ABSTRACT
Background
The US Food and Drug Administration (FDA) and European Medicines Agency (EMA) have incentives to stimulate the development and marketing of orphan drugs. Health Canada has none.
Methods
We identified 82 FDA and/or EMA-designated orphan drugs approved by one or both agencies between 2015 and 2020 that were also authorized in Canada. We tracked the drugs through health technology assessments (HTAs), price negotiations, and listing in government drug plans to assess the time required for these processes.
Results
Median times for HTAs and price negotiations suggest a delay of around a year, but the median wait time between marketing authorization and price negotiation completion was over 18 months.
Conclusions
Listing of orphan drugs in Canadian government drug plans is closely aligned with reimbursement recommendations and outcomes of price negotiations. Medicines with unsuccessful price negotiations are not listed. However, not all drugs with successful negotiations are listed by all provinces and listing does not guarantee patient access. Compared with Americans and some western Europeans, Canadians with rare disorders continue to suffer from a lack of timely and equitable access to innovative treatments. A comprehensive orphan drug policy would improve Canadians’ access to the innovative treatments on the research horizon.
1. Introduction
Unlike other comparable countries [Citation1–3], Canada has no orphan drug act nor any incentives to encourage drug developers to launch medicines for small numbers of patients, and only recently has committed to developing a national strategy to treat rare disorders [Citation4]. Some but not all medicines with US Food and Drug Administration (FDA) and/or European Medicines Agency (EMA) orphan designation are submitted to Health Canada for marketing authorization. However, considerable uncertainty among drug developers around the regulation of drug prices that has existed in Canada since 2015 was anticipated to reduce the number of medicines launched in Canada [Citation5] and this has subsequently transpired. Almost 80% of 247 medicines submitted to the FDA and/or the EMA between 2006 and 2020 and given orphan designation by one or both of these agencies were also submitted to Health Canada, but the percentage of FDA or EMA orphan medicines submitted to Health Canada decreased between 2015 and 2020 and was only 39.3% for orphan drugs submitted to the FDA and/or the EMA in 2020 [Citation6].
The broad objective of this analysis was to assess the processes involved in FDA and/or EMA orphan-designated medicines authorized for marketing in Canada moving through health technology assessment (HTA) by the Canadian Agency for Drugs and Technologies in Health (CADTH), price negotiations with government drug plans via the pan-Canadian Pharmaceutical Alliance (pCPA), and their listing in government drug plan formularies.
CADTH provides ‘evidence, analysis, advice and recommendations to healthcare decision-makers in Canada, except in Quebec, so that they can make informed decisions’ [Citation7]. The agency has two committees (one for oncology medicines and another for all other drugs) that make non-binding HTA-based reimbursement recommendations to government drug plans. Following a positive recommendation, biopharmaceutical developers usually look to be invited by the pCPA into a collective negotiating process with federal, provincial and territorial government drug plans – usually all plans for innovative costly medicines. If an agreement is reached, a letter of intent is signed that implies the drug will be listed in any subsequent agreement with government drug plans with an established price and listing criteria. However, the plans are not mandated to reimburse a medicine that has been successfully negotiated with the pCPA. Using the terms of the pCPA’s letter of intent, manufacturers negotiate individual product listing agreements or equivalents with each participating plan. The result is an uneven patchwork (sometimes called a postal code lottery) of drug access across Canada – one of the consequences of the country being a federation of provinces and territories and not a unitary nation.
2. Methods
outlines the processes and responsibilities for the approval and listing of drugs in public drug plans in Canada. With the exception of drug developers’ ability to submit to CADTH up to 180 days before the anticipated regulatory approval date, the processes are performed in succession.
Table 1. Processes and responsibilities for the listing of medicines in government drug plans.
Data on therapeutic medicines (vaccines and diagnostic products were excluded) with FDA and/or EMA orphan designation and approved by one or both of the regulatory agencies between 2015 and 2020 and approved in Canada were identified from a previous analysis [Citation6], which used publicly available data [Citation8,Citation25–29].
Data on HTA outcomes – dates of submission and recommendation, reimbursement recommendation, access conditions and price comments – were obtained from CADTH’s website as at the middle of December 2023 [Citation9]. CADTH frequently compares itself and collaborates with the United Kingdom’s National Institute for Health and Care Excellence (NICE), although their processes have some differences. For example, NICE does not evaluate every drug but when it does and recommends a drug, the National Health Service (NHS) is legally required to make it available within three months [Citation30]; in Canada, a developer may submit its drug to CADTH (most do) and receive a positive reimbursement recommendation to be considered by government drug plans, but the plans are not mandated to list it in their formularies. HTA guidance reports from NICE [Citation31] were reviewed to identify whether any of the orphan medicines were evaluated for the NHS to assess concordance between the two agencies.
Information available at the middle of December 2023 on price negotiations between the pCPA and manufacturers, including dates when negotiations began and ended or a decision was made not to pursue a negotiation, were identified from the pCPA’s website [Citation10].
All provincial drug plan formularies were reviewed to identify which orphan drugs were listed and reimbursed by mid-December 2023 [Citation11–20]. Drugs for cancer are included in the formularies of New Brunswick, Newfoundland and Labrador, Nova Scotia, Prince Edward Island and Quebec. Alberta, British Columbia, Ontario and Saskatchewan have separate cancer drug lists [Citation21–24], which were reviewed to identify listed oncology medicines. Manitoba’s cancer drug list is not publicly available.
The information on medicines reviewed and approved by Health Canada, the FDA and the EMA was part of a data collection by one of the authors that has been ongoing for many years [Citation32]. Data on HTAs, price negotiations and provincial listing were double-checked. Statistical analyses of numeric data used appropriate non-parametric tests, since the data were not normally distributed, and Cohen’s kappa for concordance.
