ABSTRACT
Beside digesting nutrients and absorbing solutes and electrolytes, the intestinal epithelium with its barrier function is in charge of a tightly controlled antigen trafficking from the intestinal lumen to the submucosa. This trafficking dictates the delicate balance between tolerance and immune response causing inflammation. Loss of barrier function secondary to upregulation of zonulin, the only known physiological modulator of intercellular tight junctions, leads to uncontrolled influx of dietary and microbial antigens. Additional insights on zonulin mechanism of action and the recent appreciation of the role that altered intestinal permeability can play in the development and progression of chronic inflammatory disorders has increased interest of both basic scientists and clinicians on the potential role of zonulin in the pathogenesis of these diseases.
This review focuses on the recent research implicating zonulin as a master regulator of intestinal permeability linked to the development of several chronic inflammatory disorders.
Introduction
Increased intestinal permeability has been recently proposed to be an integral element, along with genetic makeup and environmental triggers, in the pathogenesis of chronic inflammatory diseases (CID), including allergic, autoimmune, and metabolic diseases.Citation1-3 The incidence of these conditions in industrialized countries has been on a steady rise since the 1950s,Citation4 leading to the formulation of the hygiene hypothesis.Citation4,5 The rate and timeline of these epidemics imply that genetic factors are necessary but not sufficient in determining which individuals will develop these diseases, pointing to a key role of environmental factors as driving forces to cause CID in genetically predisposed individuals. Increased hygiene in developing countries was not paralleled by similar epidemics of CID, questioning the validity of the hygiene hypothesis, while pointing out to a more complex dynamic of host-environment interaction centered on the possible epigenetic role of the microbial ecosystem with which we co-exist since birth. The appreciation of the microbiome composition as a key “transductor” of pre-, peri-, and post-natal environmental factors affecting clinical outcome has led to the formulation of the microbiota hypothesis, which postulates the key lifestyle changes, including modality of birth, overuse of antibiotics and, most importantly, dietary differences in industrialized countries causes changes in the microbiome composition and ultimately fuel the onset of CID ().Citation6,7 It is now clear there is a symbiotic relationship between the microbiome and the host. As early as 2001, it was described that commensal bacteria have an effect on intestinal permeability.Citation8
Figure 1. Proposed effect of environmental stimuli causing changes in microbiome composition leading to CIDs. Pre-, peri-, and/or post-natal environmental factor can affect microbiota composition causing loss of barrier function, increased antigen trafficking, and altered immune response in genetically susceptible individuals eventually leading to the onset of CID.
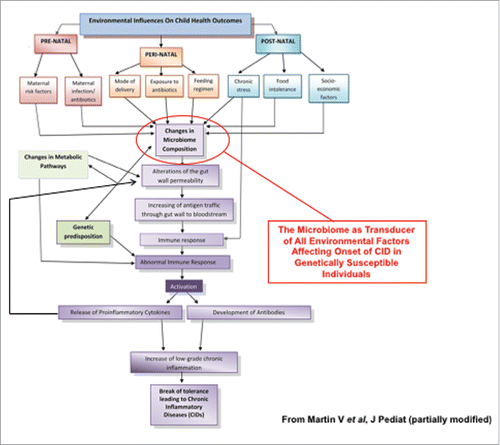
Evidence has shown the impaired gut barrier function is a key pathogenic component rather than the epiphenomenon of several CID.Citation9-11 In this review we will discuss the role of zonulin, the only physiologic modulator of intercellular on tight junctions discovered so far, in the development of several CID.Citation12
Intestinal physiology and tight junctions
The human intestine is lined up by a single layer of epithelial cells that represents the largest interface between the environment and the host. The structural arrangement of the intestinal mucosa suggests an intimate cross talk between epithelial cells and the underneath immune system for the coordinated surveillance of the content of the intestinal lumen. The intestinal mucosa is charged with task of maintaining the balance between the absorption of nutrients and ions, the secretion of fluids, and the protection from microorganisms, toxins, and dietary antigens present in the lumen. The epithelial cells are held together by tight junctions, adherens junctions and desmosomes.Citation13 Historically, tight junctions were thought to be an impermeable barrier blocking paracellular passage of macromolecules. We now know that tight junctions are dynamic structures involved in both physiologic and pathologic regulation of intestinal epithelial antigen tafficking.Citation14
Structure of tight junctions
Tight junctions are the most apical junctional complex connecting both neighboring epithelial and endothelial cells, first described in 1963 by Farquhar and Paladeand, and are comprised of transmembrane proteins including occludin,Citation15 claudins,Citation16 junctional adhesion molecules (JAM),Citation17 tricellulin,Citation18 and angulins.Citation19 These transmembrane proteins interact between themselves (both homophilic and heterophilic interactions) and with intracellular scaffolding proteins, including zonula occludins (ZOs), which are anchored to the actin cytoskeleton. The interaction of occludins, claudins, JAMs and tricellulin between cells and with ZOs maintain the integrity of the tight junction and control the passage of molecules through the paracellular space.
Regulation of intestinal tight junctions
Regulation of tight junctions is essential in maintaining barrier homeostasis, both in between body compartments and between body and external environment. Most of the research on intercellular tight junction regulation has been focused on cytokine-mediated dysfunction in the context of established chronic inflammation, particularly those affecting the intestinal mucosa. TNF-α and IFN-γ have been extensively studied for their effects on the tight junction barrier in the gut. The effect of TNF-α on the intestinal barrier has been associated to IBD,Citation20 graft-versus-host disease,Citation21 and celiac disease (CD).Citation22 In patients with Crohn's disease (CrD) anti-TNF treatment is able to correct barrier disruption seen in the colon.Citation20
The mechanism of TNF-α barrier disruption has been shown to be mediated by myosin-light-chain kinase (MLCK). MLCK activation alone has been shown to decrease tight junction permeability both in vitro and in vivo.Citation23,24 IFN-γ increases intestinal permeability through changes in expression and localization of tight junction proteins as well as rearrangement of the cytoskeleton.Citation25
Pattern recognition receptors (PRRs) are important in the early innate immune response in the intestine. Toll-like receptors (TLRs) are a class of transmembrane PRRs that are important for microbial recognition and control of immune responses. TLR2 is one member of the TLR family, which recognizes conserved patterns on both gram-negative and gram-positive bacteria. TLR2 is expressed on many cell types through the intestine including epithelial cells.Citation26 Stimulation of TLR2 in vitro increased trans epithelial electrical resistance through PKC activation and translocation of ZO-1 to the tight junction complex.Citation27 ZO-1 is controlled by the PI3K/Akt pathway in a MYD88 dependent manner.Citation27 Additional studies provide evidence on the protective effect of TLR2 against DSS-induced colitis. The TLR2 stimulation did not cause increase in FITC-dextran passage or redistribution of ZO-1 away from the tight junction.Citation28
Proteinase activated receptor (PARs) are a family of g-protein-couple-receptors that are activated by proteolytic cleavage of their N-terminus revealing a tether ligand. PAR2 is found on both the apical and baso-lateral side of enterocytes.Citation29 Stimulation of basolateral PAR2 results in increase permeability through redistribution of ZO-1, occludins, and F-actin.Citation30 In addition, Coelho et al. demonstrated that in vivo the apical stimulation with a PAR2 activating peptide (SLIGRL) causes a dose dependent increase in intestinal permeability.Citation31 Stimulation of PAR1 has also been shown to increase intestinal permeability.Citation32
Role of intestinal permeability in disease
A large number of CID have been described to have alterations in intestinal permeability including IBD,Citation33 CD,Citation12,34-36 IBS,Citation37 multiple sclerosis (MS),Citation38 rheumatoid arthritis (RA),Citation33 type-1-diabetes (T1D),Citation39 asthma,Citation40,41 necrotizing enterocolitisCitation42-44 and autism spectrum disorders (ASD).Citation45 Interestingly, less than 10% of subjects with compatible genetic makeup advance to clinical disease, suggesting that environment stimuli play a key role in determining those that progress to disease. Since the intestinal epithelium is the largest mucosal surface that provides an interface between host and environment, inappropriate antigen trafficking through the intestinal mucosa may be involved.
Under normal physiological conditions, the majority (∼90%) of antigens that pass through the intestinal epithelium travel through the transcellular pathway. The transcellular pathway is regulated and leads to lysosomal degradation of antigens into small non-immunogenic peptides. The remaining ∼10% of proteins cross the epithelium through the paracellular pathway as full intact proteins or partially digested peptides as a tightly regulated antigen trafficking through intestinal tight junction modulation which leads to antigenic tolerance.Citation12
The role of epithelial cells in maintaining mucosal homeostasis was postulated by Hermiston and Gordon using chimeric mice with a defective cadherin.Citation46 These mice developed profound epithelial defects including incomplete cell polarization, inappropriate actin cytoskeleton distribution, increased migration of enterocytes along crypt-villous axis and premature apoptosis.Citation46 These mice went on to develop an inflammatory bowel disease resembling CrD without additional external stimuli.Citation47 Additional studies show transgenic mice with constitutively active MLCK show increased intestinal permeability due to tight junction disassembly.Citation24 Although, these mice show increased permeability they do not manifest any signs of overt disease.Citation24 The increased permeability observed in these mice is considered similar to the barrier dysfunction seen in healthy relatives of patients with CrD, CD, and T1D. Experiments performed on JAM-A knockout mice revealed that these animals have increased intestinal permeability but only low grade colonic inflammation and normal epithelial architecture.Citation48 Similar results were obtained with an intestinal specific non muscle myosin IIA heavy chain knockout mice (NM IIA cKO).Citation49 Both JAM-A−/− and NM IIA cKO mice also show increased susceptibility to DSS induced colitis. Together these data suggest intestinal permeability may contribute to the development of several CID, provided that additional genetic traits regulating immune response and exposure to an environmental trigger are present. This hypothesis is consistent with a case report of a healthy first degree relative of a CrD patient who displayed signs of increased intestinal permeability 8 y prior to her own development of CrD.Citation50
Additional experiments on transgenic mice with constitutively active MLCK shed additional light on the role of increase permeability on disease development. The MLCK transgenic mice were crossed with recombination-activation gene (rag)-1 knockout mice (which lack mature B and T cells). The Rag-1−/− and constitutively active MLCK mice as well as their Rag1−/− with normal MLCK received CD4+CD45RBhi naive T cells from wild-type mice. While both groups of mice went on to develop colitis, the colitis in mice with constitutively active MLCK was accelerated and more clinically severe.Citation24
Intestinal barrier disruption has been shown to have a role in disease development, but it's also been shown not be sufficient for disease development. Another key piece of the puzzle seems to be the involvement of the mucosal immune system. It has been reported that barrier dysfunction can influence the immunoregulatory process of the mucosa.Citation51 Induction of mucosal erosion and barrier dysfunction through intrarectal ethanol was followed by increased IFN-γ and IL-10 producing mononuclear cells and CD4+CD25+, latency-associated peptide (LAP) expressing T cells in the lamina propria. The ethanol administration and subsequent presence of the LAP+ T-cells protected against trinitrobenzene sulphonic acid (TNBS) acid induced colitis. The induction of LAP+ T cells was dependent on CD11c+DCs, TL2, and normal microbiome.Citation51 Interestingly, CD11c+ DCs are able to interact with epithelial cells and increase their ability to induce T-regulatory cells.Citation52 These observations suggest a key role for the interaction between the epithelial cells, immune cells, and the luminal microenvironment in the maintenance of intestinal homeostasis.
