Abstract
Objective
To assess the relationship among measurements of strength, function, and quality of life in an amyotrophic lateral sclerosis (ALS) clinical trial.
Methods
In the FORTITUDE-ALS clinical trial (NCT03160898), 456 participants in the full-analysis set were treated with either reldesemtiv or placebo for 12 weeks; this post hoc analysis included all participants regardless of treatment assignments. Assessments included slow vital capacity (SVC), the ALS Functional Rating Scale-Revised (ALSFRS-R), and the 5-item ALS Assessment Questionnaire (ALSAQ-5). Muscle strength was measured quantitatively with hand-held dynamometry, and grip strength with a dedicated dynamometer. The relationship between strength and ALSFRS-R fine and gross motor domain scores, or responses to ALSAQ-5 questions on hand function and walking, was assessed with Spearman’s rank correlation. The relationship between mean upper- or lower-extremity muscle strength and specific ALSFRS-R domains was modeled using principal-components analysis.
Results
Upper-extremity muscle strength and hand grip were highly correlated with ALSFRS-R fine motor scores and the ALSAQ-5 hand function question. Similarly, lower-extremity strength correlated well with ALSFRS-R gross motor domain and the ALSAQ-5 walking question. For SVC, correlation was poor with the ALSFRS-R respiratory domain, but stronger with the total score, potentially reflecting the insensitivity of the respiratory questions in the scale. Upper- and lower-extremity strength were both strong predictors of ALSFRS-R domain scores.
Conclusions
In this analysis of data from an ALS clinical trial, muscle strength quantified by dynamometry was strongly correlated with functional capacity. These results suggest that muscle strength directly relates to specific functions of importance to people with ALS.
Introduction
Amyotrophic lateral sclerosis (ALS) is a progressive neurodegenerative disease primarily affecting motor neurons in the cerebral cortex, brainstem, and spinal cord (Citation1). The primary manifestation of this degenerative process is weakness, which typically begins focally but ultimately spreads to include extremity, bulbar, and respiratory muscles (Citation1). The strength of extremity muscles can be measured quantitatively using a hand-held dynamometer and is known to decline monotonically over time (Citation2). For ventilatory muscles, strength is assessed by vital capacity, established as a reliable measure that declines reproducibly in ALS (Citation3). To date, however, the relationship between such declines and loss of functional capacity has not been demonstrated. Unfortunately, this has left muscle strength measurement as a supportive secondary outcome in ALS clinical trials, and limited the conclusions that can be drawn regarding treatment efficacy based on preserved strength. This is regrettable, as there is a pressing need for objective measures of disease activity that can be used as an alternative to survival (Citation4).
Of the various measures of physical function for ALS, the ALS Functional Rating Scale-Revised (ALSFRS-R) is by far the most widely used in clinical practice, as well as the most frequently used primary outcome measure in ALS clinical trials (Citation5). The ALSFRS-R includes 12 items grouped into four domains assessing bulbar, fine motor (upper extremity), gross motor (trunk and lower extremity), and respiratory function (Citation6). As six of the 12 items relate to upper- or lower-extremity function – three each in the fine motor and gross motor domains – one might expect a strong relationship between these domains and strength measurements. The 5-item ALS Assessment Questionnaire (ALSAQ-5) is also widely used, and has two questions primarily related to upper- and lower-extremity function (Citation7) that might be anticipated to show a relationship with strength.
Reldesemtiv is a small-molecule fast skeletal muscle troponin activator that shows high specificity to fast skeletal muscle and leaves cardiac and slow skeletal muscle unaffected. It sensitizes the sarcomere to calcium and increases muscle force in preclinical models and healthy people (Citation8–10). In the phase 2b trial of reldesemtiv in people with ALS, FORTITUDE-ALS, investigators prospectively assessed muscle strength, utilizing slow vital capacity (SVC) and hand-held dynamometry (HHD), and function with the ALSFRS-R (Citation11). Given the size of this study, with more than 400 participants randomized, the dataset provided an opportunity to explore the extent to which conclusions about motor function could be made on the basis of strength evaluation.
In this post hoc analysis of FORTITUDE-ALS, we evaluate the relationships between outcome measures – regardless of treatment assignment in the trial – to determine the extent to which quantitative strength assessments predict clinically meaningful functional outcomes.
