receive it provided the regimen remained unchanged.
Study design
Eligible patients were randomised in a 1:1 ratio to parallel groups receiving 60 mg edaravone or placebo. A minimisation method of dynamic randomisation was used to achieve balance across important prognostic factors. In Cycle 1, the study drug was administered for 14 consecutive days followed by a two-week drug-free period. In Cycle 2 and thereafter, the study drug was administered for 10 d within a 14-d period followed by a two-week drug-free period. The double-blind period was six cycles.
After the double-blind period, all patients who had completed Cycle 6 and wanted to continue were offered open-label, active extension treatment with edaravone for an additional six cycles (up to Cycle 12).
The investigators were responsible for any final medical judgment and also evaluated efficacy and safety.
Because we thought breathing management should take first priority when respiratory function is decreased, we set a discontinuation criterion of %FVC ≤50% and PaCO2 (blood gas) ≥45 mmHg.
Study medication
In the active-treatment period, treatment was given for a total of 10 d per two weeks, followed by a two-week drug-free period. Edaravone (60 mg) was administered once a day (60-min intravenous infusion). Mitsubishi Tanabe Pharma Corporation provided edaravone in ampoules.
Efficacy evaluation
The efficacy endpoints were change in the ALSFRS-R total score, %FVC, Modified Norris Scale score (Citation13,Citation14), ALSAQ-40 score (Citation15,Citation16), and time to death or a specified state of disease progression that included disability of independent ambulation, loss of upper-limb function, tracheotomy, use of a respirator, use of a tube feeding, and loss of useful speech. The evaluations were carried out at the following times: before pre-observation, before the start of the first treatment cycle and at the end of each treatment cycle (after 14 d observation and before the first dosage of the next cycle).
Safety evaluation
The safety evaluation included incidence of adverse events (AEs), adverse drug reactions (ADRs), serious adverse events (SAEs), and laboratory tests. SAEs were identified from AEs according to the Good Clinical Practice (GCP) guideline.
Statistical analysis
A statistical test was not performed, and the distribution of efficacy and safety for the extension period was only confirmed by intent. Efficacy was analysed in the full-analysis set (FAS), defined as all patients with ALS who received at least one dose of edaravone and had at least one efficacy data point during the active-treatment period. Safety was analysed in the safety analysis set, defined as any patient who had received at least one dose of edaravone and had at least one safety data point during the active-treatment period. Descriptive statistics and frequency count with percentage were used for continuous endpoint and categorical data, respectively, in order to mainly confirm the distributions for each endpoint. Subsequently, baseline in Cycle 1 was used for statistical analysis. For time to the first event date of death or a specified state of disease progression, a Kaplan-Meier curve was implemented using the date of censoring, which was defined as the last observation date. Statistical analysis was performed using SAS software (version 9.2, SAS Institute, Cary, NC).
Ethics conduct
Thirty-one study sites in Japan participated in this study from November 2011 to September 2014. Each patient provided written informed consent before any study procedures. The study was conducted in compliance with the Japanese Ministerial Ordinance on GCP and in accordance with the ethics principles of the Declaration of Helsinki. An institutional review board approved the protocol at each site. The study was registered with ClinicalTrials.gov (NCT01492686).
Results
Patients
Overall, 213 patients were screened, and 137 patients were randomised to receive edaravone (n = 69) or placebo (n = 68) and 127 patients completed the double-blind period (edaravone n = 67 and placebo n = 60). Of these, two patients in the E-E group and two patients in the P-E group did not participate in the active-treatment period. One hundred and twenty-three patients continued into the active-treatment period; 65 patients had previously received edaravone (E-E group), and 58 patients had previously received placebo (P-E group). All patients were included in the FAS and safety analysis set (defined as the patients who received at least one dose of study drug and had at least one set of safety data during the active-treatment period). Patient disposition is shown in . The demographics and baseline characteristics of patients are shown in .
Figure 1. Trial profile. *Patient’s request, 2; **Patient’s request, 2; ***Patient’s request, 6; investigator’s decision due to AE, 1 (pneumonia aspiration); investigator’s decision due to worsening of ALS, 1; %FVC of ≤50% and PaCO2 (blood gas) of ≥45 mmHg, 4; ****patient’s request, 7; investigator’s decision due to AE, 2 (pneumonia aspiration, blood in urine/protein in urine/blood pressure increased); all-day respiratory support required, 3; %FVC of ≤50% and PaCO2 (blood gas) of ≥45 mmHg, 6. AE: adverse event; %FVC: % forced vital capacity.
