ABSTRACT
The gut-brain axis hypothesis suggests that interactions in the intestinal milieu are critically involved in regulating brain function. Several studies point to a gut–microbiota–brain connection linking an impaired intestinal barrier and altered gut microbiota composition to neurological disorders involving neuroinflammation. Increased gut permeability allows luminal antigens to cross the gut epithelium, and via the blood stream and an impaired blood–brain barrier (BBB) enters the brain impacting its function. Pre-haptoglobin 2 (pHP2), the precursor protein to mature HP2, is the first characterized member of the zonulin family of structurally related proteins. pHP 2 has been identified in humans as the thus far only endogenous regulator of epithelial and endothelial tight junctions (TJs). We have leveraged the Zonulin-transgenic mouse (Ztm) that expresses a murine pHP2 (zonulin) to determine the role of increased gut permeability and its synergy with a dysbiotic intestinal microbiota on brain function and behavior. Here we show that Ztm mice display sex-dependent behavioral abnormalities accompanied by altered gene expression of BBB TJs and increased expression of brain inflammatory genes. Antibiotic depletion of the gut microbiota in Ztm mice downregulated brain inflammatory markers ameliorating some anxiety-like behavior. Overall, we show that zonulin-dependent alterations in gut permeability and dysbiosis of the gut microbiota are associated with an altered BBB integrity, neuroinflammation, and behavioral changes that are partially ameliorated by microbiota depletion. Our results suggest the Ztm model as a tool for the study of the cross-talk between the microbiome/gut and the brain in the context of neurobehavioral/neuroinflammatory disorders.
Introduction
Over the past decade, research into the gut–brain axis (GBA) has grown exponentially. It has become evident that other elements besides genetic susceptibility and immune system dysfunction, such as the gut microbiota and changes in gut permeability, play a role in the development of inflammatory diseases in the intestine and the brain.Citation1–17 These changes in the gut environment appear to have a profound influence on brain function and behavior.Citation18 In the pediatric population, attention has been focused on neurodevelopmental and neurobehavioral disorders in children and adolescents, such as autism spectrum disorder (ASD), attention deficit hyperactivity disorder (ADHD), schizophrenia, anxiety and depression, and their frequent gastrointestinal comorbidities.Citation19–27 Paracellular antigen trafficking is strictly controlled by intercellular tight junctions (TJs) that play a critical role in immune tolerance and whose mechanisms of action are still incompletely understood. Zonulin peptides are members of a family of structurally and functionally related proteins that reversibly regulate intestinal permeability by modulating intercellular TJs.Citation28–30 The first member of this family, pre-haptoglobin 2 (pHP2) (referred in this paper as zonulin, for simplicity) is the precursor of the mature haptoglobin 2 and has been identified in humans.Citation31 Elevated zonulin serum levels positively correlate with loss of gut barrier functionCitation32 and have been reported in ASDCitation33 and associated with hyperactivity and social dysfunctions in children with ADHD.Citation34 In neurobehavioral disorders, including ASD and schizophrenia clinical observations of gut hyperpermeability have also been described.Citation35–38
Zonulin release is triggered by several environmental stimuli, including gluten and gut dysbiosis,Citation39–41 both of which are interestingly implicated in ASD.Citation42–49
Several species of commensal gut bacteria have the ability to strengthen or weaken gut barrier function,Citation50–57 thereby altering intestinal permeability.Citation58 Intestinal dysbiosis and inflammation are associated with a dysfunctional epithelial barrier.Citation59–65 Increased antigen trafficking through an impaired gut barrier due to a shift in microbiota composition allows harmful substances from the intestinal lumen into the bloodstream,Citation52,Citation66,Citation67 leading to the activation of immune cells and subsequent systemic inflammation that can, in turn, affect the integrity of the blood–brain barrier (BBB) and promote neuroinflammation and disease.Citation68–70 Defects in gut barrier function and/or gut microbiota dysbiosis, often associated with defects in BBB integrity, have been reported in several inflammatory neurological diseases,Citation9,Citation71–73 including ASD and schizophrenia, epilepsy, multiple sclerosis, Parkinson’s and Alzheimer’s disease.Citation9,Citation17,Citation74–100 Furthermore, it has been shown that zonulin increases the permeability of the BBB in vitroCitation101 and seems to be involved in the degradation of the BBB in the context of brain tumors.Citation102,Citation103 We and others have reported a dysfunctional gut-brain axis associated with neuroinflammation in ASD, adding to the evidence that the BBB is breached in this group of patients.Citation100,Citation104–107 Overall, our previous results and data from the literature suggest a role of gut microbiota dysbiosis and hyperpermeability of gut and BBBs in influencing brain function. However, despite accumulating pieces of evidence, the role that increased gut permeability plays in behavioral alterations that characterize neurobehavioral disorders is still unknown. In this study, we used the Zonulin transgenic mouse (Ztm) model of increased gut permeability to assess the role of a zonulin-dependent dysfunctional intestinal barrier and increased trafficking of bacteria and/or their products from a dysbiotic microbiota on BBB integrity, brain inflammation, and behavior. To this aim, we characterized the composition of the gut microbiota of Ztm male and female mice and assessed their brain function and behavior at baseline and after antibiotic depletion of gut commensal bacteria. We also looked at changes in the small intestinal TJs gene expression profiles in antibiotic-treated mice to assess the impact of bacteria depletion on the integrity of the intestinal barrier.
Materials and Methods
Animals
To work on a murine model expressing the pHP2/zonulin gene, we acquired a mouse (C57Bl/6 background) in which the native mouse haptoglobin 1 (HP1) allele was substituted with a murine HP2 allele by targeted insertion to generate mice with the HP2-2 genotype under the control of its own promoter.Citation108 A breeding pair was donated by Andrew Levy and kept in our Massachusetts General Hospital (MGH) facility. A colony of C57Bl/6 WT mice was housed in our animal facility as well. Both colonies were reared and maintained under standard conditions (12 h light: 12 h dark cycle, standard humidity and temperature). WT and Ztm male and female mice 8–12 weeks of age were used in this study. Animal studies were approved by the Institutional Animal Care and Use committee at MGH (2013N000013).
Experiment 1: Previously, we have shown that Ztm mice have high small intestinal permeability that is paralleled by a reduction in intestinal TJs gene expression, the presence of pro-inflammatory gut microbiota dysbiosis and an immune system prone to inflammation when exposed to environmental inflammatory stimuli.Citation109,Citation110 In Experiment 1, we sought to extend our previous findings and assess whether the observed gut and immune system alterations in the Ztm mouse affected BBB function, brain inflammation, and behavior. We also collected stool samples from male and female WT and ZTM mice to examine the gut microbiota and confirm that gut dysbiosis accompanies brain changes in both sexes. For Experiment 1 we used 13–15 WT males and 13–14 WT females, 16–17 Ztm males and 16–17 females for behavioral tests. Of these, we collected stools from 7 WT males and 9 WT females, 6 Ztm males and 10 females for microbiota analysis.
Experiment 2: We then sought to examine whether the BBB, brain, and behavioral alterations observed in Ztm mice could be ameliorated by depletion of gut bacteria. To this aim, we administered a cocktail of antibiotics in the mice drinking water starting at 3–4 weeks of age to deplete gut bacteriaCitation111 and eliminate pro-inflammatory gut microbiota, followed by BBB, brain and behavior assays. For this set of experiments, we used 13 WT males and 8 WT females, 8 Ztm males and 8 Ztm females for behavioral assays and microbiota analysis with the exception of Ztm males for which 7 males were used for behavior. All mice in experiment 2 were between 9–11 weeks.
Behavioral assays
To assess if there were behavioral differences between our experimental groups, we used three standard behavioral tests that included marble burying, open field, and elevated zero maze. Mice were tested during their light cycle, over three consecutive days, at the same time of the day (in the mornings within the same 3-hour window) and participated in one behavioral test per day. Tests were run in sequential order from the least stressful to the most stressful assay [marble burying (day 1), open field (day 2), elevated zero maze (day 3)]. Ethovision XT, an automated software for detection and quantification, was used for behavioral scoring of the open field and elevated zero maze assays. Mice were moved to the testing room at least 30 minutes prior to start the testing to allow for acclimation to the animal behavioral room. Males and females were always tested and acclimated separately.
Marble burying
The Marble Burying test measures obsessive/compulsive/repetitive behavior, as a paradigm of the ethologically natural behavior of defensive burying.Citation112–114 Regular housing cages (28x20x12 cm) were filled with 7 cm of autoclaved, and evenly distributed bedding material (Sani-chips®). Twenty Marbles (Fisher Science Education TM Glass Marbles #S04581, 1.42 cm) were placed equidistant in 4 parallel lines with alternating colors of black and blue. Mice were placed in the center of the cage and free to interact with the marbles. After 20 minutes, animals were removed carefully so as not to disturb the bedding and/or the marbles and the number of buried marbles was counted. Marbles were considered buried when at least 2/3 of their volume was covered by bedding material.
Open field
The Open Field test measures anxiety and hyperactivity in a low anxiety settingCitation115–117 based on the idea that mice naturally prefer to be near a protective wall rather than in an open field where they are exposed to predators (in nature). Each animal was placed in a square (28x28x28 cm) white opaque plexiglass box for 10 minutes, and the animal’s movements were tracked with an infrared video camera. Time spent in the center of the box (defined as a central square that covered 20% of the field) versus closer to the walls was tracked and total distance traveled was calculated using the EthoVision XT Software. Males and females were never present in the behavioral testing room at the same time and the testing apparatus was thoroughly cleaned between test animals.
Elevated zero maze
The Elevated Zero Maze measures anxiety. It is a modification of the elevated plus maze first described by Shepherd et al.Citation118 and consists of an elevated annular arena with two open areas and two closed areas (both of equal area). During the test the animal was placed in the open arm and allowed to explore freely for 5 minutes. While the open areas are, by design, anxiogenic due to the increased exposure and height of the maze, the animal is free to move into the closed areas. Time spent in the open arm vs. closed arm was measured. Both parameters were automatically tracked by an overhead infrared camera and analyzed by EthoVision XT Software.
Antibiotic treatment
We adopted antibiotic depletion protocols widely used in the literature, showing depletion of commensal bacteria.Citation111,Citation119,Citation120 Mice at weaning (3–4 weeks of age) were treated with a mix of antibiotics in their drinking water (ad libitum) containing Ampicillin (1 gr/L), vancomycin (500 mg/L), metronidazole (1 g/L), and neomycin (1 g/L). All antibiotics were purchased from Sigma. Since mice were not drinking when the full dose was administered because of the taste given by metronidazole, to ensure mice would drink regularly and consistently, the antibiotic cocktail was introduced at 1/8 of the final concentration and increased to 1/4, 1/2, and 3/4 every 5 days, until reaching full concentration. Mice were treated with a full antibiotic dose for 2 weeks. At the end of the 2 weeks, DNA was extracted from stools and bacterial load was assessed by 16S qPCR. Both antibiotic-treated WT and Ztm mice showed a 70% reduction in bacterial load (data not shown).
Total RNA extraction and quantitative PCR (real-time PCR)
Mice were anesthetized using Forane (isoflurane, USP, Baxter, US) and euthanized by cervical dislocation. Tissues were immediately collected, fresh frozen and kept at −80 C until processed. Total RNA was extracted from frozen tissues using TRIzol™ reagent (Thermo Fisher, US) and the Direct-zol RNA mini prep plus Kit (ZymoReserach, US) following the manufacturer’s protocol. To ensure full removal of genomic DNA, extracted RNA from each sample was treated with the DNA free™ Kit (Thermo Fisher, US). Total RNA was quantified and A260/280 and A260/230 ratios measured using a Nanodrop 2000 (Thermo Fisher, US). cDNA was made using the Maxima H− First Strand cDNA Synthesis Kit (Thermo Fisher, US) according to manufacturer’s instructions. Quantitative real-time PCR was performed using PerfeCTa SYBR® Green SuperMix (Quanta, US) and a Bio Rad CFX connect thermocycler. All primers were designed in-house, obtained from Integrated DNA Technologies (US) and listed in .
Table 1. List of primers and FASTA accession numbers of the genes analyzed by real time qPCR analysis. All primers were designed in house using NIH’s Primer Blast and acquired from Integrated DNA Technologies (USA).
