Abstract
Objective: Lunasin, a soy peptide that reportedly alters histone acetylation in vitro, was associated with a single ALS reversal in the media. Following an ALSUntangled report, we sought to determine whether Lunasin altered histone acetylation and improved progression in people with ALS, and whether patient-centric trial design features might improve enrollment and retention. Methods: This single-center, year-long trial (NCT02709330) featured broad inclusion criteria, historical controls, primarily virtual data collection, and real-time results. Participants measured their own ALSFRS-R score, weight and perceived efficacy, and recorded these monthly on PatientsLikeMe. Blood tests at screening and month 1 assessed alterations in histone H3 and H4 acetylation. The protocol was published online, empowering patients outside the study to self-experiment. Results: Fifty participants enrolled in 5.5 months. Although this population had more advanced disease compared to other trials, retention and adherence were very high. There was no significant effect of Lunasin treatment on histone acetylation or disease progression. A cohort following our protocol outside the trial reported similar side effects and perceived effectiveness; however, their compliance with data entry was markedly lower. Conclusions: While Lunasin’s lack of efficacy is disappointing, our novel trial design had the highest ALS trial enrollment rate ever recorded, with excellent retention and adherence. Low data density from patients who are self-experimenting outside a formal protocol casts doubt on the possibility of gathering useful information from unsupervised expanded access programs or “right to try” initiatives.
Trial registration: ClinicalTrials.gov identifier: NCT02709330.
Introduction
Lunasin is a peptide first isolated from soybeans (Citation1). It is reported to alter histone acetylation patterns (Citation2–5) which are abnormal in PALS (Citation6), may contribute to epigenetic transcriptional and translational dysregulation (Citation7) and have been the target of previous (Citation6,Citation8) and ongoing (NCT03127514) clinical trials. In 2014 a US television news story reported that a person diagnosed with ALS experienced a dramatic improvement in his speech, swallowing, and limb strength while on a Lunasin regimen (Citation9). As part of an ALSUntangled report, this person’s lower-motor-neuron-predominant ALS (sometimes called progressive muscular atrophy) and improvements were independently validated (Citation10). In our review of the available literature, Lunasin was generally regarded as safe, with no previously known serious adverse effects (Citation10). Based on this and a high level of interest from patients, we decided to perform a pilot trial of Lunasin in people with ALS (PALS).
Traditional clinical trials in ALS suffer from low enrollment, due in part to patients’ and families’ frustrations with common ALS trial design features (Citation11). These include restrictive inclusion criteria, frequent study visits, use of a placebo, and the long time it takes to receive results. In response, we designed this trial to have wider inclusion criteria, minimal travel burdens by making most of the visits virtual (via the PatientsLikeMe platform), historical controls (rather than placebo), and results made available in real time. In addition to traditional registration on ClinicalTrials.gov (NCT02709330) we also published our protocol on a website (Citation12) so that PALS all over the world with plans to self-experiment (Citation13) could be empowered to try a new treatment with a sound rationale at reasonable dose and to record their own outcome measures online, albeit outside a traditional trial infrastructure.
Materials and methods
Hypotheses
This trial was designed to determine whether Lunasin (i) decreased ALSFRS-R progression, (ii) increased the frequency of “ALS reversals”, (iii) altered histone H3 and H4 acetylation, and (iv) was safe. We also sought to explore whether the trial design features employed were associated with improvement in enrollment and retention compared to historical reports from more traditional ALS trials.