Materials and methods
FORTITUDE-ALS trial design
The design of the FORTITUDE-ALS trial (NCT03160898) has been described in detail previously (Citation11). In brief, it was a double-blind, placebo-controlled, international trial that randomized people living with ALS to placebo or reldesemtiv for 12 weeks of dosing. The trial was conducted in accordance with the Declaration of Helsinki and Good Clinical Practice. All individual trial sites received institutional review board approval before they began enrolling patients, and all patients provided written informed consent.
Assessments
Assessments occurred at baseline (using the value collected on day 1, or from the screening visit if day 1 assessments were not available), weeks 2, 4, 8, and 12, and at a follow-up visit 4 weeks after the last dose. Outcome measurements of interest for this post hoc analysis were SVC, ALSFRS-R, the ALSAQ-5 questionnaire, and quantitative muscle strength testing, all measured as previously described (Citation11). In the upper extremities, muscles tested were first dorsal interosseous, wrist extensors, and elbow flexors; in the lower extremities, muscles tested were ankle dorsiflexors, knee extensors, and hip flexors. These muscles were tested with a hand-held dynamometer (microFET 2; Hoggan Scientific, Salt Lake City, UT) and the mean strength of each muscle measured bilaterally was used for analysis. Grip strength was measured with a dedicated grip dynamometer and the mean score for the left and right hands was used for analysis.
Analyses
The analysis included data from all participants in the FORTITUDE-ALS clinical trial full analysis set, defined as all randomized participants who received any study drug and had a baseline and at least one post-baseline efficacy assessment during the double-blind period. Of 458 people who were randomized, one withdrew before receiving any study drug and one did not have any post-baseline efficacy assessments. Therefore, in the full analysis set, 456 people were randomized and treated with either placebo (n = 115), reldesemtiv 150 mg twice daily (n = 112), reldesemtiv 300 mg twice daily (n = 113), or reldesemtiv 450 mg twice daily (n = 117). Overall correlation between outcomes was evaluated using Spearman’s rank correlation coefficient with positive scores showing a positive correlation and negative scores showing an inverse correlation. In addition to testing the statistical significance of correlation, we defined coefficients in the range 0 to ±0.300 as very weak correlations, ±0.300 to ±0.499 as weak, ±0.500 to ±0.699 as moderate, ±0.700 to ±0.800 as strong, and above 0.800 or below −0.800 as very strong correlations. Strength values for hand grip, individual muscles of the upper and lower extremities, and combined strength values for bilateral upper- and lower-extremity muscle groups were correlated with the fine or gross motor domain sub-scores of the ALSFRS-R at the associated time points. Similar correlations were calculated between individual and combined muscle strength measurements and responses to ALSAQ-5 question 1 (“I have found it difficult to stand up”) and question 2 (“I have had difficulty using my arms and hands”). SVC was correlated with the ALSFRS-R total score, as well as the respiratory domain and sub-items.
To further explore the relationship between strength and function, a principal-components analysis was performed using all available upper- or lower-extremity strength data, adjusting for demographic information (sex and site of disease onset). An analysis of covariance (ANCOVA) model was generated to evaluate the correlation between scores on the ALSFRS-R fine motor domain and the scores derived from the quantitative strength testing of the three upper-extremity muscles. Similarly, an equivalent model evaluated the correlation between ALSFRS-R gross motor domain scores and the strength of the three lower-extremity muscles.
Analyses were conducted in Statistical Analysis System software version 9.4 or higher (SAS Institute, Cary, NC). All analyses were post hoc and exploratory, with no adjustment for multiplicity; p-values were all 2-sided, and deemed significant at <0.05.
Results
Characteristics of the dataset
During the trial, there were more than 2500 measurements of function and strength. The average score and range for each variable evaluated is shown in . Across all measurements in the dataset, the mean ± standard deviation (SD) for the ALSFRS-R fine motor domain score was 7.7 ± 3.0 and for the ALSFRS-R gross motor domain score was 7.1 ± 3.1. Mean ± SD upper-extremity muscle strength was 45.9 ± 29.5 lbs, lower-extremity muscle strength 99.0 ± 52.0 lbs, and hand grip strength 37.3 ± 25.3 lbs.
Table 1 Characteristics of the dataset.
