ABSTRACT
Background
Rare cancers account for approximately one in eight of all cancers diagnosed in the United States (US) every year. Remarkable scientific advances in cancer research over the past 40 years, in addition to financial incentives provided by the Orphan Drug Act (ODA), have led to hundreds of drug approvals for rare cancers.
Research design and methods
Using an internal US Food and Drug Administration (FDA) database, we classified and analyzed all orphan drug designations and approvals specifically designed to prevent, diagnose, or treat rare cancers, from 1983 to 2022 by affected organ system.
Results
In the past 40 years, more than 180 rare cancers have had at least one product that has been developed and shown promise in its treatment, diagnosis, or prevention resulting in nearly 100 approved products for rare oncologic indications. Rare hematologic cancers were the most frequently designated and approved cancers, but nearly every organ system was represented by an orphan drug-designated product.
Conclusions
Orphan drug designations and approvals target a wide variety of rare cancer types and disease locations suggesting an expanding base of research activity, discovery, and options for patients with these often-fatal diseases.
1. Introduction
Cancer remains one of the leading causes of morbidity and mortality in the US [Citation1]. In 2022, almost 2 million new diagnoses of cancer were expected, with around 600,000 deaths from cancer-related causes [Citation2]. This trend increased over the last several decades, up from 1.6 million new cases and 580,000 deaths in 2012–2013 [Citation3]. However, while the number of cancer diagnoses and deaths have increased, the death rate has decreased by almost 30% in the last 20 years [Citation4,Citation5]. The improved survival rate in cancers could be due to multiple factors, including improvements in cancer risk factor identification, screening methods, diagnostic procedures, and therapeutic interventions [Citation6]. In addition, new and innovative therapeutics are being developed with target-directed mechanisms of action [Citation7]. Targeted therapies have emerged as the standard-of-care in many cancer treatment guidelines, providing safer and more effective alternatives to conventional chemotherapy agents [Citation8].
Many of these advances are being seen in rare cancers [Citation9]. While individually rare, as a whole, rare cancers account for nearly 13% (1 in 8) of all cancers diagnosed annually in the US [Citation10]. Supporting drug and biologic (henceforth ‘drug’) development for these rare cancers has been a policy priority for decades.
The Orphan Drug Act (ODA) of 1983 was created in response to a dearth in pharmaceutical investment to prevent, diagnose, and treat rare diseases in the US [Citation11]. The law defines a rare disease or condition as one that affects fewer than 200,000 patients in the US [Citation12]. For rare cancers, this population estimate includes patients who have ever had the cancer in their lifetimes, even if they are in remission [Citation13].
Over the last 40 years, the ODA has helped to fundamentally alter the market of rare disease therapeutics through financial incentives for pharmaceutical developers. These benefits include a 25% tax credit on applicable development costs; exemption from US Food and Drug Administration (FDA) user fees (specifically those fees that companies pay to the FDA to offset the cost of application review); and the potential for 7 years of marketing exclusivity for an approved orphan-designated indication or use [Citation14]. Prior to receiving any of these benefits, developers must first receive orphan drug designation from FDA. In the most common pathway to receive designation, developers provide (1) evidence of scientific rationale (a medically plausible basis for expecting the drug to be effective in the rare disease or condition), either via in vivo preclinical or clinical data; and (2) a population estimate demonstrating that the disease or condition affects fewer than 200,000 people in the US at the time of the request [Citation15].
Consistent with the overall trends in oncology drug development, previous research has demonstrated that the number of annual orphan drug designations and approvals for management of rare cancers has increased significantly [Citation16,Citation17]. In fact, oncologic diseases have the largest proportion of orphan drug designations and approvals when analyzed by therapeutic area [Citation18]. Remarkably, rare cancers comprise nearly 60% of the most designated and approved rare diseases [Citation19].
To date, there has been no comprehensive summary of oncologic orphan drug designations. This article will provide an overview and analysis of the characteristics of oncologic orphan drug designations and approvals granted by the FDA from 1983 to 2022. As the public and policymakers are eager to engage in discussions regarding rising healthcare costs, patient access, and outcomes, an evaluation of the ODA’s impact on cancer drug development is relevant. At the 40-year anniversary of this legislation, it is an appropriate time to evaluate the historical landscape of oncologic orphan drug designations.