The specific objectives of this analysis were to evaluate:
Extent of time required for Health Canada to review orphan drugs, CADTH to assess them, and the pCPA to complete its work, together with the time between regulatory approval and the outcome of the price negotiation process.
Whether the inclusion of results from one or more randomized clinical trials in the submission to CADTH impacted the outcome or the duration of CADTH’s review.
Whether a recommendation from CADTH for a major price reduction impacted the duration of the pCPA price negotiation.
Concordance between CADTH and NICE recommendations, where available.
Alignment between CADTH recommendations and pCPA outcomes.
Listing of the orphan medicines in provincial drug plan formularies.
3. Results
A total of 132 therapeutic medicines with FDA and/or the EMA orphan designation and approved by one or both of the regulatory agencies between 2015 and 2020 were identified. Since medicines for blood disorders, such as hemophilia, are dealt with by Canadian Blood Services on a national basis, the five products of this type were excluded, which left 127 drugs. Five medicines for tropical diseases, such as Ebola fever, malaria and onchocerciasis, and two for anthrax and orthopoxvirus were also excluded because they were unlikely to be assessed by CADTH, leaving 120 orphan drugs. Eighty-two of these drugs (68.3%) were authorized for marketing by Health Canada by the end of June 2023.
3.1. Regulatory approval
The 82 orphan medicines are listed in . All were approved by Health Canada and the FDA, while 77 were approved by the EMA. The exceptions were cedazuridine/decitabine, lurbinectedin and triheptanoin, which were under review, and edaravone and enasidenib whose submissions to the EMA were withdrawn by their manufacturers.
Table 2. 41 oncology orphan drugs by Health Canada approval date.
Table 3. 41 non-oncology orphan drugs by Health Canada approval date.
The median wait time between submission of the drugs to the FDA or the EMA, whichever was first, and submission to Health Canada was almost 12.5 months; for a quarter of the medicines, the wait was at least 20 months. Once the drugs were submitted to Health Canada, they were generally authorized for use by the regulator in a similar time frame as the FDA but more quickly than the EMA (p < 0.001) (). A similar pattern existed in both oncology and non-oncology orphan drugs, although significant differences were only found in the review times of the oncology drugs where the time required by the FDA was shorter than that required by Health Canada (p < 0.001) which, in turn, was shorter than the time required by the EMA (p < 0.001).
Table 4. Health Canada, FDA and EMA regulatory review times.
3.2. Health technology assessment
Seventy-eight of the 82 orphan drugs had submissions to CADTH. The initial recommendation for the 78 drugs was positive (reimburse or reimburse with conditions) for 62 (79.5%) and negative (do not reimburse) for 16 (20.5%) (). Resubmissions made for six oncology drugs (alectinib, brigatinib, daratumumab, ixazomib, larotrectinib and lorlatinib) and two non-oncology drugs (caplacizumab and ivacaftor/lumacaftor) resulted in alectinib, brigatinib, daratumumab, larotrectinib and lorlatinib receiving positive recommendations. Thus, 67 orphan drugs (85.9%) ultimately had a positive recommendation and 11 (14.1%) had a negative one.
Table 5. Flow of the 82 orphan drugs through CADTH and the pCPA.
The time required by CADTH to review the 78 orphan drugs with a completed HTA is summarized in . The median time for HTAs was over seven months (consistent for both oncology and non-oncology drugs, and not significantly different), although a quarter of the assessments took considerably longer.
Table 6. Time for CADTH and pCPA processes & between approval and pCPA completion.
Fifty-two (66.7%) of the 78 drugs had at least one randomized clinical trial as part of the submission to CADTH. There was an important difference between submissions that received a reimbursement recommendation and those that did not. A significantly higher proportion with a positive recommendation had at least one randomized trial (74.6%) compared with those that received a negative recommendation (33.3%) (p = 0.006). No significant difference was found between review times of submissions with or without a randomized clinical trial.
CADTH’s positive recommendations are usually conditional on clinical and/or cost-reduction criteria. Until the end of 2020, reviews of oncology medicines normally only stated that an improvement in cost-effectiveness was required; 80.0% of the positive recommendations for the oncology drugs published between 2015 and 2020 were qualified in this way (none mentioned a percentage reduction). In contrast, reviews of non-oncology drugs have specified percentage price reductions required to achieve a cost-effectiveness threshold of $50,000 per quality-adjusted life year for several years. The format of oncology reviews changed in 2021 to become consistent with non-oncology reviews. The impact of this can be seen in the fact that 73.7% of the positive recommendations for the oncology drugs published after 2020 specified a percentage price reduction. Specific price reductions were included in 72.2% and 87.5% of the positive recommendations for the non-oncology drugs released in 2015–2020 and after 2020, respectively. In 73.1% of the orphan drug reviews in which a price decrease was recommended, the specified reduction could exceed 70%.
3.3. Price negotiation
Price negotiations were in progress for three of the 78 orphan drugs, negotiations were under consideration for four, and no pCPA record was identified for two. The initial negotiation led to a successful outcome for 49 (71.0%) of the other 69 drugs and an unsuccessful outcome for six (8.7%), while a decision not to negotiate was made for the remaining 14 drugs (20.3%). After further negotiations, 56 drugs (81.2%) had a successful outcome and four (5.8%) had an unsuccessful result; none was pursued for nine (13.0%) ().
The time required by the pCPA for the 60 drugs that had a completed negotiation (successful or not) is summarized in . The median time for a completed pCPA price negotiation for all the orphan drugs was six months. However, price negotiations took significantly less time for the oncology drugs at five months than for the non-oncology drugs at eight months (p = 0.002).
No significant difference was identified between the proportion of drugs with a CADTH recommendation for a price reduction of 90% or more and those with a lower price reduction recommendation in either the percentage successfully negotiated with the pCPA (p = 0.41) or the duration of the negotiation (p = 0.46).
