Abstract
Objective: Neurotoxic chemicals are suggested in the etiology of amyotrophic lateral sclerosis (ALS). We examined the association of environmental and occupational risk factors including persistent organochlorine pesticides (OCPs) and ALS risk among cases from the Centers for Disease Control and Prevention National ALS Registry and age, sex, and county-matched controls. Methods: Participants completed a risk factor survey and provided a blood sample for OCP measurement. ALS cases were confirmed through the Registry. Conditional logistic regression assessed associations between ALS and risk factors including OCP levels. Results: 243 matched case-control pairs (61.7% male, mean [SD] age = 62.9 [10.1]) were included. Fifteen of the 29 OCPs examined had sufficient detectable levels for analysis. Modest correlations of self-reported years of exposure to residential pesticide mixtures and OCP serum levels were found (p<.001). Moreover, occupational exposure to lead including soldering and welding with lead/metal dust and use of lead paint/gasoline were significantly related to ALS risk (OR = 1.77, 95% CI: 1.11-2.83). Avocational gardening was a significant risk factor for ALS (OR = 1.57, 95% CI: 1.04-2.37). ALS risk increased for each 10 ng/g of α-Endosulfan (OR = 1.42, 95% CI: 1.14-1.77) and oxychlordane (OR = 1.24, 95% CI: 1.01-1.53). Heptachlor (detectable vs. nondetectable) was also associated with ALS risk (OR = 3.57, 95% CI: 1.50-8.52). Conclusion: This national case-control study revealed both survey and serum levels of OCPs as risk factors for ALS. Despite the United States banning many OCPs in the 1970s and 1980s, their use abroad and long half-lives continue to exert possible neurotoxic health effects.
Introduction
Amyotrophic lateral sclerosis (ALS) is fatal and the most common motor neuron disease. The 2018 United States (U.S.) annual incidence of ALS is 2-3 per 100,000 persons and the age-adjusted prevalence is 6.6 per 100,000, which has been increasing in recent decades (Citation1,Citation2). ALS has few known risk factors and 90-95% of cases are considered non-familial/sporadic with 5-10% familial. The median survival is 3-5 years and there is no current reversible treatment for ALS. Major advances have been made in identification of genetic causes of the disease (Citation3). Several environmental and occupational factors have been investigated as potential risk factors for ALS (Citation4–7). In particular, long-term exposure to air pollutants, military service, and hazardous agents such as pesticides and solvents have been studied (Citation4,Citation5,Citation8,Citation9). Associations between ALS and exposure to heavy metals and other trace elements including lead, particularly from occupational activities, are supported by epidemiological evidence (Citation10,Citation11). Similarly, environmental exposure to the inorganic metalloid selenium has been linked to increased ALS incidence (Citation9).
Neurotoxic chemicals including pesticides are suggested to play a role in the etiology of ALS (Citation6,Citation12–15). Numerous studies examined self-reported exposure to pesticides and risk of ALS. Three studies to date have compared persistent organic pesticide (POPs) blood levels in ALS cases and controls. Su et al. (Citation16) measured 122 environmental toxins focusing on POPs in 126 ALS cases and 130 controls in Michigan. Increased ALS risk was reported with elevated serum levels of pentachlorobenzene and cis-chlordane after adjustment for age, sex, and educational levels. Several residential and occupational self-reported exposures were associated with increased serum POPs levels. Su et al. extended their work with a second case-control study of POPs and ALS risk to replicate earlier findings and to evaluate the association with an environmental risk score and survival within the ALS case group (Citation17). Their data continued to support POPs as important factors for ALS risk as well as to be an important factor for enhanced ALS mortality rate (1.65 times, p = 0.008). Vinceti et al. (Citation18) measured six dichlorodiphenyltrichloroethane (DDT) metabolites and hexachlorobenzene levels in cerebrospinal fluid of 38 ALS cases and controls in Italy. Among males aged >60 years, a slight but statistically unstable increased ALS risk was noted with higher levels of the metabolite, dichlorodiphenyldichloroethylene (p,p’-DDE).