Correlations with ALSFRS-R and ALSAQ-5
For the ALSFRS-R, strength measurements for the individual upper-extremity muscles – first dorsal interosseus, elbow flexion and elbow extension – showed a moderate correlation with the fine motor domain (). When the muscles were combined, they showed a strong correlation with the fine motor domain. For the gross motor domain, strength in these individual upper-extremity muscles showed a very weak correlation and a weak correlation when combined. Grip strength was strongly correlated with the fine motor domain, and weakly correlated with the gross motor domain. Lower-extremity muscle strength correlated mostly moderately with gross motor domain scores, although the strengths of these associations were slightly lower than the correlations between upper-extremity muscles and the fine motor domain. Nevertheless, all correlations were statistically significant (p < 0.05).
Table 2 Correlation of muscle strength measurements with ALSFRS-R domains.
For ALSAQ-5, scores increase as quality of life decreases, and correlation coefficients for strength with ALSAQ-5 scores were generally inverse, giving negative correlation scores (). Strength in upper-extremity muscles and hand grip demonstrated moderate correlation with the score for question 2 (arm/hand use), and a similar pattern was seen with lower-extremity strength and question 1 (standing up). All correlations were statistically significant, although coefficient values were higher (around −0.6) for upper-extremity strength and arm/hand use than for lower-extremity strength and standing up (around −0.5). Weak correlations were also observed between strength in upper-extremity muscles and standing up, but lower-extremity muscle strength was not significantly correlated with arm/hand use.
Table 3 Correlation of muscle strength with ALSAQ-5 items.
For SVC, values correlated significantly with results for the respiratory domain of the ALSFRS-R, but the relationship was weaker than for extremity muscles and their respective domains (). In fact, the relationship between SVC and the respiratory domain was weaker than the correlation with the ALSFRS-R total score. Correlations with the respiratory domain items of dyspnea, orthopnoea, and respiratory insufficiency were also very weak.
Table 4 Correlation of SVC with ALSFRS-R respiratory scores.
Principal-components analysis
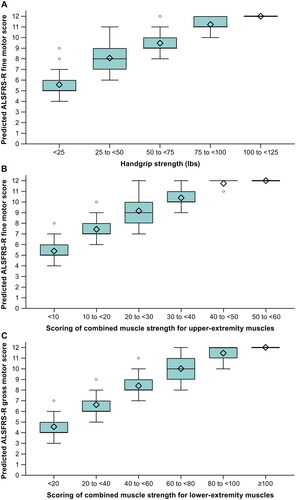
In the adjusted model, grip strength was predictive of the ALSFRS-R fine motor domain sub-score (); overall, those with weaker grip strength had lower fine motor sub-scores. Strength scores were grouped into five categories utilizing 25-pound intervals ranging from the highest category of 100–125 pounds down to <25 pounds, and the fine motor domain sub-score was ∼2 points less with each downward shift in category. In a second analysis, all upper-extremity strength data was transformed and used to predict score values of the ALSFRS-R fine motor domain (). Strength and ALSFRS-R score were strongly related throughout the range of ALSFRS-R values; a change of ≥2 points in the ALSFRS-R was generally associated with strength ranges that were not overlapping. A similar pattern was seen in relating ALSFRS-R gross motor domain to lower-extremity muscle strength ().
Figure 1 Predicted ALSFRS-R domain sub-scores versus categories of (A) hand grip strength, (B) upper-extremity muscle strength, and (C) lower-extremity muscle strength.
Diamonds indicate the mean. Box edges indicate the 25th to 75th percentiles (the interquartile range), horizontal lines in between the edges indicate the medians, whiskers extend to the upper and lower adjacent values, and circles represent outlier values. The adjacent values are the lowest and highest observations that are still inside the region, defined by the following limits: lower limit, Q1 minus 1.5 x (Q3 minus Q1); upper limit, Q3 plus 1.5 x (Q3 minus Q1).
The maximum score is 12 for either the fine motor or gross motor domain. Upper-extremity muscles include wrist extension, first dorsal interosseous, and elbow flexion strength. Lower-extremity muscles include ankle dorsiflexion, hip flexor, and knee extension strength.
ALSFRS-R: ALS Functional Rating Scale–Revised.
Discussion
It seems intuitive that quantitative measurements of muscle strength should reflect clinically important symptoms of ALS, yet there are no published datasets definitively confirming this relationship. If the relationship was established, muscle strength could provide an objective, quantitative measure of disease progression that would complement outcomes such as ALSFRS-R scores. Similarly, interpretations of changes in the fine and gross motor domains of the ALSFRS-R would be strengthened by knowledge that they are directly related to changes in strength of upper- and lower-extremity muscles. In this post hoc analysis of data collected in the well-controlled setting of a phase 2 clinical trial, we observed the relationship of muscle strength to functional attributes assessed by ALSFRS-R domains, showing that upper-extremity strength measurements are related to scores on the fine motor domain while lower-extremity strength correlates with the gross motor domain. A relationship was also demonstrated between muscle strength in upper or lower extremities and responses on the corresponding items of the ALSAQ-5. When muscles were evaluated individually, hand grip demonstrated the highest correlation with the fine motor domain while correlations between upper-extremity strength and fine motor domain as well as those between lower-extremity strength and gross motor domain were generally strong, with correlation coefficients >0.6.
