Abstract
Aim
This study aims to evaluate the safety and efficacy of salvage HDR brachytherapy in patients who have undergone a thorough diagnostic process.
Materials and methods
100 prostate cancer patients – locally relapsed after previous radiotherapy – were treated with salvage HDR brachytherapy to a total dose of 24 Gy. Before treatment, the patients underwent PET imaging, prostate MRI, and prostate biopsies to confirm local relapse and exclude systemic disease. Concomitant ADT was applied in 69 patients. Toxicity and efficacy data were collected as a patient chart review. Toxicity was graded using Common Terminology Criteria for Adverse Events (CTCAE 5.0).
Results
The 3-year bDFS and OS were 74% (confidence interval [CI] 95%: 60–87%) and 93% (CI 95%: 84–100%), respectively. Acute Grade 1–2 genitourinary toxicity was observed in 70 patients, 58 patients with Grade 1 and 12 patients with Grade 2, respectively. Acute Grade 1 gastrointestinal toxicity was observed in 8 patients.
Conclusions
This retrospective study shows that salvage HDR brachytherapy is a well-tolerated and effective treatment for histologically proven, local radio-recurrent disease.
Introduction
External beam radiotherapy is widely used for treating prostate cancer. Up to a third of patients receiving EBRT as primary therapy will undergo biochemical failure, which may be linked with local failure and/or metastases and death [Citation1]. Salvage therapy options including radical prostatectomy, stereotactic radiotherapy, high intensity focused ultrasound, and brachytherapy have gained interest in the management of recurrent prostate cancer [Citation2,Citation3]. Among these techniques, salvage HDR brachytherapy has been shown to be a promising option for treating local recurrence [Citation4,Citation5].
The efficacy and safety of salvage brachytherapy has been demonstrated in earlier studies. An 86–92% 5-year overall survival and 51–71% bDFS have been reported [Citation6–8]. The most recent studies show that GU and GI toxicity is low [Citation9].
In earlier studies the diagnostics for recurrence have varied, thus introducing uncertainty for the evaluation of oncologic outcomes. The aim of this retrospective study was to evaluate the safety and efficacy of salvage HDR brachytherapy in patients who have undergone a thorough diagnostic process confirming local relapse and excluding systemic disease.
Materials and methods
All patients (n = 100) who were treated with salvage HDR brachytherapy in Helsinki University Hospital Comprehensive Cancer Center between February 2015 and April 2020 were included in this retrospective study. No patient treated with salvage HDR brachytherapy during that time interval was excluded from this study. The study was approved by the Ethics Committee of Helsinki University Hospital. Data were collected as a patient chart review.
Almost all patients had undergone a thorough diagnostic evaluation before brachytherapy. 98 patients had a prostate MRI, of which 91 showed a suspicious focus. 96 patients had a Gallium68-PSMA-PET-CT, and 8 patients had a Choline-PET-CT (). All patients had prostate biopsies, of which 99 showed prostate cancer. An IPSS questionnaire was performed before treatment to assess pre-treatment urinary function, but there was no symptom threshold to exclude the patients.
Table 1. Characteristics of patients treated with salvage HDR brachytherapy in Helsinki University Hospital between February 2015 and April 2020.
Median age during salvage brachytherapy was 72 years (58–80). At the time of primary radiotherapy, 90 patients had Gleason 6–7 disease and 10 patients had Gleason 8–10 disease, whereas at the time of salvage brachytherapy, 45 patients had Gleason 6–7 disease and 54 patients had Gleason 8–10 disease. The primary treatment was EBRT in 82 patients and LDR brachytherapy in 18 patients. The median time between primary treatment and salvage brachytherapy was 10 years (1–19). 69 patients were under androgen deprivation therapy during brachytherapy.
All patients received three fractions of salvage HDR brachytherapy targeting the whole prostate under general anesthesia. Each fraction was 8 Gy, leading to a total dose of 24 Gy with 3 week treatment intervals. Target dose parameters (EQD2) used in the planning of the treatment were V100 > 97% and D90 > 105%. The following OAR dose constraints were used: Urethra D100m3 <110%, Rectum D10 < 75%, D2000mm3 <65%, Rectal wall D10 < 75%, D2000mm3 <75%.