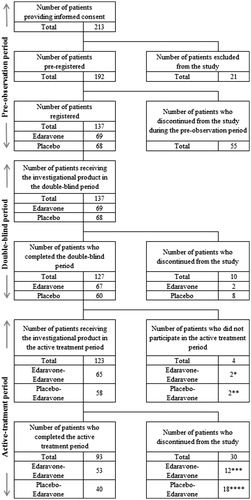
Table 1. Subject demographic characteristics in the FAS.
During the active-treatment period, 53 patients in the E-E group and 40 in the P-E group received all infusions in compliance with the study protocol. Twelve patients in the E-E group and 18 in the P-E group discontinued treatment, in accordance with a discontinuation criterion, before completion of Cycle 12.
Efficacy
The change in the ALSFRS-R score from the start of the double-blind period to the end of the active-treatment period is shown in . The mean change ± SD in the ALSFRS-R score from baseline in Cycle 1 to the end of Cycle 6 was –4.1 ± 3.4 in the E group vs. –6.9 ± 5.1 in the P group. Throughout, the change from baseline in Cycle 1 to the end of Cycle 12 was –8.0 ± 5.6 in the E-E group vs. –10.9 ± 6.9 in the P-E group.
Figure 2. The efficacy endpoints in the FAS (Mean ± SD). (a) ALSFRS-R score (b) %FVC (c) Modified Norris Scale (d) ALSAQ-40; ALSFRS-R score: Total =48, Worst =0, Best =48; ALSFRS-R score (Limb function): Total =24, Worst =0, Best =24; ALSFRS-R score (Bulbar function): Total =12, Worst =0, Best =12; ALSFRS-R score (Respiratory function): Total =12, Worst =0, Best =12; Modified Norris Scale score: Total =102, Worst =0, Best =102; Modified Norris Scale score (Limb scale): Total =63, Worst =0, Best =63; Modified Norris Scale score (Bulbar scale): Total =39, Worst =0, Best =39; ALSAQ-40 score: Total =200, Worst =200, Best =40. BL: baseline; FAS: full analysis set; SD: standard deviation; ALSFRS-R: Revised ALS Functional Rating Scale; %FVC: % forced vital capacity; ALSAQ-40: ALS assessment questionnaire 40; E: Edaravone group; P: placebo group.
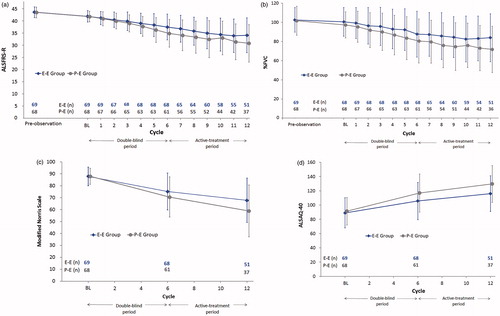
Changes in %FVC, Modified Norris Scale (total) and ALSAQ-40 from the start of the double-blind period to the end of the active-treatment period are shown in , and , respectively.
The Kaplan-Meier curve representing time to death or a specified state of disease progression over the 48 weeks from the start of the double-blind period to the end of the active-treatment period is shown in . There were 15 events in 10 patients in the E-E group and 20 events in 19 patients in the P-E group.
Figure 3. Survival rate, by death or a specified state of disease progression* in the FAS. A Kaplan-Meier curve was constructed, with any of ‘Death, Disability of independent ambulation, Loss of upper limbs function, Tracheotomy, Use of respirator, Use of tube feeding and Loss of useful speech’ defined as an event, and the date of censoring, which was defined as the last observation date. For patients with multiple events, the day of onset of the first event was considered the event onset day. *Event description. Death. Disability of independent ambulation: Rating of 0 points (‘No purposeful leg movement’) for ALSFRS-R item ‘8. Walking’ was used as criterion. Loss of upper limbs function: Criterion was ALSFRS-R rating of 0 points for all of the following items: ‘Handwriting,’ ‘Eating motion,’ and ‘Dressing and hygiene’ (i.e., ‘Handwriting’ [‘Unable to grip pen’]; ‘Eating motion: Handling utensils (patients without gastrostomy)’ [‘Needs to be fed’]; ‘Eating motion: Finger motion (patients with gastrostomy)’ [‘Unable to perform any aspect of task’]; and ‘Dressing and hygiene’ [‘Total dependence’]). Tracheotomy. Use of respirator: Did not include the use of BiPAP. Use of tube feeding: Criterion was ALSFRS-R rating of 0 points for ‘Swallowing’ (Exclusively parenteral or enteral feeding). Loss of useful speech: Criterion was ALSFRS-R rating of 0 points for ‘Speech’ (Loss of useful speech). Abbreviations: ALSFRS-R: ALS functional rating scale-revised; BiPAP: bilevel positive airway pressure; FAS: full analysis set; E: edaravone group; P: placebo group.