Zonulin as a master regulator of intercellular tight junction in health and disease
Research while developing a vaccine for Vibrio cholera, led our group to the discovery of zonula occludens toxins (Zot), an enterotoxin which is able to reversibly open intracellular tight junctions.Citation53 Subsequent research led to the appreciation of the complexity of the signaling cascades triggered by Zot involved in its regulation of the paracellular pathway.
Zot causes polymerization of actin of targeted cells leading to disassembly of tight junction complexes through a protein kinase C (PKC)-dependent mechanism.Citation54 Immunofluorescent studies have shown that Zot is able to interact with epithelial cells along the GI tract with the highest binding in the jejunum and distal ileum and also decreasing along the villous to crypt axis.Citation55 These binding studies confirm data on the regional effect of Zot along the intestine.
Given the complexity of the intracellular signaling activated by Zot leading to tight junction modulation, it was hypothesized that the toxin may mimic an endogenous protein which is able to regulate the epithelial tight junctions. The combination of Ussing chamber experiments and anti-Zot antibodies led to the identification a ∼47 kDa human analog to Zot, named zonulin.Citation35 Ex vivo studies show endogenous human zonulin is able to increase permeability in both the jejunum and ileum.Citation56
Studies on human sera from CD patients, who have increased zonulin levelsCitation35 as determined by ELISA measurement using polyclonal zonulin cross reacting anti-Zot antibodies,Citation57 revealed that zonulin is pre-haptoglobin(Hp)-2, the pro-protein of Hp2 before enzymatic cleavage into its mature form. Recombinant zonulin produced by expressing the HP2 cDNA in a baculovirus system, was detected by the anti-Zot antibodiesCitation57 and showed the expected permeating effect on gut mucosa when tested ex vivo in C57BL/6 small intestine. When mice were gavaged with recombinant zonulin and subjected to sucrose and lactulose/mannitol tests they showed increased gastroduodenal and small intestine permeability measured within 24 hours of zonulin exposure, which returned to baseline level after 48 hours.Citation57 To confirm the increase in permeability was specific to zonulin (pre-Hp2), recombinant zonulin was subject to proteolytic cleavage, resulting in the mature α and β chains of Hp. The effects of zonulin observed in both the in vivo and ex vivo experiments failed to cause changes in permeability after proteolytic cleavage.Citation57 Together these results confirmed zonulin to be pre-Hp2 and when cleaved in its mature Hp2 form, loses its effect on paracellular permeability.
Haptoglobin
Haptoglobin is an ancient protein which has been genetically mapped to first appear ∼450 million years ago in bony fish.Citation58 Wicher and Fries suggested that Hp evolved from the complement-associated protein mannose-binding-lectin-associated serine proteinase (MASP). Hp's primary function is to bind free hemoglobin (Hb) in order to prevent the oxidative stress caused by free intravascular Hb. The Hp-Hb complex is cleared through binding of the scavenger receptor CD163 on monocytes/macrophages.Citation59
In humans, Hp is found in 2 genetic variants, HP1 and HP2. The Human HP1 gene is homologous to the HP gene found in other mammals. Genetically human HP1 is made up of 5 exons and 4 introns. HP2 arose from an uneven crossover that occurred ∼2 million years ago causing the duplication of exons 3 and 4 of HP1 giving rise to a gene with 7 exons and 6 introns.Citation60
Hp is translated as a pro-protein before enzymatic cleavage into an α and β chain by C1r-like protein in the endoplasmic reticulum (ER).Citation61 The β chain (∼35kDa) is conserved in Hp1 and Hp2 and contains an inactive chymotrypsin-like serine protease domain, while the α chain exists in 2 forms α-1 (9kDa) and α-2 (18kDa), corresponding to the HP1 and HP2 gene respectively, and contain a complement control domain.Citation62,63 These 2 α chains give rise to 3 different possible genetic combinations in humans, Hp1-1 (Hp1homozygous), Hp2-1(Hp2-1 heterozygous), and Hp2-2 (Hp2 homozygous). After pre-Hp is cleaved, the α and β chains form polymers which are the mature functional form of Hp. The α and β chains form disulfide bridges and heterodimerize. The heterodimer α and β pair is able to then form polymers with other α and β pairs.
Before the discovery of zonulin as pre-Hp2, no biological function had been described for either form of Hp precursors, as they are cleaved in the endoplasmic reticulum and minimal pre-Hp is found circulating in the plasma. Interestingly, Hp was historically used clinically as a marker of general inflammation, similar to C-reactive protein today, as it is an acute phase protein. The discovery of zonulin as pre-Hp2 added a mechanistic mean to the elevated Hp levels in the course of inflammation.
Additionally, the distribution of Hp genotypes in a wide variety of disease has been extensively studied. In 2007 Carter and Worwood reviewed all the disorders in which Hp was reported to be associated. Of the 23 disorders that were linked to Hp, 11 were shown to be more common in patients with the Hp2-1 or Hp2-2 genotype.Citation64 The Hp2-2 phenotype has also been associated with worse prognosis of infectious diseases such as HIVCitation65 and tuberculosis.Citation66 Hp2 has also been shown to been associated with autoimmune disorders, CDCitation57,67 and CrD,Citation68,69 neurological disorders, epilepsyCitation70 schizophrenia,Citation71 complications in diabetes (including diabetic nephropathyCitation72 and diabetic retinopathyCitation73), and Chagas' disease.Citation74,75 This association of Hp2 with CID was postulated to be related to the less efficient capability of Hp2 to bind Hb, which will cause oxidative stress from free Hb, and therefore inflammation. While this could be a plausible hypothesis, increased hemolysis and subsequent oxidative tissue damage have never been reported in these diseases.
Zonulin signaling
Sequential and structural analysis of zonulin revealed an epidermal growth factor (EGF)-like motif. It was therefore hypothesized that zonulin may disassemble TJ through EGF activation, since it has been described EGF can modulate the actin cytoskeleton,Citation76,77 similar to the effects seen with zonulin.Citation56,78 In vitro studies in Caco-2 cells showed zonulin caused EGFR phosphorylation and subsequent increases in permeability which was blocked by an EGFR inhibitor.Citation57 To confirm the effect was due to zonulin and not mature Hp2, trypsin digested zonulin was tested and showed no EGFR activation.Citation57 Additionally, it was shown that EGFR activation was dependent on PAR2 as demonstrated both in Caco2 cells in which the receptor was silenced, and in PAR2−/− mice.Citation57 Zonulin contains a PAR2 activating peptide-like sequence in its β-chain (FCAGMS) very similar to the PAR2 Zot activating peptide AT1002 (FCIGRL). It had been reported previously that several GPCRs including PAR2 are able to transactivate EGFR.Citation79
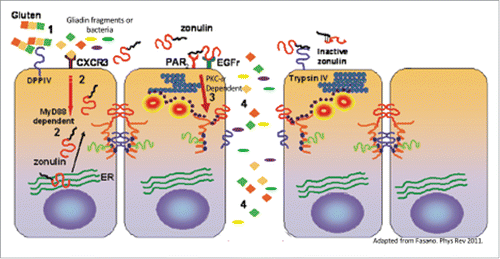
The signaling pathways triggered by Zot and zonulin leading to tight junction disassembly have been extensively studied and resulted being similar ().Citation80 Indeed, as shown with zonulin, Zot also binds to PAR2 through its AT1002 active domain generated during Zot trafficking in V. cholera.Citation81 AT1002 (FCIGRL) structurally resembles the PAR2 activating peptide tethering motif (SLIGRL) and causes increased permeability through displacement of ZO-1 and occludin from the cell junctions that occurred only if PAR2 was expressed in the target cells ().Citation82 The displacement of ZO-1 and occludin was shown to be secondary to PCKα-dependent phosphorylation of ZO-1, causing decreased tight junction protein-protein interactions, and of myosin-1C that, together with the cytoskeletal rearrangement, temporarily removes ZO-1 and occludin from the junctional complex ().Citation82 While ZO-1 displacement per se is not sufficient to cause a barrier defect,Citation83 the combination with other intracellular signaling events affecting TJ, including occludin displacement, actin polymerization, and myosin-1C phosphorylationCitation54,82 may contribute to a more profound rearrangement of the junctional complex that ultimately cause transient TJ disassembly.
Figure 2. Mechanism of gliadin- and bacteria-induced zonulin release and subsequent increase in intestinal permeability. Gliadin specific peptides or bacteria (1) cause a CXCR-3-mediated, MyD88-dependent zonulin release (2). Zonulin transactivates EGFR through PAR2 leading to PCK-α dependent tight junction disassembly (3). Increased intestinal permeability leads to paracellular passage of non-self antigens (4) into the lamina propria where they are able to interact with the immune system.
Zonulin release
The two major triggers of zonulin release that have been described so far are bacteria and gliadin. It is well described that many enteric pathogens are able to produce enterotoxins that affect the intestinal tight junction of the host. In addition to enteroxins, several enteric pathogens, including commensal Eschericha coli, lab E. coli, virulent E. coli, and Salmonella typhi have been shown to cause a release of zonulin from the intestine when applied to the apical surface.Citation84 Following the release of zonulin, the intestine showed increased permeability and disassembly of ZO-1 from the tight junction complex.Citation84
Gliadin is the other trigger that has been described to release zonulin.Citation85,86 Gliadin, only when applied to the apical surface, caused a release of zonulin, and subsequent increase in permeability, in both cell culture models and ex vivo studies of intestinal tissue.Citation87,88 The increase in permeability, but not the release of zonulin was blocked with pretreatment of the zonulin inhibitor AT-1001.Citation85 Lammers et al. described that specific non-digestible gliadin peptides are able to bind the CXCR3 receptor on the apical surface of enterocytes with subsequent MyD88-dependent zonulin release.Citation88 The CXCR3 receptor is also overexpressed on the apical surface of CD patients,Citation88 which may explain the increased levels of zonulin detected in intestinal explant obtained from CD patients when exposed to gliadin.Citation34 While the full signaling cascade following gliadin binding to CXCR3 leading to release of zonulin is not completely understood, it has been shown to be dependent on MyD88, a key adapter molecule in the TLR signaling pathwayCitation88 (). Gliadin is also able to cause a release of zonulin and pro-inflammatory cytokines from macrophages similar to the response seen after bacterial exposure.Citation86 The zonulin release from macrophages is also MyD88 dependent, but TLR2 and TLR4 independent.Citation88
Role of zonulin in specific diseases
Zonulin has been implicated in many CIDs (). Independent from the CID considered, the steps leading to break of tolerance and subsequent development of CID seem to be similar (). Under physiological circumstances there is a tightly control of mucosal antigen trafficking (antigen sampling) that, in concert with specific immune cells and chemokine and cytokine mediators lead to anergy and, therefore, to mucosal tolerance (). The inappropriate production of increased amount of zonulin causes a functional loss of barrier function, with subsequent inappropriate and uncontrolled antigen trafficking instigating an innate immune response by the submucosal immune compartment. If this process continues, an adaptive immune response is mounted causing production of pro-inflammatory cytokines, including IFN-γ and TNF-α that cause further opening of the paracellular pathway to the passage of antigens, creating a vicious cycle. Ultimately, these processes lead to break of tolerance with subsequent onset of CID () whose nature is influenced by the specific host genetic background that dictates which organ or tissue will be targeted by the inflammatory process. Below we will review the CID that have been associated with dysregulation of the zonulin pathway, with special emphasis on CD and T1D, the 2 conditions in which the role of zonulin in disease pathophysiology and gut barrier disruption has been demonstrated, while in many of the other CIDs zonulin has only been shown to be upregulated and the mechanism has not been described.