Design
This was a 12-month open-label clinical trial designed to be semi-virtual. Participants made three in-person visits to Duke’s ALS clinic (at enrollment, month 1 and month 12) and “virtual visits” were made using the PatientsLikeMe platform. In-person visits included ALSFRS-R and PatientsLikeMe registration and training, and vital signs, clinician-rated ALSFRS-R, weight, concomitant medications, adverse events, and compliance were measured by a study coordinator. “Virtual visits” occurred at weeks 2 and 3, and then once per month for months 2–11. During these check-ins, participants signed into their PatientsLikeMe account and were asked to enter their self-measured ALSFRS-R, weight, perceived efficacy (from options of “can’t tell”, “none”, “slight”, “moderate”, and “major”) and perceived side effects (with severity on a scale of “none”, “mild”, “moderate”, or “severe” plus optional side effect reporting from an auto-completing list of MedDRA-coded side effects from prior reports of all treatments on PatientsLikeMe). Finally, there were telephone visits at weeks 2 and 3, co-occurring with the virtual visits that served the dual purposes of trouble-shooting any problems using the website and also continuing ALSFRS-R training. Telephone visits also occurred throughout the study as needed for participants who reported problems or missed a virtual visit.
Ethics approval and registration
This protocol was approved by the Duke Healthcare System Institution Review Board (Pro00063754) and the protocol uploaded to ClinicalTrials.gov (NCT02709330). In addition, the US Food and Drug Administration (FDA) approved an Investigational New Drug (IND) application for this trial (128306).
Population
Participants were recruited through the Duke ALS Clinic. Inclusion and exclusion criteria are listed in . Unlike most ALS trials which tend to recruit ALS patients with a recent onset (e.g. less than 2 years post diagnosis) and well-preserved breathing function (e.g. FVC >70%), this study had no cutoff for disease duration or FVC, and allowed patients with feeding tubes, tracheostomy, noninvasive and invasive ventilation to participate.
Controls
For each enrolled participant, PatientsLikeMe matched three controls from their existing online population according to pretreatment ALSFRS-R progression, as previously described for an observational study of lithium carbonate (Citation14). Use of each participant’s symptom onset date (with inferred ALSFRS-R = 48) and score at first study visit allowed calculation of pretreatment ALSFRS-R progression rate and thus application of the algorithm for generating controls. Three controls per treated participant was selected, as this value minimized pretreatment progression bias between treated and control. Given that we already matched participants and controls in terms of pretreatment ALSFRS-R progression, we did not consider additional demographic comparisons.
Treatment
All participants were asked to take the same Lunasin-containing products at the same dosages as the initial index patient from our ALS Untangled report (Citation9,Citation10). These products included Lunarich X Capsules (Citation15), Provantage (Citation16), and Reliv Now (Citation17). Participants titrated up to a target dose of 6 Lunarich capsules twice a day, 1 scoop of Provantage twice a day and 1.5 scoops of Reliv Now twice a day. All products were donated by Reliv International and provided to participants free of charge for the duration of the trial.
Outcomes
ALSFRS-R
Participants were taught to measure their own ALSFRS-R scores over the first three weekly in-person and telephone visits. Participant-measured ALSFRS-R scores were thereafter utilized for the primary efficacy measurements. When interim data points were required, data were linearly interpolated from nearest neighbor ALSFRS-R as described previously (Citation14). The Kolmogorov–Smirnov statistical test was used to compare ALSFRS-R progression in participants and controls. Participants had access to their own “profile” page on PatientsLikeMe which graphed their ALSFRS-R longitudinally along with their treatments, symptoms, lab values, weight, and treatment evaluations. All members of the site could access the shared data of all members; therefore, the participants were unblinded and most of the information recorded during the study in real time was available to them.
ALS reversals
We looked for participants that had “ALS reversals” over the course of this study. Based on analysis of data from prior studies and members of PatientsLikeMe, these were defined as having an improvement of at least four ALSFRS-R points over 12 months. This degree of ALSFRS-R improvement occurs spontaneously in less than 1% of all ALS patients (Citation18).
Histone acetylation
All 50 participants on Lunasin, as well as 5 healthy and 5 ALS controls, had blood drawn at enrollment and month 1 time points. Histones were extracted from peripheral blood mononuclear cells using previously published protocols (Citation19–21). Western blots were used to measure alterations in acetylated histones H3 (H3K9K14ac2 (AcH3)) and H4 (H4K5K8K12K16 (AcH4)), previously reported to be decreased in response to Lunasin treatment (Citation2–5) as previously described (Citation19–21). Integrated density values (IDV) for AcH3 and AcH4 protein bands were normalized to IDV for total histone H3 (%H3). Percent H3 values for the 1-month time point were normalized to that of the enrollment visit values. Results were analyzed using a one-way ANOVA.