3.4. Regulatory approval to price negotiation completion
The times taken for CADTH reviews and pCPA negotiations suggest delays of a little over a year, but the median of the time between the date of authorization from Health Canada and the date of the price negotiation completion, including a decision not to pursue negotiation, was over 18 months due to gaps between processes, resubmissions to CADTH and more than one pCPA negotiation (). For a quarter of the drugs, the time from regulatory approval to price negotiation completion was over two years.
Only 34 (43.6%) of the 78 submissions to CADTH took advantage of the opportunity to submit prior to the anticipated Health Canada authorization date; they were submitted a median of 94 days (inter-quartile range: 58–147 days) before authorization.
3.5. Concordance between CADTH and NICE
Sixty-two orphan drugs in had reimbursement recommendations from both CADTH and NICE (34 oncology drugs and 28 non-oncology drugs). CADTH and NICE were in agreement for 51 drugs (82.3%) based on the initial assessment from CADTH. For seven oncology drugs (alectinib, brigatinib, daratumumab, ixazomib, larotrectinib, lorlatinib and pemigatinib) and two non-oncology drugs (caplacizumab and fostamatinib), the NICE guidance was positive and CADTH’s recommendation was negative, while CADTH’s recommendation was positive for pralsetinib and ripretinib and that from NICE was negative. The clinical evidence submitted to both CADTH and NICE was the same for six of these drugs (caplacizumab, fostamatinib, ixazomib, larotrectinib, pemigatinib and ripretinib). Resubmissions to CADTH with different or additional clinical evidence led to positive recommendations for alectinib, brigatinib, daratumumab, larotrectinib and lorlatinib, but resubmissions for caplacizumab and ixazomib with the same evidence received further negative recommendations. The resubmissions increased the agreement between the two agencies to 90.3% (Cohen’s kappa = 0.45 indicating moderate concordance).
3.6. Concordance between CADTH and pCPA
Close alignment between CADTH and the pCPA was found for the 69 orphan drugs with a reimbursement recommendation and a price negotiation completed or not pursued. Fifty-five drugs received a positive recommendation and had a successful price negotiation, and nine received a negative recommendation and had either no price negotiation or an unsuccessful one (94.2% agreement; Cohen’s kappa = 0.79 indicating substantial concordance). One drug with a negative CADTH recommendation (ivacaftor/lumacaftor) had a successful price negotiation after two pCPA decisions not to pursue negotiation and three oncology drugs with a positive CADTH recommendation (dacomitinib, olaratumab and pralsetinib) had unsuccessful negotiations.
3.7. Listing in provincial drug plans
Eighty-two of the 120 orphan drugs approved by the FDA and/or the EMA were approved by Health Canada by the end of June 2023. The 10 drugs approved in Canada after June 2022 were, as the previous results have demonstrated, unlikely to be listed in provincial drug plans because the HTA and price negotiation processes take at least a year. Consequently, the analysis of listings was limited to the other 72 drugs.
Listing of the drugs in provincial drug plans was aligned with CADTH recommendations and pCPA price negotiations. A price negotiation was not pursued by the pCPA for nine drugs with a negative HTA recommendation and none were listed in provincial drug plans. All four drugs with an unsuccessful pCPA negotiation were also not listed by any plan.
On the other hand, not all medicines with a successfully completed price negotiation were listed. Seven provinces (British Columbia, Alberta, Saskatchewan, Manitoba, Ontario, New Brunswick and Nova Scotia) listed 70% or more of the drugs with a successful price negotiation, while the other three listed only 19.6% to 62.5% (). The same seven provinces listed 50% or higher of the 72 orphan drugs approved in Canada, while only three provinces (British Columbia, Saskatchewan and Ontario) listed 40% or more of the drugs approved by the FDA and/or the EMA.
Figure 1. Listing rates for orphan drugs with successful price negotiations in Canada, approved in Canada, and approved by the FDA and/or the EMA.
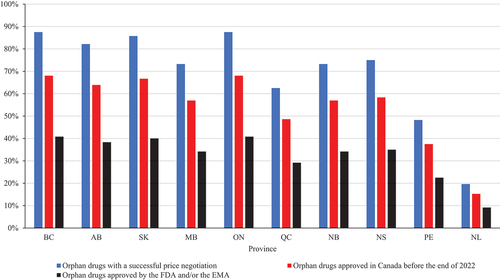
The 72 drugs had at least 35 months in which HTAs, price negotiations and listing decisions could occur. However, only 18 oncology and 19 non-oncology drugs (48.6% and 54.3%, respectively) were listed in eight provincial drug plans by mid-December 2023.
4. Discussion
This analysis tracked drugs given orphan status by the FDA and/or the EMA that were approved by one or both agencies between 2015 and 2020 and also authorized in Canada by June 2023 through Canada’s processes for HTA, price negotiation and listing in government drug plans. Some of the drugs are indicated for what may appear to be common disorders (e.g. asthma and non-small cell lung cancer), but they are actually for specific subtypes of these illnesses. As medical science advances, it has been realized that several diseases originally considered to be a single entity are, in fact, a spectrum of rare disorders [Citation33,Citation34].
4.1. Regulatory approval
Only 68.3% of the orphan medicines approved by the FDA and/or the EMA between 2015 and 2020 were authorized by Health Canada by the end of June 2023 and submission in Canada was over a year later for more than half of the drugs and over 19 months later for a quarter. Once the drugs were submitted to Health Canada, they were generally authorized for use by the regulator in a similar time frame as the FDA but more quickly than the EMA.
4.2. Health technology assessment and price negotiation
Before resubmissions, CADTH’s positive reimbursement recommendation rate for the 82 orphan drugs was 79.5%. The corresponding positive recommendation rate for drugs for common disorders over the same time period was 86.5%. The difference could be due to submissions for orphan drugs being less likely to include randomized trial results.