2. Materials and methods
To perform our data analysis, we used an internal FDA database that contained all orphan drug designations and associated approvals from 1983 to 2022. This internal dataset contains all orphan drug designations that were ever granted, regardless of the current status of the drug (e.g. development discontinued) or designation (e.g. withdrawn) (A database that contains all data considered relevant to this study, including orphan drug designations, approvals, the respective designation dates, and company names, is also available publicly on the FDA’s webpage [Citation20]). All designations and approvals related to oncology, either directly for diagnosis, prevention, or treatment of cancer, were extracted from the database and gathered into a separate dataset.
Several major variables were considered from the internal database for analysis, including designation date, product type (drug or biologic), intended use of the product (therapeutic, prophylactic, or diagnostic), and disease for which the product received orphan drug designation.
We also created several variables for this analysis. As orphan-designated diseases are typically longer phrases that may be difficult to aggregate, we converted all orphan-designated disease ‘phrases’ into simplified, uniform disease ‘terms.’ For example, an orphan-designated disease phrase of ‘diagnostic for the management of malignant rhabdoid tumors,’ was converted into the disease term ‘rhabdoid tumor.’
Using a standardized disease terminology designed for optimal data integration and harmonization to align disease naming across multiple sources (‘Mondo’), we assigned discrete disease labels based on the most granular information available in the original orphan-designated disease phrases [Citation21]. Infrequently, this resulted in seemingly related cancers being separated into different types of cancers. One example of this distinction occurred between glioma and glioblastoma, with glioma representing any tumor originating in the glial cells while glioblastoma referring specifically to a grade IV astrocytoma or glioblastoma multiforme (GBM). Each designation was considered individually and assigned to the lowest hierarchical term in the original orphan-designated phrase. Two percent of the oncologic designations analyzed could not be classified into a discrete disease label via the Mondo ontology. In these cases, we included them in the dataset, but aggregated disease terms according to our own discrete disease label based on the details provided in the designation phrase.
Our dataset includes all approvals that are associated with an orphan drug designation. Drug approvals, including orphan drug approvals, can be granted to a variety of types of approvals, including novel drugs or biologics, new indications, and new formulations. For ease of comparison, and to provide a more precise and relevant assessment of the actual frequency of drugs approved for a specific orphan drug–disease combination, we limit our dataset to focus on ‘initial approvals’ which we define as the first approval associated with an orphan designation. This is in contrast to ‘subsequent approvals,’ which occur after the initial approval and are expansions of the initial approval, e.g. age expansions based on clinical studies, new formulations. For instance, ofatumumab, a drug designated for treatment of chronic lymphocytic leukemia, received five approvals between 1983 and 2022: one was the first approval under this orphan designation and the subsequent four expanded the population eligible to receive ofatumumab. However, for this study, we counted one approval (the ‘initial approval’) and did not include the four ‘subsequent approvals’ in our approval counts.
Based on the targeted disease, each designation and initial approval was also classified into an affected ‘site group’ and associated organ based on the International Classification of Diseases for Oncology Site Recode ICD-0-3/WHO 2008 Definition [Citation22]. However, the designations did not always precisely align with this classification system, and we therefore added groupings, when necessary. We created the site group ‘Blood’ to group all hematologic malignancies, and for ‘Brain and Nervous Tissue,’ the organ of ‘Brain’ was broadened to two categories: ‘Central Nervous System (CNS)’ and ‘Peripheral Nervous System (PNS).’ The subcategory of ‘Neuroendocrine’ was created within the site group of ‘Endocrine’ diseases. Additionally, some orphan drug designations (and subsequent approvals) did not fit into established categories or organ systems and were therefore subdivided into a ‘Miscellaneous’ category consisting of ‘Genetic cancer syndromes’ (e.g. von Hippel-Lindau disease) and ‘Tissue agnostic/Biomarker-defined cancers’ – multiple cancer types related to one or more specific molecular alterations (e.g. Neurotrophic Tyrosine Receptor Kinase [NTRK] fusion-positive solid tumors) [Citation23].
Finally, we investigated the number of orphan drug designations and approvals for cancer- or therapy-related complications (e.g. tumor lysis syndrome, neoplastic meningitis, and chemotherapy-induced toxicity). We present this as a separate analysis because, while these drugs are not designed for a particular rare cancer, they are related to the innate response to malignancy or to the complications arising from the treatment of cancer.
2.1. Limitations
While the longitudinal nature of the data provides for a substantial time period to assess trends, the drug development process is complex and sponsors experience many obstacles and delays prior to drug approval, which lead to the two central limitations of this study. First, not all designations are currently in development. The dataset used for this analysis cannot assess whether a drug continues to be in development, and therefore, there may be many designations in our dataset that will not be translated into a drug approval.