Enrollment and retention
Enrollment rate was calculated as the number of participants enrolled divided by the number of months it took to enroll them. This was qualitatively compared to the historical mean ALS trial enrollment rate of two participants per site per month (Citation22). Retention rate was calculated as percentage of surviving participants who completed the month 12 visit. This was qualitatively compared to the historical mean ALS trial retention rate of 78% (Citation23).
Other outcomes
Weight, adverse events, perceived effectiveness, and perceived side effects were also assessed. Patients provided their most recent self-obtained weight and were independently weighed by study coordinators during in-person visits. Adverse events were recorded by the coordinator throughout the trial. At every monthly visit, patients recorded their own perceived effectiveness and perceived side effects on PatientsLikeMe.
Accuracy, adherence, and compliance with treatment
To check for accuracy in participant-obtained ALSFRS-R and weight, these were compared to coordinator-obtained measures at month 1 and again at month 12 using Lin’s concordance (Citation24). Adherence was measured as the percentage of enrolled participants completing at least two of the required study outcomes on PatientsLikeMe (ALSFRS-R, perceived effectiveness and perceived side-effects) at each time point. Compliance with treatment was measured in two ways. At the month 1 visit it was assessed objectively by having the coordinator count the number of Lunasin capsules consumed, converting this to a “percent of expected”. At the last visit, it was assessed subjectively by having each participant rate it as “always”, “usually”, “sometimes” or “never”.
“Playing along at Home Cohort”
In addition to registration on ClinicalTrials.gov, we posted our IRB-approved protocol on the Internet, and anticipated that, as with previous studies (Citation14) many patients might try this Lunasin regimen outside our study. We decided to examine selected outcomes in a “playing along at home” cohort of PatientsLikeMe users who reported starting this Lunasin regimen during our trial enrollment period. This group was not enrolled or consented in the formal part of the study, and this activity was not overseen by an ethics committee, given that participants might self-select to take Lunasin anywhere in the world and under their own volition. Members of PatientsLikeMe were informed when they joined as part of the terms of use that participation in data sharing is voluntary. The “playing along at home” cohort had to source their own Lunasin and was provided no more information or prompting to enter data than any other PatientsLikeMe members.
Power calculation
Prior to the beginning of the study, a power analysis was performed using G*Power version 3.1. Ninety percent power to observe a relative effect size of 0.65 (moderately large effect, consistent with observing a slowing of progression by 50%) at significance level 0.05 for one-sided t-test, is achieved with N = 43 participants in each arm. Allowing for a dropout of 7 participants, the decision was made to recruit 50 participants.
Results
Population, enrollment, and retention
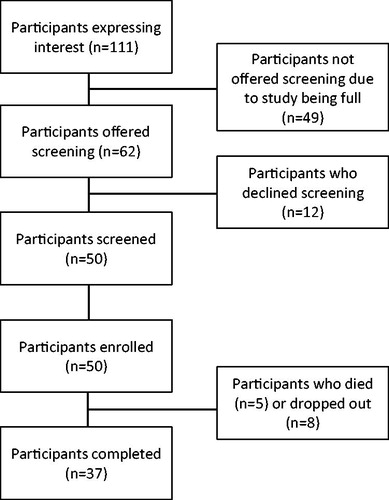
shows a diagram of study participant flow. We enrolled all 50 participants in 5.5 months, for a trial enrollment rate of 9.1 participants per month. shows participant demographics. As with most ALS trials, our participants were mostly white (94%), males (58%), with limb onset disease (82%), mean age of 60 years and on riluzole (60%). However, our participants differed from those in most ALS trials due to their longer disease duration (mean 3.7 years, range 0.5–13 years), use of noninvasive ventilation (n = 20), PEG (n = 8), or tracheostomy with invasive ventilation (n = 3). Thirteen participants dropped out of the study early, five due to deaths (all deaths assessed as unrelated to the study). Thus, our survivor retention rate was 84%.