Our aim was to examine the association of persistent organochlorine pesticides (OCPs), a form of POPs, and ALS risk among a national sample of cases from the Centers for Disease Control and Prevention Agency for Toxic Substances and Disease Registry (CDC/ATSDR) National ALS Registry and matched controls using serum samples for OCP analysis and a survey assessment for comparison.
Methods
Case selection
To enter the National ALS Registry through its web portal, patients must answer a series of validation (screening) questions. These validation questions were obtained from the Veterans Administration’s ALS Registry and were found to be effective; 93.4% of those who passed the screening questions were determined by a neurologist to have ALS/motor neuron disease (Citation19,Citation20).
The OCP analyses were conducted for 300 of the ALS cases enrolled in the National ALS Registry as well as the National ALS Biorepository arms from the 500 participants meeting the study criteria. For our study, CDC identified a convenience sample of 280 of these 500 cases distributed equally between four geographic areas. Full survey information was important for participants, so it was used as one of the selection criteria. These cases enrolled in the Registry and provided survey and serum samples for OCP analyses performed by SGS AXYS Analytical Services Ltd. (British Columbia).
The current study included 80 Biorepository Pilot cases from 2013-2015 and 200 cases (2017-2018) from the ongoing ALS Biorepository. In addition to CDC obtaining a representative age and sex distribution for the 280 cases, cases were randomly assigned from within each of the four Census regions of the U.S.
The University of Pittsburgh, under a data use agreement with CDC, received the case survey information and serum OCP results. Controls were identified to collect survey data and non-fasting blood samples (N = 243), which were measured at the same lab as cases. The University of Pittsburgh Institutional Review Board approved the study.
Control selection
We recruited age, sex, and county-matched controls for ALS cases between 2018 and 2021. Potential controls were identified using a national sample of individuals through MSG (Marketing Systems Group, Horsham, Pennsylvania) based on commercial/consumer databases; details of which are published elsewhere (Citation21). Exclusion criteria included a self-reported diagnosis of ALS, Parkinson’s disease, Parkinsonism, or post-polio syndrome. Information for cases was based on the ALS Registry’s Risk Factor Survey which was created and validated by the Stanford University School of Medicine’s ALS Consortium of Epidemiologic Studies (ACES) (Citation22). Controls were administered a similar survey to collect self-reported information by trained interviewers using a computer-assisted questionnaire available on the web. https://pitt.co1.qualtrics.com/jfe/form/SV_3gcOXnQ54PMfjwh
Survey-Based Occupational, Residential and Hobby Exposure: The survey consisted of personal demographics, lifestyle, residential, military, and occupational history sections as well as detailed information on lifetime household, occupational and hobby exposure to herbicides and pesticides, metals, aromatics, and other solvents (Citation23). Total years of exposure to pesticides was calculated (age of first use to age of last use minus years of nonuse).
To capture occupational exposures for participants who responded “Yes,” the survey asked, “Over your lifetime (at least 100 days or more), have you ever had a JOB where you handled [exposure of interest]?” Duration in years of occupational exposures included handling herbicides, fungicides, insecticides, rodenticides, glues or adhesives, solvents, or degreasers, as well as unleaded/leaded gasoline or paint, and using soldering or welding in metal fabricating resulting in metal dust or fumes. There were 27 jobs categorized into six groups based on the participant’s longest held position: professional; managerial specialty; technical/sales; service, operators, fabricators, and laborers; and other. Similarly, the survey included a checklist of industries based on the 2012 North American Industry Classification System (Citation24).