Second, not all diseases that have had orphan drugs designated for them in the past would still be considered rare today. Designation determinations rely on population estimates at the time of a sponsor’s request and these calculations can change over time. For many cancers, the prevalence is now over 200,000, and therefore our results may overestimate current oncology rare disease drug development trends by including these designations. However, it is important to note that drugs to treat certain rare subtypes of non-rare cancers may still be granted orphan drug designation under the regulations as an orphan-subset, which may lead to a high number of designations and approvals associated with a non-rare cancer [Citation14,Citation24].
3. Results
There were 2,355 orphan drug designations related to cancer between 1983 and 2022 and 317 (13%) of these designations resulted in at least one initial approval (first approval under one orphan drug designation). There were 181 rare cancers that were represented by the 2,355 designations and 92 rare cancers had at least one FDA approval. We found that five cancers had more than 100 associated designations each, 29 cancers had 20 or more designations each, and 54 rare cancers had only one associated designation. The maximum number of initial approvals was 19 (for multiple myeloma designations).
The vast majority of oncologic orphan products (n = 2289; 97.2%) were designated as a therapeutic, while the remaining designations were diagnostic (n = 57; 2.4%) or prophylactic (n = 9; 0.4%). Around 60% of all oncologic designations were for small-molecule drugs, and 40% were biologic products. Among the approvals, the estimates were similar: 67% drugs and 33% biologics. The proportions for both designations and approvals have been roughly the same over the 40-year period.
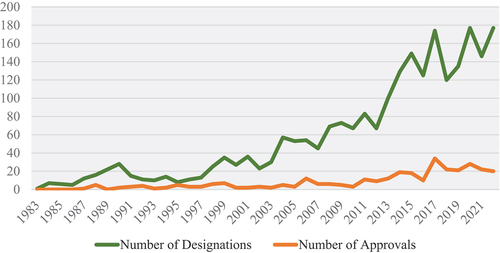
Over the last four decades since the passage of the ODA, oncologic orphan drug designations grew in number annually, as detailed in . In the first 10 years after the ODA passed, from 1983 to 1992, there were 123 designations for rare cancers, and that number almost doubled to 202 in the following decade. This growth was surpassed in the next 10 years, from 2003 to 2012, with a 196% increase to 598 designations. This growth trend slowed slightly in the most recent 10 years, from 2013 to 2022, with a 140% increase to 1,432 designations. This is mirrored by orphan drug approvals, which saw their greatest increase from 2003 to 2012 (n = 62) to 2013–2022 (n = 206) with a 232% increase in approvals.
Figure 1. Number of oncologic orphan drug designations (n=2,355) and approvals (n=317) from 1983–2022.
We also identified the rare cancers with the most designated and approved products ( and ). Within the top designated cancers, there are three hematologic malignancies: acute myeloid leukemia (AML), multiple myeloma, and B-cell chronic lymphocytic leukemia (CLL). The remaining diseases represent a diverse array of systems affected by cancer, including the nervous, digestive, and reproductive systems, in addition to the skin.
Table 1. Top 10 rare cancers with the most orphan drug designations (1983–2022).
Table 2. Top 10 orphan drug-designated cancers with the most initial approvals (1983–2022).
This diversity is consistent with the rare cancers with the highest numbers of approved products, with an increased representation of hematologic malignancies, including multiple myeloma, AML, follicular lymphoma, and chronic myelogenous leukemia (CML). Overall, these figures depict that a wide variety of cancers have had products designated and approved for them, with relatively more hematologic malignancies represented in the list of rare cancers with the most (initial) approved products.
The affected systems receiving the most designations are hematologic, gastrointestinal, and nervous systems (). To further identify and analyze the characteristics of oncologic orphan drug designations, we classified each rare cancer represented by designations by the site group (organ system) and site (organ) when applicable (). The three cancer sites most represented by designations are hematologic leukemias, lymphomas, and CNS tumors. Outside of these groups, pancreatic and hepatic malignancies, melanomas of the skin, and ovarian cancers share significant numbers of designations.