Only 43.6% of the orphan drug submissions were made to CADTH before regulatory approval from Health Canada by a median time of 94 days. Nevertheless, developers of these medicines appear to take advantage of this opportunity at a higher rate and apply earlier than was found for medicines in general [Citation35]. The median time for completion of CADTH reviews was seven months; the time was considerably longer for some drugs. Just 10.3% of the oncology and 7.7% of the non-oncology assessments were completed within CADTH’s ‘typical timeline’ for reviews of ≤180 days [Citation36]; the longest assessment took over a year. The median time for pCPA price negotiations for the orphan drugs was six months. Furthermore, the median time between regulatory authorization and price negotiation completion was over 18 months; for a quarter of the drugs, the time was over two years ().
4.3. Listing in provincial drug plans
Before patients can obtain access to medicines through Canadian government plans, drugs must be approved for funding by those plans. Time between pCPA outcome and listing in provincial drug plans is unavailable. However, at least three and a half years elapsed before about 50% of the orphan drugs approved in Canada made it through the HTA, price negotiation and provincial listing processes to where Canadians in some provinces might be considered eligible to access them via their government drug plan. Canadians are frequently waiting for years as innovative orphan drugs work through the ‘system,’ while Americans and some western Europeans [Citation37] have access. Similar findings have been demonstrated by others [Citation38–41].
CADTH and the pCPA have been aligning their processes for several years [Citation42,Citation43] so that they are now closely integrated, but this does not translate into shorter review times or improved patient access [Citation44]. Medicines that receive a negative reimbursement recommendation do not have a price negotiation and, consequently, are not listed in provincial drug formularies, while those that receive a positive recommendation commonly have a successful price negotiation and several are listed in provincial formularies, but not all. It is unsurprising that the governments that own, govern and fund CADTH and the pCPA [Citation45] and run the public plans want only drugs with a positive reimbursement recommendation to be listed as long as their developers are willing to negotiate an acceptable price. Negotiated prices are not made public because they are deemed business confidential by agreement between manufacturers and governments, but it seems reasonable to assume that developers did not reduce their prices by the 70–99% recommended for some of the drugs by CADTH. Limited information available for the Ontario provincial drug plan suggests that manufacturer rebates reduce prices by 35% or less [Citation46].
4.4. Concordance between CADTH and NICE
Success of the CADTH-pCPA integration depends on the proficiency of CADTH’s reviews. CADTH collaborates and compares itself with NICE in the United Kingdom [Citation47]. The comparison of reviews by the two agencies indicates that, for most of the orphan drugs, they were in agreement. Differences in recommendations can occur due to differences in HTA cultures and priorities between countries. Nevertheless, it is troubling when they exist where the same clinical evidence was reviewed. This was the case for five medicines where CADTH’s initial recommendation was negative but the NICE guidance was positive. Prices of the drugs were unlikely to have played a role in these recommendations because, after currency conversion, they were within 15% of each other.
Submissions to CADTH with randomized clinical trial data had a greater chance of a positive recommendation. Two-thirds of the submissions with a negative recommendation had no randomized trial data compared with only a quarter of those with a positive recommendation. This is of concern because randomized trials are often impractical for orphan drugs, especially drugs for ultra-rare conditions. As an example, NICE recommended funding for pemigatinib for cholangiocarcinoma based on a non-randomized, phase 2 trial and noted that the drug is ‘likely to be more effective than the comparators’ [Citation48]. CADTH gave pemigatinib a negative recommendation because reviewers found the evidence from the trial to be unconvincing. CADTH’s review stated ‘clinical experts consulted by CADTH noted that, despite the high unmet need [for pemigatinib], conducting a randomized controlled trial in this setting with a targeted therapy, such as pemigatinib, compared to currently available therapies in second-line in Canadian clinical practice would likely not be feasible’ (about 400 new cholangiocarcinoma cases occur each year in Canada of which 10–16% would be potential candidates for pemigatinib) [Citation49]. Nevertheless, CADTH reviewers and its advisory committee disregarded their clinical experts and, instead, recommended that pemigatinib not be reimbursed. Since a randomized trial is unlikely to be performed due to difficulties enrolling sufficient patients with this rare condition, CADTH’s negative recommendation will likely result in Canadians without private insurance being denied access to pemigatinib [Citation50]. Although views on pemigatinib vary [Citation51], Canadian experts have commented that there is a ‘lack of effective therapeutic options for patients with advanced biliary tract cancer’ [Citation52].
The rate of listing of the orphan medicines in seven provincial drug plans after a positive pCPA outcome was 70% or more, but only 35 (48.6%) of the 72 drugs approved in Canada before the end of June 2022 were listed in a majority of drug plans by mid-December 2023. Despite a minimum of 30 months having elapsed in which HTAs, price negotiations and listing decisions could have taken place, at least 20 (27.8%) of the 72 orphan drugs remained unlisted by all provinces. The wait until listing of orphan medicines in provincial drug plans can be extensive.
Furthermore, listing does not necessarily mean all patients can access drugs through the plan. More than half of the non-oncology drugs listed in British Columbia are only accessible on a case-by-case basis [Citation53]. Other provinces have lists of medicines that are only available through exceptional or special access programs [Citation54–56]. Patient experiences indicate that orphan medicines are frequently only accessible through these programs on a case-by-case basis, but this is often not discovered until patients try to access medicines. Access criteria specified by CADTH and implemented by provincial drug plans have become more detailed and stringent in recent years [Citation42]. Some criteria deny access to patients in the first stages of a disorder who might benefit most but provide access to patients at a much later stage in disease progression when patients are much sicker and may not benefit as much. Other criteria can lead to patients taking harmful action to maximize their opportunity of gaining access when restrictive conditions are applied [Citation57].