Five questions were included about exposure to OCPs: herbicides, insecticides, fumigants, and fungicides in the home, insecticides in the yard and lawn, and use of chemical pet soaps. We used similar definitions of duration in years for self-reported exposure to pesticides in the home and exposure to occupational and hobby related pesticides, also including exposure to metals related to jobs and hobbies involving lead and organic solvents. Due to small numbers of affirmative responses to hobby survey questions, these were grouped into ever/never categories (e.g. participation for one hour per month for at least one year).
After survey administration, a blood specimen was collected for OCP measurement. Consent was obtained from each control by mail and a blood draw conducted by ExamOne (www.examone.com, Lenexa, KS), an in-home national biospecimen collection company, during which the phlebotomist obtained a 10 ml non-fasting blood sample. Upon arrival at the University of Pittsburgh, the sample was centrifuged and the serum aliquoted and stored at −35 °C until shipment on dry ice to SGS AXYS.
Blood Sample Analysis: Serum samples were sent in batches of 20-40 specimens of 2 ml serum each to the lab for analysis. The samples, including method blank and quality control samples, were fortified with isotopically labeled pesticides and liquid-liquid extracted using a 1:1:3 mixture of ethanol: saturated ammonium sulfate: hexane. The resulting extract was chromatographically cleaned using a series of size exclusion gel permeation and florisil columns. Extracts were fractionated into non-polar (E1) and polar (E2) portions and separately instrumentally analyzed. The E1 and E2 portions were analyzed by high resolution gas chromatography/high resolution mass spectrometry (HRGC/HRMS). The high-resolution mass spectrometer (HRMS) was equipped with a J&W DB5 chromatography column (60 m, 0.25 mm i.e. 0.10 µm film thickness) coupled directly to the HRMS source. The HRMS was operated at a static (8000) mass resolution (10% valley) in the electron ionization (EI) mode using multiple ion detection (MID) acquiring two characteristic ions for each target analyte and surrogate standard. Target concentrations were calculated using isotope dilution quantification and results were adjusted to a lipid-based sample weight.
OCP Analytical Methods: SGS AXYS Analytical Services Ltd. (British Columbia) used analytical procedures described in the MLA-028 Method, the same methodology used by CDC for the NHANES Study of OCPs, to determine concentrations of OCPs using isotope dilution HRGC/HRMS by EPA Method 1699 (https://www.epa.gov/sites/default/files/2015-10/documents/method_1699_2007.pdf). This method also allows for the optional analysis of technical Toxaphene and Toxaphene congeners. C12-PCB 159 is the surrogate standard for both analyses, but different ions are monitored.
Case and control samples were analyzed by SGS AXYS using the same methodology with the same instruments and lab procedures for both the cases and the controls within a similar time period. They were not done in parallel, but cases were measured in 2018-2019 and controls 2019-2021. Twenty nine OCPs were measured using lipid-adjusted analyses for 280 cases and 243 controls that included: α-HCH, β-HCH, γ-HCH, δ-HCH, Heptachlor, Heptachlor Epoxide, Hexachlorobenzene, Aldrin, cis-Chlordane, trans-Chlordane, oxy-Chlordane, trans-Nonachlor, cis-Nonachlor, 2,4′-DDD, 4,4′-DDD, 2,4′-DDE, 4,4′-DDE, 2,4′-DDT, 4,4′-DDT, Mirex, Toxaphene, α-Endosulfan, Dieldrin, Endrin, β-Endosulfan, Endosulfan sulfate, Endrin aldehyde, Endrin ketone, and Methoxychlor.
Statistical Analysis: Personal, environmental, and occupational characteristics are presented as frequencies or means/standard deviations (SD). Demographic characteristics, smoking status, and exposure to pollutants were cross classified by case-control status. Differences between cases and controls for continuous variables were assessed using t-tests and Mann–Whitney U tests (when non-normally distributed), and Chi-square for categorical variables. We chose not to adjust for multiple comparisons in this study as all the analyses in the paper were performed according to planned hypotheses. The data analyses therefore were not corrected for multiple comparisons.