Zonulin qPCR gene expression in the small intestine and the brain
Only for the zonulin gene expression profile, qPCR data from Experiment 2 (Abx-treated Ztm mice) were compared to Experiment 1 (Ztm mice) to assess whether depletion of the gut microbiota affected the expression of zonulin in the gut and/or brain.
Microbiota analysis
Sample collection and DNA extraction
Collected fecal pellets were flash frozen and kept at −80 C until further processing. Genomic DNA for each sample was extracted using the DNeasy powersoil extraction kit (Qiagen, US) following Qiagen’s instructions. DNA was quantified using Nanodrop. In order to carry out the phylogenetic profiling, the hypervariable V4 region of the 16S rRNA gene was amplified by PCR using 5X prime master mix (Prime, US), reverse 806 primers were barcoded and a unique forward 515 primer (Integrated DNA Technologies, US) was used. To confirm correct amplification of the V4 regions, a regular gel electrophoresis was run. PCR products were purified using the QIAquick PCR purification kit (Qiagen, US) and their concentration was measured by Quant-iT Picogreen dsDNA kit following manufacturer’s instructions. Sequencing of the samples was done at the MGH NextGen Sequencing Core facility (Boston, US), on the Illumina system using the MiSeq v2 500 cycles reagent kit as per manufacturer’s instructions. To allow maximum coverage of the amplicon, the system sequenced a total of 250 paired-end cycles. The following primers were used for the sequencing:Citation121
read 1 (TATGGTAATT GT GTGYCAGCMGCCGCGGTAA)
read 2 (AGTCAGCCAGCCGGACTACNVGGGTWTCTAAT)
index (AATGATACGGCGACCACCGAGATCTACACGCT).
Microbiota data analysis
Sequencing data were processed and analyzed with QIIME2 software package v. 2018.2.0.Citation122 The sequencing reads with low-quality score (average Q < 25) were truncated to 240 bp followed by filtering using deblur algorithm with default settings.Citation123 The remaining high-quality reads were aligned to the reference library using mafft.Citation124 Next, the aligned reads were masked to remove highly variable positions, and a phylogenetic tree was generated from the masked alignment using the FastTree method.Citation125 Alpha and beta diversity metrics and Principal Component Analysis plots based on Jaccard distance were generated using default QIIME2 plugins.Citation122 Taxonomy assignment was performed using feature-classifier method and naïve Bayes classifier trained on the Greengenes 13_8 99% operational taxonomic units (OTUs). Differential abundance analysis of OTUs was performed using ANCOM.Citation126
Statistical analysis
Statistical analyses for behavioral experiments and real-time PCR were performed in Graph Pad Prism 9. Behavioral experiments are expressed as means ± SEM using unpaired t-test to assess statistical significance. Comparisons between 2 groups for the qPCR analysis are expressed as means ± SEM and analyzed with the non-parametrical Mann–Whitney U test for statistical significance. p < .05 was considered statistically significant in both tests. Statistical analysis of the microbiome was performed using the Kruskal–Wallis test to assess the statistical significance of abundance differences, with multiple testing corrections performed using Benjamini–Hochberg false discovery rate (FDR). The FDR cutoff was set at 0.05.
Results
Ztm male and female mice show altered repetitive and anxiety-like behavior compared to WT mice
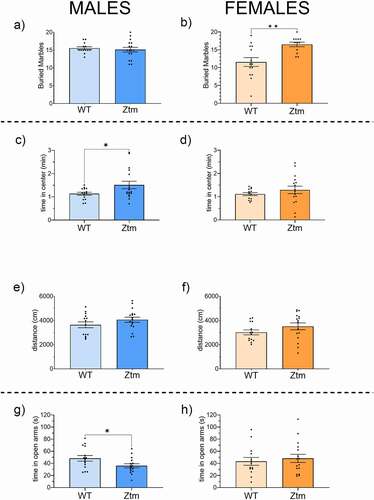
No differences were observed in the number of marbles buried in Ztm males and WT males (). In contrast, Ztm females buried significantly more marbles than WT females (). In the open field assay, both Ztm and WT males traveled similar distances (), yet Ztm males spent significantly more time in the center of the open field compared to WT males (). Within females, no significant differences were observed for total distanced traveled or time in the center of the open field between Ztm and WT mice (, f). For the elevated zero maze behavioral assay, Ztm males spent significantly less time in the open arms of the maze than WT males () while there was no difference in open arm time between Ztm females versus WT females ().
Figure 1. Behavior profile of Ztm mice compared to WT mice. Marble Burying (a, b): Total number of marbles buried is significantly higher in Ztm females than wt females (b), no differences seen in males (a). Open field (C, D, E, F): Time spent in the center of the field is significatively higher in Ztm males than wt males (c). No significant difference was seen on females (d). Distance traveled was similar for both males (e) and females (f). Track images are representative of each group. Elevated Zero Maze (g, h): Ztm mice spent significant less time in the open arm than WT mice (g), no difference seen in the female group (h). N = 13–17.
Ztm male and female mice show altered gene expression profiles for BBB TJs and markers of neuroinflammation compared to WT mice
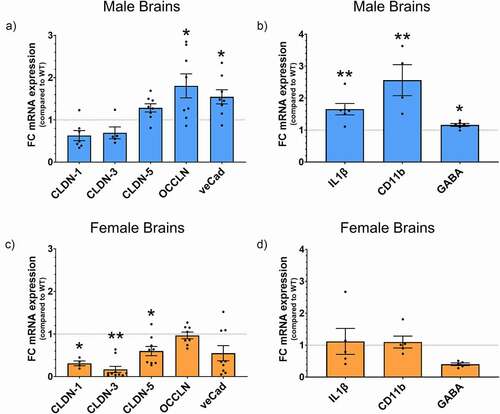
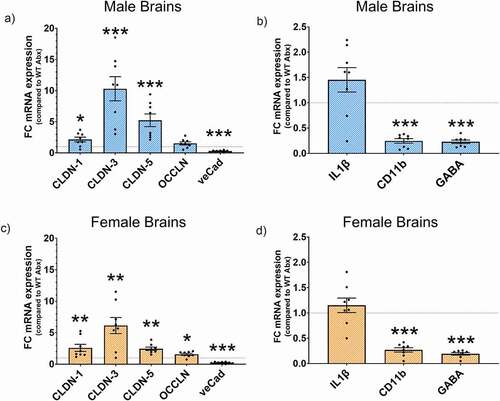
To determine whether the behavioral changes observed in the Ztm mice were linked to molecular changes in the BBB and the brain, we examined TJs and neuroinflammation-associated genes in the prefrontal cortex of mice. We observed significant differences in BBB-associated TJs gene expression in Ztm mice compared to WT mice, with each sex displaying a unique pattern of changes within the Ztm mouse model (). Ztm males exhibited a significant increase in the expression of TJs occludin (OCCLN) and ve-Cadherin (veCad) that was accompanied by significantly higher levels of pro-inflammatory markers Interleukin (IL)1β and CD11b (, B; ). Ztm males were also characterized by a significant increase in GABA gene expression (, ). Unlike males, Ztm females showed a significant decrease in key TJs components of the BBB, such as claudin (CLDN)-5, 3, and 1 and no change in the neuroinflammatory genes IL-1β and CD11b (, D; ).
Table 2. List of genes analyzed by real time PCR in the brain of adult mice (n = 4–10). Expressed as Fold Change (FC), statistics calculated with the non- parametric Mann-Whitney test (p).
Figure 2. Gene expression profile for BBB TJs and neuroinflammation markers. mRNA expression was analyzed by qPCR normalized to WT and expressed as fold change ± SEM. (a) Increased OCCLN and veCad in Ztm males compared to WT males in the BBB. (b) Altered neuroinflammatory profile in the Ztm male brain. (c) Significant downregulation of BBB TJs in the Ztm female brain. (d) No change in the neuroinflammatory profile of the Ztm females.
The gut microbiota of Ztm male and female mice is shifted toward a pro-inflammatory phenotype
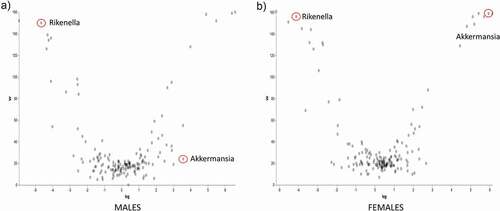
Analysis of the 16s rRNA sequencing of the gut microbiota showed a high degree of beta diversity (between groups) in PCoA plot between Ztm and WT males (). The Shannon index of alpha diversity showed that Ztm males harbored a less diverse and less uniform gut microbiota compared to WT males (). Beta diversity analysis of the microbiota from female mice also revealed that Ztm females clustered separately from WT females in the PCoA plot (), indicating a different microbiota composition compared to WT. However, the Shannon index was not different between female Ztm compared to female WT mice (). At the phylum level, Ztm males had lower Proteobacteria (class Alphaproteobacteria) and Verrucomicrobiota () with a drastic decreased Akkermansia (sp. muciniphila) sp. On the other hand, they showed an increased abundance of the inflammatory-associated Rikenella sp. (class Bacteroidia)(). Similarly, Ztm females also harbored dramatically reduced Verrucomicrobiota and decreased abundance of Proteobacteria (Alphaproteobacteria) and Cyanobacteria in their gut () with a dramatic reduction in the relative abundance of A. muciniphila sp. and significantly greater abundance of Rikenella sp. compared to WT females, as shown in the ANCOM volcano plot analysis ().
Figure 3. Gut microbiota dysbiosis in males and females Ztm mice. Stools from WT (males n = 7, females n = 9) and Ztm mice (males n = 6, females n = 10) were analyzed. (a, b): Principal Component Analysis (PCA) of Jaccard distances for Ztm and WT mice show clustering between Ztm and WT males (a) and females (b). The closer the spatial distance of the sample, the more similar the species composition of the sample. (c, d): Shannon Index expression of alpha diversity (variation/complexity of the microbiome within the group). WT males show a less diverse microbiota than Ztm males (c). Similar alpha diversity observed int the Ztm and WT females (d).
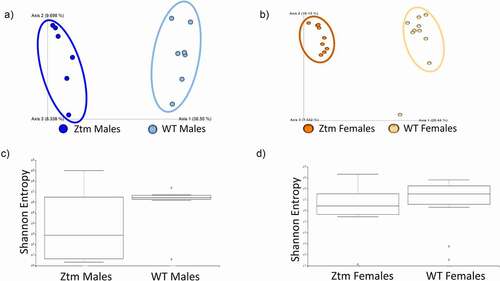
Figure 4. Histogram of the community composition of gut microbiota at the class level. The abscissa represents the group, and the ordinate represents the relative abundance. The figures show species with a relative abundance of 1% or more. (a): Lower abundance of Alphaproteobacteria and Verrucomicrobiae in Ztm males when compared to WT males. (b): Ztm females show lower abundance of Proteobacteria, Verrucomicrobiae and Cyanobacteriia in their stools.
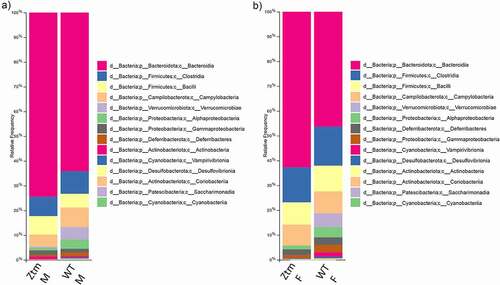
Figure 5. ANCOM volcano plot of statistical differences between Ztm and WT at genus level. (a): Ztm males harbor a significant increased abundance of Rikenella sp. vs. an abundance of A. muciniphila in WT males. (b): Ztm females show an abundance of Rikenella sp. and a reduction of A. muciniphila when compared to WT females.
Abx-treatment shifts gut microbiota composition
As expected, Abx-treatment drastically reduced bacterial load (70% reduction; data not shown) and shifted the gut microbiota composition in all groups from baseline conditions ( compared to ). PCoA showed that Abx-treated Ztm mice did not cluster separately from Abx-treated WT animals (,b). Alpha-diversity in Abx-treated mice was considerably reduced and no significant differences were observed between Abx-treated Ztm and Abx-treated WT (,d).