4.5. General comments
Drug developers must overcome several obstacles to bring new medicines to Canadians [Citation58,Citation59]. Other barriers are weaker intellectual property protection than in comparable countries [Citation60] and officials working for these governments who have no motivation to add new drugs to their formularies because they add additional costs to drug budgets. The combined impact of these impediments particularly deters small developers of orphan medicines. As an example, five drugs for Duchenne muscular dystrophy developed by small companies were approved by the FDA in the last six years, but none has been submitted to Health Canada.
Patient advocates have attempted to get the Canadian federal government to implement a national strategy [Citation61,Citation62], but they have had limited success at engaging policy-makers. The federal government has now committed $1.5 billion over three years to ‘increase access to, and affordability of, effective drugs for rare diseases to improve the health of patients across Canada’ [Citation4]; $1.4 billion has been earmarked for bilateral agreements between Health Canada and each of the provinces and territories. This will only work fully if incentives are introduced to encourage more orphan drug developers to submit their products to Health Canada, require all government drug plans to list all orphan medicines with a successful pCPA negotiation, and loosen the restrictive and, in some cases, harmful access criteria that patients and clinicians must satisfy before being able to obtain coverage. Canadian provinces and territories should also make great use of risk-sharing, managed access agreements as many European countries do [Citation63].
4.6. Limitations
This analysis is dependent upon publicly available sources, but data on listings by provincial drug plans are limited. No province provides dates on which listings were approved. Some provinces update their benefit lists daily, while others update monthly and, in one province, only half-yearly. Moreover, Alberta, British Columbia, Manitoba, Ontario and Saskatchewan have separate cancer drug formularies (Manitoba’s is not publicly available), while the other provinces do not. British Columbia, Manitoba, Ontario, Newfoundland and Labrador and Saskatchewan have separate lists of exceptional access drugs, but the others do not. Manitoba Health has bulletins that inform residents of new drugs added to or removed from coverage. Provinces may have lists of orphan medicines which may be covered in some cases that are not made public.
We did not attempt to evaluate the therapeutic value of medicines. This type of assessment, especially using pre-marketing clinical studies, is based on limited evidence and may not be applicable when drugs are used in everyday medical practice. Evaluations by the clinical committee that provides advice on the potential value of new medicines to the Canadian federal government’s tribunal assessing whether drug list prices are excessive and by Prescrire International (a French organization that reviews new medicines) have been used to evaluate therapeutic value [Citation35]; few medicines are considered by these assessors to be breakthroughs or significant developments. Patients are likely to see and experience new drugs differently. Any new medicine for a debilitating or life-threatening disease, such as cancer or amyotrophic lateral sclerosis, for which only symptom treatment or palliative care is available, may be expected to be considered an important medical advance. More effective or safer drugs are also likely to seen by patients as significant improvements. Similarly, less invasive methods of administration, such as changing from a medicine delivered by intrathecal injection to an oral medication, may be considered a valuable therapeutic advance. Clinical expertise on therapeutic benefit may be regarded the ‘gold standard’ [Citation35], but there are other considerations for patients.
5. Conclusions
Four decades after the passing of the US Orphan Drug Act and over 20 years after the European Union passed its comparable legislation, Canada has only just begun to introduce a partial strategy to help Canadians who require access to innovative orphan drugs. Unmet needs are huge; just 500 disorders – less than 5% [Citation64] – have any approved treatments so far. Only 60% of the orphan medicines authorized by the FDA and/or the EMA since 2015 were approved in Canada by the end of June 2022 and many of those are not reimbursed by government drug plans or only reimbursed for some patients who satisfy restrictive access criteria. Thus, many barriers to accessing novel medicines for rare disorders exist in Canada [Citation65] so that Canadians with these disorders continue to suffer from a lack of timely and equitable access to new therapies. They need a comprehensive orphan drug policy that includes improved intellectual property protection, marketing exclusivity and data protection incentives and funding to encourage developers to launch orphan drugs in Canada, a mandatory requirement that all government drug plans list all orphan medicines with a successful price negotiation within a short period of time, such as 90 days, and less restrictive and burdensome access criteria that patients must satisfy before being able to obtain coverage. Without these actions, Canadians with rare disorders will continue to lack timely access to the many innovative treatments on the research horizon that can reduce suffering and improve and even save lives.
Declaration of interest
Over the past three years, NSB Rawson received research and consultation fees from AbbVie Canada, Canadian Cancer Survivor Network, Canadian Health Policy Institute, Fraser Institute, Macdonald-Laurier Institute and 3Sixty Public Affairs, and an article processing expense from RAREi (a network of Canadian biopharmaceutical companies committed to improving the lives of rare disease patients by researching, developing and commercializing rare disease treatments). During the same period, J Adams received research and consulting fees from the Alliance for Safe Online Pharmacies, Canadian PKU and Allied Disorders Inc. and Macdonald-Laurier Institute, and a fee from BioMarin Pharmaceuticals for speaking to European PKU patient leaders. No conflict of interest exists between these activities and the present work. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or material discussed in the manuscript apart from those disclosed.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Author contribution statement
Both authors made a significant contribution to the work reported in the conception, study design, acquisition of data, analysis and interpretation. Both take responsibility and accountability for the contents of the article and share responsibility to resolve any questions raised about the accuracy or integrity of the work.
Data availability statement
The data used in this analysis are available from publicly accessible resources [Citation8–23, Citation25–31,Citation53–56].