Figure 3. Proposed mechanism of zonulin in causing loss of barrier function leading to development of CID. Normal barrier trafficking of non-self antigens (antigen sampling), together with specific gut-associated lamina propria cells and cytokines micro milieu leads to mucosal tolerance (1). Environmental stimuli cause microbiome imbalance triggering zonulin release (2) leading to increased antigen influx from gut lumen to the lamina propria (3). Antigens in the lamina propria activate the immune system causing IFN-γ and TNF-α release further exacerbating the increased gut permeability and immune response (4). This leads to a vicious cycle which causes break of tolerance and ultimately, onset of CIDs in genetically predisposed individuals.
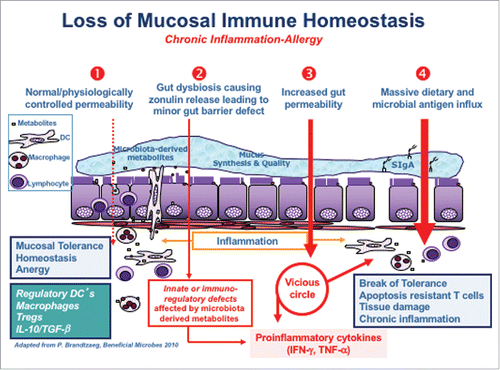
Table 1. List of CIDs in which zonulin has been implicated.
Autoimmune disorders
Celiac disease
CD is an autoimmune enteropathy triggered by the ingestion of gluten-containing grains in genetically susceptible individuals. CD is a complex genetic disorder in which many gene associations have been identified. The HLA status has been identified as accounting for up to 40% of the genetic load. The presence of either DQ2 (HLA-DQA1*05-DQB1*02) or DQ8 (HLA-DQA1*03-DQB1*0302) is necessary but not sufficient to develop CD as ∼40% of the general population also has either DQ2 or DQ8. The ingestion of gluten in CD patients causes destruction of the intestinal villi through a mechanism only partially established. Diagnosis is based on serological screening showing presence of auto-antibodies to tissue transglutaminase enzyme, followed by an upper endoscopy with duodenal biopsy showing the typical celiac autoimmune enteropathy characterized by the presence of intraepithelial lymphocytes, crypt hyperplasia, and villous blunting. Current treatment options are limited to dietary restriction of gluten from the diet, gluten free diet (GFD). The GFD allows the intestinal mucosa to heal and villous architecture to return to normal in many cases. Although, diagnosis is based on an intestinal biopsy, CD is a systemic disease which can affect many different organs and cause several extra-intestinal symptoms.
Gluten is a complex molecule consisting of gliadin and glutenins. Gliadin is a subunit of the protein gluten which is found in wheat, rye, and barley. Through extensive research at least 50 toxic epitopes have been identified. Their effects include cytotoxicity, immunomodulatory, and barrier disruption. The α-gliadin fragment has been mapped with specific domains exerting different effects on the body. The 31-43 peptide exerts a cytotoxic effect, the 57-89 (referred to as 33mer) exerts an immunomodulatory effect, the 111-130 and 151-170 are able to bind CXCR3 and release zonulinCitation88 and the 261-277 causes interleukin (IL)-8 release.Citation89
CD has been used as a model disorder to study the effect of zonulin since its involvement in the development and pathogenesis of the disease has been well documented.Citation2,12,34,35,57,78,85,86,88,90-92 In CD patients, zonulin is produced after gluten ingestion as shown by Drago et al.Citation34 Exposure of intestinal biopsies to pepsin-trypsin(PT)-digested gliadin fragments in the Ussing chamber caused an increase in zonulin release. Interesting, zonulin release could be measured in both CD in remission and healthy control patients, although in healthy controls the level of zonulin release was low and tightly regulated, as shown by the short-term release, with zonulin returning to baseline within 20 minutes.Citation34 Conversely, CD patients displayed a much more robust and prolonged zonulin release following gliadin stimulation,Citation34 followed by a significant increase in gut permeability. The release of zonulin and subsequent increase in intestinal permeability was blocked using the zonulin antagonist AT-1001.Citation34 Also, noteworthy was the observation that CD patients in full remission without PT-gliadin stimulation had constitutively increased zonulin produced by the intestine which correlated to an increase in permeability compared to healthy controls.Citation34
AT1001 (now named Larazotide acetate), is a synthetic 8 amino acid peptide that antagonize the zonulin pathway.Citation93 Pre-clinical trials have shown larazotide acetate to be able to prevent the zonulin permeating activity.Citation11,94,95 Larazotide acetate was also able to block horseradish peroxidase passage and suppress the innate immune response following gliadin challenge in HLA-HCD4/DQ8 mice,Citation94 a double transgenic used to study responses to gliadin before severe inflammation and intestinal damage.Citation96-99
Larazotide acetate is currently in clinical trials as a treatment for CD. Phase II clinical trial results have shown that following a gluten challenge larazotide acetate is able to decrease permeability (only in inpatient setting),Citation100 decrease GI and extra-intestinal symptoms,Citation100-102 and block in increase of tTG antibodies.Citation101,102 Additionally, a study on patients who had persistent symptoms despite following a strict GFD showed larazotide was able to reduce their symptoms.Citation103 Larazotide acetate is now entering phase III clinical trials in CD patients.
Type-1-diabetes
T1D is an autoimmune condition caused by the destruction of the insulin producing β-cell of the pancreas.Citation104 The exact pathogenesis of T1D is not completely understood, however both genetic and environment factors seem to be at play. T1D shares a genetic association with CD with the HLA locus, specifically HLA DQ2 and DQ8. The trigger of T1D has not been discovered but many possible environmental factors have been scrutinized, although none have been confirmed as a clear causative agent of T1D. T1D has similar pathogenic challenges to other autoimmune disorders, as the environmental trigger must cross the intestinal barrier and interact with the immune system.
Gastrointestinal symptoms have been well documented to occur in T1D patients but thought to be due to altered intestinal motility secondary to autonomic neuropathy.Citation105 Recent studies have described increased intestinal permeability to prelude these GI symptoms and the development of T1D.Citation106,107 These studies, including those performed in BioBreeding diabetic-prone (BBDP) rats, which spontaneously develop T1D, suggest a possible pathogenic role for intestinal barrier defects in T1D. The BBDP rats have increase intestinal permeability in the small intestine (but not in the colon) which precedes the loss of tolerance to glucose by at least one month.Citation108 In addition, histological analysis of the pancreatic islets cells at the time of the loss of barrier function, was normal.Citation108 These studies show the loss of intestinal barrier function occurs before the histological damage or loss of glucose tolerance seen in T1Ds. Subsequent experiments confirmed these findings and reported the increased intestinal permeability was zonulin-dependent.Citation11 Furthermore, oral administration of the zonulin blocker AT1001 (larazotide acetate) in the BBDP rats corrected the gut barrier defect and reduced the incidence of diabetes.Citation11
The involvement of zonulin in T1D seen in BBDP rats was confirmed in human studies showing ∼50% of T1D patients have increased serum zonulin levels, some of them showing these changes in the pre-diabetic phase of the disease.Citation3 Interestingly, a subset (∼25%) of first degree relatives of T1D patients also showed increased serum zonulin.Citation3 These data suggest zonulin may play a role in the pathogenesis of T1D in a subset of patients.
Inflammatory bowel disease
Increased intestinal permeability has been shown to play a crucial role in the pathogenesis of both CrD and UC.Citation109-113 Arrieta et al. used the IL-10 knockout (IL-10−/−) colitis model to show a direct relationship between increases in small intestinal permeability and development of colitis.Citation9 IL-10−/− mice were shown to have increased permeability in the small intestine that preluded the development of colitis. Treatment with the zonulin inhibitor AT-1001 caused a significant reduction in the severity of colitis.Citation9 Together, these experiments suggest a role for increased small intestinal permeability in causing aberrant antigen trafficking with subsequent activation of the gut immune cell that, ultimately migrate to the large intestine where they cause more severe colitis. Therefore, restoration of a normal small intestinal barrier function may be an effective treatment option for colitis.
Multiple sclerosis
Multiple sclerosis (MS) patients show increased permeability of both the blood-brain barrier (BBB) and the intestine. Interestingly, patients with progressive MS showed increased levels of serum zonulin, while those with relapsing-remitting MS who were in remission showed serum zonulin levels similar to controls.Citation12 A study focused on the experimental autoimmune encephalomyelitis (EAE) mouse model of MS has further described how zonulin is involved in MS.Citation114 Intestinal permeability and intestinal zonulin are increased during the pre-clinical phase of neurological symptoms, suggesting a role for zonulin in disease development.Citation114
Metabolic disorders and obesity
Obesity
Obesity has recently been shown to be associated with chronic inflammation,.Citation115-117 In an obesity mouse model, increased intestinal permeability and absorption of macromolecules were observed.Citation118 Additionally, obese patients are at risk for developing secondary complications to their obesity such as high cholesterol, type-2-diabets (T2D), coronary heart disease, high blood pressure, and stroke. Three studies have shown that serum zonulin level is increased in obese vs non obese subjects.Citation119-121 Zak-Golab et al. have shown correlation between total bacteria and serum zonulin levels. They suggest that the gut microbiota may cause increased zonulin levels, with subsequent abnormal gut permeability to endotoxin (lipopolysaccharide - LPS) and, ultimately micro-inflammation seen in obesity.Citation120
Evidence has also been provided suggesting that zonulin not only is associated to obesity, but also with its metabolic complications. Serum zonulin has been shown to be increased in T2D patients,Citation121,122 and it has been suggested, through multivariate analysis, that the relationship between insulin sensitivity and serum zonulin may be modulated through IL-6.Citation119 In it interesting to note that zonulin promoter is under IL-6 controlCitation123 and, therefore, zonulin modulation by IL-6 may be mechanistically related to its expression. In addition, serum zonulin was increased in obese children with non-alcoholic fatty liver disease (NAFLD) compared to obese children without NAFLD and correlated with the severity of steatosis.Citation124
Polycystic ovary syndrome
Polycystic ovary syndrome (PCOS) is an endocrinopathy in women of reproductive age due to elevated levels of androgens. It has been reported that both genetic makeup and the environment contribute to the development of PCOS.Citation125 The majority of women with PCOS are overweight and insulin resistant. It is well described that PCOS is characterized by a chronic state of inflammation. Studies have suggested increased zonulin associated to altered gut permeability,Citation126 as key pathogenic elements, together with intestinal microbiota, for development of PCOS.Citation127
Lung diseases
In addition to the GI tract, the lung is another mucosal surface where altered tight junctions function can play a role in a variety of diseases. The mechanisms of airway inflammation are still incompletely established.
Acute lung injury
Leakage of plasma contents into the lungs is observed in acute lung injury (ALI) and acute respiratory distress syndrome.Citation128 Zonulin has been implicated in the disassembly of lung tight junctions in ALI.Citation129 Blocking of the zonulin pathway by AT-1001 or by zonulin neutralizing antibodies reduced the severity of ALI.Citation129 Additionally, both zonulin and its agonist peptide, AT-1002, intensified ALI and increased lung permeability. The mechanism is thought to be through zonulin dependent complement activation in the lung.Citation129
Asthma
In addition to increases in lung permeability, intestinal permeability has also been implicated in other lung diseases like asthma.Citation40,41 Preliminary data suggest that a subset of asthmatic patients have increased serum zonulin levels and ∼40% have increased intestinal permeability.Citation12
These data suggest that both the lung and intestinal mucosa may be routes through which specific antigens can gain access to the submucosa with subsequent exposure to the immune system leading to lung inflammation.