![Figure 3. Survival rate, by death or a specified state of disease progression* in the FAS. A Kaplan-Meier curve was constructed, with any of ‘Death, Disability of independent ambulation, Loss of upper limbs function, Tracheotomy, Use of respirator, Use of tube feeding and Loss of useful speech’ defined as an event, and the date of censoring, which was defined as the last observation date. For patients with multiple events, the day of onset of the first event was considered the event onset day. *Event description. Death. Disability of independent ambulation: Rating of 0 points (‘No purposeful leg movement’) for ALSFRS-R item ‘8. Walking’ was used as criterion. Loss of upper limbs function: Criterion was ALSFRS-R rating of 0 points for all of the following items: ‘Handwriting,’ ‘Eating motion,’ and ‘Dressing and hygiene’ (i.e., ‘Handwriting’ [‘Unable to grip pen’]; ‘Eating motion: Handling utensils (patients without gastrostomy)’ [‘Needs to be fed’]; ‘Eating motion: Finger motion (patients with gastrostomy)’ [‘Unable to perform any aspect of task’]; and ‘Dressing and hygiene’ [‘Total dependence’]). Tracheotomy. Use of respirator: Did not include the use of BiPAP. Use of tube feeding: Criterion was ALSFRS-R rating of 0 points for ‘Swallowing’ (Exclusively parenteral or enteral feeding). Loss of useful speech: Criterion was ALSFRS-R rating of 0 points for ‘Speech’ (Loss of useful speech). Abbreviations: ALSFRS-R: ALS functional rating scale-revised; BiPAP: bilevel positive airway pressure; FAS: full analysis set; E: edaravone group; P: placebo group.](/cms/asset/c1c10845-2338-4de3-b0f4-3ac6a9d44f9d/iafd_a_1364269_f0003_c.jpg)
Safety
During the active-treatment period, 81.5% of patients in the E-E group and 82.8% of patients in the P-E group reported at least one AE. Of these, 26.2% and 39.7% were reported as serious, respectively.
During the active treatment period, eight AEs led to death in six patients. In the E-E group, two events in two patients were reported: respiratory disorder and respiratory failure. In the P-E group, six events in four patients were reported: respiratory disorder in one patient, pneumonia aspiration in two patients, respiratory failure in two patients, and stress cardiomyopathy in one patient. All were considered ‘not reasonably possible’ with regard to a relationship to the study drug.
AEs reported by ≥5% of either group are listed in , along with SAEs reported by at least two patients in either group. The most commonly reported AEs (≥5% of patients in both treatment groups) were nasopharyngitis, respiratory disorder, constipation, dysphagia, and contusion. The most commonly reported SAEs (≥3 events in either treatment group) were dysphagia, respiratory disorder, musculoskeletal disorder, pneumonia aspiration, and respiratory failure. No differences in laboratory measurement changes were observed between the groups.
Table 2. Adverse events (≥5%) and serious adverse events (more than one patient)Table Footnotea in the safety analysis set.
The incidence of ADRs was 6.2% in the E-E group (four of 65 patients experienced six events: vertigo, eczema, eczema asteatotic, back pain, pollakiuria, white blood cell count increased) and 5.2% in the P-E group (three of 58 patients experienced four events: arrhythmia, gamma-glutamyltransferase increased, blood urine present, protein urine present). There were no serious ADRs in either the edaravone group or the placebo group.
Discussion
The 24-week, active-treatment extension period was used to explore longer term efficacy in patients who received edaravone for the whole 48-week period (the E-E group) in terms of time-dependent change in the ALSFRS-R score. Mean summary statistics showed that the ALSFRS-R score changed almost linearly throughout Cycles 1–12 in the E-E group. In accordance with this result, there was no sudden deterioration in %FVC, Modified Norris Scale score or ALSAQ-40 during the period.
The incidences of AEs and serious ADRs, as well as changes in laboratory test values, were all similar in the E-E and P-E groups. Although the incidence of SAEs was slightly higher in the P-E group, the difference was considered attributable to the progression of ALS. Consequently, the extension study uncovered no concerns regarding the safety of edaravone.
The lack of statistical test and a placebo group during the active-treatment extension period represents a limitation compared with the preceding rigorous double-blind, placebo-controlled period. However, it is an important finding that no causes for concern emerged during the overall 48-week treatment period in the E-E group.