Additional information
Funding
References
- Medical products for rare diseases and conditions [internet]. Silver Spring (MD): Food and Drug Administration; 2022 May 12 [cited 2023 Dec 18]. Available from: https://www.fda.gov/industry/medical-products-rare-diseases-and-conditions
- Orphan incentives [Internet]. Amsterdam: European Medicines Agency;no date [cited 2023 Dec 18]. Available from: https://www.ema.europa.eu/en/human-regulatory/research-development/orphan-designation/orphan-incentives
- Orphan drug designation [Internet]. Woden (ACT): Australian Government; 2018 Aug [cited 2023 Dec 18]. Available from: https://www.tga.gov.au/sites/default/files/orphan-drug-designation.pdf
- Government of Canada improves access to affordable and effective drugs for rare diseases [internet]. Ottawa (ON): Government of Canada; 2023 Mar 22 [cited 2023 Dec 18]. Available from: https://www.canada.ca/en/health-canada/news/2023/03/government-of-canada-improves-access-to-affordable-and-effective-drugs-for-rare-diseases.html
- Impact of PMPRB pricing changes: final research report [Internet]. Toronto (ON): Research Etc; 2020 Feb 2 [cited 2023 Dec 18]. Available from: https://lifesciencesontario.ca/wp-content/uploads/2020/02/Research-Etc.-PMPRB-Survey-02-03-20.pdf
- Rawson NSB. Canada falls behind in new drug submissions compared with the United States and Europe [internet]. Can Health Policy J. Toronto (ON): Canadian Health Policy Institute. 2023 [cited 2023 Dec 18]. Available from:2023(JAN):1–11. doi: 10.54194/HKBH7107
- What we do [Internet]. Ottawa (ON): Canadian Agency for Drugs and Technologies in Health; 2015 Mar 3 [cited 2023 Dec 18]. Available from: https://www.cadth.ca/what-we-do
- Notice of Compliance search [Internet]. Ottawa (ON): Government of Canada, 2022 Nov 25 [cited 2023 Dec 18]. Available from: https://health-products.canada.ca/noc-ac/?lang=eng
- Reimbursement review reports [Internet]. Ottawa (ON): Canadian Agency for Drugs and Technologies in Health; 2023 Dec 18 [cited 2023 Dec 18]. Available from: https://www.cadth.ca/reimbursement-review-reports
- Brand name drug negotiations status [Internet]. Toronto (ON): pan-Canadian Pharmaceutical Alliance; 2023 [cited 2023 Dec 18]. Available from: https://www.pcpacanada.ca/negotiations
- BC PharmaCare formulary search [Internet]. Victoria (BC): Government of British Columbia; 2023 Dec 5 [cited 2023 Dec 18]. Available from: https://pharmacareformularysearch.gov.bc.ca/Search.xhtml
- Welcome to the iDBL [internet]. Edmonton (AB): Government of Alberta; 2023 Dec 18 [cited 2023 Dec 18]. Available from: https://idbl.ab.bluecross.ca/idbl/load.do
- Home – search formulary [internet]. Regina (SK): Government of Saskatchewan; 2023 [cited 2023 Dec 18]. Available from: https://formulary.drugplan.ehealthsask.ca/SearchFormulary
- Drug formulary lookup [Internet]. Winnipeg (MB): Manitoba Health; 2023 Dec 18 [cited 2023 Dec 18]. Available from: https://web22.gov.mb.ca/eFormulary/
- Formulary search [Internet]. Toronto (ON): Government of Ontario; 2023 Jul 31 [cited 2023 Dec 18]. Available from: https://www.formulary.health.gov.on.ca/formulary/
- List of medications [Internet]. Québec (QC): Gouvernement de Québec; 2023 Sep 25 [cited 2023 Dec 18]. Available from: https://www.ramq.gouv.qc.ca/sites/default/files/documents/non_indexes/liste-med-2023-09-27-en.pdf
- New Brunswick drug plan formulary [Internet]. Fredericton (NB): Government of New Brunswick; 2023 Dec [cited 2023 Dec 18]. Available from: https://www2.gnb.ca/content/dam/gnb/Departments/h-s/pdf/en/NBDrugPlan/NewBrunswickDrugPlansFormulary.pdf
- Formulary [Internet]. Halifax (NS): Government of Nova Scotia; 2023 Dec [cited 2023 Dec 18]. Available from: https://novascotia.ca/dhw/pharmacare/documents/formulary.pdf
- PEI Pharmacare formulary [Internet]. Charlottetown (PE): Health PEI; 2023 Dec [cited 2023 Dec 18]. Available from: https://www.princeedwardisland.ca/sites/default/files/publications/pei_pharmacare_formulary.pdf
- Newfoundland and Labrador interchangeable drug products formulary [internet]. St. John’s (NL): Government of Newfoundland and Labrador; 2023 Oct 1 [cited 2023 Dec 18]. Available from: https://www.gov.nl.ca/hcs/files/nlpdp-formularyvol89.pdf
- BC Cancer benefit drug list [Internet]. Vancouver (BC): BC Cancer Agency; 2023 Dec [cited 2023 Dec 18]. Available from: http://www.bccancer.bc.ca/systemic-therapy-site/Documents/Policy%20and%20Forms/Benefit%20Drug%20List.pdf
- Outpatient cancer drug benefit program [Internet]. Edmonton (AB): Alberta Health Services; 2023 Nov 23 [cited 2023 Dec 18]. Available from: https://www.albertahealthservices.ca/assets/programs/ps-1025651-drug-benefit-list.pdf
- Saskatchewan Cancer Agency drug formulary [Internet]. Regina (SK): Saskatchewan Cancer Agency; 2023 Sep 1 [cited 2023 Dec 18]. Available from: http://www.saskcancer.ca/images/pdfs/health_professionals/drug_formulary/drug_formulary/SCA_Drug_Formulary_-_2023-09-01.pdf
- Drug formulary [Internet]. Toronto (ON): Cancer Care Ontario; no date [cited 2023 Dec 18]. Available from: https://www.cancercareontario.ca/en/drugformulary/drugs