Heart diseases
Coronary artery disease
Coronary artery disease (CAD) is a major cause of death throughout the world. Several studies have shown a link between infectious pathogens and CAD.Citation130-132 Additionally, enterobacteria have been detected in atherosclerotic plaque biopsies. CAD patients have been shown to have increased serum zonulin levels and high levels of Enterobacteriaceaes in their blood.Citation133 These data suggest that zonulin-dependent bacterial translocation may cause increase levels of bacteria in the circulation, with subsequent onset of atherosclerotic plaque leading to CAD.
Neurological disorders
The BBB is formed by endothelial cells and separates the circulating blood from the brain. Since zonulin can also modulate endothelial tight junctions, it was hypothesized zonulin dysregulation may be involved in the pathogenesis of neurological disorders.
Zonulin has also been shown to be involved brain tumors, specifically gliomas.Citation134,135 Skardelly et al. showed there was increased expression of zonulin in gliomas which correlated with the degree of malignancy and degradation of the BBB.Citation135 In vitro studies on a glioma cell line showed zonulin was expressed in high amounts compared to non-glioma control cells.Citation134 Additionally, zonulin has been shown to induce transmigration of neuronal progenitor cells across the BBB.Citation134
Systemic infectious diseases
Septicemia
Intestinal barrier dysfunction has been implicated in the pathogenesis and progression of septicemia. Yoseph et al. have shown in an experimental model of sepsis that tight junction proteins expression is altered.Citation136 In patients with septicemia serum zonulin levels were found to be increased.Citation137 Post-surgical septicemia continues to be a common complication despite advances in surgical techniques and perioperative care. It was hypothesized that zonulin could be a key contributor to post-surgical septicemia.Citation138 Liu et al. described how treatments with probiotics can decrease post-surgical septicemia and is correlated with decreased serum zonulin levels.Citation138 These data suggest increased release of zonulin from enterocytes leads to the migration of bacteria across the epithelium which can lead to septicemia.
HIV
It is widely accepted that the intestine plays an integral role in the immunopathogenesis of human immunodeficiency virus (HIV).Citation139-141 Interestingly, it has been reported that decreased zonulin levels correlated with increased mortality in HIV patients.Citation142 Additionally, treatment with HIV treatment drugs, maraviroc and raltegracir (CCR5 receptor antagonist and integrase inhibitor), increased serum zonulin levels.Citation143 Combined, these data suggest that the zonulin pathway in its innate immunity function can be protective against HIV infection.
Intestinal diseases
Irritable bowel syndrome
The pathophysiology leading to the development of irritable bowel syndrome (IBS) is unknown, but it has been reported that patients with IBS have increased gut permeability.Citation37 Recent data show patients with diarrhea associated IBS (IBS-D) having increased serum zonulin levels.Citation144 Interestingly, PAR2 has been suggested to be involved in the increased permeability detected in IBS-D patients.Citation145 Serine-proteases, which activate PAR2, are found to be increased in the lumal contents of IBS-D patients but not in constipated or alternating IBS patients.Citation146 Diluted fecal supernatants of human IBS-D patients increased permeability of mouse mucosa when added to the apical surface. These changes were not detected in PAR2−/− mice.Citation147 Additionally, IBS-D patients who carry either the HLA-DQ2 or DQ8 genotype have increased gut permeability compared to IBS-D who do not carry one of those HLA genotypes.Citation148 Taken together, these data suggest zonulin signaling through PAR2 may be involved in the pathogenesis of IBS-D.
Non-celiac gluten sensitivity
Non-celiac gluten sensitivity (NCGS) is a newly described condition that clinically presents similar to CD but does not have the intestinal damage seen in CD.Citation149 It has been reported patients with NCGS may have an increase in intestinal permeability following gluten exposure.Citation36 Preliminary evidence also suggest increased serum zonulin levels in patients with NCGS.Citation144
Environmental enteropathy
Environmental enteropathy (EE) is a disease of unknown etiology seen in developing countries. It is hypothesized that constant exposure to infectious agents in the proximal intestine causes increased gut permeability, excess of macromolecules and endotoxin trafficking that trigger chronic inflammation leading to an enteropathy that structurally resembles CD. Like in CD, the enteropathy causes decreased absorption of key nutrients eventually leading to stunted growth. We have recently shown that serum zonulin levels and other markers of barrier dysfunction are correlated with stunted growth in EE patients.Citation150 This data provided additional evidence that a functional loss of barrier function may play a key role in the pathophysiology and EE.
Necrotizing enterocolitis
Necrotizing enterocolitis (NEC) is a devastating disease that affects premature infants resulting in bacteria translocation causing local infection, inflammation and eventually necrosis in portions of the intestine. Changes in tight junction protein expression and localization during NEC have been described in several studies.Citation42-44,151,152 A recently published study implicated the role of zonulin in NEC. Pre-treatment of Caco2 cells with Bifidobacterium before LPS exposure decreased release of zonulin and preserves tight junction competency.Citation153 Ling et al. went further to confirm these results in an in vivo rat model of NEC.Citation153 This study highlights a model in which changes in the microbiome can influence the release of zonulin and, subsequently intestinal barrier function.
Conclusions
Zonulin is a protein involved in the functional regulation of both epithelial and endothelial barrier functions whose role is health and disease is still object of active research. In this review we have provided an overview for its role in the development and pathogenesis of several CIDs. While the specific pathophysiological role of zonulin in many of these diseases is poorly understood, we propose the loss of gut barrier function, through increased zonulin, as an essential step to initiate the inflammatory process. In CD and possibility T1D, research shows gliadin as the trigger of zonulin release leading to gut barrier dysfunction. In other CIDs the specific instigator causing increased zonulin release is not known, but an imbalanced microbiome or its inappropriate distribution along the gastrointestinal tract may be the triggering factors as described in . Dysbiosis of the microbiome may cause the release of zonulin leading to the passage of luminal contents across the epithelial barrier causing the release of pro-inflammatory cytokines. The presence of cytokines eventually sustains the increased permeability causing massive influx of dietary and microbial antigens leading to the activation of T-cells. Depending on the genetic makeup of the host, these T-cells can remain within the GI tract causing CID of the gut (IBD, CD) or migrate to several different organs to cause systemic CID. Research using larazotide acetate, a zonulin antagonist, in animal models and now in clinical trials, not only confirmed the pathogenic role of zonulin in many CIDs, but also opens the possibility for its possible therapeutic use not only in CD, but also in other CIDs in which a pathogenic role for zonulin has been hypothesized or proved. Further research is needed to completely understand the mechanism zonulin plays in the development, pathogenesis, and progression of several CIDs.
Disclosure of potential conflicts of interest
CS has no potential conflict of interest. AF is co-founder and stock holder of Alba Therapeutics.
Funding
This work was supported by NIH grant DK048373.
References
- Arrieta MC, Bistritz L, Meddings JB. Alterations in intestinal permeability. Gut 2006; 55:1512-20; PMID:16966705; http://dx.doi.org/10.1136/gut.2005.085373
- Fasano A, Shea-Donohue T. Mechanisms of disease: the role of intestinal barrier function in the pathogenesis of gastrointestinal autoimmune diseases. Nat Clin Pract Gastroenterol Hepatol 2005; 2:416-22; PMID:16265432; http://dx.doi.org/10.1038/ncpgasthep0259
- Sapone A, de Magistris L, Pietzak M, Clemente MG, Tripathi A, Cucca F, Lampis R, Kryszak D, Cartenì M, Generoso M, et al. Zonulin upregulation is associated with increased gut permeability in subjects with type 1 diabetes and their relatives. Diabetes 2006; 55:1443-9; PMID:16644703; http://dx.doi.org/10.2337/db05-1593
- Bach JF. The effect of infections on susceptibility to autoimmune and allergic diseases. N Engl J Med 2002; 347:911-20; PMID:12239261; http://dx.doi.org/10.1056/NEJMra020100
- Strachan DP. Hay fever, hygiene, and household size. BMJ 1989; 299:1259-60; PMID:2513902; http://dx.doi.org/10.1136/bmj.299.6710.1259
- Noverr MC, Huffnagle GB. The ‘microflora hypothesis’ of allergic diseases. Clin Exp Allergy 2005; 35:1511-20; PMID:16393316; http://dx.doi.org/10.1111/j.1365-2222.2005.02379.x
- Martin VJ, Leonard MM, Fiechtner L, Fasano A. Transitioning from descriptive to mechanistic understanding of the microbiome: the need for a prospective longitudinal approach to predicting disease. J Pediatr 2016; 30736-3; PMID: 27634626; http://dx.doi.org/10.1016/j.jpeds.2016.08.049
