Abstract
We have prepared a novel nanobiotherapeutic, Poly-[hemoglobin-superoxide dismutase-catalase-carbonic anhydrase], which not only transports both oxygen and carbon dioxide but also a therapeutic antioxidant. Our previous study in a severe sustained 90 min hemorrhagic shock rat model shows that it has a hepatoprotective effect. We investigate its hepatoprotective effect further in this present report using an alcohol-damaged primary hepatocyte culture model. Results show that it significantly reduced ethanol-induced AST release, lipid peroxidation, and ROS production in rat primary hepatocytes culture. It also significantly enhanced the viability of ethanol-treated hepatocytes. Thus, the result shows that Poly-[hemoglobin-superoxide dismutase-catalase-carbonic anhydrase] also has some hepatoprotective effects against alcohol-induced injury in in vitro rat primary hepatocytes cell culture. This collaborate our previous observation of its hepatoprotective effect in a severe sustained 90-min hemorrhagic shock rat model.
Introduction
Red blood cells have three major functions: transport oxygen, transport carbon dioxide, and remove oxygen radicals. Poly-[hemoglobin-superoxide dismutase-catalase] (Poly-[Hb-SOD-CAT]) was prepared to carry oxygen and removal oxygen radicals (D’Agnillo and Chang Citation1998). It could protect the brain from ischemia-reperfusion injuries in a severe hemorrhagic shock-cerebral ischemia rat model (Powanda and Chang Citation2002). Others showed that it could also prevent ischemia-reperfusion injury in liver and kidney transplantation in rats (Chang Citation2007).
Recent study shows that increase in tissue CO2 may also be related to organ ischemia (Tronstad et al. Citation2010). Thus, we have more recently devised a novel nanobiotherapeutic with enhancement of all 3 RBC functions in the form of Poly-[Hemoglobin-Superoxide dismutase-catalase-carbonic anhydrase] (Poly-[Hb-SOD-CAT-CA]) (Bian et al. Citation2011). Our study in a severe 90-min sustained hemorrhagic shock rat model shows that it is more effective than whole blood in the protective effect for the heart, liver, kidney, and intestine (Bian and Chang Citation2015). The purpose of the present study is to further investigate the hepatoprotective effect of Poly-[Hb-SOD-CAT-CA] using an alcohol-damaged primary rat hepatocytes culture model. Alcohol-dependent liver disease (ALD) ranges in severity from simple steatosis (fatty liver) to steatohepatitis, cirrhosis, and hepatocellular carcinoma (Tome and Lucey Citation2004). Although the exact mechanism of alcohol-induced hepatotoxicity is not clear yet, this adverse effect has been closely related to reactive oxygen species (ROS) generation during ethanol metabolism (Ishii et al. Citation1997).
Materials and methods
Materials
The stroma-free hemoglobin (SFHb) used in our experiments was extracted from bovine blood purchased from McGill University McDonald Campus Cattle Complex (Sainte-Anne-de-Bellevue, Canada). Glutaraldehyde (25%) was purchased from Polysciences (Warrington, PA). Drabkin’s Reagent, L-lysine (monohydrochloride, Sigma (Ontaria, Canada) Ultra >99%), SOD, CAT, and bovine CA were purchased from Sigma-Aldrich (Ontario, Canada). All other chemicals or reagents of analytical grade were purchased from Sigma-Aldrich.
Preparation of PolySFHb and Poly-[Hb-SOD-CAT-CA]
PolySFHb and Poly-[Hb-SOD-CAT-CA] were prepared using the glutaraldehyde methods described by Bian and Chang (Citation2015) and Chang (Citation2007). For the preparation of Poly-[Hb-SOD-CAT-CA], SOD (1050 units/mL), catalase (21 000 units/mL), and carbonic anhydrase (1070 units/mL) were added to the 20 mL solution containing SFHb (7 g/dL) in 50 mM sodium phosphate buffer (pH 7.4). For the preparation of PolySFHb, an equivalent volume of buffer containing no enzymes was added. The Hb concentration in the PolySFHb and Poly-[Hb-SOD-CAT-CA] preparations was colorimetrically determined by reacting the samples with Drabkin’s Reagent (Sigma-Aldrich).
Determination of CAT and SOD Activity: The method for catalase measurement was according to the study of D’Agnillo and Chang (Citation1998) and Chang (Citation2007). The catalase activity was expressed in units per grams of hemoglobin. The measurement for SOD activity was based on the reduction of Cytochrome c by superoxide.