- New drugs at FDA: CDER’s new molecular entities and new therapeutic biological products [internet]. Silver Spring (MD): Food and Drug Administration; 2023 May 22 [cited 2023 Dec 18]. Available from: https://www.fda.gov/drugs/development-approval-process-drugs/new-drugs-fda-cders-new-molecular-entities-and-new-therapeutic-biological-products
- Biological approvals by year [internet]. Silver Spring (MD): Food and Drug Administration; 2023 Jul 3 [cited 2023 Dec 18]. Available from: https://www.fda.gov/vaccines-blood-biologics/development-approval-process-cber/biological-approvals-year
- Drugs@FDA: FDA-approved drugs [Internet]. Silver Spring (MD):Food and Drug Administration; no date [cited 2023 Dec 18]. Available from: https://www.accessdata.fda.gov/scripts/cder/daf/index.cfm
- Human regulatory: marketing authorization [Internet]. Amsterdam: European Medicines Agency; no date [cited 2023 Dec 18]. Available from: https://www.ema.europa.eu/en/human-regulatory/marketing-authorisation
- Drug products: what information can you find here? [internet]. Ottawa (ON): Government of Canada; 2023 Jun 23 [cited 2023 Dec 18]. Available from: https://www.canada.ca/en/health-canada/services/drugs-health-products/reports-publications/drug-products.html
- Technology appraisal guidance [Internet]. London: National Institute for Health and Care Excellence; 2023 [cited 2023 Dec 18]. Available from: https://www.nice.org.uk/about/what-we-do/our-programmes/nice-guidance/nice-technology-appraisal-guidance
- Guidance, NICE standards and quality standards [Internet]. London: National Institute for Health and Care Excellence; 2023 [cited 2023 Dec 18]. Available from: https://www.nice.org.uk/guidance/published?ndt=Guidance&ndt=Quality%20standard
- Rawson NSB. Canadian, European and United States new drug approval times now relatively similar. Regul Toxicol Pharmacol. 2018;96:121–126 doi: 10.1016/j.yrtph.2018.05.002
- Oppenheimer J, Hoyte FCL, Phipatanakul W, et al. Allergic and eosinophilic asthma in the era of biomarkers and biologics: similarities, differences and misconceptions. Ann Allergy Asthma Immunol. 2022;129(2):169–80. doi: 10.1016/j.anai.2022.02.021
- Rodak O, Peris-Diaz MD, Olbromski M, et al. Current landscape of non-small cell lung cancer: epidemiology, histological classification, targeted therapies, and immunotherapy. Cancers (Basel). 2021;13(18):4705. doi: 10.3390/cancers13184705
- Lexchin J. Time to potential listing of new drugs on public and private formularies in Canada: a cross-sectional study. CMAJ Open. 2022;10(4):E993–9. doi: 10.9778/cmajo.20220063
- Procedures for CADTH reimbursement reviews [Internet]. Ottawa (ON): Canadian Agency for Drugs and Technologies in Health; 2023 Nov [cited 2023 Dec 18]. Available from: https://www.cadth.ca/sites/default/files/Drug_Review_Process/CADTH%20Drug%20Reimbursement%20Review%20Procedures.pdf
- Norton M, Stoddart K, Travaglio M, et al. EFPIA patients WAIT indicator 2022 survey [internet]. IQVIA, 2023 Apr [cited 2023 Dec 18]. Available from: https://www.efpia.eu/media/s4qf1eqo/efpia_patient_wait_indicator_final_report.pdf
- Salek S, Lussier Hoskyn S, Johns JR, et al. Factors influencing delays in patient access to new medicines in Canada: a retrospective study of reimbursement processes in public drug plans. Front Pharmacol. 2019;10:196. doi: 10.3389/fphar.2019.00196.
- Barua B, Westcott W, Vo VN Timely access to new pharmaceuticals in Canada, the United States and the European Union [Internet]. Vancouver (BC): Fraser Institute, 2021 [cited 2023 Dec 18]. Available from: https://www.fraserinstitute.org/sites/default/files/timely-access-to-new-pharmaceuticals-in-canada-US-and-EU.pdf
- Ward LM, Chambers A, Mechichi E, et al. An international comparative analysis of public reimbursement of orphan drugs in Canadian provinces compared to European countries. Orphanet J Rare Dis. 2022;17(1):113. doi: 10.1186/s13023-022-02260-6
- Skinner B Patent term erosion and the availability of new medicines in Canada, 2000-2022 [internet]. Can Health Policy J. Toronto (ON): Canadian Health Policy Institute; 2022 [cited 2023 Dec 18]. Available from: https://www.canadianhealthpolicy.com/product/patent-term-erosion-and-the-availability-of-new-medicines-in-canada-2000-2022/
- Rawson NSB. Alignment of health technology assessments and price negotiations for new drugs for rare disorders in Canada: does it lead to improved patient access? J Popul Ther Clin Pharmacol. 2020;27(1):e48–64. doi: 10.15586/jptcp.v27i1.658
- Rawson NSB. Health technology assessment and price negotiation alignment for rare disorder drugs in Canada: who benefits? Orphanet J Rare Dis. 2022;17(1):218. doi: 10.1186/s13023-022-02390-x
- Fontrier AM, Kanavos P. Do reimbursement recommendations by the Canadian Agency for Drugs and Technologies in Health translate into coverage decisions for orphan drugs in the Canadian province of Ontario? Value Health. 2023;26(7):1011–1021.
- Rawson NSB, Adams J. Do reimbursement recommendation processes used by government drug plans in Canada adhere to good governance principles? Clinicoecon Outcomes Res. 2017;9:721–730.