Figure 6. Effect of Abx treatment on gut microbiota composition in Abx-treated Ztm and WT mice. Stools from 21 WT mice (males n = 13, females n = 8) and 16 Ztm (males n = 8, females n = 8) were analyzed. (a, b): In Principal Component Analysis (PCA) of Jaccard distances for Abx -treated Ztm (ZtmAbx) and Abx-treated WT (WTAbx) mice revealed no distinct microbiota clustering between Abx-treated Ztm and WT males (a) or females (b). (c, d): Shannon Index, expression of alpha diversity (variation/complexity of the microbiome within the group). Alpha diversity was reduced in Abx-treated Ztm males when compared to Abx-treated WT males (c), and in Abx-treated Ztm females when compared to Abx-treated WT females.
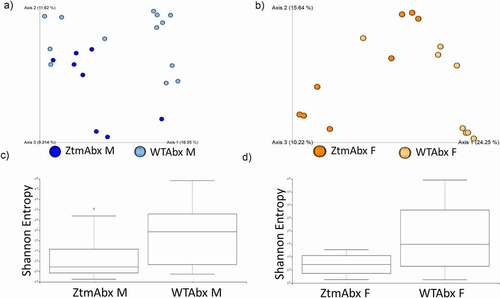
Figure 7. Histogram of the community composition of gut microbiota at the class level after Abx treatment. Abx-treated Ztm (ZtmAbx) males have a higher abundance of Firmicutes (Bacilli), and severely decreased Alphaproteobacteria and Cyanobacteriia composition compared to Abx-treated WT (WTAbx) mice. (b): Abx-treated Ztm females show a dramatic increase in Gammaproteobacteria and a drastic reduction of Alphaproteopacteria and Cyanobateriia when compared to WTAbx females.
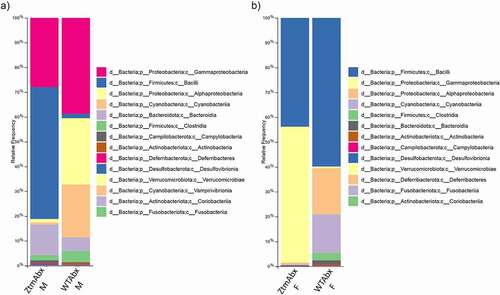
Abx-treated WT and Abx-treated Ztm males showed a drastic reduction of Bacteroidota versus a higher prevalence of Firmicutes (Bacilli) and Proteobacteria (Gammaproteobacteria) in Abx-treated Ztm and Proteobacteria (Gamma- and Alphaproteobacteria) and Cyanobacteria in Abx-treated WT ().
In females, we also observed a drastic reduction of Bacteroidota in both Abx-treated Ztm and Abx-treated WT concurrent with an expansion of Firmicutes (Bacilli). While Abx-treated Ztm females also showed a prevalence of Proteobacteria of the class Gammaproteobacteria, Abx-treated WT females had a prevalence of Proteobacteria of the class Alphaproteobacteria as well as Cyanobacteria (). Analysis of the gut microbiota at the genus level showed that almost 80% of the microbiota of Ztm mice treated with Abx was predominantly composed of Ureaplasma (Firmicutes, Bacilli; 42.5% in females and 52.1% in males) and Escherichia-Shigella (Proteobacteria, Gamma proteobacteria; 35.9% in females and 23.2% in males) with a drastic reduction of Bacteroidota (including the genus Rikenella) that was noticeably more pronounced in females (retained only 0.3%) than in males (retained only 12.3%). Ztm females also retained 18.7% of the genus Parasutterella while it was reduced to a 4.6% in males.
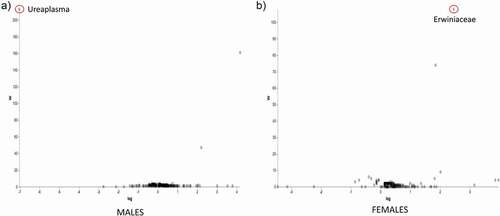
In Abx-treated WT males, Proteobacteria were represented by the genus Ralstonia and Mitochondria that contributed 64.4% of the total microbiota composition. Another 21.3% was made of Cyanobacteria of the genus Chloroplast. In Abx-treated WT females we observed a considerable expansion of Firmicutes of the genus Ureaplasma (58.1%), while 18.3% were Proteobacteria (genus Mitochondria) and 15.5% were Cyanobacteria (genus Chloroplast). Of note, Abx treatment severely depleted the genus Akkermansia from the Abx-treated WT microbiota. Volcano plot analysis did not show significant differences between Abx-treated Ztm and Abx-treated WT mice except for the Ureaplasma genus that was significantly more abundant in Abx-treated Ztm males and the Erwiniaceae family of the class Gammaproteobacteria, which was more abundant in Abx-treated WT females (, b).
Figure 8. ANCOM volcano plot of statistical differences between Abx-treated Ztm and Abx-treated WT mice at genus level. (a): Abx-treated Ztm (ZtmAbx) males harbor a significantly higher abundance of Ureaplasma sp. compared to Abx-treated WT (WTAbx) males. (b): Abx-treated WT (WTABX) females show significantly more abundant Erwiniaceae sp. when compared to Abx-treated Ztm (ZtmAbx) females.
Abx treatment restores small intestinal gut barrier integrity
We previously reported decreased gene expression of several TJs components in Ztm males compared to WT male mice.Citation110 In contrast, Abx-treated Ztm male mice did not show differences in TJs gene expression compared to Abx-treated WT male mice (). In Abx-treated females, we only detected a significantly decreased CLDN-4 in Ztm mice compared to WT females (Fig. B9, ). Noteworthy, in both Abx-treated Ztm males and females (from Experiment 2), we detected reduced levels of zonulin expression compared to Ztm males and females (from Experiment 1) ().
Table 3. List of genes analyzed by real time PCR in the brain of Abx-treated Ztm mice (n = 8 per group), normalized to Abx-treated WT mice. Expressed as Fold Change (FC), statistics calculated with the non- parametric Mann-Whitney test (p).
Figure 9. Effect of Abx gut microbiota depletion on the gene expression profile of TJs in the duodenum of Abx-treated mice. mRNA expression was analyzed in ZtmAbx treated mice by qPCR, normalized to WTAbx, and expressed as fold change ± SEM. (a): No differences were seen in the Abx-treated Ztm male mice when compared to Abx-treated WT males. (b): Abx-treated Ztm females show a significantly decreased CLDN-4 mRNA expression when compared with Abx-treated WT females.
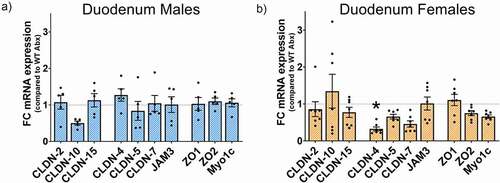
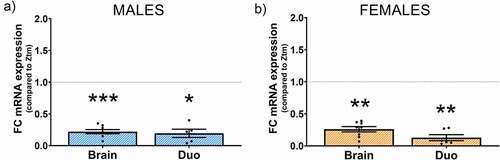
Figure 10. Effect of Abx gut microbiota depletion on Zonulin mRNA expression in the brain prefrontal cortex and the duodenum of Abx-treated Ztm mice. mRNA expression was analyzed by qPCR in Abx-treated Ztm mice, normalized to Ztm mice (non Abx-treated), and expressed as fold change ± SEM. Microbiota depletion significantly decreased mRNA zonulin expression in the brain and duodenum of (a): males and (b): female Abx-treated Ztm mice when compared to Ztm.
Abx-treatment affects gene expression profiles of BBB TJs and markers of neuroinflammation
We analyzed the gene expression profile of BBB TJs as well as neuroinflammatory markers in the prefrontal cortex, as we had done at baseline, to assess the role of gut bacteria in brain function in Abx-treated mice. In Abx-treated Ztm male mice we observed a significant higher expression of CLDN-1, −3 and −5, while ve-Cad, Cd11b and GABA expression were significantly decreased (, B, ), as compared to Abx-treated WT male mice. No significant changes were observed for IL-1β and OCCLN between Abx-treated Ztm and Abx-treated WT male mice (, B; ). The exact same changes in gene expression were observed in Abx-treated Ztm female mice compared to Abx-treated WT females but unlike males, OCCLN expression was significantly increased (,d ). Notably, we detected reduced expression levels of zonulin in the prefrontal cortex of Abx-treated Ztm (from Experiment 2) compared to not treated mice (from Experiment 1) ().
Figure 11. Effect of Abx gut microbiota depletion on the gene expression profile for BBB TJs and neuroinflammation markers on Abx-treated Ztm. mRNA expression was analyzed in Abx-treated Ztm (ZtmAbx) mice by qPCR, normalized to Abx-treated WT (WTAbx) mice, and expressed as fold change ± SEM. (a): Abx-treated Ztm males show an increased mRNA expression of CLND-1, CLDN-3, and CLDN-5 in the BBB, whereas veCad is significantly decreased when compared to Abx-treated WT males. (b): Altered neuroinflammatory profile in the Abx-treated Ztm male brain prefrontal cortex, showing a significant decrease in CD11b and GABA. (c): mRNA expression in Abx-treated Ztm females shows a generalized increase, except for veCad that is significantly decreased when compared to Abx-treated WT females. (d): CD11b and GABA are decreased in Abx-treated Ztm females when normalized against Abx-treated WT females.
Gut microbiota depletion affects behavior and ameliorates some anxiety-like traits in the Ztm mouse
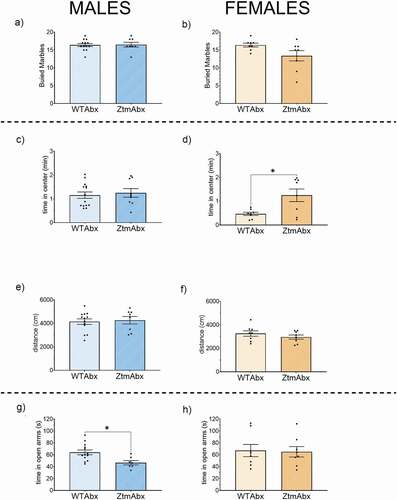
To assess whether the behavioral patterns we observed in the Ztm mouse were dependent on the composition of the gut microbiota, we administered antibiotics (Abx) to deplete intestinal bacteria within Ztm and WT mice and tested for repetitive and anxiety-like behavior. We did not observe any difference between WT and Ztm mice treated with Abx in the number of buried marbles (). Within the open field test, we did not observe significant differences between Abx-treated Ztm males and Abx-treated WT males in terms of time spent in the center of the field or total distance traveled (,e). Conversely, Abx-treated Ztm females showed a significant increase in time spent in the center of the open field compared to Abx-treated WT females (), with no difference in the distance traveled (). In the elevated zero maze, Abx-treated Ztm mice spent significantly less time in the open arms compared to Abx-treated WT males () while no significant differences were observed in either Abx-treated Ztm female mice compared to Abx-treated WT females ().
Figure 12. Effect of Abx gut microbiota depletion on the behavior profile in Abx-treated Ztm (ZtmAbx) mice compared to Abx-treated WT (WTAbx) mice. Marble Burying (a, b): No differences in the number of marbles buried were observed in Abx-treated males (a) and Abx treated females (b). Open field (C, D, E, F): No significative difference in the time spent in the center of the filed was observed in Abx-treated Ztm males compared to Abx-treated WT mice (c). Abx treated Ztm females spend significantly more time in the center of the field than Abx-treated WT females (d). Distance traveled was similar for both males (e) and females (f). Track images are representative of each group. Elevated Zero Maze (g, h): Abx-treated Ztm male mice spend significatively less time in the open arm than Abx-treated WT mice (g). No differences were detected in the female group (h). N = 8–13.
Discussion
We have previously reported that the zonulin-expressing mouse (Ztm) has an intrinsic increased gut permeability in the small intestine at baseline that influences the development of an immune system and a gut microbiota composition predisposed to inflammation.Citation109,Citation110
In the present study, we sought to understand whether a primary defect in small intestinal gut permeability caused by increased zonulin expression in the Ztm mouse synergizes with a dysbiotic, pro-inflammatory microbiota to affect brain function and behavior and whether these changes are sex dependent. We found that Ztm mice exhibited behavioral abnormalities and that these alterations were often different in males versus females. Compared to WT mice, female Ztm mice buried more marbles, suggesting an increase in obsessive/compulsive/repetitive behavior, while male Ztm mice exhibited lower or higher anxiety dependent on the test performed. In the open field, male Ztm mice showed lower anxiety by spending more time in the center of the field compared to WT males, yet they exhibited an increased level of anxiety when introduced to the elevated zero maze, suggesting a differential response associated with the anxiogenic level of the stimulus. However, the increased time spent by Ztm males in the center of the open field can be the result of the slightly increased hyperactivity showed by these mice, that might be interpreted as an overall increased anxiety-like behavior.