Table 1 Demographics of participants and “playing along at home” cohort.
ALSFRS-R, reversals, and perceived efficacy
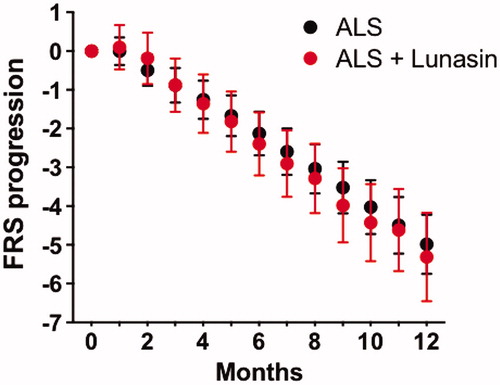
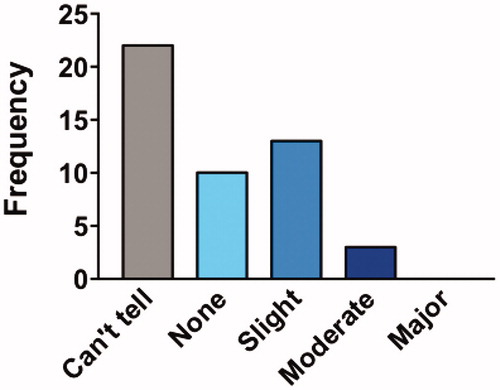
One participant deleted their PLM account and two others did not enter ALSFRS-R data, leaving 47 participants available for ALSFRS-R analyses. Each participant was matched with 3 controls, resulting in a total of 141 controls. As illustrates, there was no detectable difference (p = 0.99) in ALSFRS-R progression between participants taking Lunasin (0.44 points per month, standard deviation 1.6 points) and matched historical controls (0.42 points per month standard deviation 1.5 points) using the two-sample Kolmogorov–Smirnov test. No participant experienced an “ALS reversal”. Most participants perceived little or no effectiveness, or could not tell ().
Histone acetylation
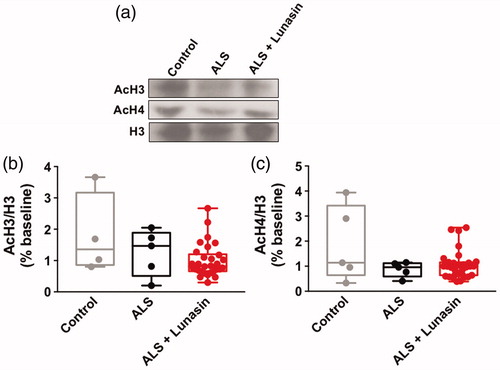
Previous studies demonstrate that Lunasin alters histone H3 and H4 acetylation (Citation2–5). Therefore, we sought to determine if histone acetylation is altered in blood samples from ALS patients following Lunasin treatment. Unfortunately, not all participants had extractable histone data. One-way ANOVA analysis of acetylated histone H3 (H3K9K14ac2; AcH3) levels in healthy control (n = 4), ALS controls (n = 5), and ALS + Lunasin (n = 30) PBMC samples demonstrated no significant effect overall [F(2, 36) = 2.789, p = 0.0748] (). One-way ANOVA analysis of acetylated histone H4 (H4K5K8K12K16ac5; AcH4) levels in healthy control (n = 5), ALS controls (n = 5), and ALS + Lunasin (n = 31) PBMCs demonstrated no significant effect overall [F(2, 38)=3.119, p = 0.0557] ().
Figure 5 AcH3 and AcH4 levels in PBMCs. Representative immunoblot images of AcH3, AcH4, and H3 levels in healthy controls, ALS controls and ALS + Lunasin (a). There was no significant difference in AcH3 levels in PBMCs between groups (b). There was no significant difference in AcH4 in PBMCs between groups (c).