Figure 2. Oncologic orphan drug designations (and approvals) by organ system, 1983–2022.*Miscellaneous category includes tissue agnostic/biomarker-defined cancers, and genetic cancer syndromes
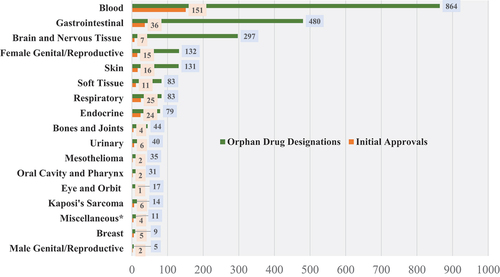
Table 3. Classification of oncologic orphan drug designations (and Approvals) by site group and site, 1983—2022 (Designations, n = 2,355) (Approvals, n = 317).
The systems receiving the most orphan drug approvals were hematologic, gastrointestinal, and respiratory system malignancies. However, hematologic malignancies received significantly more approvals relative to designations than any other body system. While the ratio of designations to approvals for the rare cancers with the most designated products is greater than 10:1, reaching almost 40:1 for nervous system tumors, the ratio for hematologic cancers is around 5:1.
Overall, we see that almost every organ and organ system has been targeted by at least one orphan drug-designated oncologic product.
In a separate analysis, we found that drugs for cancer- and therapy-related complications account for a greater number of designations (56) and approvals (21) than most cancer sites.
4. Discussion
This research uses a comprehensive 40-year dataset of orphan drug designations and approvals to assess trends in rare cancer drug development. The results highlight three key trends: 1) oncology is an active and growing area for rare disease drug development, 2) a wide variety of systems and pathologies have been targeted, and 3) rare cancers of the blood have the highest approval rates among rare cancers.
First, oncology appears to be an active therapeutic area for rare disease drug development. While previous research has demonstrated that rare cancers represent the largest disease category of orphan drug designations, this research also shows that there has been rapid growth over time in the number of orphan drug designations granted in this area, as demonstrated in [Citation16].
We propose two key factors that may have contributed to such growth. First, we recognize that advancements in the understanding of cancer pathophysiology along with technological advancements have enabled development of targeted therapies, including small-molecule inhibitors, chimeric antigen receptor (CAR) T-cell therapy, and monoclonal antibodies. With the ability to classify and target specific proteins or genetic markers within a tumor, there are now greater opportunities to combat rare cancers [Citation25]. These technologies and molecular targets can also be shared across a variety of different cancers, presenting the opportunity to use one drug for different types of cancers [Citation25]. We also propose that several policy and funding initiatives within the last 20 years have likely significantly contributed to the development of oncologic orphan drugs. This includes the funding of the National Cancer Institute (NCI), which in recent years has topped $5B annually [Citation26]. Reforms that increased Medicare reimbursements for cancer drugs in the early 2000s likely also contributed to the drug industry’s activity in this area [Citation27]. Finally, the Cancer Moonshot Initiative, funded through the 21st Century Cures Act of 2016, authorized $1.8B in funding for cancer research over 7 years [Citation28].
Second, our analysis in demonstrates that nearly every organ system is targeted by at least one orphan-designated product for rare cancer, thus representing a wide variety of systems and pathologies. From a societal perspective, drug development has not been limited to certain rare cancers. It is likely that this wide representation is due to a previously mentioned fact – that technologies and targets can be shared across cancers. It is likely that developers have translated their experience from cancers with greater development to other rare cancers with previously less development. This appears to have helped bolster the entire rare cancer drug development pipeline.
Third, we found that hematologic malignancies, including leukemias, lymphomas, and myelomas, have the highest number of approvals relative to designations. Additionally, among rare cancers with the most orphan drug-designated products, cancers of the nervous system and pancreas proportionally have the fewest number of associated approvals.
These findings may be explained by several scientific and clinical factors. First, hematologic cancers may have higher approval rates because of the success of drug cocktails in improving outcomes for specific subgroups [Citation29]. Second, approval rates for brain cancers may be lower than average because of challenges inherent in developing therapies that cross the blood-brain barrier (a natural protective structure). In addition to a formidable natural resistance to therapies, the high degree of intra-tumoral cellular heterogeneity and plasticity along with the migratory nature of glioblastoma cells contribute to the poor outcomes experienced by patients with glioblastoma [Citation30–32]. Finally, pancreatic cancer may have lower approval rates because almost half of these cancers are metastatic at diagnosis, leading to difficulty in demonstrating a treatment benefit for pancreatic cancer [Citation33]. Clinical trials of drugs have consistently failed to show a therapeutic benefit due to cell-specific and extracellular resistance mechanisms that inhibit drug delivery and efficacy, and targeted therapy approaches have been largely unsuccessful [Citation33,Citation34].