These sex-dependent behavioral anomalies observed in Ztm mice were accompanied by differential expression of critical BBB TJ associated and neuroinflammatory genes. Ztm females showed decreased expression of essential BBB claudins such as CLDN-5 and CLDN-3, pointing to defects in BBB integrity, yet they did not display increased levels of the inflammatory markers IL-1β and CD11b or changes in GABA within the prefrontal cortex. In contrast, Ztm males showed increased gene expression of some barrier forming BBB components (veCad and OCCLN), as well as neuroinflammation-associated markers (IL1b, CD11b and GABA) in the prefrontal cortex. Increased gene expression of some of the TJs might suggest a more competent BBB or be explained as compensatory mechanism for post-translational destruction/degradation of the protein leading to a sustained gene expression and protein accumulation. A role of zonulin has been suggested in the regulation of vascular endothelial cells and/or the BBB and in neuroinflammation,Citation101–103,Citation127–131 so zonulin expression might be responsible for the altered BBB TJs gene expression profile in Ztm mice. Increased CD11b, the β-integrin marker of microglia and elevated IL-1β in the prefrontal cortex of Ztm male mice suggest microglia activation. Interleukin-1β is a major microglial pro-inflammatory cytokine that acts on endothelial cells and studies have shown that by modulating TJ or by driving leukocyte recruitment IL-1β increases BBB permeability.Citation132–134 GABA is the major inhibitory neurotransmitter of the central nervous system and is involved in behavioral disorders, such as anxiety and depression.Citation135–138 Several studies indicate that increased production and/or synaptic availability of GABA can have anti-inflammatory effects.Citation139–146 The elevated GABA expression in the Ztm male mice at baseline might act as a compensatory mechanism to neuroinflammation.
We also found that the gut microbiota in both male and female Ztm mice harbor an abundance of pro-inflammatory species and is skewed toward a more maladaptive and pathogenic profile. Expansion of Rikenellaceae has been reported in obesity and diabetes and is associated with the pathological progression of inflammatory bowel disease.Citation147–150 Moreover, low levels of A. muciniphila are positively correlated with several human diseases and this species has been shown to be a critical contributor to epithelial barrier integrity and strength.Citation46,Citation151–156 It is conceivable that the intrinsic defect in gut permeability in the Ztm mice allows the passage of pathobionts (commensal organisms with pathological potential) or their products into the bloodstream and across a compromised BBB, thereby contributing to the onset of a neuroinflammatory status that leads to behavioral abnormalities. Furthermore, because microbiota dysbiosis triggers zonulin secretion,Citation39–41 the abundance of pathobionts in the gut of Ztm mice might further induce expression of zonulin in a vicious cycle.
To assess synergy between the intestinal microbiota and a zonulin-dependent increase in gut permeability in triggering behavioral alterations, we used antibiotics to drastically reduce intestinal bacteria within mice. We hypothesized that a reduction of microbial products within the intestinal lumen following antibiotic treatment could decrease their trafficking through a compromised intestinal barrier and eliminate and/or ameliorate the observed behavioral differences between WT and Ztm mice. In addition, the behavioral differences may likely be attributed to other pathways of communication between the gut microbiota and the brain and we cannot exclude cytokine regulation in the brain or other antibiotic treatment effects on neuroinflammation that are not related to gut barrier permeability.
When we assessed behavior in Abx-treated mice, we did not observe significant differences in the marble burying test between Abx-treated Ztm and Abx-treated WT females in contrast to baseline conditions where untreated Ztm females buried more marbles. These results suggest that Abx treatment of intestinal bacteria ameliorated obsessive/compulsive/repetitive behavior in Ztm females. Similarly, no differences in open field behavior were observed between Abx-treated Ztm and Abx-treated WT males in contrast to baseline behavior where Ztm males spent more time in the center of the open field than WT males, indicating that Abx depletion of gut microbiota affected the level of anxiety in Ztm males. On the other hand, in the elevated zero maze, in a higher anxiety setting, Abx-treated Ztm males still spent significantly less time in the open arms than Abx-treated WT males, consistent with their behavior at baseline, suggesting that Abx treatment did not restore their anxiety-like behavior to WT levels for this test. Interestingly, whereas there was no difference between Ztm and WT female mice in open field behavior at baseline, Abx depletion of the gut microbiota resulted in Abx-treated Ztm females spending significantly more time in the center of the open field than Abx-treated WT females.
While some of these differences might be attributable directly to the reduced bacterial translocation from the gut into the bloodstream of Ztm mice, other differences may be due to the absence/reduction of bacteria and/or their metabolites affecting specific pathways of communication between the gut and the brain. For instance, microorganisms can communicate with the brain via neurotransmitters and neuromodulators like serotonin, dopamine, and bacterial metabolites like short-chain fatty acids (SCFAs).Citation157–161 The gut microbiota can also induce the secretion of a variety of gut peptides, such as leptin and neuropeptide Y, by enteroendocrine cells located in the gut epithelium. Several of these peptides are known to affect anxiety levels and behavior via the host nervous system.Citation162–164
It has been reported that the absence of microbiota in the gut of germ-free mice reduces anxietyCitation165–167 while other studies have reported increased anxiety in germ-free mice,Citation168,Citation169 together suggesting that the absence of gut bacteria affects brain function and behavior. Our data suggest that while zonulin and increased trafficking through an impaired gut barrier might be involved in some of the behavioral traits we observed in the Ztm mouse, such as obsessive/compulsive/repetitive behavior in females (marble burying) and some of the anxiety traits in males (open field), other behaviors might be influenced by alternative pathways of communication between the gut and the brain.
It is interesting to note that in both male and female Abx-treated Ztm mice there was an increased expression of key TJ genes of the BBB and decreased expression of neuroinflammation-associated markers CD11b and GABA, as compared to Abx-treated WT, suggesting that the interaction of a dysbiotic gut microbiota with zonulin-dependent trafficking of bacterial products might influence BBB integrity and the inflammatory state of the brain. Of note, Abx microbiota depletion reduces the expression level of zonulin in the prefrontal cortex and the small intestine of Ztm mice, further stressing the effect of dysbiosis on gut and BBBs’ integrity.
Taken together, these data demonstrated that microbiota depletion in the gut of Ztm mice ameliorated some behavioral traits that characterize Ztm mice, likely via a reduction in BBB impairment and neuroinflammation, likely secondary to reduced zonulin expression.
Abx treatment drastically reduced Bacteroidota in both WT and Ztm mice against an expansion of Firmicutes that we observed in all Abx-treated groups except for AbxWT males that showed a growth of Alphaproteobacteria and Cyanobacteria, instead. While Ureaplasma spp. are commensals isolated from the human male and female urogenital tractsCitation170 and have been associated with urethritis, cervicitis or bacterial vaginosis, fetal chorioamnionitis and adverse pregnancy outcome,Citation171–174 not much is known about the Erwiniaceae family and we cannot associate either of these species with a pathogenic role in mice.
Furthermore, Abx treatment had a similar effect on the gene expression profile of TJs in the small intestine of males and females Abx-treated Ztm and Abx-treated WT mice. Differently from our published data showing reduced gene expression of several TJs in the small intestine of Ztm male mice, in Abx-treated Ztm mice we did not observe differences in the duodenum compared to Abx-treated WT animals, suggesting that Abx treatment depleted the Ztm gut of pathobionts responsible of defects in small intestinal gut permeability. This is further corroborated by the reduced expression of zonulin in the duodenum of Abx treated Ztm mice compared to Ztm animals.
The interaction of gut bacteria with the brain is bidirectional and involves multiple routes of communications. Gut bacteria can influence brain function via several pathways including the enteric nervous system (ENS), the vagus nerve, the immune system and cytokines release, and the metabolic products of gut bacteria (SCFAs).Citation157,Citation162,Citation164,Citation175–182 Our data strongly suggest that in the Ztm animal model, increased trafficking of bacteria through an impaired gut barrier affect BBB/brain function and behavior and that by decreasing the antigen trafficking in the small intestine (or by shifting the microbiota composition), the differences between the WT and Ztm phenotypes are reduced.
It is clear from this study that the gut-microbiota-brain crosstalk happens via several pathways, and that the microbiota dysbiosis-zonulin pathway might contribute to neuroinflammatory disorders. Overall, we have shown that in the Ztm mouse, a zonulin dependent increased gut permeability synergizes with a pro-inflammatory dysbiotic gut microbiota contributing to brain function and behavioral alterations. More studies are needed to dissect the specific role of zonulin and of distinct microbial species/products in the gut-brain crosstalk in Ztm mice.
Ethics Statement
This study was carried out in accordance with the recommendations and guidelines of the Institutional Animal Care and Use Committee at the MGH. The protocol was approved by the Institutional Animal Care and Use Committee at the MGH (2013N000013).
Author Contributions
MRF and AM-R conceived and designed the experiments. AM-R, GS, and JL carried out the experiments. MRF, AM-R, MAK, and AF contributed to the interpretation of the results. AM-R, MRF, MAK, and AF wrote the manuscript. All authors provided critical feedback and helped shape the research, analysis, and manuscript.
Disclosure statement
AF is a stockholder at Alba Therapeutics, serves as a consultant for Inova Diagnostics and Innovate Biopharmaceuticals, is an advisory board member for Axial Biotherapeutics and Ubiome, and has a speaker agreement with Mead Johnson Nutrition. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Additional information
Funding
References
- Arrieta MC, Bistritz L, Meddings JB. Alterations in intestinal permeability. Gut. 2006;55(10):1–25. doi:10.1136/gut.2005.085373.
- Fasano A, Shea-Donohue T. Mechanisms of disease: the role of intestinal barrier function in the pathogenesis of gastrointestinal autoimmune diseases. Nat Clin Pract Gastroenterol Hepatol. 2005;2(9):416–422. doi:10.1038/ncpgasthep0259.
- Wapenaar MC, Monsuur AJ, Van Bodegraven AA, Weersma RK, Bevova MR, Linskens RK, Howdle P, Holmes G, Mulder CJ, Dijkstra G, et al. Associations with tight junction genes PARD3 and MAGI2 in Dutch patients point to a common barrier defect for coeliac disease and ulcerative colitis. Gut. 2008;57(4):463–467. doi:10.1136/gut.2007.133132.
- Mowat AM, Millington OR, Chirdo FG. Anatomical and cellular basis of immunity and tolerance in the intestine. J Pediatr Gastroenterol Nutr. 2004;39(Suppl 3):S723–4. doi:10.1097/00005176-200406003-00003.
- Rescigno M. Intestinal microbiota and its effects on the immune system. Cell Microbiol. 2014;16(7):1004–1013. doi:10.1111/cmi.12301.
- Rescigno M, Lopatin U, Chieppa M. Interactions among dendritic cells, macrophages, and epithelial cells in the gut: implications for immune tolerance. Curr Opin Immunol. 2008;20(6):669–675. doi:10.1016/j.coi.2008.09.007.
- Brandtzaeg P. Homeostatic impact of indigenous microbiota and secretory immunity. Benef Microbes. 2010;1(3):211–227. doi:10.3920/BM2010.0009.
- Turner JR. Intestinal mucosal barrier function in health and disease. Nat Rev Immunol. 2009;9(11):799–809. doi:10.1038/nri2653.
- Fang X. Potential role of gut microbiota and tissue barriers in Parkinson’s disease and amyotrophic lateral sclerosis. Int J Neurosci. 2016;126(9):771–776. doi:10.3109/00207454.2015.1096271.
- Fasano A, Visanji NP, Liu LWC, Lang AE, Pfeiffer RF. Gastrointestinal dysfunction in Parkinson’s disease. Lancet Neurol. 2015;14(6):625–639. doi:10.1016/S1474-4422(15)00007-1.
- Rowin J, Xia Y, Jung B, Sun J. Gut inflammation and dysbiosis in human motor neuron disease. Physiol Rep. 2017;5(18):e13443. doi:10.14814/phy2.13443.