Follow-up data on oncologic outcomes and toxicity was available in the 55 patients who were living in our hospital district, whereas the 45 patients who were referred to our center from 13 other Finnish hospitals were lost to follow-up after brachytherapy. The patients had a follow-up visit and a PSA test done 6 months after the salvage-brachytherapy. The subsequent follow-up was determined by the treating physician. Toxicity was graded using Common Terminology Criteria for Adverse Events (CTCAE 5.0). The maximum toxicity grade ≥3 months following the salvage therapy was reported as a long-term toxicity.
Biochemical disease-free survival (bDFS) was defined by Phoenix criteria of nadir PSA plus ≥2mg/ml. BDFS was then calculated according to the Kaplan–Meier curves, and the log-rank test was used to compare curves between different Gleason groups, with p < 0.05 considered statistically significant.
Results
Acute toxicity data were available on all patients (n = 100) (). Acute Grade 1-2 genitourinary toxicity was observed in 70 patients. Acute Grade 1 gastrointestinal toxicity was observed in 8 patients (). Acute grade 2 nervous system toxicity (cardiogenic cerebral stroke) was observed in one patient. Late toxicity data were available on 55 patients. Cumulative late Grade 1-2 genitourinary toxicity was observed in 12 (22%) patients. Cumulative late Grade 1 gastrointestinal toxicity was observed in 4 (7%) patients. There was no late Grade ≥3 GU or GI toxicity.
Table 2. Acute and late toxicity in patients treated with salvage HDR brachytherapy in Helsinki University Hospital between February 2015 and April 2020.
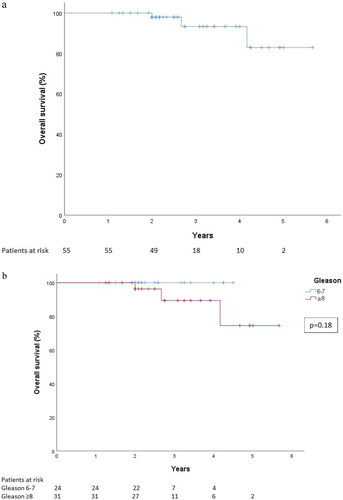
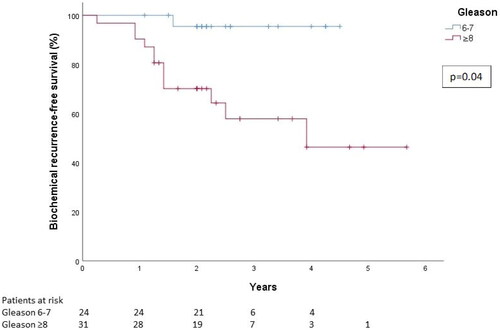
The oncologic outcome data were available on 55 patients. Median follow-up time was 28 months (13–68). Concomitant ADT was used in 41 (74.5%) patients in this follow-up cohort. In the Gleason 6–7 group ADT was used in 12 (50%) patients and in the Gleason 8–10 group in 29 (94%) patients. The 3-year overall survival was 93% (CI 95%: 84–100%) for all patients (). The 3-year overall survival was 100% for patients with Gleason 6–7 disease, and 89% (CI 95%: 75–100%) for patients with Gleason 8–10 disease (). For all patients, the 2- and 3-year bDFS were 8108 and 74%, respectively. The 3-year bDFS was 96% (CI 95%: 87–100%) for patients with Gleason 6–7 disease, and 56% (CI 95%: 37–78%) for patients with Gleason 8–10 disease ().
Discussion
The current study includes 100 consecutive patients and represents a real-life scenario of outcomes of whole-gland salvage HDR brachytherapy across all Gleason grades.
Recurrence of prostate cancer after primary radiotherapy is a therapeutic challenge. Several treatment options are available, but none of them has become a golden standard [Citation2]. Furthermore, the development of more accurate diagnostic methods (PSMA-PET-CT, prostate MRI) allows a more precise staging [Citation10,Citation11], thus enabling to offer salvage therapy only for patients with proven intraprostatic recurrence without distant metastases.
The toxicity of salvage radiotherapy is low in this study. Unfortunately, we did not have late toxicity data available for all patients and, additionally, the data was physician-rated. In other studies, the risk of late toxicity has been low. Our radiation dose was conservative compared to many published series, where total fractionated dose has been at least 30 Gy [Citation4–6]. This conservative radiation dose in our study may contribute to the low early and late toxicity in our study. One patient experienced a stroke after brachytherapy. It shows that there is a general risk of complications as in all invasive procedures.