The current increasing trend in oncologic orphan drug designations suggests that we will continue to see approvals for rare cancer treatments [Citation35]. Further scientific understanding of the underlying pathophysiologic and genetic bases of cancer may lead to identification of potential new targets for drug development that, along with advances in technologies like artificial intelligence, machine learning, and CRISPR-based genome engineering, may contribute to advances in this space [Citation7]. Future research could explore specific areas where these advances may lead in rare cancer drug development, as well as investigating any differences between orphan-designated and non-orphan-designated oncology approvals.
5. Conclusion
This study represents the first comprehensive overview of oncology orphan drug designations and approvals and can serve as a baseline for assessment of future analyses. In the 40 years since enactment of the ODA, 2,355 oncologic orphan drug designations were granted for 181 rare cancers. Additionally, 317 of those designations resulted in at least one FDA approval for use in 92 rare cancers. Rare hematologic cancers were the most frequently represented cancers, but nearly every organ system was represented by an orphan drug-designated product. Interest and investment in drug development for rare cancers, as measured by the number of orphan drug designations and associated approvals, has been stimulated by policy and funding initiatives coinciding with exponential scientific advances in recent years.
Declaration of interests
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Disclaimer
This article reflects the views of the authors and should not be construed to represent FDA’s or HHS’s views or policies.
Author contributions
LJF and KLM conceived the study. AVI, LJF, SK, CM, TL, and KLM designed the study. AVI and LJF collected the data. AVI, LJF, SK, CM, and KLM analyzed and interpreted the data. AVI and KLM drafted the manuscript. LJF, SK, CM, and TL provided substantive edits to the manuscript. All authors read and approved the final manuscript.
Acknowledgments
The authors would like to thank Sandra Retzky and Aaron Friedman for their helpful comments on the manuscript.
Additional information
Funding
References
- National cancer institute surveillance E, and end results program cancer stat facts: common cancer sites. 2023. Available from: https://seer.cancer.gov/statfacts/html/common.html
- American Cancer Society. Cancer facts & figures. 2022. Available from: https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/annual-cancer-facts-and-figures/2022/2022-cancer-facts-and-figures.pdf
- Henley SJ, Singh SD, King J, et al. Invasive cancer incidence and survival-united states 2012 morbidity and mortality weekly report (MMWR): Centers for Disease Control and Prevention; 2015. Available from: https://www.cdc.gov/mmwr/preview/mmwrhtml/mm6449a1.htm
- Centers for Disease Control and Prevention. An update on cancer deaths in the United States. 2022. Available from: https://www.cdc.gov/cancer/dcpc/research/update-on-cancer-deaths/index.htm
- McDowell S. Understanding cancer death rates: American Cancer Society; 2019. Available from: https://www.cancer.org/research/acs-research-news/understanding-cancer-death-rates.html
- American Cancer Society. Cancer prevention & early detection facts & figures 2023–2024. 2023. Available from: https://www.cancer.org/research/cancer-facts-statistics/cancer-prevention-early-detection.html
- Scott EC, Baines AC, Gong Y, et al. Trends in the approval of cancer therapies by the FDA in the twenty-first century. Nat Rev Drug Discov. 2023;22(8):625–640. doi: 10.1038/s41573-023-00723-4
- American Cancer Society. How targeted therapies are used to treat cancer. 2021. Available from: https://www.cancer.org/cancer/managing-cancer/treatment-types/targeted-therapy/what-is.html
- Miller KL, Lanthier M. Trends in orphan new molecular entities, 1983–2014: half were first in class, and rare cancers were the most frequent target. Health Aff. 2016;35(3):464–470.
- American Cancer Society. Cancer facts & fi gures 2017-special section: rare cancers in adults. 2017. Available from: https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/annual-cancer-facts-and-figures/2017/cancer-facts-and-figures-2017-special-section-rare-cancers-in-adults.pdf
- Pub. L. No. 97-414(Jan 4, 1983).
- 21 U.S.C. 360bb(a)(2).
- National Cancer Institute Division of Cancer Control & Population Sciences. Measures of cancer prevalence. 2023. Available from: https://surveillance.cancer.gov/prevalence/measures.html#complete
- Patel S, Miller Needleman KI. FDA’s office of orphan products development: providing incentives to promote the development of products for rare diseases. J Pharmacokinet Pharmacodyn. 2019;46(5):387–393. doi: 10.1007/s10928-019-09645-4
- 21CFR316.20.