Evaluation of long-term treatment for efficacy and safety remains for a future issue.
Declaration of interests
Mr. Abe reports personal fees from Mitsubishi Tanabe Pharma Corporation unrelated to the submitted work. Mr. Aoki reports grants from Research on Psychiatric and Neurological Diseases and Mental Health from the Japanese Ministry of Health Labour and Welfare, Grants-in-Aid for Scientific Research, grants from An Intramural Research Grant for Neurological Psychiatric Disorders from NCNP, grants from Grants-in-Aids for Scientific Research from the Japanese Ministry of Education, Culture, Sports, Science and Technology (MEXT), grants from Practical Research Project for Rare/Intractable Diseases from Japan Agency for Medical Research and Development (AMED), personal fees from Eisai Inc., Mitsubishi Tanabe Pharma Corporation, Astellas Pharma Inc., Takeda Pharmaceutical Company Ltd., Sanofi K.K., Novartis Pharma K.K., and Dainippon Sumitomo Pharma Co. Ltd., unrelated to the submitted work. Mr. Tsuji reports grants from the Ministry of Education, Culture, Sports, Science and Technology of Japan, grants from Ministry of Health, Welfare and Labor, Japan, grants from Japan Agency for Medical Research and Development, and personal fees from Mitsubishi Tanabe Pharma Corporation unrelated to the submitted work. Mr. Itoyama reports grants from Health and Labour Sciences Research Grant and personal fees from Mitsubishi Tanabe Pharma Corporation unrelated to the submitted work. Mr. Sobue reports personal fees from Mitsubishi Tanabe Pharma Corporation, Shionogi & Co., Ltd., Bristol-Myers Squibb, Sumitomo Dainippon Pharma Co., Ltd., Novartis Pharma KK, Bayer Yakuhin, Ltd., Pfizer Japan Inc., Boehringer Ingelheim Japan, Inc., Kissei Pharmaceutical Co., Ltd., Janssen Pharmaceutical K.K., Teijin Pharma Ltd., FP Pharmaceutical Corporation, Nihon Pharmaceutical Co., Ltd., Japan Blood Products Organisation, Kowa Pharmaceutical Co., Ltd., Ono Pharmaceutical Co., Ltd., Otsuka Pharmaceutical Co., Ltd., Eisai Co., Ltd., as well as grants from Grants-in-Aid from the Ministry of Health, Labor and Welfare Japan, grants from Grant-in-Aid, Japan's Ministry of Education, Culture, Sports, Science and Technology, grants from Grant-in-Aid for Scientific Research, and the Japan Society for the Promotion of Science, unrelated to the submitted work. Mr. Hamada reports personal fees from Mitsubishi Tanabe Pharma Corporation unrelated to the submitted work. Mr. Togo, Mr. Tanaka, Mr. Akimoto, Ms. Nakamura, Mr. Ueda, Mr. Takahashi and Mr. Kondo report personal fees from Mitsubishi Tanabe Pharma Corporation during the conduct of the study as well as personal fees from Mitsubishi Tanabe Pharma Corporation unrelated to the submitted work. Mr. Yoshino reports personal fees from Mitsubishi Tanabe Pharma Corporation with whom he had co-owned a patent unrelated to the submitted work.
The study was funded by Mitsubishi Tanabe Pharma Corporation.
Open access publication of this article and editorial support were funded by Mitsubishi Tanabe Pharma America, Inc.
Acknowledgements
We thank all participating patients and their family members and the Data Safety Monitoring Committee (Manabu Doyu, Department of Neurology, Aichi Medical University Hospital, Aichi; Fumio Kanda, Integrated Clinical Education Center, Kobe University Hospital, Kobe; Toshimitsu Hamasaki, Osaka University, Graduate School of Medicine, Osaka (present affiliation is National Cerebral and Cardiovascular Center, Osaka); participating staff at all study sites; Teresa Oblak of Covance Market Access, Inc., for editorial assistance; Aya Tokaji and Marika Ogasawara of MDS-CMG Inc., for publication assistance; Richard Steele of WRS Steele Scientific and Technical Editing for proofreading the manuscript; Koji Takei and Kikumi Tsuda of Mitsubishi Tanabe Pharma Development America for critical review of the manuscript.
Additional information
Funding
References
- Watanabe T, Yuki S, Egawa M, Nishi H. Protective effects of MCI-186 on cerebral ischemia: possible involvement of free radical scavenging and antioxidant actions. J Pharmacol Exp Ther. 1994;268:1597–604.