The proportion of detectable levels in serum samples varied dramatically by OCP. Thus, the exposure metric used in the logistic regression models for the different OCPs included one of the three classifications:
For OCPs having measurable levels in ≥70% of the samples, the exposure was treated as a continuous measure. Additionally, samples with nondetectable values were imputed as LOD/sqrt (2), where LOD is the limit of detection (Citation25).
For OCPs having measurable levels in 25-<70% of the samples, the metric had three categories: undetectable, below and above the median of the measurable exposures.
For OCPs with measurable levels in ∼5-<25% of the samples, the metric had two categories: undetectable/detectable. Finally, OCPs with too few measurable samples (i.e. <5%) were not fit to regression models and descriptives by case-control status and are provided in Table S1.
For OCPs analyzed as having a continuous exposure metric (e.g. 1), Spearman rank-order correlations assessed the association of self-reported exposures (years when personally handled herbicides, fungicides, insecticides, and use of chemical pet soaps at home) and OCP serum levels in cases and controls.
Both unadjusted and adjusted conditional regression and adjusted unconditional logistic regression models (including matching variables: age and sex), were fit with case-control status as the dependent variable and OCP serum level as the independent variable. From previous work and the literature, a base multivariable model was fit with the covariates of education level and smoking (Citation26,Citation27). Odds ratios (OR) and 95% confidence intervals (CI) from the resulting regression models are estimates of the relative risk of ALS. Multivariable models with self-reported occupational and personal exposures to insecticides, herbicides, solvents, and lead, were also separately fit using conditional logistic models. All analyses were performed using SAS 9.4.
Results
Of the 280 cases with surveys and blood, one individual had an OCP analysis but lacked lipid adjustments, resulting in 279 cases. A matched control with a survey was obtained for 267 of the 279 cases (95.7% participation rate). Among the 267 controls, 243 (91%) consented to the University of Pittsburgh on the followed request of an in-home blood draw. See Figure S1.
Demographic Characteristics: presents characteristics of the cases and matched controls. The male/female distribution of cases and controls was similar to the national norm for ALS with 61.7% males and 38.3% females. The mean (SD) age at ALS diagnosis was 61.2 (9.9) years, and their age at blood draw was 62.9. Controls were individually matched to cases on year of birth; with 72% born between 1940 and 1959. Most participants were white (98.8%). A total of 34.2% of cases and 39.5% of controls attained a college degree. Regional distribution of cases and controls was similar with slightly less proportion of cases and controls living in the Northeast region of the U.S. Military enrollment and deployment outside the U.S. were comparable between cases and controls. A greater proportion of ALS cases reported ever smoking than controls (43.2% vs 34.2%, p=.04). Occupation and industry for the longest job held showed no significant increased risk of ALS (Table S1).
Table 1. Demographic characteristics at survey of 243 ALS cases and controls.
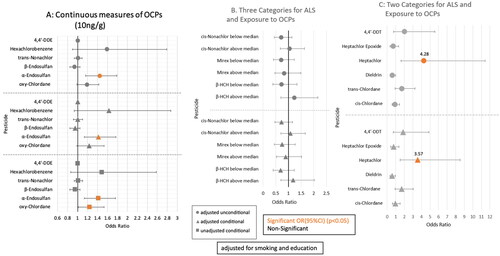
OCP Serum Levels Distribution: presents percentile distributions of the 243 cases and 243 controls for the 29 OCPs. Six of the OCPs had detectable levels in ≥70% of their samples (4,4′-DDE, Hexachlorobenzene, trans-Nonachlor, β-Endosulfan, α-Endosulfan, and oxychlordane). Three additional OCPs had detectable levels in 25-52% of their samples (cis-Nonachlor, Mirex, and β-HCH). The third group consisting of cis-Chlordane, trans-Chlordane, Dieldrin, Heptachlor, Heptachlor Epoxide, and 4,4′-DDT had detectable levels in 5-<25% of their samples. Of the remaining 14 OCPs, 11 had detectable levels in <5% of samples (Table S2), with three having no detectable levels (Toxaphene, Methoxychlor, δ-HCH) thus limiting analysis. Table S3 presents summary statistics for serum OCP levels for the 6 pesticides analyzed as a continuous measure (classification 1) for observed and LOD adjusted data by case-control status.