The time elapsed between primary and salvage brachytherapy was long in our study, possibly because in recent years we have been treating more primary prostate cancer patients with combination radiotherapy (HDR + external beam radiotherapy) which may contribute to fewer recurrences. As shown in this study, however – analogously with salvage radiotherapy after radical prostatectomy – brachytherapy is effective even if cancer recurs after primary external beam radiotherapy, thus maintaining the chance of a cure for patients primarily treated with EBRT or LDR brachytherapy while sparing other patients from unnecessary brachytherapy.
Our 3-year bDFS and OS results are in line with previous reports (4–6). The 3-year overall survival was high (93%), which was to be expected as even a recurrent prostate cancer has a good prognosis: A retrospective analysis of 2694 males with localized prostate cancer treated with external beam RT showed median time of 10.5 years to prostate cancer-specific mortality following biochemical failure [Citation1].
The 3-year bDFS was 96% for patients with Gleason 6–7 disease, and 56% for patients with Gleason 8–10 disease. It should be noted that we are reporting the Gleason grade in the presalvage treatment biopsies. It is still uncertain how well the Gleason grade in post-irradiation biopsies reflect the clinical behavior of the tumor, especially when the time from RT is long [Citation12,Citation13].
Interestingly, only one Gleason 6–7 cancer patient experienced biochemical recurrence during follow-up. In this patient group salvage brachytherapy might therefore represent the new standard therapy for post-RT local recurrence. In Gleason 8–10 patients, on the other hand, salvage brachytherapy may delay the need for medical therapies even when the therapy is non-curative, thus enabling a better quality of life by prolonging the time to additional – often toxic – therapies.
As mentioned previously, there are other salvage therapy options. Corkum et al. performed a systematic review and meta-analysis on re-irradiation using stereotactic radiation therapy including 13 cohorts with 428 patients in total [Citation14]. With mean follow-up of 26.1 months the local control rate was 83.2% and bRFS was 59.3%. In these cohorts, only 30.1% of the patients were receiving androgen deprivation therapy with re-RT.
In the current study the patients were thoroughly examined to verify prostatic recurrence and exclude metastases, which may also partly explain the excellent bDFS and OS outcomes. The diagnostic ‘trifecta’ – PSMA-PET-CT, prostate MRI, and biopsy – is also useful when planning the follow-up and timing of potential additional therapies after brachytherapy. Nevertheless, there is no clear consensus about diagnostic evaluation of patients considered for a salvage treatment in prostate cancer. In our patient cohort almost all the patients had a prostate MRI, prostate biopsy to confirm the local relapse and a PET-CT to exclude metastases. Recently an ESTRO ACROP Delphi consensus project investigated expert opinion on prostate re-irradiation with SBRT [Citation15]. A consensus or major agreement was achieved on 68% of the questions. Among these was the imaging methodology to detect eventual metastases with choline positron emission tomography (PET indicated by 89% of responders, although only 28% voted for PSMA-PET). Unfortunately, because of a retrospective design of our study we do not have data on how many patients were excluded by metastases shown on PET-scan but there are reports how the PET-imaging is guiding the planning of the treatment in recurrent prostate cancer using F-18 fluciclovine PET/CT [Citation16,Citation17] or PSMA PET/CT [Citation11]. Interestingly, only 22% of participants agreed that a prostate biopsy was always needed for diagnosis of recurrence. However, in the EAU guidelines histological confirmation before local salvage treatment is recommended [Citation2]. In our patient cohort prostate biopsy was performed on all patients. We feel this approach is supported by the fact that re-irradiation with curative-intent dose is potentially associated with significant risk of toxicity and the treatment decision should be therefore carefully estimated.
It should be also noted that the quality of HDR brachytherapy is very operator-sensitive. It should therefore be centralized to high-volume centers such as ours to minimize operator- and brachytherapy-specific toxicity and optimize oncologic outcomes.
This study has some limitations essentially being a retrospective study with relatively short follow-up time. This may contribute to the fact that the incidence of long-term side effects was low in this study. Also, the fact that we did not see many relapses in the Gleason 6–7 patients may be related to short follow-up time, as the natural course of the disease is much slower in these patients. The oncologic outcome and late side effect data was available only on 55 patients. We concluded that this would be too small a cohort for analyzing predicting factors for oncologic outcome. We also had incomplete data on primary radiotherapy and ADT use, so we were not able to analyze the effect of these factors to the results of this study. Additionally, there were no control group or population level data on treatment of similar patients.