Although strength in any individual muscle or muscle group showed a statistically significant correlation with both fine and gross motor domains, the correlations between upper-extremity muscles and lower-extremity function, as well as those between lower-extremity muscles and upper-extremity function, were quite low. Correlation coefficients between lower-extremity strength values and the fine motor domain were ∼0.1, while those between upper-extremity strength and the gross motor domain were ∼0.3. This discrepancy probably results from the differences in specificity between the domain questions: fine motor domain questions are related to the upper extremity (using utensils, handwriting, and activities of daily living), whereas two of the gross motor domain questions (turning in bed, climbing stairs) involve both upper- and lower-extremity muscles. Similarly, on the ALSAQ-5, correlation coefficients of upper-extremity strength with arm/hand use were higher than those between lower-extremity strength and standing up, most likely because the upper-extremity question asks only about hand and arm strength, while the lower-extremity question asks about standing up, which typically requires the use of upper- and lower-extremity muscles.
The principal-components analysis demonstrates the ranges of strength required to achieve various scores in both fine and gross motor domains of the ALSFRS-R. While there is some overlap of strength in the various ALSFRS-R scores, differences of ≥2 points in either domain are associated with non-overlapping strength values throughout the entire range of both the fine motor and gross motor domains. Overall, the data present compelling evidence that strength in upper and lower extremities is strongly related to function in those areas.
The data evaluated here are not intended to “validate” muscle strength against the ALSFRS-R. The ALSFRS-R is not a validated scale, so that validating any measure against this does not add useful information. When the ALSFRS-R was initially proposed, it was validated against the original scale, the ALSFRS, and shown to be closely related (Citation6). The United States Food and Drug Administration (FDA) has proposed a process for outcome measure validation (Citation12). The process is onerous, and, to date, no outcome measure or biomarker has received qualification. However, qualification of outcomes has not proved necessary for drug approval. The ALSFRS-R is not qualified, but it was the primary outcome measure in studies leading to approval of both edaravone and sodium phenylbutyrate/taurursodiol (Citation13,Citation14). Similarly, cerebrospinal fluid neurofilament light chain, an unvalidated biomarker, was used as the basis for approval of tofersen for patients with ALS and SOD1 mutations (Citation15). The goal of this paper is to improve our understanding of muscle strength as an outcome in ALS trials, and to enrich our understanding of the ALSFRS-R.
Quantitative measurements of strength have been frequently employed as secondary outcomes in ALS trials (Citation16), but have not served as primary outcome measures in recent studies (Citation2). In phase 3 studies, the primary outcome measure is mandated to be an assessment that is clinically relevant; that is, a measure of “how a patient feels, functions, or survives” (Citation17). In the United States, the FDA has defined outcome measures that would meet criteria as clinical outcomes assessments (COAs). A COA can be a measure that is patient reported, observer reported, clinician reported, or a performance measurement (Citation18). For ALS, the FDA lists the ALSFRS-R as a COA, and designates it a clinician-reported outcome (Citation19). Strength might be regarded as a performance measurement, but for strength to be eligible for consideration as a COA, the relationship between specific values and a patient-, observer-, or clinician-reported outcome must be clear. The findings reported here contribute to this effort.
In our analysis, SVC, another quantitative measure of muscle strength, correlated much less well to the respiratory domain of the ALSFRS-R than extremity muscles did to their relevant domains. In some scenarios, lack of correlation could result from the disease status of the population under study; however, in this case, it is most likely due to the relative insensitivity of the respiratory questions on the ALSFRS-R. This supposition is supported by the observed decline in respiratory domain scores in the placebo group in FORTITUDE-ALS, as the respiratory domain fell at less than half the rate of either the gross or fine motor domains (Citation11). The observation that SVC correlated better with the ALSFRS-R total score than the respiratory domain sub-score is also consistent with this interpretation. Both because of decreased activity related to worsening extremity weakness and a gradual rather than abrupt decline in SVC, it may be that patients with ALS do not report dyspnea or orthopnoea until their SVC has fallen fairly extensively. A weak correlation between SVC and the respiratory sub-score of the ALSFRS-R has previously been reported (Citation3) and of the four domains of the ALSFRS-R, the sub-score of the respiratory domain has the least steep decline (Citation20).