- Garcia-Lafuente A, Antolin M, Guarner F, Crespo E, Malagelada JR. Modulation of colonic barrier function by the composition of the commensal flora in the rat. Gut 2001; 48:503-7; PMID:11247894; http://dx.doi.org/10.1136/gut.48.4.503
- Arrieta MC, Madsen K, Doyle J, Meddings J. Reducing small intestinal permeability attenuates colitis in the IL10 gene-deficient mouse. Gut 2009; 58:41-8; PMID:18829978; http://dx.doi.org/10.1136/gut.2008.150888
- Visser JT, Lammers K, Hoogendijk A, Boer MW, Brugman S, Beijer-Liefers S, Zandvoort A, Harmsen H, Welling G, Stellaard F, et al. Restoration of impaired intestinal barrier function by the hydrolysed casein diet contributes to the prevention of type 1 diabetes in the diabetes-prone BioBreeding rat. Diabetologia 2010; 53:2621-8; PMID:20853098; http://dx.doi.org/10.1007/s00125-010-1903-9
- Watts T, Berti I, Sapone A, Gerarduzzi T, Not T, Zielke R, Fasano A. Role of the intestinal tight junction modulator zonulin in the pathogenesis of type I diabetes in BB diabetic-prone rats. Proc Natl Acad Sci U S A 2005; 102:2916-21; PMID:15710870; http://dx.doi.org/10.1073/pnas.0500178102
- Fasano A. Zonulin and its regulation of intestinal barrier function: the biological door to inflammation, autoimmunity, and cancer. Physiol Rev 2011; 91:151-75; PMID:21248165; http://dx.doi.org/10.1152/physrev.00003.2008
- Farquhar MG, Palade GE. Junctional complexes in various epithelia. J Cell Biol 1963; 17:375-412; PMID:13944428; http://dx.doi.org/10.1083/jcb.17.2.375
- Turner JR. Intestinal mucosal barrier function in health and disease. Nat Rev Immunol 2009; 9:799-809; PMID:19855405; http://dx.doi.org/10.1038/nri2653
- Furuse M, Hirase T, Itoh M, Nagafuchi A, Yonemura S, Tsukita S. Occludin: a novel integral membrane protein localizing at tight junctions. J Cell Biol 1993; 123:1777-88; PMID:8276896; http://dx.doi.org/10.1083/jcb.123.6.1777
- Furuse M, Fujita K, Hiiragi T, Fujimoto K, Tsukita S. Claudin-1 and -2: novel integral membrane proteins localizing at tight junctions with no sequence similarity to occludin. J Cell Biol 1998; 141:1539-50; PMID:9647647; http://dx.doi.org/10.1083/jcb.141.7.1539
- Martin-Padura I, Lostaglio S, Schneemann M, Williams L, Romano M, Fruscella P, Panzeri C, Stoppacciaro A, Ruco L, Villa A, et al. Junctional adhesion molecule, a novel member of the immunoglobulin superfamily that distributes at intercellular junctions and modulates monocyte transmigration. J Cell Biol 1998; 142:117-27; PMID:9660867; http://dx.doi.org/10.1083/jcb.142.1.117
- Ikenouchi J, Furuse M, Furuse K, Sasaki H, Tsukita S, Tsukita S. Tricellulin constitutes a novel barrier at tricellular contacts of epithelial cells. J Cell Biol 2005; 171:939-45; PMID:16365161; http://dx.doi.org/10.1083/jcb.200510043
- Higashi T, Tokuda S, Kitajiri S, Masuda S, Nakamura H, Oda Y, Furuse M. Analysis of the ‘angulin’ proteins LSR, ILDR1 and ILDR2–tricellulin recruitment, epithelial barrier function and implication in deafness pathogenesis. J Cell Sci 2013; 126:966-77; PMID:23239027; http://dx.doi.org/10.1242/jcs.116442
- Noth R, Stuber E, Hasler R, Nikolaus S, Kuhbacher T, Hampe J, Bewig B, Schreiber S, Arlt A. Anti-TNF-α antibodies improve intestinal barrier function in Crohn's disease. J Crohns Colitis 2012; 6:464-9; PMID:22398062; http://dx.doi.org/10.1016/j.crohns.2011.10.004
- Brown GR, Lindberg G, Meddings J, Silva M, Beutler B, Thiele D. Tumor necrosis factor inhibitor ameliorates murine intestinal graft-vs.-host disease. Gastroenterology 1999; 116:593-601; PMID:10029618; http://dx.doi.org/10.1016/S0016-5085(99)70181-2
- Marafini I, Monteleone I, Di Fusco D, Cupi ML, Paoluzi OA, Colantoni A, Ortenzi A, Izzo R, Vita S, De Luca E, et al. TNF-α Producing Innate Lymphoid Cells (ILCs) Are Increased in Active Celiac Disease and Contribute to Promote Intestinal Atrophy in Mice. PLoS One 2015; 10:e0126291; PMID:25950701; http://dx.doi.org/10.1371/journal.pone.0126291
- Shen L, Black ED, Witkowski ED, Lencer WI, Guerriero V, Schneeberger EE, Turner JR. Myosin light chain phosphorylation regulates barrier function by remodeling tight junction structure. J Cell Sci 2006; 119:2095-106; PMID:16638813; http://dx.doi.org/10.1242/jcs.02915
- Su L, Shen L, Clayburgh DR, Nalle SC, Sullivan EA, Meddings JB, Abraham C, Turner JR. Targeted epithelial tight junction dysfunction causes immune activation and contributes to development of experimental colitis. Gastroenterology 2009; 136:551-63; PMID:19027740; http://dx.doi.org/10.1053/j.gastro.2008.10.081
- Bruewer M, Utech M, Ivanov AI, Hopkins AM, Parkos CA, Nusrat A. Interferon-gamma induces internalization of epithelial tight junction proteins via a macropinocytosis-like process. FASEB J 2005; 19:923-33; PMID:15923402; http://dx.doi.org/10.1096/fj.04-3260com
- Cario E, Rosenberg IM, Brandwein SL, Beck PL, Reinecker HC, Podolsky DK. Lipopolysaccharide activates distinct signaling pathways in intestinal epithelial cell lines expressing Toll-like receptors. J Immunol 2000; 164:966-72; PMID:10623846; http://dx.doi.org/10.4049/jimmunol.164.2.966
- Cario E, Gerken G, Podolsky DK. Toll-like receptor 2 enhances ZO-1-associated intestinal epithelial barrier integrity via protein kinase C. Gastroenterology 2004; 127:224-38; PMID:15236188; http://dx.doi.org/10.1053/j.gastro.2004.04.015
- Cario E, Gerken G, Podolsky DK. Toll-like receptor 2 controls mucosal inflammation by regulating epithelial barrier function. Gastroenterology 2007; 132:1359-74; PMID:17408640; http://dx.doi.org/10.1053/j.gastro.2007.02.056
- Kong W, McConalogue K, Khitin LM, Hollenberg MD, Payan DG, Bohm SK, Bunnett NW. Luminal trypsin may regulate enterocytes through proteinase-activated receptor 2. Proc Natl Acad Sci U S A 1997; 94:8884-9; PMID:9238072; http://dx.doi.org/10.1073/pnas.94.16.8884
- Darmoul D, Marie JC, Devaud H, Gratio V, Laburthe M. Initiation of human colon cancer cell proliferation by trypsin acting at protease-activated receptor-2. Br J Cancer 2001; 85:772-9; PMID:11531266; http://dx.doi.org/10.1054/bjoc.2001.1976
- Coelho AM, Vergnolle N, Guiard B, Fioramonti J, Bueno L. Proteinases and proteinase-activated receptor 2: a possible role to promote visceral hyperalgesia in rats. Gastroenterology 2002; 122:1035-47; PMID:11910355; http://dx.doi.org/10.1053/gast.2002.32387
- Chin AC, Vergnolle N, MacNaughton WK, Wallace JL, Hollenberg MD, Buret AG. Proteinase-activated receptor 1 activation induces epithelial apoptosis and increases intestinal permeability. Proc Natl Acad Sci U S A 2003; 100:11104-9; PMID:12960392; http://dx.doi.org/10.1073/pnas.1831452100
- Mielants H, De Vos M, Goemaere S, Schelstraete K, Cuvelier C, Goethals K, Maertens M, Ackerman C, Veys EM. Intestinal mucosal permeability in inflammatory rheumatic diseases. II. Role of disease. J Rheumatol 1991; 18:394-400; PMID:1906939
- Drago S, El Asmar R, Di Pierro M, Grazia Clemente M, Tripathi A, Sapone A, Thakar M, Iacono G, Carroccio A, D'Agate C, et al. Gliadin, zonulin and gut permeability: Effects on celiac and non-celiac intestinal mucosa and intestinal cell lines. Scand J Gastroenterol 2006; 41:408-19; PMID:16635908; http://dx.doi.org/10.1080/00365520500235334
- Fasano A, Not T, Wang W, Uzzau S, Berti I, Tommasini A, Goldblum SE. Zonulin, a newly discovered modulator of intestinal permeability, and its expression in coeliac disease. Lancet 2000; 355:1518-9; PMID:10801176; http://dx.doi.org/10.1016/S0140-6736(00)02169-3
- Hollon J, Puppa EL, Greenwald B, Goldberg E, Guerrerio A, Fasano A. Effect of gliadin on permeability of intestinal biopsy explants from celiac disease patients and patients with non-celiac gluten sensitivity. Nutrients 2015; 7:1565-76; PMID:25734566; http://dx.doi.org/10.3390/nu7031565
- Camilleri M, Gorman H. Intestinal permeability and irritable bowel syndrome. Neurogastroenterol Motil 2007; 19:545-52; PMID:17593135; http://dx.doi.org/10.1111/j.1365-2982.2007.00925.x
- Yacyshyn B, Meddings J, Sadowski D, Bowen-Yacyshyn MB. Multiple sclerosis patients have peripheral blood CD45RO+ B cells and increased intestinal permeability. Dig Dis Sci 1996; 41:2493-8; PMID:9011463; http://dx.doi.org/10.1007/BF02100148
- Mooradian AD, Morley JE, Levine AS, Prigge WF, Gebhard RL. Abnormal intestinal permeability to sugars in diabetes mellitus. Diabetologia 1986; 29:221-4; PMID:3519337; http://dx.doi.org/10.1007/BF00454879
- Benard A, Desreumeaux P, Huglo D, Hoorelbeke A, Tonnel AB, Wallaert B. Increased intestinal permeability in bronchial asthma. J Allergy Clin Immunol 1996; 97:1173-8; PMID:8648009; http://dx.doi.org/10.1016/S0091-6749(96)70181-1
- Hijazi Z, Molla AM, Al-Habashi H, Muawad WM, Molla AM, Sharma PN. Intestinal permeability is increased in bronchial asthma. Arch Dis Child 2004; 89:227-9; PMID:14977697; http://dx.doi.org/10.1136/adc.2003.027680
- Bergmann KR, Liu SX, Tian R, Kushnir A, Turner JR, Li HL, Chou PM, Weber CR, De Plaen IG. Bifidobacteria stabilize claudins at tight junctions and prevent intestinal barrier dysfunction in mouse necrotizing enterocolitis. Am J Pathol 2013; 182:1595-606; PMID:23470164; http://dx.doi.org/10.1016/j.ajpath.2013.01.013
- Clark JA, Doelle SM, Halpern MD, Saunders TA, Holubec H, Dvorak K, Boitano SA, Dvorak B. Intestinal barrier failure during experimental necrotizing enterocolitis: protective effect of EGF treatment. Am J Physiol Gastrointest Liver Physiol 2006; 291:G938-49; PMID:16798726; http://dx.doi.org/10.1152/ajpgi.00090.2006
- Hogberg N, Stenback A, Carlsson PO, Wanders A, Lilja HE. Genes regulating tight junctions and cell adhesion are altered in early experimental necrotizing enterocolitis. J Pediatr Surg 2013; 48:2308-12; PMID:24210204; http://dx.doi.org/10.1016/j.jpedsurg.2013.06.027