- Toepfer M, Folwaczny, C, Klauser, A, Riepl, RL, Müller-Felber, W, and Pongratz, D, Gastrointestinal dysfunction in amyotrophic lateral sclerosis. Amyotroph Lateral Scler Other Motor Neuron Disord. 1999;1(1):15–19. doi:10.1080/146608299300079484.
- Zhang R, Miller RG, Gascon R, Champion S, Katz J, Lancero M, Narvaez A, Honrada R, Ruvalcaba D, McGrath MS, et al. Circulating endotoxin and systemic immune activation in sporadic amyotrophic lateral sclerosis (sALS). J Neuroimmunol. 2009;206(1–2):121–124. doi:10.1016/j.jneuroim.2008.09.017.
- Hill JM, Bhattacharjee S, Pogue AI, Lukiw WJ. The Gastrointestinal Tract Microbiome and Potential Link to Alzheimer’s Alzheimer’s Disease. Front Neurol. 2014;5:43. doi:10.3389/fneur.2014.00043.
- Kirby TO, and Ochoa-Reparaz J. The Gut Microbiome in Multiple Sclerosis: a Potential Therapeutic Avenue. Med Sci (Basel). 2018;6(3 69 doi:10.3390/medsci6030069).
- Buscarinu MC, et al. The Contribution of Gut Barrier Changes to Multiple Sclerosis Pathophysiology. Front Immunol. 2019;10:1916. doi:10.3389/fimmu.2019.01916.
- Rose DR, Yang H, Serena G, Sturgeon C, Ma B, Careaga M, Hughes HK, Angkustsiri K, Rose M, Hertz-Picciotto I, et al. Differential immune responses and microbiota profiles in children with autism spectrum disorders and co-morbid gastrointestinal symptoms. Brain Behav Immun. 2018;70:354–368. doi:10.1016/j.bbi.2018.03.025.
- Morais LH, Schreiber HL, Mazmanian SK. The gut microbiota-brain axis in behaviour and brain disorders. Nat Rev Microbiol. 2020.
- Motil KJ, Caeg E, Barrish JO, Geerts S, Lane JB, Percy AK, Annese F, McNair L, Skinner SA, Lee H-S, et al. Gastrointestinal and nutritional problems occur frequently throughout life in girls and women with Rett syndrome. J Pediatr Gastroenterol Nutr. 2012;55(3):292–298. doi:10.1097/MPG.0b013e31824b6159.
- Strati F, Cavalieri D, Albanese D, De Felice C, Donati C, Hayek J, Jousson O, Leoncini S, Renzi D, Calabrò A, et al. New evidences on the altered gut microbiota in autism spectrum disorders. Microbiome. 2017;5(1):24. doi:10.1186/s40168-017-0242-1.
- Kang V, Wagner GC, Ming X. Gastrointestinal dysfunction in children with autism spectrum disorders. Autism Res. 2014;7(4):501–506. doi:10.1002/aur.1386.
- Duel BP, et al. A survey of voiding dysfunction in children with attention deficit-hyperactivity disorder. J Urol. 2003;170(4 Pt 2):1521–1523. discussion 1523-4. doi:10.1097/01.ju.0000091219.46560.7b.
- Jameson ND, et al. Medical Comorbidity of Attention-Deficit/Hyperactivity Disorder in US Adolescents. J Child Neurol. 2016;31(11):522–529. doi:10.1177/0883073816653782.
- Ming X, et al. A Gut Feeling: a Hypothesis of the Role of the Microbiome in Attention-Deficit/Hyperactivity Disorders. Child Neurol Open. 2018;5:2329048X18786799. doi:10.1177/2329048X18786799.
- Severance EG, Dickerson F, Yolken RH. Complex Gastrointestinal and Endocrine Sources of Inflammation in Schizophrenia. Front Psychiatry. 2020;11:549. doi:10.3389/fpsyt.2020.00549.
- Severance EG, Prandovszky E, Castiglione J, Yolken RH. Gastroenterology issues in schizophrenia: why the gut matters. Curr Psychiatry Rep. 2015;17(5):27. doi:10.1007/s11920-015-0574-0.
- Molloy CA, Manning-Courtney P. Prevalence of chronic gastrointestinal symptoms in children with autism and autistic spectrum disorders. Autism. 2003;7(2):165–171. doi:10.1177/1362361303007002004.
- Fasano A, Not T, Wang W, Uzzau S, Berti I, Tommasini A, Goldblum SE. Zonulin, a newly discovered modulator of intestinal permeability, and its expression in coeliac disease. Lancet. 2000;355(9214):1518–1519. doi:10.1016/S0140-6736(00)02169-3.
- Fasano A. Regulation of intercellular tight junctions by zonula occludens toxin and its eukaryotic analogue zonulin. Ann N Y Acad Sci. 2000;915(1):214–222. doi:10.1111/j.1749-6632.2000.tb05244.x.
- Wang W, Uzzau S, Goldblum SE, Fasano A. Human zonulin, a potential modulator of intestinal tight junctions. J Cell Sci. 2000;113(Pt 24):4435–4440. doi:10.1242/jcs.113.24.4435.
- Tripathi A, Lammers KM, Goldblum S, Shea-Donohue T, Netzel-Arnett S, Buzza MS, Antalis TM, Vogel SN, Zhao A, Yang S, et al. Identification of human zonulin, a physiological modulator of tight junctions, as prehaptoglobin-2. Proc Natl Acad Sci U S A. 2009;106(39):16799–16804. doi:10.1073/pnas.0906773106.
- Sapone A, De Magistris L, Pietzak M, Clemente MG, Tripathi A, Cucca F, Lampis R, Kryszak D, Carteni M, Generoso M, et al. Zonulin upregulation is associated with increased gut permeability in subjects with type 1 diabetes and their relatives. Diabetes. 2006;55(5):1443–1449. doi:10.2337/db05-1593.
- Esnafoglu E, Cırrık S, Ayyıldız SN, Erdil A, Ertürk EY, Daglı A, Noyan T. Increased Serum Zonulin Levels as an Intestinal Permeability Marker in Autistic Subjects. J Pediatr. 2017;188:240–244. doi:10.1016/j.jpeds.2017.04.004.
- Ozyurt G, Öztürk Y, Appak YÇ, Arslan FD, Baran M, Karakoyun İ, Tufan AE, Pekcanlar AA. Increased zonulin is associated with hyperactivity and social dysfunctions in children with attention deficit hyperactivity disorder. Compr Psychiatry. 2018;87:138–142. doi:10.1016/j.comppsych.2018.10.006.
- D’Eufemia P, Celli M, Finocchiaro R, Pacifico L, Viozzi L, Zaccagnini M, Cardi E, Giardini O. Abnormal intestinal permeability in children with autism. Acta Paediatr. 1996;85(9):1076–1079. doi:10.1111/j.1651-2227.1996.tb14220.x.
- De Magistris L, Familiari V, Pascotto A, Sapone A, Frolli A, Iardino P, Carteni M, De Rosa M, Francavilla R, Riegler G, et al. Alterations of the intestinal barrier in patients with autism spectrum disorders and in their first-degree relatives. J Pediatr Gastroenterol Nutr. 2010;51(4):418–424. doi:10.1097/MPG.0b013e3181dcc4a5.
- Lau NM, Green PHR, Taylor AK, Hellberg D, Ajamian M, Tan CZ, Kosofsky BE, Higgins JJ, Rajadhyaksha AM, Alaedini A, et al. Markers of Celiac Disease and Gluten Sensitivity in Children with Autism. PLoS One. 2013;8(6):e66155. doi:10.1371/journal.pone.0066155.
- Maes M, Sirivichayakul S, Kanchanatawan B, Vodjani A. Upregulation of the Intestinal Paracellular Pathway with Breakdown of Tight and Adherens Junctions in Deficit Schizophrenia. Mol Neurobiol. 2019;56(10):7056–7073. doi:10.1007/s12035-019-1578-2.
- El Asmar R, Panigrahi P, Bamford P, Berti I, Not T, Coppa GV, Catassi C, Fasano A. Host-dependent zonulin secretion causes the impairment of the small intestine barrier function after bacterial exposure. Gastroenterology. 2002;123(5):1607–1615. doi:10.1053/gast.2002.36578.
- Alaish SM, Smith AD, Timmons J, Greenspon J, Eyvazzadeh D, Murphy E, Shea-Donahue T, Cirimotich S, Mongodin E, Zhao A, et al. Gut microbiota, tight junction protein expression, intestinal resistance, bacterial translocation and mortality following cholestasis depend on the genetic background of the host. Gut Microbes. 2013;4(4):292–305. doi:10.4161/gmic.24706.
- Drago S, et al. Gliadin, zonulin and gut permeability: effects on celiac and non-celiac intestinal mucosa and intestinal cell lines. Scand J Gastroenterol. 2006;41(4):408–419. doi:10.1080/00365520500235334.
- Parracho HM, et al. Differences between the gut microflora of children with autistic spectrum disorders and that of healthy children. J Med Microbiol. 2005;54(Pt 10):987–991.
- Finegold SM. Desulfovibrio species are potentially important in regressive autism. Med Hypotheses. 2011;77(2): 16799–4. doi:10.1016/j.mehy.2011.04.032.
- Finegold SM, Dowd SE, Gontcharova V, Liu C, Henley KE, Wolcott RD, Youn E, Summanen PH, Granpeesheh D, Dixon D, et al. Pyrosequencing study of fecal microflora of autistic and control children. Anaerobe. 2010;16(4):444–453. doi:10.1016/j.anaerobe.2010.06.008.
- Adams JB, et al. Gastrointestinal flora and gastrointestinal status in children with autism–comparisons to typical children and correlation with autism severity. BMC Gastroenterol. 2011;11(1):22. doi:10.1186/1471-230X-11-22.
- Wang L, et al. Low relative abundances of the mucolytic bacterium Akkermansia muciniphila and Bifidobacterium spp. in feces of children with autism. Appl Environ Microbiol. 2011;77(18):6718–6721. doi:10.1128/AEM.05212-11.
- Williams BL, Hornig M, Buie T, Bauman ML, Cho Paik M, Wick I, Bennett A, Jabado O, Hirschberg DL, Lipkin WI, et al. Impaired carbohydrate digestion and transport and mucosal dysbiosis in the intestines of children with autism and gastrointestinal disturbances. PLoS One. 2011;6(9):e24585. doi:10.1371/journal.pone.0024585.
- Zou R, et al. Dysbiosis of Gut Fungal Microbiota in Children with Autism Spectrum Disorders. J Autism Dev Disord. 2020.
- Zou R, Xu F, Wang Y, Duan M, Guo M, Zhang Q, Zhao H, Zheng H. Changes in the Gut Microbiota of Children with Autism Spectrum Disorder. Autism Res. 2020;13(9):1614–1625. doi:10.1002/aur.2358.
- Jakobsson HE, Rodríguez‐Piñeiro AM, Schütte A, Ermund A, Boysen P, Bemark M, Sommer F, Bäckhed F, Hansson GC, Johansson ME, et al. The composition of the gut microbiota shapes the colon mucus barrier. EMBO Rep. 2015;16(2):164–177. doi:10.15252/embr.201439263.
- Alvarez CS, Badia J, Bosch M, Giménez R, Baldomà L. Outer Membrane Vesicles and Soluble Factors Released by Probiotic Escherichia coli Nissle 1917 and Commensal ECOR63 Enhance Barrier Function by Regulating Expression of Tight Junction Proteins in Intestinal Epithelial Cells. Front Microbiol. 2016;7:1981. doi:10.3389/fmicb.2016.01981.
- Bischoff SC, et al. Intestinal permeability–a new target for disease prevention and therapy. BMC Gastroenterol. 2014;14:189.
- Everard A, Belzer C, Geurts L, Ouwerkerk JP, Druart C, Bindels LB, Guiot Y, Derrien M, Muccioli GG, Delzenne NM, et al. Cross-talk between Akkermansia muciniphila and intestinal epithelium controls diet-induced obesity. Proc Natl Acad Sci U S A. 2013;110(22):9066–9071. doi:10.1073/pnas.1219451110.
- Deng H, Yang S, Zhang Y, Qian K, Zhang Z, Liu Y, Wang Y, Bai Y, Fan H, Zhao X, et al. Bacteroides fragilis Prevents Clostridium difficile Infection in a Mouse Model by Restoring Gut Barrier and Microbiome Regulation. Front Microbiol. 2018;9:2976. doi:10.3389/fmicb.2018.02976.