Perceived limitations of measuring strength quantitatively include variability when measured by different evaluators and the requirement for evaluator training. Coefficient of variation for rate of change, a measure that incorporates both the rate of change and the inter-subject variability of a measure, is in general slightly greater for strength as measured by HHD than for the ALSFRS-R (Citation2). This is in the context of the use of programs for standardized training and validation of evaluators in the use of both measures. Despite this potential limitation, HHD has shown reproducible declines in overall function in multiple studies (Citation15,Citation21,Citation22). As noted above, another supposed limitation of strength as an ALS outcome measure is its lack of evidence for a relationship to clinically important outcomes. The data reported here address that issue, demonstrating that strength evaluation closely correlates with and predicts function as measured using the ALSFRS-R domains or relevant questions of the ALSAQ-5. These findings support the continued use of strength, as determined by HHD, as an ALS outcome measure, and the application of principal-components analysis to combine strength from different muscle locations to have a more efficient and representative prediction of ALSFRS-R scores.
A limitation of the analysis reported here is the 16-week trial duration. A longer study may provide additional insights into the relationship between strength and function as ALS continues to progress.
In conclusion, the findings of this post hoc analysis suggest moderate to strong correlations between objective values of grip strength or HHD in the upper and lower extremities and associated domains of physical function assessed by the ALSFRS-R, and participant-perceived quality of life as measured by the ALSAQ-5.
Acknowledgements
FORTITUDE-ALS was conducted by Cytokinetics, Incorporated in collaboration with Astellas Pharma Inc. We wish to thank the participants and their families for their contributions to the FORTITUDE-ALS clinical trial, the investigators, and members of the Data Monitoring and Steering Committees. The authors acknowledge Susan Tan, of Engage Scientific Solutions, Sydney, Australia, for editorial assistance with the preparation of this manuscript, which was funded by Cytokinetics, Incorporated.
Declaration of interest
JMS reports compensation received as a consultant from Amylyx, Apic Biosciences, NeuroSense Therapeutics, Cytokinetics, Denali Therapeutics, GSK, Mitsubishi Tanabe Pharma America, Orphazyme, Orthogonal, Pinteon Therapeutics, RRD, SwanBio, Helixmith, Novartis, Sanofi, PTC, and EMD Serono; and research support from Amylyx, Biogen, Biotie Therapies (now Acorda Therapeutics), Cytokinetics, Mitsubishi Tanabe Pharma America, Alexion, MediciNova Inc, Ionis, Alector, and Orphazyme. BJ has no conflicts of interest to declare. SK, FIM, LM, JW, AAW, and SAR are employees and stockholders of Cytokinetics, Incorporated.
Data availability statement
Data reported herein are part of a sponsor-led clinical development programme that is ongoing, and thus complete datasets for the trial will not be made available with this report.
References
- Brown RH, Al-Chalabi A. Jr. Amyotrophic lateral sclerosis. N Engl J Med. 2017;377:162–72.
- Shefner JM, Liu D, Leitner ML, Schoenfeld D, Johns DR, Ferguson T, et al. Quantitative strength testing in ALS clinical trials. Neurology 2016;87:617–24.
- Andrews JA, Meng L, Kulke SF, Rudnicki SA, Wolff AA, Bozik ME, et al. Association between decline in slow vital capacity and respiratory insufficiency, use of assisted ventilation, tracheostomy, or death in patients with amyotrophic lateral sclerosis. JAMA Neurol. 2018;75:58–64.
- Proudfoot M, Jones A, Talbot K, Al-Chalabi A, Turner MR. The ALSFRS as an outcome measure in therapeutic trials and its relationship to symptom onset. Amyotroph Lateral Scler Frontotemporal Degener. 2016;17:414–25.
- van Eijk RPA, de Jongh AD, Nikolakopoulos S, McDermott CJ, Eijkemans MJC, Roes KCB, et al. An old friend who has overstayed their welcome: the ALSFRS-R total score as primary endpoint for ALS clinical trials. Amyotroph Lateral Scler Frontotemporal Degener. 2021;22:300–7.