Safety
There were 15 serious adverse events over the course of the study (); of these, 2 were possibly related to Lunasin (obstipation/fecal impaction requiring hospitalization). Many participants experienced non-serious adverse events over the course of the trial (). The most common adverse events attributed to Lunasin were gastrointestinal in nature, including constipation (n = 11) and fullness/early satiety (n = 8). While only a single participant complained of weight loss, 32 participants actually lost weight over the course of the study, with an average weight loss of 4.1 pounds. Participants own ratings of their side effects were similar to those obtained by our coordinator, with most noting mild or no side effects, but a few noting moderate to severe effects ().
Figure 6 Participant ratings of side effects. Percentage of participants that rated their overall side effects in different categories. Most participants reported their side effects as “mild” or “none” but a few reported these as “severe”.
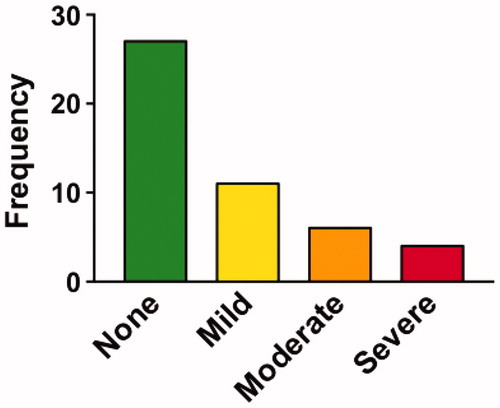
Table 2 Adverse events.
Accuracy, adherence, and compliance with treatment
Lin’s concordance showed statistically significant (p < 0.001) agreement between available coordinator- and participant-obtained weights and ALSFRS-R scores at month 1 and month 12 visits (). Adherence was very high for the first 6 months of the trial, and then dropped off thereafter (). Objectively, compliance with treatment was assessed at month 1 as 100%. At their last study visit, 52% of participants stated they were always compliant and 35% said they were usually compliant.
Figure 7 Adherence in participants versus those “playing along at home”. This figure shows that participant adherence (% completing at least 2 out of 3 PLM outcome measures) was high for the first 6 months of the study, then dropped off after that (solid line). On the other hand, a “play along at home” cohort (54 PLM users who started self-experimentation with Lunasin during our trial enrollment period) had much lower adherence (dashed line).
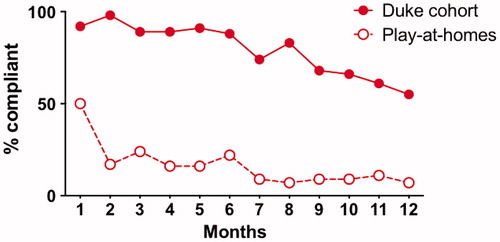
Table 3 Outcome measure agreement.
“Playing along at Home” cohort
Independent of our trial, 34 PLM users reported starting this same Lunasin regimen during our enrollment period. Compared to our enrolled participants, this “playing along at home” cohort had similar demographics () and reported similar perceived effectiveness and side effects (data not shown), but they had much lower adherence throughout the study ().
Discussion
Unfortunately, we found no evidence that Lunasin slowed, stopped or reversed ALS progression in this study, nor that it significantly influenced AcH3 or AcH4 histone acetylation patterns. Lunasin was associated with unexpected and common GI side effects in PALS, including two cases of obstipation/fecal impaction requiring hospitalization. Based on our results, we now doubt that the ALS reversal that originally caught our attention (Citation9) was caused by Lunasin. This person may have had an undetected ALS mimic syndrome, or some endogenous resistance to the disease (Citation25). It is more difficult to explain why we could not confirm previous reports of Lunasin’s effect on AcH3 or AcH4 levels (Citation2–5) or even previous reports of altered H3 acetylation in association with ALS itself relative to healthy controls (Citation6). Possible explanations include the small sample size in our study, differences in the types of antibodies used to detect histone acetylation in the different studies and differences in the way the histone data were analyzed (we normalized our AcH3 and AcH4 findings to total histone H3 levels while a previous study used β-actin levels to normalize acetylation of histone H3).