In conclusion, salvage HDR brachytherapy with a total dose of 24 Gy in 3 fractions every 3 weeks is an effective salvage treatment for locally recurrent prostate cancer, with low acute and late toxicity. Furthermore, we consider that PET/CT plays an important part in the detection of metastatic disease and should be a standard imaging methodology to exclude metastases in salvage patients. Prospective trials between different modalities for patients with local recurrence are warranted.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Zumsteg ZS, Spratt DE, Romesser PB, et al. The natural history and predictors of outcome Following biochemical relapse in the dose escalation era for prostate cancer patients undergoing definitive external beam radiotherapy. Eur Urol. 2015;67(6):1009–1016.
- Cornford P, van den Bergh RCN, Briers E, et al. EAU-EANM-ESTRO-ESUR-SIOG guidelines on prostate cancer. Part II-2020 update: treatment of relapsing and metastatic prostate cancer. Eur Urol. 2021;79(2):263–282.
- Uchida T, Shoji S, Nakano M, et al. High-intensity focused ultrasound as salvage therapy for patients with recurrent prostate cancer after external beam radiation, brachytherapy or proton therapy. BJU Int. 2011;107(3):378–382.
- Henríquez López I, González-San Segundo C, Vegas JO, et al. Salvage brachytherapy for locally-recurrent prostate cancer after radiation therapy: a comparison of efficacy and toxicity outcomes with high-dose rate and low-dose rate brachytherapy. Radiother Oncol. 2019;141:156–163.
- Łyczek J, Kawczyńska MM, Garmol D, et al. HDR brachytherapy as a solution in recurrences of locally advanced prostate cancer. J Contemp Brachytherapy. 2009;1(2):105–108.
- Wojcieszek P, Szlag M, Głowacki G, et al. Salvage high-dose-rate brachytherapy for locally recurrent prostate cancer after primary radiotherapy failure. Radiother Oncol. 2016;119(3):405–410.
- Chen CP, Weinberg V, Shinohara K, et al. Salvage HDR brachytherapy for recurrent prostate cancer after previous definitive radiation therapy: 5-year outcomes. Int J Radiat Oncol Biol Phys. 2013;86(2):324–329.
- Wu SY, Wong AC, Shinohara K, et al. Salvage high-dose-rate brachytherapy for recurrent prostate cancer after definitive radiation. Pract Radiat Oncol. 2021;11(6):515–526.
- Valle LF, Lehrer EJ, Markovic D, et al. A systematic review and meta-analysis of local salvage therapies after radiotherapy for prostate cancer (MASTER). Eur Urol. 2021;80:280–292.
- Rouvière O, Vitry T, Lyonnet D. Imaging of prostate cancer local recurrences: why and how? Eur Radiol. 2010;20(5):1254–1266.
- Perera M, Papa N, Christidis D, et al. Sensitivity, specificity, and predictors of positive 68Ga-prostate-specific membrane antigen positron emission tomography in advanced prostate cancer: a systematic review and meta-analysis. Eur Urol. 2016;70:926–937.
- Evans AJ. Treatment effects in prostate cancer. Mod Pathol. 2018;31:110–121.
- Goldstein NS, Martinez A, Vicini F, et al. The histology of radiation therapy effect on prostate adenocarcinoma as assessed by needle biopsy after brachytherapy boost: correlation With biochemical failure. Am J Clin Pathol. 1998;110(6):765–775.
- Corkum MT, Mendez LC, Chin J, et al. A novel salvage option for local failure in prostate cancer, reirradiation using external beam or stereotactic radiation therapy, systematic review and meta-analysis. Adv Radiat Oncol. 2020;5:965–977.
- Jereczek-Fossa BA, Marvaso G, Zaffaroni M, on Behalf of the European Society for Radiotherapy, Oncology Advisory Committee on Radiation Oncology Practice (ESTRO ACROP), et al. Salvage stereotactic body radiotherapy (SBRT) for intraprostatic relapse after prostate cancer radiotherapy: an ESTRO ACROP delphi consensus. Cancer Treat Rev. 2021;98:102206.
- Andriole GL, Kostakoglu L, Chau A, et al. The impact of positron emission tomography with 18F-Fluciclovine on the treatment of biochemical recurrence of prostate cancer. Results from the LOCATE Trial. J Urol. 2019;201:322–331.
- Scarsbrook AF, Bottomley D, Teoh EJ, et al. Effect of 18F-fluciclovine positron emission tomography on the management of patients with recurrence of prostate cancer: results from the Falcon Trial. Int J Radiat Oncol Biol Phys. 2020;107:316–324.