- D'Eufemia P, Celli M, Finocchiaro R, Pacifico L, Viozzi L, Zaccagnini M, Cardi E, Giardini O. Abnormal intestinal permeability in children with autism. Acta Paediatr 1996; 85:1076-9; PMID:8888921; http://dx.doi.org/10.1111/j.1651-2227.1996.tb14220.x
- Hermiston ML, Gordon JI. In vivo analysis of cadherin function in the mouse intestinal epithelium: essential roles in adhesion, maintenance of differentiation, and regulation of programmed cell death. J Cell Biol 1995; 129:489-506; PMID:7721948; http://dx.doi.org/10.1083/jcb.129.2.489
- Hermiston ML, Gordon JI. Inflammatory bowel disease and adenomas in mice expressing a dominant negative N-cadherin. Science 1995; 270:1203-7; PMID:7502046; http://dx.doi.org/10.1126/science.270.5239.1203
- Laukoetter MG, Nava P, Lee WY, Severson EA, Capaldo CT, Babbin BA, Williams IR, Koval M, Peatman E, Campbell JA, et al. JAM-A regulates permeability and inflammation in the intestine in vivo. J Exp Med 2007; 204:3067-76; PMID:18039951; http://dx.doi.org/10.1084/jem.20071416
- Naydenov NG, Feygin A, Wang D, Kuemmerle JF, Harris G, Conti MA, Adelstein RS, Ivanov AI. Nonmuscle Myosin IIA Regulates Intestinal Epithelial Barrier in vivo and Plays a Protective Role During Experimental Colitis. Sci Rep 2016; 6:24161; PMID:27063635; http://dx.doi.org/10.1038/srep24161
- Irvine EJ, Marshall JK. Increased intestinal permeability precedes the onset of Crohn's disease in a subject with familial risk. Gastroenterology 2000; 119:1740-4; PMID:11113095; http://dx.doi.org/10.1053/gast.2000.20231
- Boirivant M, Amendola A, Butera A, Sanchez M, Xu L, Marinaro M, Kitani A, Di Giacinto C, Strober W, Fuss IJ. A transient breach in the epithelial barrier leads to regulatory T-cell generation and resistance to experimental colitis. Gastroenterology 2008; 135:1612-23 e5; PMID:18765239; http://dx.doi.org/10.1053/j.gastro.2008.07.028
- Iliev ID, Mileti E, Matteoli G, Chieppa M, Rescigno M. Intestinal epithelial cells promote colitis-protective regulatory T-cell differentiation through dendritic cell conditioning. Mucosal Immunol 2009; 2:340-50; PMID:19387433; http://dx.doi.org/10.1038/mi.2009.13
- Fasano A, Baudry B, Pumplin DW, Wasserman SS, Tall BD, Ketley JM, Kaper JB. Vibrio cholerae produces a second enterotoxin, which affects intestinal tight junctions. Proc Natl Acad Sci U S A 1991; 88:5242-6; PMID:2052603; http://dx.doi.org/10.1073/pnas.88.12.5242
- Fasano A, Fiorentini C, Donelli G, Uzzau S, Kaper JB, Margaretten K, Ding X, Guandalini S, Comstock L, Goldblum SE. Zonula occludens toxin modulates tight junctions through protein kinase C-dependent actin reorganization, in vitro. J Clin Invest 1995; 96:710-20; PMID:7635964; http://dx.doi.org/10.1172/JCI118114
- Fasano A, Uzzau S, Fiore C, Margaretten K. The enterotoxic effect of zonula occludens toxin on rabbit small intestine involves the paracellular pathway. Gastroenterology 1997; 112:839-46; PMID:9041245; http://dx.doi.org/10.1053/gast.1997.v112.pm9041245
- Wang W, Uzzau S, Goldblum SE, Fasano A. Human zonulin, a potential modulator of intestinal tight junctions. J Cell Sci 2000; 113 Pt 24:4435-40; PMID:11082037
- Tripathi A, Lammers KM, Goldblum S, Shea-Donohue T, Netzel-Arnett S, Buzza MS, Antalis TM, Vogel SN, Zhao A, Yang S, et al. Identification of human zonulin, a physiological modulator of tight junctions, as prehaptoglobin-2. Proc Natl Acad Sci U S A 2009; 106:16799-804; PMID:19805376; http://dx.doi.org/10.1073/pnas.0906773106
- Wicher KB, Fries E. Haptoglobin, a hemoglobin-binding plasma protein, is present in bony fish and mammals but not in frog and chicken. Proc Natl Acad Sci U S A 2006; 103:4168-73; PMID:16537503; http://dx.doi.org/10.1073/pnas.0508723103
- Asleh R, Marsh S, Shilkrut M, Binah O, Guetta J, Lejbkowicz F, Enav B, Shehadeh N, Kanter Y, Lache O, et al. Genetically determined heterogeneity in hemoglobin scavenging and susceptibility to diabetic cardiovascular disease. Circ Res 2003; 92:1193-200; PMID:12750308; http://dx.doi.org/10.1161/01.RES.0000076889.23082.F1
- Maeda N, Yang F, Barnett DR, Bowman BH, Smithies O. Duplication within the haptoglobin Hp2 gene. Nature 1984; 309:131-5; PMID:6325933; http://dx.doi.org/10.1038/309131a0
- Wicher KB, Fries E. Prohaptoglobin is proteolytically cleaved in the endoplasmic reticulum by the complement C1r-like protein. Proc Natl Acad Sci U S A 2004; 101:14390-5; PMID:15385675; http://dx.doi.org/10.1073/pnas.0405692101
- Kurosky A, Barnett DR, Lee TH, Touchstone B, Hay RE, Arnott MS, Bowman BH, Fitch WM. Covalent structure of human haptoglobin: a serine protease homolog. Proc Natl Acad Sci U S A 1980; 77:3388-92; PMID:6997877; http://dx.doi.org/10.1073/pnas.77.6.3388
- Polticelli F, Bocedi A, Minervini G, Ascenzi P. Human haptoglobin structure and function–a molecular modelling study. FEBS J 2008; 275:5648-56; PMID:18959750; http://dx.doi.org/10.1111/j.1742-4658.2008.06690.x
- Carter K, Worwood M. Haptoglobin: a review of the major allele frequencies worldwide and their association with diseases. Int J Lab Hematol 2007; 29:92-110; PMID:17474882; http://dx.doi.org/10.1111/j.1751-553X.2007.00898.x
- Delanghe JR, Langlois MR, Boelaert JR, Van Acker J, Van Wanzeele F, van der Groen G, Hemmer R, Verhofstede C, De Buyzere M, De Bacquer D, et al. Haptoglobin polymorphism, iron metabolism and mortality in HIV infection. AIDS 1998; 12:1027-32; PMID:9662199; http://dx.doi.org/10.1097/00002030-199809000-00010
- Kasvosve I, Gomo ZA, Mvundura E, Moyo VM, Saungweme T, Khumalo H, Gordeuk VR, Boelaert JR, Delanghe JR, De Bacquer D, et al. Haptoglobin polymorphism and mortality in patients with tuberculosis. Int J Tuberc Lung Dis 2000; 4:771-5; PMID:10949330
- Papp M, Foldi I, Nemes E, Udvardy M, Harsfalvi J, Altorjay I, Mate I, Dinya T, Varvolgyi C, Barta Z, et al. Haptoglobin polymorphism: a novel genetic risk factor for celiac disease development and its clinical manifestations. Clinical Chemistry 2008; 54:697-704; PMID:18258668; http://dx.doi.org/10.1373/clinchem.2007.098780
- Papp M, Lakatos PL, Palatka K, Foldi I, Udvardy M, Harsfalvi J, Altorjay I, Mate I, Dinya T, Varvolgyi C, et al. Haptoglobin polymorphisms are associated with Crohn's disease, disease behavior, and extraintestinal manifestations in Hungarian patients. Dig Dis Sci 2007; 52:1279-84; PMID:17357835; http://dx.doi.org/10.1007/s10620-006-9615-1
- Vanuytsel T, Vermeire S, Cleynen I. The role of Haptoglobin and its related protein, Zonulin, in inflammatory bowel disease. Tissue Barriers 2013; 1:e27321; PMID:24868498; http://dx.doi.org/10.4161/tisb.27321
- Gloria-Bottini F, Lucarelli P, Saccucci P, Cozzoli E, Cerminara C, Curatolo P, Bottini E. Genetic polymorphism and idiopathic generalized epilepsy. Evidence of interaction between haptoglobin and ACP1 systems. Neuropediatrics 2008; 39:357-8; PMID:19569003; http://dx.doi.org/10.1055/s-0029-1202834
- Maes M, Delanghe J, Bocchio Chiavetto L, Bignotti S, Tura GB, Pioli R, Zanardini R, Altamura CA, et al. Haptoglobin polymorphism and schizophrenia: genetic variation on chromosome 16. Psychiatry Res 2001; 104:1-9; PMID:11600184; http://dx.doi.org/10.1016/S0165-1781(01)00298-0
- Nakhoul FM, Zoabi R, Kanter Y, Zoabi M, Skorecki K, Hochberg I, Leibu R, Miller B, Levy AP. Haptoglobin phenotype and diabetic nephropathy. Diabetologia 2001; 44:602-4; PMID:11380078; http://dx.doi.org/10.1007/s001250051666
- Nakhoul FM, Marsh S, Hochberg I, Leibu R, Miller BP, Levy AP. Haptoglobin genotype as a risk factor for diabetic retinopathy. JAMA 2000; 284:1244-5; PMID:10979109; http://dx.doi.org/10.1001/jama.284.10.1244-a
- Calderoni DR, Andrade Tdos S, Grotto HZ. Haptoglobin phenotype appears to affect the pathogenesis of American trypanosomiasis. Ann Trop Med Parasitol 2006; 100:213-21; PMID:16630378; http://dx.doi.org/10.1179/136485906X86356
- Jorge SE, Abreu CF, Guariento ME, Sonati Mde F. Haptoglobin genotypes in Chagas' disease. Clin Biochem 2010; 43:314-6; PMID:19804773; http://dx.doi.org/10.1016/j.clinbiochem.2009.09.020
- Ridley AJ, Paterson HF, Johnston CL, Diekmann D, Hall A. The small GTP-binding protein rac regulates growth factor-induced membrane ruffling. Cell 1992; 70:401-10; PMID:1643658; http://dx.doi.org/10.1016/0092-8674(92)90164-8
- Ridley AJ, Hall A. The small GTP-binding protein rho regulates the assembly of focal adhesions and actin stress fibers in response to growth factors. Cell 1992; 70:389-99; PMID:1643657; http://dx.doi.org/10.1016/0092-8674(92)90163-7
- Fasano A. Regulation of intercellular tight junctions by zonula occludens toxin and its eukaryotic analogue zonulin. Ann N Y Acad Sci 2000; 915:214-22; PMID:11193578; http://dx.doi.org/10.1111/j.1749-6632.2000.tb05244.x
- van der Merwe JQ, Hollenberg MD, MacNaughton WK. EGF receptor transactivation and MAP kinase mediate proteinase-activated receptor-2-induced chloride secretion in intestinal epithelial cells. Am J Physiol Gastrointest Liver Physiol 2008; 294:G441-51; PMID:18032480; http://dx.doi.org/10.1152/ajpgi.00303.2007
- Lu R, Wang W, Uzzau S, Vigorito R, Zielke HR, Fasano A. Affinity purification and partial characterization of the zonulin/zonula occludens toxin (Zot) receptor from human brain. J Neurochem 2000; 74:320-6; PMID:10617135; http://dx.doi.org/10.1046/j.1471-4159.2000.0740320.x
- Uzzau S, Lu R, Wang W, Fiore C, Fasano A. Purification and preliminary characterization of the zonula occludens toxin receptor from human (CaCo2) and murine (IEC6) intestinal cell lines. FEMS Microbiol Lett 2001; 194:1-5; PMID:11150657; http://dx.doi.org/10.1111/j.1574-6968.2001.tb09437.x