We know that many PALS experiment with complementary and alternative medicine and that they frequently fail to report this use to their clinicians, perhaps from fear of being admonished. Coverage of potential “breakthroughs” for ALS in the news media make compelling “human interest” stories but are rarely investigated in depth. The results of this study represent a comprehensive answer to questions raised by a large number of patients in response to such a news story. Despite concerted efforts to move quickly, this study represents a four-year delay between initial report and final refutation that Lunasin might slow disease progression in ALS for patients. In part, this was due to time spent seeking funding, regulatory submissions such as IRB reviews, FDA IND, statistical analysis, and dissemination efforts. Sadly, even this accelerated turnaround time is longer than the median survival time following ALS diagnosis.
Logistically, we were satisfied with the performance of our “hybrid virtual” trial design. Relative to published historical norms, this was the fastest enrolling trial in ALS history, with an enrollment rate of nearly 5 times the average (Citation22). In terms of costs, this trial was made possible by a $250,000 USD grant, considerably cheaper than most trial designs. Our enrolled population was more diverse than that of a typical ALS trial, suggesting that our results may be more generalizable (Citation26). Our participants demonstrated that (with training) they could accurately measure and record their own ALSFRS-R and weight. The ALSFRS-R progression rate in our participants prior to and during treatment was about half that seen in ALS trials with more traditional entry criteria (Citation27). This is not unexpected given the long disease duration of our participants. In spite of enrolling patients with longer disease duration, some of whom were using noninvasive or invasive ventilation and/or feeding tubes, participant retention and adherence were equal to or better than most ALS trials (Citation23). Therefore, we plan to use similar trial designs in future assessment of other alternative therapies, albeit potentially without the in-person visits.
By posting our IRB-approved protocol on the Internet we empowered a large number of PALS outside the trial, who were likely to self-experiment with alternative therapies (Citation13,Citation14), to test the efficacy of a new compound with a plausible rationale. This “playing along at home” group was not adherent as far as data entry, perhaps because they did not have the reminders from PLM and the trial coordinator that were built into our study. In our opinion, this lack of adherence casts doubts on the potential of unsupervised expanded access or “right to try” programs to gather useful outcome data (Citation28,Citation29). At least in the case of Lunasin this was a relatively safe supplement with reversible side effects. A similar balance of benefits and risks is unlikely to be found in expanded access to, for example, stem cell therapies, which attract much excitement and optimism from the community but also the potential for larger financial outlays and risks to human health (Citation30).
In terms of limitations, some of the features of our trial that made it more appealing to patients may have weakened its scientific rigor. By taking a wider inclusion/exclusion we may have enrolled patients too sick to have ever benefitted from a treatment to slow their progression. Some have argued that small treatment effects may easier to measure in faster ALS progressors (Citation31); this is far from proven, but we accept that we could have missed a small treatment effect in the slow progressors we enrolled in this study. We did not have a placebo arm. In the event of a positive finding this would have made interpretation of the effect more ambiguous and probably necessitated a larger, blinded, placebo-controlled pivotal study, which might have been made difficult given the widespread availability of Lunasin. Our sharing of the “play along at home” protocol may have instigated some PALS to waste time and money on an intervention that did not work and potentially expose them to side effects and inconvenience outside the protections afforded by a typical trial design. The very existence of our trial was used on social media by people wishing to promote sales of Lunasin before the results had been announced, and lending undue credence to alternative therapies may inadvertently increase their use by desperate PALS hoping to slow their disease. During the design and conduct of the trial, new consensus guidelines were being developed as the “Airlie House Clinical Trial Guidelines” (Citation32). While we were unable to incorporate all these recommendations into the current trial design we were able to feed our experiences from the Lunasin virtual hybrid study into recommendations for more patient-centric trial designs, such as having patients on the study committee, minimizing burden through “remote virtual visits”, and ensuring the timely return of results to trial participants in open access venues. Future virtual trial designs, inspired as this trial was by ALSUntangled reports, should adhere to as many of these criteria as is practical.