- Chiaro TR, Soto R, Zac Stephens W, Kubinak JL, Petersen C, Gogokhia L, Bell R, Delgado JC, Cox J, Voth W, et al. A member of the gut mycobiota modulates host purine metabolism exacerbating colitis in mice. Sci Transl Med. 2017;9(380). doi:10.1126/scitranslmed.aaf9044.
- Martin R, Laval L, Chain F, Miquel S, Natividad J, Cherbuy C, Sokol H, Verdu EF, Van Hylckama Vlieg J, Bermudez-Humaran LG, et al. Bifidobacterium animalis ssp. lactis CNCM-I2494 Restores Gut Barrier Permeability in Chronically Low-Grade Inflamed Mice. Front Microbiol. 2016;7:608. doi:10.3389/fmicb.2016.00608.
- Karczewski J, Troost FJ, Konings I, Dekker J, Kleerebezem M, Brummer RJM, Wells JM. Regulation of human epithelial tight junction proteins by Lactobacillus plantarum in vivo and protective effects on the epithelial barrier. Am J Physiol Gastrointest Liver Physiol. 2010;298(6):G851–9. doi:10.1152/ajpgi.00327.2009.
- Alhasson F, et al. Altered gut microbiome in a mouse model of Gulf War Illness causes neuroinflammation and intestinal injury via leaky gut and TLR4 activation. PLoS One. 2017;12(3):e0172914. doi:10.1371/journal.pone.0172914.
- Wang S, Li Q, Zang Y, Zhao Y, Liu N, Wang Y, Xu X, Liu L, Mei Q. Apple Polysaccharide inhibits microbial dysbiosis and chronic inflammation and modulates gut permeability in HFD-fed rats. Int J Biol Macromol. 2017;99:282–292. doi:10.1016/j.ijbiomac.2017.02.074.
- Thevaranjan N, Puchta A, Schulz C, Naidoo A, Szamosi JC, Verschoor CP, Loukov D, Schenck LP, Jury J, Foley KP, et al. Age-Associated Microbial Dysbiosis Promotes Intestinal Permeability, Systemic Inflammation, and Macrophage Dysfunction. Cell Host Microbe. 2018;23(4):570. doi:10.1016/j.chom.2018.03.006.
- Martinez-Oca P, et al. Gut DYSBIOSIS and altered barrier function precedes the appearance of metabolic syndrome in a rat model of nutrient-induced catch-up growth. J Nutr Biochem. 2020;81:108383. doi:10.1016/j.jnutbio.2020.108383.
- Brandl K, Schnabl B. Is intestinal inflammation linking dysbiosis to gut barrier dysfunction during liver disease? Expert Rev Gastroenterol Hepatol. 2015;9(8):1069–1076. doi:10.1586/17474124.2015.1057122.
- Gasmi A, et al. Relationship between gut microbiota, gut hyperpermeability, and obesity. Curr Med Chem. 2020.
- Marizzoni M, Provasi S, Cattaneo A, Frisoni GB. Microbiota and neurodegenerative diseases. Curr Opin Neurol. 2017;30(6):630–638. doi:10.1097/WCO.0000000000000496.
- Sochocka M, Donskow-Łysoniewska K, Diniz BS, Kurpas D, Brzozowska E, Leszek J. The Gut Microbiome Alterations and Inflammation-Driven Pathogenesis of Alzheimer’s Disease-a Critical Review. Mol Neurobiol. 2019;56(3):1841–1851. doi:10.1007/s12035-018-1188-4.
- Potgieter M, Bester J, Kell DB, Pretorius E. The dormant blood microbiome in chronic, inflammatory diseases. FEMS Microbiol Rev. 2015;39(4):567–591. doi:10.1093/femsre/fuv013.
- Konig J, Wells J, Cani PD, García-Ródenas CL, MacDonald T, Mercenier A, Whyte J, Troost F, Brummer R-J. Human Intestinal Barrier Function in Health and Disease. Clin Transl Gastroenterol. 2016;7(10):e196. doi:10.1038/ctg.2016.54.
- Wardill HR, Mander KA, Van Sebille YZA, Gibson RJ, Logan RM, Bowen JM, Sonis ST. Cytokine-mediated blood brain barrier disruption as a conduit for cancer/chemotherapy-associated neurotoxicity and cognitive dysfunction. Int J Cancer. 2016;139(12):2635–2645. doi:10.1002/ijc.30252.
- Elahy M, Jackaman C, Mamo JC, Lam V, Dhaliwal SS, Giles C, Nelson D, Takechi R. Blood-brain barrier dysfunction developed during normal aging is associated with inflammation and loss of tight junctions but not with leukocyte recruitment. Immun Ageing. 2015;12(1):2. doi:10.1186/s12979-015-0029-9.
- Wang J, Song Y, Chen Z, Leng SX. Connection between Systemic Inflammation and Neuroinflammation Underlies Neuroprotective Mechanism of Several Phytochemicals in Neurodegenerative Diseases. Oxid Med Cell Longev. 2018;2018:1972714. doi:10.1155/2018/1972714.
- Wu S, Yi J, Zhang Y-G, Zhou J, Sun J. Leaky intestine and impaired microbiome in an amyotrophic lateral sclerosis mouse model. Physiol Rep. 2015;3(4):e12356. doi:10.14814/phy2.12356.
- Buscarinu MC, et al. Altered intestinal permeability in patients with relapsing-remitting multiple sclerosis: a pilot study. Mult Scler. 2017;23(3):442–446.
- Buscarinu MC, Romano S, Mechelli R, Pizzolato Umeton R, Ferraldeschi M, Fornasiero A, Reniè R, Cerasoli B, Morena E, Romano C, et al. Intestinal Permeability in Relapsing-Remitting Multiple Sclerosis. Neurotherapeutics. 2018;15(1):68–74. doi:10.1007/s13311-017-0582-3.
- Moor AC, De Vries HE, De Boer AG, Breimer DD. The blood-brain barrier and multiple sclerosis. Biochem Pharmacol. 1994;47(10):1717–1724. doi:10.1016/0006-2952(94)90297-6.
- Soon D, Tozer DJ, Altmann DR, Tofts PS, Miller DH. Quantification of subtle blood-brain barrier disruption in non-enhancing lesions in multiple sclerosis: a study of disease and lesion subtypes. Mult Scler. 2007;13(7):884–894. doi:10.1177/1352458507076970.
- Minagar A, Alexander JS. Blood-brain barrier disruption in multiple sclerosis. Mult Scler. 2003;9:540–549.
- Gray MT, Woulfe JM. Striatal blood-brain barrier permeability in Parkinson’s disease. J Cereb Blood Flow Metab. 2015;35:747–750.
- Wada H. Blood-brain barrier permeability of the demented elderly as studied by cerebrospinal fluid-serum albumin ratio. Intern Med. 1998;37(6):509–513. doi:10.2169/internalmedicine.37.509.
- Bell RD, Zlokovic BV. Neurovascular mechanisms and blood–brain barrier disorder in Alzheimer’s disease. Acta Neuropathol. 2009;118(1):103–113. doi:10.1007/s00401-009-0522-3.
- Weissberg I, et al. Blood-brain barrier dysfunction in epileptogenesis of the temporal lobe. Epilepsy Res Treat. 2011;2011:143908.
- Heinemann U, Kaufer D, Friedman A. Blood-brain barrier dysfunction, TGFbeta signaling, and astrocyte dysfunction in epilepsy. Glia. 2012;60(8):1251–1257. doi:10.1002/glia.22311.
- Cassani E, Barichella M, Cancello R, Cavanna F, Iorio L, Cereda E, Bolliri C, Zampella Maria P, Bianchi F, Cestaro B, et al. Increased urinary indoxyl sulfate (indican): new insights into gut dysbiosis in Parkinson’s disease. Parkinsonism Relat Disord. 2015;21(4):389–393. doi:10.1016/j.parkreldis.2015.02.004.
- Keshavarzian A, Green SJ, Engen PA, Voigt RM, Naqib A, Forsyth CB, Mutlu E, Shannon KM. Colonic bacterial composition in Parkinson’s disease. Mov Disord. 2015;30(10):1351–1360. doi:10.1002/mds.26307.
- Scheperjans F, Aho V, Pereira PAB, Koskinen K, Paulin L, Pekkonen E, Haapaniemi E, Kaakkola S, Eerola‐Rautio J, Pohja M, et al. Gut microbiota are related to Parkinson’s disease and clinical phenotype. Mov Disord. 2015;30(3):350–358. doi:10.1002/mds.26069.
- Unger MM, et al. Short chain fatty acids and gut microbiota differ between patients with Parkinson’s disease and age-matched controls. Parkinsonism Relat Disord. 2016;32:66–72. doi:10.1016/j.parkreldis.2016.08.019.
- Zhuang Z-Q, et al. Gut Microbiota is Altered in Patients with Alzheimer’s Disease. J Alzheimers Dis. 2018;63(4):1337–1346. doi:10.3233/JAD-180176.
- Miyake S, et al. Dysbiosis in the Gut Microbiota of Patients with Multiple Sclerosis, with a Striking Depletion of Species Belonging to Clostridia XIVa and IV Clusters. PLoS One. 2015;10(9):e0137429. doi:10.1371/journal.pone.0137429.
- Chen J, Chia N, Kalari KR, Yao JZ, Novotna M, Paz Soldan MM, Luckey DH, Marietta EV, Jeraldo PR, Chen X, et al. Multiple sclerosis patients have a distinct gut microbiota compared to healthy controls. Sci Rep. 2016;6(1):28484. doi:10.1038/srep28484.
- Jangi S, Gandhi R, Cox LM, Li N, Von Glehn F, Yan R, Patel B, Mazzola MA, Liu S, Glanz BL, et al. Alterations of the human gut microbiome in multiple sclerosis. Nat Commun. 2016;7(1):12015. doi:10.1038/ncomms12015.
- Vogt NM, Kerby RL, Dill-mcfarland KA, Harding SJ, Merluzzi AP, Johnson SC, Carlsson CM, Asthana S, Zetterberg H, Blennow K, et al. Gut microbiome alterations in Alzheimer’s disease. Sci Rep. 2017;7(1):13537. doi:10.1038/s41598-017-13601-y.
- Cattaneo A, et al. Association of brain amyloidosis with pro-inflammatory gut bacterial taxa and peripheral inflammation markers in cognitively impaired elderly. Neurobiol Aging. 2017;49:60–68. doi:10.1016/j.neurobiolaging.2016.08.019.
- Kang DW, Park JG, Ilhan ZE, Wallstrom G, LaBaer J, Adams JB, Krajmalnik-Brown R. Reduced incidence of Prevotella and other fermenters in intestinal microflora of autistic children. PLoS One. 2013;8(7):e68322. doi:10.1371/journal.pone.0068322.
- Wang L, Christophersen CT, Sorich MJ, Gerber JP, Angley MT, Conlon MA. Increased abundance of Sutterella spp. and Ruminococcus torques in feces of children with autism spectrum disorder. Mol Autism. 2013;4(1):42. doi:10.1186/2040-2392-4-42.
- Tomova A, Husarova V, Lakatosova S, Bakos J, Vlkova B, Babinska K, Ostatnikova D. Gastrointestinal microbiota in children with autism in Slovakia. Physiol Behav. 2015;138:179–187. doi:10.1016/j.physbeh.2014.10.033.
- Luna RA, Oezguen N, Balderas M, Venkatachalam A, Runge JK, Versalovic J, Veenstra-vanderweele J, Anderson GM, Savidge T, Williams KC, et al. Distinct Microbiome-Neuroimmune Signatures Correlate With Functional Abdominal Pain in Children With Autism Spectrum Disorder. Cell Mol Gastroenterol Hepatol. 2017;3(2):218–230. doi:10.1016/j.jcmgh.2016.11.008.
- Kang DW, Ilhan ZE, Isern NG, Hoyt DW, Howsmon DP, Shaffer M, Lozupone CA, Hahn J, Adams JB, Krajmalnik-Brown R, et al. Differences in fecal microbial metabolites and microbiota of children with autism spectrum disorders. Anaerobe. 2018;49:121–131. doi:10.1016/j.anaerobe.2017.12.007.