- Goldblum SE, Rai U, Tripathi A, Thakar M, De Leo L, Di Toro N, Not T, Ramachandran R, Puche AC, Hollenberg MD, et al. The active Zot domain (aa 288-293) increases ZO-1 and myosin 1C serine/threonine phosphorylation, alters interaction between ZO-1 and its binding partners, and induces tight junction disassembly through proteinase activated receptor 2 activation. FASEB J 2011; 25:144-58; PMID:20852064; http://dx.doi.org/10.1096/fj.10-158972
- McNeil E, Capaldo CT, Macara IG. Zonula occludens-1 function in the assembly of tight junctions in Madin-Darby canine kidney epithelial cells. Mol Biol Cell 2006; 17:1922-32; PMID:16436508; http://dx.doi.org/10.1091/mbc.E05-07-0650
- El Asmar R, Panigrahi P, Bamford P, Berti I, Not T, Coppa GV, Catassi C, Fasano A. Host-dependent zonulin secretion causes the impairment of the small intestine barrier function after bacterial exposure. Gastroenterology 2002; 123:1607-15; PMID:12404235; http://dx.doi.org/10.1053/gast.2002.36578
- Clemente MG, De Virgiliis S, Kang JS, Macatagney R, Musu MP, Di Pierro MR, Drago S, Congia M, Fasano A. Early effects of gliadin on enterocyte intracellular signalling involved in intestinal barrier function. Gut 2003; 52:218-23; PMID:12524403; http://dx.doi.org/10.1136/gut.52.2.218
- Thomas KE, Sapone A, Fasano A, Vogel SN. Gliadin stimulation of murine macrophage inflammatory gene expression and intestinal permeability are MyD88-dependent: role of the innate immune response in Celiac disease. J Immunol 2006; 176:2512-21; PMID:16456012; http://dx.doi.org/10.4049/jimmunol.176.4.2512
- Clemente MG, Musu MP, Troncone R, Volta U, Congia M, Ciacci C, Neri E, Not T, Maggiore G, Strisciuglio P, et al. Enterocyte actin autoantibody detection: a new diagnostic tool in celiac disease diagnosis: results of a multicenter study. Am J Gastroenterol 2004; 99:1551-6; PMID:15307876; http://dx.doi.org/10.1111/j.1572-0241.2004.30296.x
- Lammers KM, Lu R, Brownley J, Lu B, Gerard C, Thomas K, Rallabhandi P, Shea-Donohue T, Tamiz A, Alkan S, et al. Gliadin induces an increase in intestinal permeability and zonulin release by binding to the chemokine receptor CXCR3. Gastroenterology 2008; 135:194-204 e3; PMID:18485912; http://dx.doi.org/10.1053/j.gastro.2008.03.023
- Lammers KM, Khandelwal S, Chaudhry F, Kryszak D, Puppa EL, Casolaro V, Fasano A. Identification of a novel immunomodulatory gliadin peptide that causes interleukin-8 release in a chemokine receptor CXCR3-dependent manner only in patients with coeliac disease. Immunology 2010; 132(3):432-40; PMID:21091908
- Fasano A. Intestinal zonulin: open sesame! Gut 2001; 49:159-62; PMID:11454785; http://dx.doi.org/10.1136/gut.49.2.159
- Fasano A. Physiological, pathological, and therapeutic implications of zonulin-mediated intestinal barrier modulation: living life on the edge of the wall. Am J Pathol 2008; 173:1243-52; PMID:18832585; http://dx.doi.org/10.2353/ajpath.2008.080192
- Smecuol E, Sugai E, Niveloni S, Vazquez H, Pedreira S, Mazure R, Moreno ML, Label M, Mauriño E, Fasano A, et al. Permeability, zonulin production, and enteropathy in dermatitis herpetiformis. Clin Gastroenterol Hepatol 2005; 3:335-41; PMID:15822038; http://dx.doi.org/10.1016/S1542-3565(04)00778-5
- Di Pierro M, Lu R, Uzzau S, Wang W, Margaretten K, Pazzani C, Maimone F, Fasano A. Zonula occludens toxin structure-function analysis. Identification of the fragment biologically active on tight junctions and of the zonulin receptor binding domain. J Biol Chem 2001; 276:19160-5; PMID:11278543; http://dx.doi.org/10.1074/jbc.M009674200
- Gopalakrishnan S, Tripathi A, Tamiz AP, Alkan SS, Pandey NB. Larazotide acetate promotes tight junction assembly in epithelial cells. Peptides 2012; 35:95-101; PMID:22401910; http://dx.doi.org/10.1016/j.peptides.2012.02.016
- Gopalakrishnan S, Durai M, Kitchens K, Tamiz AP, Somerville R, Ginski M, Paterson BM, Murray JA, Verdu EF, Alkan SS, et al. Larazotide acetate regulates epithelial tight junctions in vitro and in vivo. Peptides 2012; 35:86-94; PMID:22401908; http://dx.doi.org/10.1016/j.peptides.2012.02.015
- Black KE, Murray JA, David CS. HLA-DQ determines the response to exogenous wheat proteins: a model of gluten sensitivity in transgenic knockout mice. J Immunol 2002; 169:5595-600; PMID:12421937; http://dx.doi.org/10.4049/jimmunol.169.10.5595
- de Kauwe AL, Chen Z, Anderson RP, Keech CL, Price JD, Wijburg O, Jackson DC, Ladhams J, Allison J, McCluskey J. Resistance to celiac disease in humanized HLA-DR3-DQ2-transgenic mice expressing specific anti-gliadin CD4+ T cells. J Immunol 2009; 182:7440-50; PMID:19494267; http://dx.doi.org/10.4049/jimmunol.0900233
- Marietta E, Black K, Camilleri M, Krause P, Rogers RS, 3rd, David C, Pittelkow MR, Murray JA. A new model for dermatitis herpetiformis that uses HLA-DQ8 transgenic NOD mice. J Clin Invest 2004; 114:1090-7; PMID:15489956; http://dx.doi.org/10.1172/JCI200421055
- Verdu EF, Huang X, Natividad J, Lu J, Blennerhassett PA, David CS, McKay DM, Murray JA. Gliadin-dependent neuromuscular and epithelial secretory responses in gluten-sensitive HLA-DQ8 transgenic mice. Am J Physiol Gastrointest Liver Physiol 2008; 294:G217-25; PMID:18006603; http://dx.doi.org/10.1152/ajpgi.00225.2007
- Paterson BM, Lammers KM, Arrieta MC, Fasano A, Meddings JB. The safety, tolerance, pharmacokinetic and pharmacodynamic effects of single doses of AT-1001 in coeliac disease subjects: a proof of concept study. Aliment Pharmacol Ther 2007; 26:757-66; PMID:17697209; http://dx.doi.org/10.1111/j.1365-2036.2007.03413.x
- Kelly CP, Green PH, Murray JA, Dimarino A, Colatrella A, Leffler DA, Alexander T, Arsenescu R, Leon F, Jiang JG, et al. Larazotide acetate in patients with coeliac disease undergoing a gluten challenge: a randomised placebo-controlled study. Aliment Pharmacol Ther 2013; 37:252-62; PMID:23163616; http://dx.doi.org/10.1111/apt.12147
- Leffler DA, Kelly CP, Abdallah HZ, Colatrella AM, Harris LA, Leon F, Arterburn LA, Paterson BM, Lan ZH, Murray JA. A randomized, double-blind study of larazotide acetate to prevent the activation of celiac disease during gluten challenge. Am J Gastroenterol 2012; 107:1554-62; PMID:22825365; http://dx.doi.org/10.1038/ajg.2012.211
- Leffler DA, Kelly CP, Green PH, Fedorak RN, DiMarino A, Perrow W, Rasmussen H, Wang C, Bercik P, Bachir NM, et al. Larazotide acetate for persistent symptoms of celiac disease despite a gluten-free diet: a randomized controlled trial. Gastroenterology 2015; 148:1311-9 e6; PMID:25683116; http://dx.doi.org/10.1053/j.gastro.2015.02.008
- Krumbhaar EB. Spontaneous Diabetes in a Dog. J Exp Med 1916; 24:361-5; PMID:19868047; http://dx.doi.org/10.1084/jem.24.4.361
- Feldman M, Schiller LR. Disorders of gastrointestinal motility associated with diabetes mellitus. Ann Intern Med 1983; 98:378-84; PMID:6402969; http://dx.doi.org/10.7326/0003-4819-98-3-378
- Carratu R, Secondulfo M, de Magistris L, Iafusco D, Urio A, Carbone MG, Pontoni G, Cartenì M, Prisco F. Altered intestinal permeability to mannitol in diabetes mellitus type I. J Pediatr Gastroenterol Nutr 1999; 28:264-9; PMID:10067726; http://dx.doi.org/10.1097/00005176-199903000-00010
- De Magistris L, Secondulfo M, Iafusco D, Carbone AG, Urio A, Pontoni G, Carratu R. Altered mannitol absorption in diabetic children. Ital J Gastroenterol 1996; 28:367; PMID:8891852
- Meddings JB, Jarand J, Urbanski SJ, Hardin J, Gall DG. Increased gastrointestinal permeability is an early lesion in the spontaneously diabetic BB rat. Am J Physiol 1999; 276:G951-7; PMID:10198339
- Buhner S, Buning C, Genschel J, Kling K, Herrmann D, Dignass A, Kuechler I, Krueger S, Schmidt HH, Lochs H. Genetic basis for increased intestinal permeability in families with Crohn's disease: role of CARD15 3020insC mutation? Gut 2006; 55:342-7; PMID:16000642; http://dx.doi.org/10.1136/gut.2005.065557
- Buning C, Geissler N, Prager M, Sturm A, Baumgart DC, Buttner J, Bühner S, Haas V, Lochs H. Increased small intestinal permeability in ulcerative colitis: rather genetic than environmental and a risk factor for extensive disease? Inflamm Bowel Dis 2012; 18:1932-9; PMID:22344959; http://dx.doi.org/10.1002/ibd.22909
- Peeters M, Geypens B, Claus D, Nevens H, Ghoos Y, Verbeke G, Baert F, Vermeire S, Vlietinck R, Rutgeerts P. Clustering of increased small intestinal permeability in families with Crohn's disease. Gastroenterology 1997; 113:802-7; PMID:9287971; http://dx.doi.org/10.1016/S0016-5085(97)70174-4
- Teahon K, Smethurst P, Levi AJ, Menzies IS, Bjarnason I. Intestinal permeability in patients with Crohn's disease and their first degree relatives. Gut 1992; 33:320-3; PMID:1568650; http://dx.doi.org/10.1136/gut.33.3.320
- Wyatt J, Vogelsang H, Hubl W, Waldhoer T, Lochs H. Intestinal permeability and the prediction of relapse in Crohn's disease. Lancet 1993; 341:1437-9; PMID:8099141; http://dx.doi.org/10.1016/0140-6736(93)90882-H
- Nouri M, Bredberg A, Westrom B, Lavasani S. Intestinal barrier dysfunction develops at the onset of experimental autoimmune encephalomyelitis, and can be induced by adoptive transfer of auto-reactive T cells. PLoS One 2014; 9:e106335; PMID:25184418; http://dx.doi.org/10.1371/journal.pone.0106335
- Olszanecka-Glinianowicz M, Chudek J, Kocelak P, Szromek A, Zahorska-Markiewicz B. Body fat changes and activity of tumor necrosis factor α system–a 5-year follow-up study. Metabolism 2011; 60:531-6; PMID:20580040; http://dx.doi.org/10.1016/j.metabol.2010.04.023
- Olszanecka-Glinianowicz M, Zahorska-Markiewicz B, Janowska J, Zurakowski A. Serum concentrations of nitric oxide, tumor necrosis factor (TNF)-α and TNF soluble receptors in women with overweight and obesity. Metabolism 2004; 53:1268-73; PMID:15375781; http://dx.doi.org/10.1016/j.metabol.2004.07.001