Declaration of interest
Richard Bedlack has research support from ALSA, MNDA, Cytokinetics, Neuraltus, Oirion and GSK, and consulting support from ALSA, Avanir, Biohaven, Neuraltus, Ultragenyx, Cytokinetics, ITF Pharma, Mallinkrodt and Brainstorm Cell. Paul Wicks (PW), Timothy Vaughan, Alicia Opie and Rebecca Blum are employees of PatientsLikeMe and hold stock options in the company. PW is an associate editor at the Journal of Medical Internet Research and is on the Editorial Boards of The BMJ, BMC Medicine, and Digital Biomarkers. The PatientsLikeMe Research Team has received research funding (including conference support and consulting fees) from Abbvie, Accorda, Actelion, Alexion, Amgen, AstraZeneca, Avanir, Biogen, Boehringer Ingelheim, Celgene, EMD, Genentech, Genzyme, Janssen, Johnson & Johnson, Merck, Neuraltus, Novartis, Otsuka, Permobil, Pfizer, Sanofi, Shire, Takeda, Teva, and UCB. The PatientsLikeMe R&D team has received research grant funding from Kaiser Permanente, the Robert Wood Johnson Foundation, Sage Bionetworks, The AKU Society, and the University of Maryland. PW has received speaker fees from Bayer and honoraria from Roche, ARISLA, AMIA, IMI, PSI, and the BMJ.
Acknowledgments
We thank the many patients and families who participated in and raised money to fund this trial, especially those working through the Larry Vance Hughes ALS Foundation. We thank Reliv International for donating the products.
Additional information
Funding
References
- Liu J, Jia S, Kirberger M, Chen N. Lunasin as a promising health-beneficial peptide. Eur Rev Med Pharmacol Sci. 2014;18:2070–5.
- Hernandez-Ledesma B, Hsieh CC, de Lumen B. Relationship between lunasin’s sequence and its inhibitory activity of histones H3 and H4 acetylation. Mol Nutr Food Res. 2011;55:989–98.
- Shidal C, Inaba J, Yaddanapudi K, Davis KR. The soy-derived peptide lunasin inhibits invasive potential of melanoma initiating cells. Oncotarget. 2017;8:25525–41.
- Galvez A, Chen N, Macasieb J, de Lumen B. Chemopreventive property of a soybean peptide (lunasin) that binds to deacetylated histones and inhibits acetylation. Cancer Res. 2001;61:7473–8.
- Jeong J, Jeong J, Park J, Lee S, Lee J, Lee H, et al. Cancer-preventive peptide lunasin from Solanum nigrum L. inhibits acetylation of core histones H3 and H4 and phosphorylation of retinoblastoma protein (Rb). J Agric Food Chem. 2007;55:10707–13.
- Cudkowicz M, Andres P, Macdonald S, Bedlack R, Choudry R, Brown R, et al. Phase 2 study of sodium phenylbutyrate in ALS. Amyotroph Lateral Scler. 2009;10:99–106.
- Jimenez-Pacheco A, Franco J, Lopez S, Gomez-Zumaquero J, Magdalena Leal-Lesarte M, Caballero-Hernandez D, et al. Epigenetic mechanisms of gene regulation in amyotrophic lateral sclerosis. Adv Exp Med Biol. 2017;978:255–75.
- Piepers S, Veldink JH, de Jong SW, van der Tweel I, van der Pol W, Uijtendaal E, et al. Randomized sequential trial of valproic acid in amyotrophic lateral sclerosis. Ann Neurol. 2009;66:227–34.
- https://www.youtube.com/watch?v=2U16BK61xic. Accessed February 17, 2018. (Archived by WebCite® at http://www.webcitation.org/6xIlvJt4h)
- ALSUntangled G. ALSUntangled no. 26: lunasin. Amyotroph Lateral Scler Frontotemporal Degener. 2014;7:622–6.
- Bedlack R, Wicks P, Heywood J, Kasarskis E. Modifiable barriers to enrollment in American ALS research studies. Amyotroph Lateral Scler. 2010;11:502–7.