- Lv F, Chen S, Wang L, Jiang R, Tian H, Li J, Yao Y, Zhuo C. The role of microbiota in the pathogenesis of schizophrenia and major depressive disorder and the possibility of targeting microbiota as a treatment option. Oncotarget. 2017;8(59):100899–100907. doi:10.18632/oncotarget.21284.
- Severance EG, Gressitt KL, Stallings CR, Origoni AE, Khushalani S, Leweke FM, Dickerson FB, Yolken RH. Discordant patterns of bacterial translocation markers and implications for innate immune imbalances in schizophrenia. Schizophr Res. 2013;148(1–3):130–137. doi:10.1016/j.schres.2013.05.018.
- Melkersson K, Bensing S. Signs of impaired blood-brain barrier function and lower IgG synthesis within the central nervous system in patients with schizophrenia or related psychosis, compared to that in controls. Neuro Endocrinol Lett. 2018;39:33–42.
- Fiorentino M, Sapone A, Senger S, Camhi SS, Kadzielski SM, Buie TM, Kelly DL, Cascella N, Fasano A. Blood-brain barrier and intestinal epithelial barrier alterations in autism spectrum disorders. Mol Autism. 2016;7(1):49. doi:10.1186/s13229-016-0110-z.
- Rahman MT, Ghosh C, Hossain M, Linfield D, Rezaee F, Janigro D, Marchi N, Van Boxel-dezaire AHH. IFN-gamma, IL-17A, or zonulin rapidly increase the permeability of the blood-brain and small intestinal epithelial barriers: relevance for neuro-inflammatory diseases. Biochem Biophys Res Commun. 2018;507(1–4):274–279. doi:10.1016/j.bbrc.2018.11.021.
- Diaz-Coranguez M, Segovia J, López-Ornelas A, Puerta-Guardo H, Ludert J, Chávez B, Meraz-Cruz N, González-Mariscal L. Transmigration of neural stem cells across the blood brain barrier induced by glioma cells. PLoS One. 2013;8(4):e60655. doi:10.1371/journal.pone.0060655.
- Skardelly M, Armbruster FP, Meixensberger J, Hilbig H. Expression of Zonulin, c-kit, and Glial Fibrillary Acidic Protein in Human Gliomas. Transl Oncol. 2009;2(3):117–120. doi:10.1593/tlo.09115.
- Singer HS, Morris CM, Williams PN, Yoon DY, Hong JJ, Zimmerman AW. Antibrain antibodies in children with autism and their unaffected siblings. J Neuroimmunol. 2006;178(1–2):149–155. doi:10.1016/j.jneuroim.2006.05.025.
- Zimmerman AW, Connors SL, Matteson KJ, Lee L-C, Singer HS, Castaneda JA, Pearce DA. Maternal antibrain antibodies in autism. Brain Behav Immun. 2007;21(3):351–357. doi:10.1016/j.bbi.2006.08.005.
- Braunschweig D, Van De Water J. Maternal autoantibodies in autism. Arch Neurol. 2012;69(6):693–699. doi:10.1001/archneurol.2011.2506.
- Eshraghi RS, Davies C, Iyengar R, Perez L, Mittal R, Eshraghi AA. Gut-Induced Inflammation during Development May Compromise the Blood-Brain Barrier and Predispose to Autism Spectrum Disorder. J Clin Med. 2020;10(1):27. doi:10.3390/jcm10010027.
- Levy AP, Levy JE, Kalet-Litman S, Miller-Lotan R, Levy NS, Asaf R, Guetta J, Yang C, Purushothaman KR, Fuster V, et al. Haptoglobin genotype is a determinant of iron, lipid peroxidation, and macrophage accumulation in the atherosclerotic plaque. Arterioscler Thromb Vasc Biol. 2007;27(1):134–140. doi:10.1161/01.ATV.0000251020.24399.a2.
- Sturgeon C, Lan J, Fasano A. Zonulin transgenic mice show altered gut permeability and increased morbidity/mortality in the DSS colitis model. Ann N Y Acad Sci. 2017;1397(1):130–142. doi:10.1111/nyas.13343.
- Miranda-Ribera A, Ennamorati M, Serena G, Cetinbas M, Lan J, Sadreyev RI, Jain N, Fasano A, Fiorentino M. Exploiting the Zonulin Mouse Model to Establish the Role of Primary Impaired Gut Barrier Function on Microbiota Composition and Immune Profiles. Front Immunol. 2019;10:2233. doi:10.3389/fimmu.2019.02233.
- Rakoff-Nahoum S, Paglino J, Eslami-Varzaneh F, Edberg S, Medzhitov R. Recognition of commensal microflora by toll-like receptors is required for intestinal homeostasis. Cell. 2004;118(2):229–241. doi:10.1016/j.cell.2004.07.002.
- Thomas A, Burant A, Bui N, Graham D, Yuva-Paylor LA, Paylor R. Marble burying reflects a repetitive and perseverative behavior more than novelty-induced anxiety. Psychopharmacol (Berl). 2009;204(2):361–373. doi:10.1007/s00213-009-1466-y.
- Chang YC, Cole TB, Costa LG. Behavioral Phenotyping for Autism Spectrum Disorders in Mice. Curr Protoc Toxicol. 2017;72(1):11 22 1–11 22 21. doi:10.1002/cptx.19.
- Shinomiya K, Fujii Y, Sugimoto Y, Azuma N, Tokunaga S, Kitazumi K, Kamei C. Effect of paroxetine on marble-burying behavior in mice. Methods Find Exp Clin Pharmacol. 2005;27(10):685–687. doi:10.1358/mf.2005.27.10.948883.
- Prut L, Belzung C. The open field as a paradigm to measure the effects of drugs on anxiety-like behaviors: a review. Eur J Pharmacol. 2003;463(1–3):3–33. doi:10.1016/S0014-2999(03)01272-X.
- Kraeuter AK, Guest PC, Sarnyai Z. The Open Field Test for Measuring Locomotor Activity and Anxiety-Like Behavior. Methods Mol Biol. 2019;1916:99–103.
- Gentsch C, Lichtsteiner M, Feer H. Open field and elevated plus-maze: a behavioural comparison between spontaneously hypertensive (SHR) and Wistar-Kyoto (WKY) rats and the effects of chlordiazepoxide. Behav Brain Res. 1987;25(2):101–107. doi:10.1016/0166-4328(87)90003-9.
- Shepherd JK, Grewal SS, Fletcher A, Bill DJ, Dourish CT. Behavioural and pharmacological characterisation of the elevated “zero-maze” as an animal model of anxiety. Psychopharmacol (Berl). 1994;116(1):56–64. doi:10.1007/BF02244871.
- Brown RL, Sequeira RP, Clarke TB. The microbiota protects against respiratory infection via GM-CSF signaling. Nat Commun. 2017;8(1):1512. doi:10.1038/s41467-017-01803-x.
- Baldridge MT, Nice TJ, McCune BT, Yokoyama CC, Kambal A, Wheadon M, Diamond MS, Ivanova Y, Artyomov M, Virgin HW, et al. Commensal microbes and interferon-λ determine persistence of enteric murine norovirus infection. Science. 2015;347(6219):266–269. doi:10.1126/science.1258025.
- Caporaso JG, Lauber CL, Walters WA, Berg-Lyons D, Lozupone CA, Turnbaugh PJ, Fierer N, Knight R. Global patterns of 16S rRNA diversity at a depth of millions of sequences per sample. Proc Natl Acad Sci U S A. 2011;108(Suppl 1):4516–4522. doi:10.1073/pnas.1000080107.
- Caporaso JG, Kuczynski J, Stombaugh J, Bittinger K, Bushman FD, Costello EK, Fierer N, Peña AG, Goodrich JK, Gordon JI, et al. QIIME allows analysis of high-throughput community sequencing data. Nat Methods. 2010;7(5):335–336. doi:10.1038/nmeth.f.303.
- Amir A, McDonald D, Navas-Molina JA, Kopylova E, Morton JT, Zech Xu Z, Kightley EP, Thompson LR, Hyde ER, Gonzalez A, et al. Deblur Rapidly Resolves Single-Nucleotide Community Sequence Patterns. mSystems. 2017;2(2). doi:10.1128/mSystems.00191-16.
- Katoh K, Standley DM. MAFFT multiple sequence alignment software version 7: improvements in performance and usability. Mol Biol Evol. 2013;30(4):772–780. doi:10.1093/molbev/mst010.
- Price MN, Dehal PS, Arkin AP, Poon AFY. FastTree 2–approximately maximum-likelihood trees for large alignments. PLoS One. 2010;5(3):e9490. doi:10.1371/journal.pone.0009490.
- Mandal S, et al. Analysis of composition of microbiomes: a novel method for studying microbial composition. Microb Ecol Health Dis. 2015;26:27663.
- Camara-Lemarroy CR, et al. Biomarkers of intestinal barrier function in multiple sclerosis are associated with disease activity. Mult Scler. 2019;1352458519863133.
- Kilic F, Işık Ü, Demirdaş A, Doğuç DK, Bozkurt M. Serum zonulin and claudin-5 levels in patients with bipolar disorder. J Affect Disord. 2020;266:37–42. doi:10.1016/j.jad.2020.01.117.
- Usta A, et al. Serum zonulin and claudin-5 levels in patients with schizophrenia. Eur Arch Psychiatry Clin Neurosci. 2020.
- Barber GS, Sturgeon C, Fasano A, Cascella NG, Eaton WW, McMahon RP, Kelly DL. Elevated zonulin, a measure of tight-junction permeability, may be implicated in schizophrenia. Schizophr Res. 2019;211:111–112. doi:10.1016/j.schres.2019.07.006.
- Ciccia F, Guggino G, Rizzo A, Alessandro R, Luchetti MM, Milling S, Saieva L, Cypers H, Stampone T, Di Benedetto P, et al. Dysbiosis and zonulin upregulation alter gut epithelial and vascular barriers in patients with ankylosing spondylitis. Ann Rheum Dis. 2017;76(6):1123–1132. doi:10.1136/annrheumdis-2016-210000.
- Wang Y, Jin S, Sonobe Y, Cheng Y, Horiuchi H, Parajuli B, Kawanokuchi J, Mizuno T, Takeuchi H, Suzumura A, et al. Interleukin-1beta induces blood-brain barrier disruption by downregulating Sonic hedgehog in astrocytes. PLoS One. 2014;9(10):e110024. doi:10.1371/journal.pone.0110024.
- Ching S, He L, Lai W, Quan N. IL-1 type I receptor plays a key role in mediating the recruitment of leukocytes into the central nervous system. Brain Behav Immun. 2005;19(2):127–137. doi:10.1016/j.bbi.2004.06.001.
- Pare A, Mailhot B, Lévesque SA, Juzwik C, Ignatius Arokia Doss PM, Lécuyer M-A, Prat A, Rangachari M, Fournier A, Lacroix S, et al. IL-1β enables CNS access to CCR2 hi monocytes and the generation of pathogenic cells through GM-CSF released by CNS endothelial cells. Proc Natl Acad Sci U S A. 2018;115(6):E1194–E1203. E1194-E1203. doi:10.1073/pnas.1714948115.
- Cryan JF, Kaupmann K. Don’t worry ‘B’ happy!: a role for GABA(B) receptors in anxiety and depression. Trends Pharmacol Sci. 2005;26(1):36–43. doi:10.1016/j.tips.2004.11.004.
- Schousboe A, Waagepetersen HS. GABA: homeostatic and pharmacological aspects. Prog Brain Res. 2007;160:9–19.
- Mohler H. The GABA system in anxiety and depression and its therapeutic potential. Neuropharmacology. 2012;62(1):42–53. doi:10.1016/j.neuropharm.2011.08.040.
- Kalueff A, Nutt DJ. Role of GABA in memory and anxiety. Depress Anxiety. 1996;4(3):100–110. doi:10.1002/(SICI)1520-6394(1996)4:3<100::AID-DA2>3.0.CO;2-K.
- Song DK, et al. Central GABAA and GABAB receptor modulation of basal and stress-induced plasma interleukin-6 levels in mice. J Pharmacol Exp Ther. 1998;287(1):144–149.
- Ito Y, Banno R, Shibata M, Adachi K, Hagimoto S, Hagiwara D, Ozawa Y, Goto M, Suga H, Sugimura Y, et al. GABA type B receptor signaling in proopiomelanocortin neurons protects against obesity, insulin resistance, and hypothalamic inflammation in male mice on a high-fat diet. J Neurosci. 2013;33(43):17166–17173. doi:10.1523/JNEUROSCI.0897-13.2013.