Table 2. Distribution of observed organochlorine pesticides detection levels and analytical strategies among 243 cases and controls.
Duration of Exposure to Pesticides: Results of the comparison of years of self-reported exposure to residential-based pesticide mixtures and their correlations with OCP levels show () modest correlations with years of use and OCP serum levels. Significant spearman correlation coefficients ranged from 0.17 for cases for levels of hexachlorobenzene from exposure to insecticides in the home to 0.30 for trans-nonachlor levels and insecticides use in the yard. For controls, the lowest correlation was 0.19 for oxychlordane exposure and years of herbicide use in the yard to 0.27 for oxychlordane and use of chemical pet soaps. α- and β-endosulfan levels were not correlated to at home pesticide use and not approved for residential use.
Table 3. Spearman rank-order correlation matrix for cases and controls using self-reported total years of exposure to residential pesticides.
Logistic Regression Results: Results are displayed for adjusted and unadjusted logistic regression analyses for the 15 OCPs with sufficient detectable levels for analysis in . After controlling for smoking and education, α-Endosulfan (OR = 1.42, 95% CI: 1.14-1.77; adjusted conditional) and oxychlordane (OR = 1.24, 95% CI: 1.01-1.53; unadjusted conditional) showed increased risk of ALS for a 10 ng/g increase (). shows the three OCPs (cis-Nonachlor, Mirex, and β-HCH) were not significantly related to risk of ALS. Analyses of the six additional OCPs (cis-Chlordane, trans-Chlordane, Dieldrin, Heptachlor, Heptachlor Epoxide, and 4,4′-DDT) (), dichotomized by detectable or undetectable OCP levels, revealed Heptachlor was significantly associated with ALS risk (OR = 3.57, 95% CI: 1.50-8.52) in both adjusted conditional and unconditional regression models.
presents the conditional logistic regression results for self-reported occupational, residential, and avocational exposures to pesticides, solvents, and lead with ALS risk. Occupational exposures to lead including soldering and welding with lead/metal dust and use of lead paint/gasoline were significantly related to ALS risk (OR = 1.77, 95% CI: 1.11-2.83). In addition, gardening was a significant risk factor for ALS (OR = 1.57, 95% CI: 1.04-2.37).
Table 4. Occupational, residential, and avocational exposures to pesticides, solvents, and lead in 243 ALS cases and matched controlsTable Footnotea.
Discussion
Our population-based case-control study used both survey data and serum levels for measurement of 29 OCPs resulting in 15 OCPs with detectable frequencies to consider the association with risk of ALS. Three OCPs (Oxychlordane, α-endosulfan, Heptachlor) demonstrated an elevated risk for ALS. These are within a subcategory of heavily chlorinated organochlorine insecticides (Citation28). Moreover, our survey data revealed occupations involving lead exposure and gardening as a hobby were also associated with increased risk for ALS.
ALS is characterized by progressive degeneration of spinal and cortical motor neurons, and multiple pathogenic mechanisms have been suggested. These include mitochondrial dysfunction, oxidative stress, dysregulated RNA signaling, excitotoxicity, and impaired axonal transport (Citation29). A recent study by Kulic et al. investigated the neurotoxicity of cis-chlordane on motor neurons using in vitro and in vivo models. The authors found cis-chlordane is especially toxic to motor neurons in vitro- and in vivo-independent of its known antagonism of the GABA receptor (Citation30).