- Cedarbaum JM, Stambler N, Malta E, Fuller C, Hilt D, Thurmond B, et al. The ALSFRS-R: a revised ALS functional rating scale that incorporates assessments of respiratory function. BDNF ALS Study Group (Phase III). J Neurol Sci. 1999;169:13–21.
- Jenkinson C, Fitzpatrick R. Reduced item set for the amyotrophic lateral sclerosis assessment questionnaire: development and validation of the ALSAQ-5. J Neurol Neurosurg Psychiatry. 2001;70:70–3.
- Hwee DT, Kennedy AR, Hartman JJ, Ryans J, Durham N, Malik FI, et al. The small-molecule fast skeletal troponin activator, CK-2127107, improves exercise tolerance in a rat model of heart failure. J Pharmacol Exp Ther. 2015;353:159–68.
- Andrews JA, Miller TM, Vijayakumar V, Stoltz R, James JK, Meng L, et al. CK-2127107 amplifies skeletal muscle response to nerve activation in humans. Muscle Nerve. 2018;57:729–34.
- Collibee SE, Bergnes G, Chuang C, Ashcraft L, Gardina J, Garard M, et al. Discovery of reldesemtiv, a fast skeletal muscle troponin activator for the treatment of impaired muscle function. J Med Chem. 2021;64:14930–41.
- Shefner JM, Andrews JA, Genge A, Jackson C, Lechtzin N, Miller TM, et al. A phase 2, double-blind, randomized, dose-ranging trial of reldesemtiv in patients with ALS. Amyotroph Lateral Scler Frontotemporal Degener. 2021;22:287–99.
- Center for Drug Evaluation and Research; Center for Biologics Evaluation and Research. Qualification process for drug development tools. Guidance for industry and FDA staff; November 2020. Available at: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/qualification-process-drug-development-tools-guidance-industry-and-fda-staff. Accessed August 14, 2023.
- Paganoni S, Macklin EA, Hendrix S, Berry JD, Elliott MA, Maiser S, et al. Trial of sodium phenylbutyrate-taurursodiol for amyotrophic lateral sclerosis. N Engl J Med. 2020;383:919–30.
- The Writing Group on behalf of the Edaravone (MCI-186) ALS 19 Study Group. Safety and efficacy of edaravone in well defined patients with amyotrophic lateral sclerosis: a randomised, double-blind, placebo-controlled trial. Lancet Neurol 2017;16:505–12.
- Miller TM, Cudkowicz ME, Genge A, Shaw PJ, Sobue G, Bucelli RC, et al. Trial of antisense oligonucleotide tofersen for SOD1 ALS. N Engl J Med. 2022;387:1099–110.
- Paganoni S, Cudkowicz M, Berry JD. Outcome measures in amyotrophic lateral sclerosis clinical trials. Clin Investig (Lond). 2014;4:605–18.
- Hufnagel SB. Defining and assessing clinical benefit: a regulatory perspective. Presented at the FDA workshop "Developing therapies for primary mitochondrial diseases: bridging the gaps" held September 6, 2019; Silver Spring, MD, USA. Available at: https://www.fda.gov/media/131585/download. Accessed August 15, 2023.
- U.S. Food and Drug Administration. Clinical outcome assessment (COA): frequently asked questions 2020 (updated 2 December 2020). Available at: https://www.fda.gov/about-fda/clinical-outcome-assessment-coa-frequently-asked-questions. Accessed August 14, 2023.
- U.S. Food and Drug Administration Center for Drug Evaluation and Research. Clinical outcome assessment (COA) compendium 2021 (updated June 2021). https://www.fda.gov/media/130138/download. Accessed August 14, 2023.
- Thakore NJ, Lapin BR, Pioro EP, Pooled Resource Open-Access ALS Clinical Trials Consortium. Trajectories of impairment in amyotrophic lateral sclerosis: Insights from the Pooled Resource Open-Access ALS Clinical Trials cohort. Muscle Nerve. 2018;57:937–45.
- Cudkowicz ME, van den Berg LH, Shefner JM, Mitsumoto H, Mora JS, Ludolph A, et al. Dexpramipexole versus placebo for patients with amyotrophic lateral sclerosis (EMPOWER): a randomised, double-blind, phase 3 trial. Lancet Neurol. 2013;12:1059–67.
- Shefner JM, Cudkowicz ME, Schoenfeld D, Conrad T, Taft J, Chilton M, et al. A clinical trial of creatine in ALS. Neurology 2004;63:1656–61.