- Kuhn SA, Van Landeghem FKH, Zacharias R, Färber K, Rappert A, Pavlovic S, Hoffmann A, Nolte C, Kettenmann H. Microglia express GABA(B) receptors to modulate interleukin release. Mol Cell Neurosci. 2004;25(2):312–322. doi:10.1016/j.mcn.2003.10.023.
- Guyon A, Kussrow A, Olmsted IR, Sandoz G, Bornhop DJ, Nahon J-L. Baclofen and other GABAB receptor agents are allosteric modulators of the CXCL12 chemokine receptor CXCR4. J Neurosci. 2013;33(28):11643–11654. doi:10.1523/JNEUROSCI.6070-11.2013.
- Lee M, Schwab C, McGeer PL. Astrocytes are GABAergic cells that modulate microglial activity. Glia. 2011;59(1):152–165. doi:10.1002/glia.21087.
- Crowley T, Fitzpatrick J-M, Kuijper T, Cryan JF, O’Toole O, O’Leary OF, Downer EJ. Modulation of TLR3/TLR4 inflammatory signaling by the GABAB receptor agonist baclofen in glia and immune cells: relevance to therapeutic effects in multiple sclerosis. Front Cell Neurosci. 2015;9:284. doi:10.3389/fncel.2015.00284.
- Liu GX, Cai G-Q, Cai Y-Q, Sheng Z-J, Jiang J, Mei Z, Wang Z-G, Guo L, Fei J. Reduced anxiety and depression-like behaviors in mice lacking GABA transporter subtype 1. Neuropsychopharmacology. 2007;32(7):1531–1539. doi:10.1038/sj.npp.1301281.
- Kjeldsen TH, Hansen EW, Christensen JD, Moesby L. Baclofen influences lipopolysaccharide-mediated interleukin-6 release from murine pituicytes. Eur J Pharmacol. 2002;451(2):209–215. doi:10.1016/S0014-2999(02)02222-7.
- Kim KA, Gu W, Lee I-A, Joh E-H, Kim D-H. High fat diet-induced gut microbiota exacerbates inflammation and obesity in mice via the TLR4 signaling pathway. PLoS One. 2012;7(10):e47713. doi:10.1371/journal.pone.0047713.
- Leiva-Gea I, Sánchez-Alcoholado L, Martín-Tejedor B, Castellano-Castillo D, Moreno-Indias I, Urda-Cardona A, Tinahones FJ, Fernández-García JC, Queipo-Ortuño MI. Gut Microbiota Differs in Composition and Functionality Between Children With Type 1 Diabetes and MODY2 and Healthy Control Subjects: a Case-Control Study. Diabetes Care. 2018;41(11):2385–2395. doi:10.2337/dc18-0253.
- Geurts L, Lazarevic V, Derrien M, Everard A, Van Roye M, Knauf C, Valet P, Girard M, Muccioli GG, François P, et al. Altered gut microbiota and endocannabinoid system tone in obese and diabetic leptin-resistant mice: impact on apelin regulation in adipose tissue. Front Microbiol. 2011;2:149. doi:10.3389/fmicb.2011.00149.
- Alkadhi S, Kunde D, Cheluvappa R, Randall-Demllo S, Eri R. The murine appendiceal microbiome is altered in spontaneous colitis and its pathological progression. Gut Pathog. 2014;6(1):25. doi:10.1186/1757-4749-6-25.
- Grander C, Adolph TE, Wieser V, Lowe P, Wrzosek L, Gyongyosi B, Ward DV, Grabherr F, Gerner RR, Pfister A, et al. Recovery of ethanol-induced Akkermansia muciniphila depletion ameliorates alcoholic liver disease. Gut. 2018;67(5):891–901. doi:10.1136/gutjnl-2016-313432.
- Png CW, et al. Mucolytic bacteria with increased prevalence in IBD mucosa augment in vitro utilization of mucin by other bacteria. Am J Gastroenterol. 2010;105(11):2420–2428. doi:10.1038/ajg.2010.281.
- Reunanen J, et al. Akkermansia muciniphila Adheres to Enterocytes and Strengthens the Integrity of the Epithelial Cell Layer. Appl Environ Microbiol. 2015;81(11):27–62. doi:10.1128/AEM.04050-14.
- Belzer C, De Vos WM. Microbes inside–from diversity to function: the case of Akkermansia. ISME J. 2012;6(8):1449–1458. doi:10.1038/ismej.2012.6.
- Earley H, Lennon G, Balfe A, Kilcoyne M, Clyne M, Joshi L, Carrington S, Martin ST, Coffey JC, Winter DC, et al. A Preliminary Study Examining the Binding Capacity of Akkermansia muciniphila and Desulfovibrio spp., to Colonic Mucin in Health and Ulcerative Colitis. PLoS One. 2015;10(10):e0135280. doi:10.1371/journal.pone.0135280.
- Li J, Lin S, Vanhoutte PM, Woo CW, Xu A. Akkermansia Muciniphila Protects Against Atherosclerosis by Preventing Metabolic Endotoxemia-Induced Inflammation in Apoe −/− Mice. Circulation. 2016;133(24):2434–2446. doi:10.1161/CIRCULATIONAHA.115.019645.
- Yano JM, Yu K, Donaldson G, Shastri G, Ann P, Ma L, Nagler C, Ismagilov R, Mazmanian S, Hsiao E, et al. Indigenous bacteria from the gut microbiota regulate host serotonin biosynthesis. Cell. 2015;161(2):264–276. doi:10.1016/j.cell.2015.02.047.
- Tran SM, Mohajeri MH. The Role of Gut Bacterial Metabolites in Brain Development, Aging and Disease. Nutrients. 2021;13(3):732. doi:10.3390/nu13030732.
- Bojovic K, et al. Gut Microbiota Dysbiosis Associated With Altered Production of Short Chain Fatty Acids in Children With Neurodevelopmental Disorders. Front Cell Infect Microbiol. 2020;10:223. doi:10.3389/fcimb.2020.00223.
- Bhattacharjee S, Lukiw WJ. Alzheimer’s disease and the microbiome. Front Cell Neurosci. 2013;7:153. doi:10.3389/fncel.2013.00153.
- Strandwitz P. Neurotransmitter modulation by the gut microbiota. Brain Res. 2018;1693(7):128–133. doi:10.1016/j.brainres.2018.03.015.
- Frohlich EE, et al. Cognitive impairment by antibiotic-induced gut dysbiosis: analysis of gut microbiota-brain communication. Brain Behav Immun. 2016;56:140–155. doi:10.1016/j.bbi.2016.02.020.
- Borre YE, et al. The impact of microbiota on brain and behavior: mechanisms & therapeutic potential. Adv Exp Med Biol. 2014;817:373–403.
- Reichmann F, Wegerer V, Jain P, Mayerhofer R, Hassan AM, Fröhlich EE, Bock E, Pritz E, Herzog H, Holzer P, et al. Environmental enrichment induces behavioural disturbances in neuropeptide Y knockout mice. Sci Rep. 2016;6(1):28182. doi:10.1038/srep28182.
- Clarke G, Grenham S, Scully P, Fitzgerald P, Moloney RD, Shanahan F, Dinan TG, Cryan JF. The microbiome-gut-brain axis during early life regulates the hippocampal serotonergic system in a sex-dependent manner. Mol Psychiatry. 2013;18(6):666–673. doi:10.1038/mp.2012.77.
- Diaz Heijtz R. Fetal, neonatal, and infant microbiome: perturbations and subsequent effects on brain development and behavior. Semin Fetal Neonatal Med. 2016;21(6):410–417. doi:10.1016/j.siny.2016.04.012.
- Neufeld KM, Kang N, Bienenstock J, Foster JA. Reduced anxiety-like behavior and central neurochemical change in germ-free mice. Neurogastroenterol Motil. 2011;23(3):255–264 e119. doi:10.1111/j.1365-2982.2010.01620.x.
- Crumeyrolle-Arias M, Jaglin M, Bruneau A, Vancassel S, Cardona A, Daugé V, Naudon L, Rabot S. Absence of the gut microbiota enhances anxiety-like behavior and neuroendocrine response to acute stress in rats. Psychoneuroendocrinology. 2014;42:207–217. doi:10.1016/j.psyneuen.2014.01.014.
- Nishino R, et al. Commensal microbiota modulate murine behaviors in a strictly contamination-free environment confirmed by culture-based methods. Neurogastroenterol Motil. 2013;25(6):521–528. doi:10.1111/nmo.12110.
- Uuskula A, Kohl PK. Genital mycoplasmas, including Mycoplasma genitalium, as sexually transmitted agents. Int J STD AIDS. 2002;13(2):79–85. doi:10.1258/0956462021924695.
- De Francesco MA, Negrini R, Pinsi G, Peroni L, Manca N. Detection of Ureaplasma biovars and polymerase chain reaction-based subtyping of Ureaplasma parvum in women with or without symptoms of genital infections. Eur J Clin Microbiol Infect Dis. 2009;28(6):641–646. doi:10.1007/s10096-008-0687-z.
- Horner P, Thomas B, Gilroy CB, Egger M, Taylor-Robinson D. Role of Mycoplasma genitalium and Ureaplasma urealyticum in acute and chronic nongonococcal urethritis. Clin Infect Dis. 2001;32(7):995–1003. doi:10.1086/319594.
- Kafetzis DA, Skevaki CL, Skouteri V, Gavrili S, Peppa K, Kostalos C, Petrochilou V, Michalas S. Maternal genital colonization with Ureaplasma urealyticum promotes preterm delivery: association of the respiratory colonization of premature infants with chronic lung disease and increased mortality. Clin Infect Dis. 2004;39(8):1113–1122. doi:10.1086/424505.
- McGowin CL, Anderson-Smits C, Manchester M. Mycoplasma genitalium: an emerging cause of sexually transmitted disease in women. PLoS Pathog. 2011;7(5):e1001324. doi:10.1371/journal.ppat.1001324.
- Reigstad CS, Salmonson CE, Iii JFR, Szurszewski JH, Linden DR, Sonnenburg JL, Farrugia G, Kashyap PC. Gut microbes promote colonic serotonin production through an effect of short-chain fatty acids on enterochromaffin cells. FASEB J. 2015;29(4):1395–1403. doi:10.1096/fj.14-259598.
- Forsythe P, Bienenstock J. Immunomodulation by commensal and probiotic bacteria. Immunol Invest. 2010;39(4–5):429–448. doi:10.3109/08820131003667978.
- Stilling RM, Dinan TG, Cryan JF. Microbial genes, brain & behaviour - epigenetic regulation of the gut-brain axis. Genes Brain Behav. 2014;13(1):69–86. doi:10.1111/gbb.12109.
- Macfabe DF. Short-chain fatty acid fermentation products of the gut microbiome: implications in autism spectrum disorders. Microb Ecol Health Dis. 2012;23.
- Bercik P, Park AJ, Sinclair D, Khoshdel A, Lu J, Huang X, Deng Y, Blennerhassett PA, Fahnestock M, Moine D, et al. The anxiolytic effect of Bifidobacterium longum NCC3001 involves vagal pathways for gut-brain communication. Neurogastroenterol Motil. 2011;23(12):1132–1139. doi:10.1111/j.1365-2982.2011.01796.x.
- Nohr MK, Egerod KL, Christiansen SH, Gille A, Offermanns S, Schwartz TW, Møller M. Expression of the short chain fatty acid receptor GPR41/FFAR3 in autonomic and somatic sensory ganglia. Neuroscience. 2015;290:126–137. doi:10.1016/j.neuroscience.2015.01.040.
- Svensson E, Horváth-Puhó E, Thomsen RW, Djurhuus JC, Pedersen L, Borghammer P, Sørensen HT. Vagotomy and subsequent risk of Parkinson’s disease. Ann Neurol. 2015;78(4):522–529. doi:10.1002/ana.24448.
- Holmqvist S, Chutna O, Bousset L, Aldrin-Kirk P, Li W, Björklund T, Wang Z-Y, Roybon L, Melki R, Li J-Y, et al. Direct evidence of Parkinson pathology spread from the gastrointestinal tract to the brain in rats. Acta Neuropathol. 2014;128(6):805–820. doi:10.1007/s00401-014-1343-6.