Serum levels of OCPs have been decreasing over time in the general population after a period of peak production and banning of OCPs in the U.S (Citation31). However, currently background concentrations of these remaining OCPs are still higher than many organic pollutants such as polychlorinated biphenyls (PCBs) and polybrominated diphenyl ethers (PBDEs) (Citation32).
Strengths and limitations
The National ALS Registry and Biorepository provided a unique opportunity to obtain both survey data and serum for OCP analysis for our study. We were able to use the same lab, methodology and instrumentation for OCP measurement of cases and controls. Additionally, we recruited a population-based sample of controls matched to cases which strengthened the generalizability of the results.
The survey obtained self-reported occupational and residential pesticide exposure history and information on risk factors such as lead and solvents. The individual data on duration (years) of exposure to pesticides revealed a significant correlation of self-reported duration of exposure to pesticides serum levels with additional confirmation of occupational exposure to lead and ALS risk. We also noted an increased risk of ALS and gardening over time.
Limitations include the lack of information on the ages that participants self-reported personal, environmental, and occupational exposures, preventing analysis by time windows of exposures. It is also possible that some individuals who self-enrolled in the National ALS Registry were not diagnosed with ALS which could result in nondifferential classification of the exposure and bias the results toward the null.
Moreover, particularly at the start of the Registry, patients who joined the CDC Registry may have been longer survival prevalent cases. Our ALS study cases enrolled in the Registry from 2013-2018 and ALS survival is estimated to be on average two to five years. We calculated years from diagnosis of ALS to enrollment in the Registry and determined that 79.4% reported diagnosis and ALS registry survey completion within two years of their diagnosis with an additional ten percent within five years of diagnosis. The remaining ten percent of registry cases represent those with longer survival which could have introduced bias.
However, one of the study’s main objectives was to investigate persistent OCP serum levels and its association with ALS; many OCPs have a have long half-lives (DDE, DDT, 7 years, chlordane, 10 years) and are still used in other developed countries and continues to accumulate in foods (Citation33,Citation34). As most cases fall within this window for capturing blood levels of these OCPs, we believe the validity and integrity of the study has been maintained with the original hypothesis and aims.
Another potential limitation is that this study may not reflect a more standard ALS Cohort with some socioeconomic bias regarding who participates. As this was a matched case control study, controls were matched to cases by age, gender, and region of the country; which helps control for many potential confounding factors regarding the potential exposures under investigation. Moreover, education, a primary indicator of socioeconomic bias was similar within comparison groups (the cases had 34.2% with college graduation and 32.5% postgraduate compared to 39.2% of controls with college education and postgraduate was 30.0% which is similar). In addition, the male: female ratio of 61.7% and 38.3% is the same as the national norm (Citation35). We believe we have good internal validity to perform the analyses.
There was also no ability to compensate or adjust for the prodromal or preclinical period. Although the survey obtained self-reported years of exposure to lawn, garden and residential applications of insecticides, herbicides, etc., the individual compounds were not collected. We were not able to adequately address occupational exposures due to small numbers and lack of specificity in the occupational history.
Conclusion
This study found significantly increased risk of ALS with occupational exposures to lead as well as residential pesticide use in the garden. Moreover, three of the more heavily chlorinated serum OCPs, oxychlordane, α-endosulfan, and heptachlor, should be targeted for further investigation due to their long half-lives and continued use abroad, exerting possible neurotoxic health effects.
Role of the funder/sponsor
The funding agencies had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication. The content is solely the responsibility of the authors and does not necessarily represent the official views of the Centers for Disease Control and Prevention.
Supplemental Material
Download MS Word (211.9 KB)Declaration of interest
The authors have no conflicts of interest to disclose.
Additional information
Funding
References
- Longinetti E, Fang F. Epidemiology of amyotrophic lateral sclerosis: an update of recent literature. Curr Opin Neurol. 2019;32:771–6.
- Mehta P, Raymond J, Zhang Y, Punjani R, Han M, Larson T, et al. Prevalence of amyotrophic lateral sclerosis in the United States, 2018. Amyotroph Lateral Scler Frontotemporal Degener. 2023;24:1–7.