Rat primary hepatocytes culture and treatments
Primary hepatocytes were isolated from rats according to the two-step collagenase perfusion technique described by Seglen (Citation1976) with minor modifications as follows. After perfusion with a chelating agent to allow the cleavage of the hepatic desmosomes, hepatic collagen was hydrolyzed by ex situ perfusion with a collagenase solution supplemented with dispase at a rate of 35 mL/min at 37 °C for 10–12 min. The liver was excised and minced in ice-cold William’s E medium (GIBCO, Grand Island, NY) to free the hepatocytes. The hepatocytes were collected and filtered through a 100-μm nylon mesh kept on ice. Following which, sedimented by centrifugation at 50 g, resuspended, and washed 2–3 times at 4 °C for 5 min, the hepatocytes were purified by 90% Percoll solution (Sigma). The viability of hepatocytes measured by trypan blue exclusion test was consistently within the range of 88–94%.
The primary hepatocytes were cultured with complete culture medium (William’s E medium supplemented with 5% FCS, 1% penicillin/streptomycin, 4 μg/mL insulin, 2 mM glutamate, 15 mM HEPES) in previously collagen-coated culture wells at a density of 1 × 105 cells/mL. Four hours after plating, the medium was changed with fresh medium to remove the non-viable cells and cell particles. After 24 h of incubation at 37 °C in a humidified atmosphere composed of 5% CO2 in air, the cultured cells were seeded in 96-well plates at a density of 4.8 × 104 cells/mL. Cell viability evaluation was based on 12-well plates containing 200 μL per well at a density of 2.4 × 105 cells/mL. Malondialdehyde (MDA) content and AST leakage analysis was based on 6-well plates containing 600 μL per well at a density of 2.4 × 105 cells/mL. ROS in liver cell analysis was carried out in 6-well plates containing 1000 μL per well at a density of 2.4 × 105 cells/mL. The following groups of the study were carried out each of the analysis:
Add alcohol (200 mmol/L) to the hepatocyte culture and incubated for 12 h.
Three groups pre-incubated at 37 °C for 2 h, each group with respectively 5, 10, or 15 μL Poly-[Hb-SOD-CAT-CA]. After this, alcohol (200 mmol/L) was added and incubated for 12 h.
Three groups pre-incubated at 37 °C for 2 h each group with respectively 5, 10, or 15 μL PolySFHb. After this, alcohol (200 mmol/L) was added and incubated for 12 h.
Measurement of aspartate aminotransferase (AST) activity
AST leakage of the hepatocytes was used to study membrane integrity of the cells. The culture medium was centrifuged at 1600 g for 10 min and the supernatant was collected. AST was measured using commercially available assay kits (Cat. No. MAK 055, Sigma). This was based on the mechanism that AST facilitates the conversion of aspartate and alpha-ketoglutarate to oxaloacetate and glutamate. This AST activity assay is based on the transfer of an amino group from aspartate to alpha-ketoglutarate resulting in the generation of glutamate, and the production of a colorimetric product proportional to the AST enzymatic activity present. After incubation, culture supernatant was removed and the AST level was measured using a microplate reader at a wavelength of 40 nm according to the supplier instructions, the results were expressed as units/liter (U/L).
Assaying of malondialdehyde content
MDA content of the cells was measured by a OXI-TEK TBARS Assay kit (Cat. No. ALX-850-287-K101, Enzo Life Sciences, Mountain View, CA). This was based on the mechanism that the MDA can react with thiobarbituric acid (TBA) to generate a color compound with a maximal absorbance at 532 nm. In brief, 2 × 105 hepatocytes in 500 μL of phosphate-buffered saline were sonicated for 5-s intervals at 40 V setting over ice to collect the whole homogenates in the assay. 0.1 mL of the whole homogenates was mixed with 2.5 mL TBA/buffer reagent and incubated in a water bath for 60 min. The absorbance was recorded on the microplate reader at a wavelength of 532 nm. MDA content was expressed as nmol/mL to reflect lipid oxidation.
Measurement of oxidative stress
The measurement of ROS production in hepatocytes was quantified by CellRox Green (Cat. No. C10444, Invitrogen, Carlsbad, CA) staining using fluorescence microscopy (Sollinger et al. Citation2014). In brief, after incubation, hepatocytes were washed with phosphate-buffered saline and stained with 5 mmol/L CellRox Green and 10 μg/mL of Hoechst 33342 (Life Technologies, Waltham, MA) for 30 min. Then placed on fluorescence microscope (Carl Zeiss, Oberkochen, Germany) with a 10-fold magnification objective. Excitation/emission was recorded at 485/520 nm, respectively. Total nuclei and CellRox Green-positive nuclei were determined simultaneously and an overlap of staining of nuclei was performed. CellRox Green-positive nuclei were only counted in the case of a positive overlap.