- http://www.alsreversals.com/roar.html. Accessed February 17, 2018. (Archived by WebCite® at http://www.webcitation.org/6xImfmwwr).
- Wasner M, Klier H, Borasio G. The use of alternative medicine by patients with amyotrophic lateral sclerosis. J Neurol Sci. 2001;191:151–4.
- Wicks P, Vaughan T, Massagli M, Heywood J. Accelerated clinical discovery using self-reported patient data collected online and a patient-matching algorithm. Nat Biotechnol. 2011;29:411–4.
- https://reliv.com/p/lunarich-x. Accessed February 26, 2018. (Archived by WebCite® at http://www.webcitation.org/6xWmACyCc).
- https://reliv.com/p/provantage. Accessed February 26, 2018. (Archived by WebCite® at http://www.webcitation.org/6xWmb0a6l).
- https://reliv.com/p/reliv-now. Accessed February 26, 2018. (Archived by WebCite® at http://www.webcitation.org/6xWmqMMBZ).
- Bedlack R, Vaughan T, Wicks P, Heywood J, Sinani E, Selsov R, et al. How common are ALS plateaus and reversals? Neurology. 2016;86:808–12.
- Sadri-Vakili G, Bouzou B, Benn C, Kim M, Chawla P, Overland R, et al. Histones associated with downregulated genes are hypo-acetylated in Huntington’s disease models. Hum Mol Genet. 2007;16:1293–306.
- Kim MO, Chawla P, Overland RP, Xia E, Sadri-Vakili G, Cha JHJ. Altered histone monoubiquination mediated by mutant huntington induces transcriptional dysregulation. J Neurosci. 2008;28:3947–57.
- McFarland KN, Das S, Sun TT, Leyfer D, Xia E, Sangrey GR, et al. Genome-wide histone acetylation is altered in a transgenic mouse model of Huntington’s disease. PLoS One. 2012;7:e41423.
- Bedlack R, Pastula D, Welsh E, Pulley D, Cudkowicz M. Scrutinizing enrollment in ALS clinical trials: room for improvement? Amyotroph Lateral Scler. 2008;9:257–65.
- Atassi N, Yerramilli-Rao P, Szymonifka J, Yu H, Kearney M, Grasso D, et al. Analysis of start-up, retention, and adherence in ALS clinical trials. Neurology. 2013;81:1350–5.
- Lin L. A concordance correlation coefficient to evaluate reproducibility. Biometrics. 1989;45:255–68.
- Harrison D, Mehta P, van Es MA, Stommel E, Drory VE, Nefussy B, et al. ALS reversals: demographics, disease characteristics, treatments and co-morbidities. Amyotroph Lateral Scler Frontotemporal Degener. 2018;1–5. [Epub ahead of print].
- Chio A, Canosa A, Gallo S, Cammarosano S, Moglia C, Fuda G, et al. ALS clinical trials: do enrolled patients accurately represent the ALS population? Neurology. 2011;77:1432–7.
- Atassi N, Berry J, Shui A, Zach N, Sherman A, Sinani E, et al. The PRO-ACT database: design, initial analyses, and predictive features. Neurology. 2014;83:1719–25.
- Rabourn J, Bedlack R. SAPs: a different perspective. Am J Bioeth. 2014;14:19–20.
- Wicks P, Heywood J. Data donation could power the learning health care system, including special access programs. Am J Bioeth. 2014;14:27–9.
- Wicks P, Heywood J. Getting stem cell patients ‘on the grid’. Nat Biotechnol. 2016;34:1228–30.
- Beydoun S, Rosenfeld J. Edaravone in amyotrophic lateral sclerosis-lessons from the clinical development program. US Neurol. 2018;14:47–53.
- Mitsumoto H. Guidelines for ALS clinical trials (AIRLIE HOUSE 2016) and clinical criteria (the diagnosis WFN research group 2015-a critical appraisal). J Neurol Sci. 2017;381:39.