- Zufiría M, Gil-Bea FJ, Fernández-Torrón R, Poza JJ, Muñoz-Blanco JL, Rojas-García R, et al. ALS: A bucket of genes, environment, metabolism and unknown ingredients. Prog Neurobiol. 2016;142:104–129.
- Wang H, O'Reilly EJ, Weisskopf MG, Logroscino G, McCullough ML, Thun MJ, et al. Smoking and risk of amyotrophic lateral sclerosis: a pooled analysis of 5 prospective cohorts. Arch Neurol. 2011;68:207–13.
- Weisskopf MG, O'Reilly EJ, McCullough ML, Calle EE, Thun MJ, Cudkowicz M, et al. Prospective study of military service and mortality from ALS. Neurology 2005;64:32–7.
- McGuire V, Longstreth WT, Jr., Nelson LM, Koepsell TD, Checkoway H, Morgan MS, et al. Occupational exposures and amyotrophic lateral sclerosis. A population-based case-control study. Am J Epidemiol. 1997;145:1076–88.
- Fiore M, Parisio R, Filippini T, Mantione V, Platania A, Odone A, et al. Living near waterbodies as a proxy of cyanobacteria exposure and risk of amyotrophic lateral sclerosis: a population based case-control study. Environ Res. 2020;186:109530.
- Ingre C, Roos PM, Piehl F, Kamel F, Fang F. Risk factors for amyotrophic lateral sclerosis. Clin Epidemiol. 2015;7:181–93.
- Vinceti M, Bottecchi I, Fan A, Finkelstein Y, Mandrioli J. Are environmental exposures to selenium, heavy metals, and pesticides risk factors for amyotrophic lateral sclerosis? Rev Environ Health. 2012;27:19–41.
- Wang MD, Gomes J, Cashman NR, Little J, Krewski D. A meta-analysis of observational studies of the association between chronic occupational exposure to lead and amyotrophic lateral sclerosis. J Occup Environ Med. 2014;56:1235–42.
- Oskarsson B, Horton DK, Mitsumoto H. Potential Environmental Factors in Amyotrophic Lateral Sclerosis. Neurol Clin. 2015;33:877–88.
- Malek AM, Barchowsky A, Bowser R, Youk A, Talbott EO. Pesticide exposure as a risk factor for amyotrophic lateral sclerosis: a meta-analysis of epidemiological studies: pesticide exposure as a risk factor for ALS. Environ Res. 2012;117:112–9.
- Kamel F, Umbach DM, Bedlack RS, Richards M, Watson M, Alavanja MCR, et al. Pesticide exposure and amyotrophic lateral sclerosis. Neurotoxicology 2012;33:457–62.
- Weisskopf MG, Morozova N, O'Reilly EJ, McCullough ML, Calle EE, Thun MJ, et al. Prospective study of chemical exposures and amyotrophic lateral sclerosis. J Neurol Neurosurg Psychiatry. 2009;80:558–61.
- Andrew A, Zhou J, Gui J, Harrison A, Shi X, Li M, et al. Pesticides applied to crops and amyotrophic lateral sclerosis risk in the U.S. Neurotoxicology 2021;87:128–35.
- Su FC, Goutman SA, Chernyak S, Mukherjee B, Callaghan BC, (British Columbia), Batterman S, et al. Association of environmental toxins with amyotrophic lateral sclerosis. JAMA Neurol. 2016;73:803–11.
- Goutman SA, Boss J, Jang D-G, Mukherjee B, Richardson RJ, Batterman S, et al. Environmental risk scores of persistent organic pollutants associate with higher ALS risk and shorter survival in a new Michigan case/control cohort. J Neurol Neurosurg Psychiatry. 2023;95:241–8.