- Zahorska-Markiewicz B, Janowska J, Olszanecka-Glinianowicz M, Zurakowski A. Serum concentrations of TNF-α and soluble TNF-α receptors in obesity. Int J Obes Relat Metab Disord 2000; 24:1392-5; PMID:11126333; http://dx.doi.org/10.1038/sj.ijo.0801398
- Ferraris RP, Vinnakota RR. Intestinal nutrient transport in genetically obese mice. Am J Clin Nutr 1995; 62:540-6; PMID:7661115
- Moreno-Navarrete JM, Sabater M, Ortega F, Ricart W, Fernandez-Real JM. Circulating zonulin, a marker of intestinal permeability, is increased in association with obesity-associated insulin resistance. PLoS One 2012; 7:e37160; PMID:22629362; http://dx.doi.org/10.1371/journal.pone.0037160
- Zak-Golab A, Kocelak P, Aptekorz M, Zientara M, Juszczyk L, Martirosian G, Chudek J, Olszanecka-Glinianowicz M. Gut microbiota, microinflammation, metabolic profile, and zonulin concentration in obese and normal weight subjects. Int J Endocrinol 2013; 2013:674106; PMID:23970898; http://dx.doi.org/10.1155/2013/674106
- Zhang D, Zhang L, Zheng Y, Yue F, Russell RD, Zeng Y. Circulating zonulin levels in newly diagnosed Chinese type 2 diabetes patients. Diabetes Res Clin Pract 2014; 106:312-8; PMID:25238913; http://dx.doi.org/10.1016/j.diabres.2014.08.017
- Jayashree B, Bibin YS, Prabhu D, Shanthirani CS, Gokulakrishnan K, Lakshmi BS, Mohan V, Balasubramanyam M. Increased circulatory levels of lipopolysaccharide (LPS) and zonulin signify novel biomarkers of proinflammation in patients with type 2 diabetes. Mol Cell Biochem 2014; 388:203-10; PMID:24347174; http://dx.doi.org/10.1007/s11010-013-1911-4
- Oliviero S, Cortese R. The human haptoglobin gene promoter: interleukin-6-responsive elements interact with a DNA-binding protein induced by interleukin-6. EMBO J 1989; 8:1145-51; PMID:2787245
- Pacifico L, Bonci E, Marandola L, Romaggioli S, Bascetta S, Chiesa C. Increased circulating zonulin in children with biopsy-proven nonalcoholic fatty liver disease. World J Gastroenterol 2014; 20:17107-14; PMID:25493023; http://dx.doi.org/10.3748/wjg.v20.i45.17107
- Diamanti-Kandarakis E, Kandarakis H, Legro RS. The role of genes and environment in the etiology of PCOS. Endocrine 2006; 30:19-26; PMID:17185788; http://dx.doi.org/10.1385/ENDO:30:1:19
- Zhang D, Zhang L, Yue F, Zheng Y, Russell R. Serum zonulin is elevated in women with polycystic ovary syndrome and correlates with insulin resistance and severity of anovulation. Eur J Endocrinol 2015; 172:29-36; PMID:25336505; http://dx.doi.org/10.1530/EJE-14-0589
- Tremellen K, Pearce K. Dysbiosis of Gut Microbiota (DOGMA)–a novel theory for the development of Polycystic Ovarian Syndrome. Med Hypotheses 2012; 79:104-12; PMID:22543078; http://dx.doi.org/10.1016/j.mehy.2012.04.016
- Ware LB, Matthay MA. The acute respiratory distress syndrome. N Engl J Med 2000; 342:1334-49; PMID:10793167; http://dx.doi.org/10.1056/NEJM200005043421806
- Rittirsch D, Flierl MA, Nadeau BA, Day DE, Huber-Lang MS, Grailer JJ, Zetoune FS, Andjelkovic AV, Fasano A, Ward PA. Zonulin as prehaptoglobin2 regulates lung permeability and activates the complement system. Am J Physiol Lung Cell Mol Physiol 2013; 304:L863-72; PMID:23564505; http://dx.doi.org/10.1152/ajplung.00196.2012
- Eurich DT, Johnstone JJ, Minhas-Sandhu JK, Marrie TJ, Majumdar SR. Pneumococcal vaccination and risk of acute coronary syndromes in patients with pneumonia: population-based cohort study. Heart 2012; 98:1072-7; PMID:22739637; http://dx.doi.org/10.1136/heartjnl-2012-301743
- Neumann T, Lulsdorf KA, Krings P, Reinsch N, Erbel R. Coronary artery disease in HIV-infected subjects. Results of 101 coronary angiographies. Herz 2011; 36:18-23
- Vcev A, Nakic D, Mrden A, Mirat J, Balen S, Ruzic A, Persić V, Soldo I, Matijević M, Barbić J, et al. Helicobacter pylori infection and coronary artery disease. Coll Antropol 2007; 31:757-60; PMID:18041385
- Li C, Gao M, Zhang W, Chen C, Zhou F, Hu Z, Zeng C. Zonulin Regulates Intestinal Permeability and Facilitates Enteric Bacteria Permeation in Coronary Artery Disease. Sci Rep 2016; 6:29142; PMID:27353603; http://dx.doi.org/10.1038/srep29142
- Diaz-Coranguez M, Segovia J, Lopez-Ornelas A, Puerta-Guardo H, Ludert J, Chavez B, Meraz-Cruz N, González-Mariscal L. Transmigration of neural stem cells across the blood brain barrier induced by glioma cells. PLoS One 2013; 8:e60655; PMID:23637756; http://dx.doi.org/10.1371/journal.pone.0060655
- Skardelly M, Armbruster FP, Meixensberger J, Hilbig H. Expression of Zonulin, c-kit, and Glial Fibrillary Acidic Protein in Human Gliomas. Transl Oncol 2009; 2:117-20; PMID:19701495; http://dx.doi.org/10.1593/tlo.09115
- Yoseph BP, Klingensmith NJ, Liang Z, Breed ER, Burd EM, Mittal R, Dominguez JA, Petrie B, Ford ML, Coopersmith CM. Mechanisms of Intestinal Barrier Dysfunction in Sepsis. Shock 2016; 46:52-9; PMID:27299587; http://dx.doi.org/10.1097/SHK.0000000000000565
- Klaus DA, Motal MC, Burger-Klepp U, Marschalek C, Schmidt EM, Lebherz-Eichinger D, Krenn CG, Roth GA. Increased plasma zonulin in patients with sepsis. Biochem Med (Zagreb) 2013; 23:107-11; PMID:23457771; http://dx.doi.org/10.11613/BM.2013.013
- Liu Z, Li C, Huang M, Tong C, Zhang X, Wang L, Peng H, Lan P, Zhang P, Huang N, et al. Positive regulatory effects of perioperative probiotic treatment on postoperative liver complications after colorectal liver metastases surgery: a double-center and double-blind randomized clinical trial. BMC Gastroenterol 2015; 15:34; PMID:25881090; http://dx.doi.org/10.1186/s12876-015-0260-z
- Deeks SG, Tracy R, Douek DC. Systemic effects of inflammation on health during chronic HIV infection. Immunity 2013; 39:633-45; PMID:24138880; http://dx.doi.org/10.1016/j.immuni.2013.10.001
- Sandler NG, Wand H, Roque A, Law M, Nason MC, Nixon DE, Pedersen C, Ruxrungtham K, Lewin SR, Emery S, et al. Plasma levels of soluble CD14 independently predict mortality in HIV infection. J Infect Dis 2011; 203:780-90; PMID:21252259; http://dx.doi.org/10.1093/infdis/jiq118
- Tenorio AR, Zheng Y, Bosch RJ, Krishnan S, Rodriguez B, Hunt PW, Plants J, Seth A, Wilson CC, Deeks SG, et al. Soluble markers of inflammation and coagulation but not T-cell activation predict non-AIDS-defining morbid events during suppressive antiretroviral treatment. J Infect Dis 2014; 210:1248-59; PMID:24795473; http://dx.doi.org/10.1093/infdis/jiu254
- Hunt PW, Sinclair E, Rodriguez B, Shive C, Clagett B, Funderburg N, Robinson J, Huang Y, Epling L, Martin JN, et al. Gut epithelial barrier dysfunction and innate immune activation predict mortality in treated HIV infection. J Infect Dis 2014; 210:1228-38; PMID:24755434; http://dx.doi.org/10.1093/infdis/jiu238
- Serrano-Villar S, Sainz T, Ma ZM, Utay NS, Chun TW, Mann S, Kashuba AD, Siewe B, Albanese A, Troia-Cancio P, et al. Effects of Combined CCR5/Integrase Inhibitors-Based Regimen on Mucosal Immunity in HIV-Infected Patients Naive to Antiretroviral Therapy: A Pilot Randomized Trial. PLoS Pathog 2016; 12:e1005540; PMID:27015639; http://dx.doi.org/10.1371/journal.ppat.1005540
- Barbaro MR, Cremon C, Caio G, Bellacosa L, De Giorgio R, Volta U, et al. The role of zonulin in non-celiac gluten sensitivity and irritable bowel syndrome. United Euro Gastroenterol J 2015; 3:A87
- Bueno L. Protease activated receptor 2: a new target for IBS treatment. Eur Rev Med Pharmacol Sci 2008; 12 Suppl 1:95-102; PMID:18924448
- Kim JA, Choi SC, Yun KJ, Kim DK, Han MK, Seo GS, Yeom JJ, Kim TH, Nah YH, Lee YM. Expression of protease-activated receptor 2 in ulcerative colitis. Inflamm Bowel Dis 2003; 9:224-9; PMID:12902845; http://dx.doi.org/10.1097/00054725-200307000-00002
- Gecse K, Roka R, Ferrier L, Rosztoczy A, Wittman T, Fioramonti J, et al. Elevated Fecal Serine-Protease Activity: Protease-Activated Receptor 2 (PAR2) Mediated Alteration of Colonic Permeability in Diarrhea-Predominant Irritable Bowel Syndrome. Gastroenterology 2007; 132:A-402
- Vazquez-Roque MI, Camilleri M, Smyrk T, Murray JA, O'Neill J, Carlson P, Lamsam J, Eckert D, Janzow D, Burton D, et al. Association of HLA-DQ gene with bowel transit, barrier function, and inflammation in irritable bowel syndrome with diarrhea. Am J Physiol Gastrointest Liver Physiol 2012; 303:G1262-9; PMID:23042942; http://dx.doi.org/10.1152/ajpgi.00294.2012
- Volta U, Caio G, Tovoli F, De Giorgio R. Non-celiac gluten sensitivity: questions still to be answered despite increasing awareness. Cell Mol Immunol 2013; 10:383-92; PMID:23934026; http://dx.doi.org/10.1038/cmi.2013.28
- Guerrant RL, Leite AM, Pinkerton R, Medeiros PH, Cavalcante PA, DeBoer M, Kosek M, Duggan C, Gewirtz A, Kagan JC, et al. Biomarkers of Environmental Enteropathy, Inflammation, Stunting, and Impaired Growth in Children in Northeast Brazil. PLoS One 2016; 11:e0158772; PMID:27690129; http://dx.doi.org/10.1371/journal.pone.0158772
- Rentea RM, Liedel JL, Welak SR, Cassidy LD, Mayer AN, Pritchard KA, Jr., Oldham KT, Gourlay DM. Intestinal alkaline phosphatase administration in newborns is protective of gut barrier function in a neonatal necrotizing enterocolitis rat model. J Pediatr Surg 2012; 47:1135-42; PMID:22703783; http://dx.doi.org/10.1016/j.jpedsurg.2012.03.018
- Shiou SR, Yu Y, Chen S, Ciancio MJ, Petrof EO, Sun J, Claud EC. Erythropoietin protects intestinal epithelial barrier function and lowers the incidence of experimental neonatal necrotizing enterocolitis. J Biol Chem 2011; 286:12123-32; PMID:21262973; http://dx.doi.org/10.1074/jbc.M110.154625
- Ling X, Linglong P, Weixia D, Hong W. Protective Effects of Bifidobacterium on Intestinal Barrier Function in LPS-Induced Enterocyte Barrier Injury of Caco-2 Monolayers and in a Rat NEC Model. PLoS One 2016; 11:e0161635; PMID:27551722; http://dx.doi.org/10.1371/journal.pone.0161635