- Ontario public drug plans [Internet]. Toronto (ON): Office of the Auditor General of Ontario; no date [cited 2023 Dec 18]. Available from: https://www.auditor.on.ca/en/content/annualreports/arreports/en17/v1_309en17.pdf
- CADTH partners with international health technology assessment bodies to boost collaboration on shared challenges [internet]. Ottawa (ON): Canadian Agency for Drugs and Technologies in Health; 2022 Sep 6 [cited 2023 Dec 18]. Available from: https://www.cadth.ca/news/cadth-partners-international-health-technology-assessment-bodies-boost-collaboration-shared
- Pemigatinib for treating relapsed or refractory advanced cholangiocarcinoma with FGFR2 fusion or rearrangement [Internet]. London: National Institute for Health and Care Excellence; 2021 Aug 25 [cited 2023 Dec 18]. Available from: https://www.nice.org.uk/guidance/ta722/resources/pemigatinib-for-treating-relapsed-or-refractory-advanced-cholangiocarcinoma-with-fgfr2-fusion-or-rearrangement-pdf-82611190679749
- CADTH reimbursement recommendation: pemigatinib (Pemazyre) [Internet]. Can J Health Technol. 2022;2(6). [cited 2023 Dec 18]. doi: 10.51731/cjht.2022.358
- Stories from the frontlines of the fight for access to innovative therapies [internet]. Toronto (ON): Maclean’s; 2023 Aug 3 [cited 2023 Dec 18]. Available from: https://macleans.ca/longforms/the-fight-for-access-to-innovative-therapies/
- Gadaleta-Caldarola G, Rizzo A, Dadduzio V, et al. Pemigatinib in intrahepatic cholangiocarcinoma: a work in progress. Curr Oncol. 2022;29(10):7925–31. doi: 10.3390/curroncol29100626
- Tam VC, Ramjeesingh R, Burkes R, et al. Emerging systemic therapies in advanced unresectable biliary tract cancer: review and Canadian perspectives. Curr Oncol. 2022;29(10):7072–85. doi: 10.3390/curroncol29100555
- Exceptional funding of EDRDs [internet]. Victoria (BC): Government of British Columbia; 2023 Jun 22 [cited 2023 Dec 18]. Available from: https://www2.gov.bc.ca/gov/content/health/health-drug-coverage/pharmacare-for-bc-residents/what-we-cover/exceptional-funding-edrd?keyword=case&keyword=by&keyword=case&keyword=rare&keyword=disease&keyword=drugs
- Exception drug status [Internet]. Winnipeg (MB): Manitoba Health; 2023 Oct 26 [cited 2023 Dec 18]. Available from: https://www.gov.mb.ca/health/mdbif/docs/edsnotice.pdf
- Exceptional access program reimbursement criteria for frequently requested drugs [internet]. Toronto (ON): Ministry of Health; 2023 Aug 8 [cited 2023 Dec 18]. Available from: https://files.ontario.ca/moh-frequently-requested-drugs.pdf
- Criteria for the coverage of special authorization drugs [internet]. St. John’s (NL): Government of Newfoundland and Labrador; no date [cited 2023 Dec 18]. Available from: https://www.gov.nl.ca/hcs/files/prescription-special-auth-drug-products.pdf
- Begovic M Getting sick to get better: cystic fibrosis patients worry about access to ‘breakthrough’ drug [internet]. Toronto (ON): Healthing.ca; 2022 Oct 5 [cited 2023 Dec 18]. Available from: https://www.healthing.ca/diseases-and-conditions/cystic-fibrosis/trikafta-cystic-fibrosis-drug-access
- Abunassar C, Dowson JP, Fleming M, et al. Biopharmaceutical ecosystem index: where does Canada rank on its attractiveness for new medicine launch? [Internet]. Mississauga (ON): PDCI Market Access, 2022 [cited 2023 Dec 18]. Available from: https://www.pdci.ca/wp-content/uploads/2023/05/PDCI-Biopharmaceutical-Ecosystem-Index-May-2023.pdf
- Rawson N, Adams J Waiting for new drugs for rare disorders in Canada [Internet]. Ottawa (ON): Macdonald-Laurier Institute, 2023 [cited 2023 Dec 18]. Available from: https://macdonaldlaurier.ca/category/projects/rare-disorder-drugs/
- Owens RC Defending our rights: eliminating dysfunction in Canada’s intellectual property regime [internet]. Ottawa (ON): Macdonald-Laurier Institute, 2017 [cited 2023 Dec 18]. Available from: https://macdonaldlaurier.ca/mli-files/pdf/MLI_IPPaper3_06-17_webready.pdf
- Now is the time: a strategy for rare diseases is a strategy for all Canadians [internet]. Toronto (ON): Canadian Organization for Rare Disorders; 2015 May [cited 2023 Dec 18]. Available from: https://www.raredisorders.ca/content/uploads/CORD_Canada_RD_Strategy_22May15.pdf
- Developing a truly Canadian approach to improving the lives of Canadians with rare diseases [internet]. Toronto (ON): Canadian Organization for Rare Disorders; 2022 Jul 8 [cited 2023 Dec 18]. Available from: http://www.raredisorders.ca/content/uploads/CORD-letter-to-Premiers_08July2022.pdf
- Grubert N Innovative access arrangements and managed entry: what Canada can learn from Europe [internet]. Ottawa (ON): Innovative Medicines Canada; no date [cited 2023 Dec 18]. Available from: https://innovativemedicines.ca/wp-content/uploads/2023/04/6328_IMC_ME_IAA_Report_v6.pdf
- Lamoreaux K, Lefebvre S, Levine DS, et al. The power of being counted [internet]. Washington (DC): Rare-X, 2022 [cited 2023 Dec 18]. Available from: https://rare-x.org/wp-content/uploads/2022/05/be-counted-052722-WEB.pdf
- Djordjevic D, McFadyen A, Anderson JA. Ethical challenges and opportunities in the development and approval of novel therapeutics for rare diseases. J Med Access. 2023;7:27550834231177507. doi: 10.1177/27550834231177507