- Yamamoto T, Yuki S, Watanabe T, Mitsuka M, Saito K, Kogure K. Delayed neuronal death prevented by inhibition of increased hydroxyl radical formation in a transient cerebral ischemia. Brain Res. 1997;762:240–2.
- Mizuno A, Umemura K, Nakashima M. Inhibitory effect of MCI-186, a free radical scavenger, on cerebral ischemia following the rat middle cerebral artery occlusion. Gen Pharmacol. 1998;30:575–8.
- Edaravone Acute Brain Infarction Study Group. Effect of a novel free radical scavenger, edaravone (MCI-186), on acute brain infarction. Randomized, placebo-controlled, double-blind study at multicenters. Cerebrovasc Dis. 2003;15:222–9.
- Ikeda K, Iwasaki Y, Kinoshita M. Treatment of wobbler mice with free radical scavenger. In: Molecular mechanism and therapeutics of amyotrophic lateral sclerosis. Amsterdam: Elsevier Science; 2001:335–340.
- Watanabe T, Morita I, Nishi H, Murota S. Preventive effect of MCI-186 on 15-HPETE induced vascular endothelial cell injury in vitro. Prostaglandins Leukot Essent Fatty Acids. 1988;33:81–7.
- Abe K, Itoyama Y, Sobue G, Tsuji S, Aoki M, Doyu M, et al. Confirmatory double-blind, parallel-group, placebo-controlled study of efficacy and safety of edaravone (MCI-186) in amyotrophic lateral sclerosis patients. Amyotroph Lateral Scler Frontotemporal Degener. 2014;15:610–17.
- The Edaravone (MCI-186) ALS 16 Study Group. A post-hoc subgroup analysis of outcomes in the first phase III clinical study of edaravone (MCI-186) in amyotrophic lateral sclerosis. Amyotroph Lateral Scler Frontotemporal Degener. 2017;18(Suppl). [Epub ahead of print]. doi: 10.1080/21678421.2017.1363780.
- The Writing Group on behalf of the Edaravone (MCI-186) ALS 17 Study Group. Exploratory double-blind, parallel-group, placebo-controlled extension study of edaravone (MCI-186) in amyotrophic lateral sclerosis. Amyotroph Lateral Scler Frontotemporal Degener. 2017;18(Suppl). [Epub ahead of print]. doi: 10.1080/21678421.2017.1362000.
- The Writing Group on behalf of the Edaravone (MCI-186) ALS 18 Study Group. Exploratory double-blind, parallel-group, placebo-controlled study of edaravone (MCI-186) in amyotrophic lateral sclerosis (Japan ALS Severity Classification: Grade 3, requiring assistance for eating, excretion or ambulation). Amyotroph Lateral Scler Frontotemporal Degener. 2017;18(Suppl). [Epub ahead of print]. doi: 10.1080/21678421.2017.1361441.
- The Writing Group on behalf of the Edaravone (MCI-186) ALS 19 Study Group. Safety and efficacy of edaravone in well defined patients with amyotrophic lateral sclerosis: a randomised, double-blind, placebo-controlled trial. Lancet Neurol. 2017;16:505–12.
- Brooks BR, Miller RG, Swash M, Munsat T; World Federation of Neurology Research Group on Motor Neuron Diseases. El Escorial revisited: revised criteria for the diagnosis of amyotrophic lateral sclerosis. Amyotroph Lateral Scler Other Motor Neuron Disord. 2000;1:293–9.
- Norris FH,Jr., Calanchini PR, Fallat RJ, Panchari S, Jewett B. The administration of guanidine in amyotrophic lateral sclerosis. Neurology. 1974;24:721–8.
- Oda E, Ohashi Y, Tashiro K, Mizuno Y, Kowa H, Yanagisawa N. Reliability and factorial structure of a rating scale for amyotrophic lateral sclerosis. No To Shinkei. 1996;48:999–1007. [article in Japanese].
- Jenkinson C, Fitzpatrick R, Brennen C, Swash M. Evidence for the validity and reliability of the ALS assessment questionnaire: the ALSAQ40. Amyotroph Lateral Scler Other Motor Neuron Disord. 1999;1:33–40.
- Yamaguchi T, Ohbu S, Saito M, Ito Y, Moriwaka F, Tashikiro K, et al. Validity and clinical applicability of the Japanese version of amyotrophic lateral sclerosis: assessment questionnaire 40 (ALSAQ-40). No To Shinkei. 2004;56:483–94. [article in Japanese].