- Vinceti M, Violi F, Tzatzarakis M, Mandrioli J, Malagoli C, Hatch EE, et al. Pesticides, polychlorinated biphenyls, and polycyclic aromatic hydrocarbons in cerebrospinal fluid of amyotrophic lateral sclerosis patients: a case-control study. Environ Res. 2017;155:261–7.
- Kaye WE, Wagner L, Wu R, Mehta P. Evaluating the completeness of the national ALS registry, United States. Amyotroph Lateral Scler Frontotemporal Degener. 2017;19:112–7.
- Allen KD, Kasarskis EJ, Bedlack RS, Rozear MP, Morgenlander JC, Sabet A, et al. The national registry of veterans with amyotrophic lateral sclerosis. Neuroepidemiology 2008;30:180–90.
- Bear TM, Malek AM, Foulds A, Rager J, DePerrior SE, Vena JE, et al. Recruitment of population-based controls for ALS cases from the National ALS Registry. ALS FTD. 2021.
- ALS Consortium of Epidemiologic Studies (ACES). ALS Consortium of Epidemiologic Studies (ACES): Stanford School of Medicine. http://aces.stanford.edu/.
- Bryan L, Kaye W, Antao V, Mehta P, Muravov O, Horton DK. Preliminary Results of National Amyotrophic Lateral Sclerosis (ALS) Registry Risk Factor Survey Data. PLoS One. 2016;11:e0153683.
- U.S. Census Bureau. North American Industry Classification System. Office of Management and Budget, U.S. Census Bureau. 2012.
- Croghan CW, Egehy PP. Methods of Dealing with Values Below the Limit of Detection using SAS. Presented at Southeastern SAS User Group, St Petersburg, FL. 2003.
- Seelen M, Toro Campos RA, Veldink JH, Visser AE, Hoek G, Brunekreef B, et al. Long-term air pollution exposure and amyotrophic lateral sclerosis in Netherlands: A population-based case–control study. Environ Health Perspect. 2017;125:097023.
- Sutedja NA, Veldink JH, Fischer K, Kromhout H, Wokke JHJ, Huisman MHB, et al. Lifetime occupation, education, smoking, and risk of ALS. Neurology 2007;69:1508–14.
- Hastings J, Owen G, Dekker A, Ennis M, Kale N, Muthukrishnan V, et al. ChEBI in 2016: Improved services and an expanding collection of metabolites. Nucleic Acids Res. 2016 Jan;44:D1214–9.
- Ferraiuolo L, Kirby J, Grierson AJ, Sendtner M, Shaw PJ. Molecular pathways of motor neuron injury in amyotrophic lateral sclerosis. Nat Rev Neurol. 2011;7:616–30.
- Kulick D, Moon E, Riffe RM, Teicher G, Van Deursen S, Berson A, et al. Amyotrophic lateral sclerosis-associated persistent organic pollutant cis-chlordane causes GABAA-independent toxicity to motor neurons, providing evidence toward an environmental component of sporadic amyotrophic lateral sclerosis. ACS Chem Neurosci. 2022;13:3567–77.
- Stoytcheva M. Pesticides: Strategies for Pesticides Analysis. Intech Open Access Pub.; 2011. ISBN 978-953-307-460-3, Croatia.
- Woodruff T, Wolff MS, Davis DL, Hayward D. Organochlorine exposure estimation in the study of cancer etiology. Environ Res. 1994;65:132–44.
- Longnecker MP. Invited commentary: Why DDT matters now. Am J Epidemiol. 2005;162:726–8.
- Reinke EN, Deck AT. Wildlife toxicity assessment for chlordane. Wildlife Toxic Assess Chem Milit Concern 2015;385:385–411.
- Talbott EO, Malek AM, Lacomis D. The epidemiology of amyotrophic lateral sclerosis. In: Rosano CIG, ed. Handbook of clinical neurology: Neuroepidemiology. Vol 138. 3rd series. Amsterdam, Netherlands: Elsevier; 2016:225–8.