- Miller KL, Fermaglich LJ, Maynard J. Using four decades of FDA orphan drug designations to describe trends in rare disease drug development: substantial growth seen in development of drugs for rare oncologic, neurologic, and pediatric-onset diseases. Orphanet J Rare Dis. 2021;16(1):265. doi: 10.1186/s13023-021-01901-6
- Stockklausner C, Lampert A, Hoffmann GF, et al. Novel treatments for rare cancers: the U.S. orphan drug act is delivering-a cross-sectional analysis. Oncology. 2016;21(4):487–493. doi: 10.1634/theoncologist.2015-0397
- Bridget Silverman. FDA’s novel approvals again led by oncology and neurology, but dermatology also shone in 2022: pink sheet pharma intelligence. 2023. Available from: https://pink.pharmaintelligence.informa.com/PS147589/FDAs-Novel-Approvals-Again-Led-By-Oncology-And-Neurology-But-Dermatology-Also-Shone-In-2022?utm_source=dailyem&utm_medium=email&utm_term=&utm_campaign=&utm_medium=email&utm_source=sfmc&utm_campaign=Pink+Sheet+Daily+(Tues±Fri)&utm_id=4547902&sfmc_id=124906171
- Fermaglich LJ, Miller KL. A comprehensive study of the rare diseases and conditions targeted by orphan drug designations and approvals over the forty years of the orphan drug act. Orphanet J Rare Dis. 2023;18(1):163.
- U.S Food & Drug Administration. Search orphan drug designations and approvals. Available from: https://www.accessdata.fda.gov/scripts/opdlisting/oopd/index.cfm
- Vasilevsky NA, Matentzoglu NA, Toro S, et al. Mondo: unifying diseases for the world, by the world. medRxiv. 2022;2022.04.13.22273750.
- National cancer institute surveillance E, and end results program site recode ICD-0-3/WHO 2008 definition. Available from: https://seer.cancer.gov/siterecode/icdo3_dwhoheme/
- U.S Food & Drug Administration. Tissue agnostic drug development in oncology guidance for industry. 2022. p. 16.
- 21CFR316.3(b)(13).
- Zhong L, Li Y, Xiong L, et al. Small molecules in targeted cancer therapy: advances, challenges, and future perspectives. Signal Transduct Target Ther. 2021;6(1):201. doi: 10.1038/s41392-021-00572-w
- National Cancer Institute. NCI budget and appropriations. 2022. Available from: https://www.cancer.gov/about-nci/budget.
- Polite BN, Ward JC, Cox JV, et al. Payment for oncolytics in the United States: a history of buy and bill and proposals for reform. J Oncol Pract. 2014;10(6):357–362. doi: 10.1200/JOP.2014.001958
- de Souza JA, de Lima Lopes G Jr. Medicare reimbursement changes and the practice of oncology: understanding of the past is a key to the future. J Oncol Pract. 2011;7(5):306–308. doi: 10.1200/JOP.2010.000043
- Bachireddy P, Burkhardt UE, Rajasagi M, et al. Haematological malignancies: at the forefront of immunotherapeutic innovation. Nat Rev Cancer. 2015;15(4):201–215. doi: 10.1038/nrc3907
- Upton DH, Ung C, George SM, et al. Challenges and opportunities to penetrate the blood-brain barrier for brain cancer therapy. Theranostics. 2022;12(10):4734–4752. doi: 10.7150/thno.69682
- Noch EK, Ramakrishna R, Magge R. Challenges in the treatment of glioblastoma: multisystem mechanisms of therapeutic resistance. World Neurosurg. 2018;116:505–517. doi: 10.1016/j.wneu.2018.04.022
- Gimple RC, Bhargava S, Dixit D, et al. Glioblastoma stem cells: lessons from the tumor hierarchy in a lethal cancer. Genes Dev. 2019;33(11–12):591–609. doi: 10.1101/gad.324301.119
- Oberstein PE, Olive KP. Pancreatic cancer: why is it so hard to treat? Therap Adv Gastroenterol. 2013;6(4):321–337. doi: 10.1177/1756283X13478680
- Aldape K, Brindle KM, Chesler L, et al. Challenges to curing primary brain tumours. Nat Rev Clin Oncol. 2019;16(8):509–520. doi: 10.1038/s41571-019-0177-5
- DiMasi JA, Reichert JM, Feldman L, et al. Clinical approval success rates for investigational cancer drugs. Clin Pharmacol Ther. 2013;94(3):329–335. doi: 10.1038/clpt.2013.117