Analysis of cell viability
Cell viability was measured by the MTT assay that is based on the production of a blue product, formazan, by the living cells. After the cells were treated as described previously, 20 μL MTT (Sigma) solution (5 g/L) was added into each well and incubated 4 h at 37 °C. Then, 150 μL dimethyl sulfoxide was added into each well to dissolve the formazan generation. The absorbance of each well was recorded on a microplate reader (Biotek, Winooski, VT) at a wavelength of 490 nm. The data were recorded using the software package Gen 5TM. The viability was calculated as follows:
Viability = average of test wells OD – average of blank wells OD (OD = optical density; test wells = treated wells)
Statistical analysis
Values were expressed as mean ± SD. To evaluate the differences between the groups studied, one-way analysis of variance was used. Differences were considered statistically significant when P < 0.05.
Results
Molecular weight distribution of PolyHb-SOD-CAT-CA and enzyme activities
Sepacryl S-300 gel column chromatography was used to analyze the molecular weight distribution of PolyHb-SOD-CAT-CA. The molecular weight distributions for Poly-[Hb-SOD-CAT-CA] show three molecular components: (1) low (<100 kDa); (2) intermediate (100–450 kDa); and (3) high molecular weight (>450 kDa). The sample contains about 86% components with molecular weight higher than 100 kDa. The low molecular weight fraction (<100 kDa) was discarded before used in the present study. Most of the Hb, SOD, CAT, and CA activities were in the Poly-[Hb-SOD-CAT-CA] fraction with a molecular weight of >450 kDa. SOD activity was 51,608.33 and 89,678.66 (U/g) in Poly-[Hb-SOD-CAT-CA] and PolySFHb respectively, CAT activity was 12,301,682.7 and 275,983.0 (U/g) in Poly-[Hb-SOD-CAT-CA] and PolySFHb, respectively.
Effects of PolySFHb and PolyHb-SOD-CAT-CA on the levels of hepatic transaminase (AST) released into the culture medium
Incubation of rat primary hepatocytes with ethanol for 12 h, resulted in a significant increase in AST leakage compared to control cells. Pre-incubation with 5, 10, or 15 μL of PolySFHb did not have any reduction of AST release. Poly-[Hb-SOD-CAT-CA] pre-incubation significantly reduced the release of AST only at the higher dosage of 15 μL. There was no significant effect at the lower dosage of 5 and 10 μL ().
Figure 1. Effects of Poly-[Hb-SOD-CAT-CA] (pe) on ethanol-induced AST release in rat primary hepatocytes. Rat primary hepatocytes were pre-incubated with 5 μL, 10 μL, or 15 μL of Poly-[Hb-SOD-CAT-CA] (pe) or PolyHb (p) for 2 h before treated with 200 mmol/L alcohol (a) for 12 h. *P < 0.05 versus 200 mm alcohol group.
![Figure 1. Effects of Poly-[Hb-SOD-CAT-CA] (pe) on ethanol-induced AST release in rat primary hepatocytes. Rat primary hepatocytes were pre-incubated with 5 μL, 10 μL, or 15 μL of Poly-[Hb-SOD-CAT-CA] (pe) or PolyHb (p) for 2 h before treated with 200 mmol/L alcohol (a) for 12 h. *P < 0.05 versus 200 mm alcohol group.](/cms/asset/5d129b2d-3ad4-498c-9ba5-23ebe22ae4bd/ianb_a_1191229_f0001_c.jpg)
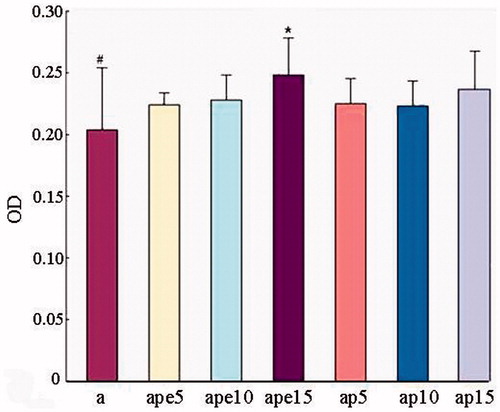
Effects of PolySFHb and PolyHb-SOD-CAT-CA on ethanol-induced lipid peroxidation
The effects of Poly-[Hb-SOD-CAT-CA] on ethanol-induced lipid peroxidation were measured by following the MDA level () in culture lysates. Ethanol exposure for 12 h significantly (P < 0.01) increased intracellular MDA level compared to control in rat primary hepatocytes. Pre-incubation with 5, 10, or 15 μL PolySFHb did not have any reduction in MDA formation of rat primary hepatocytes. However, 15 μL of PolyHb-SOD-CAT-CA resulted in a significant decrease in the MDA levels.
Poly-[Hb-SOD-CAT-CA] prohibits ethanol-induced ROS production in rat primary hepatocytes
The effects of Poly-[Hb-SOD-CAT-CA] on ethanol-induced ROS production were measured by the CellRox Green reagent. Ethanol exposure for 12 h significantly (P < 0.01) increased ROS production compared to control in rat primary hepatocytes. Pre-incubation with 5, 10, or 15 μL PolySFHb did not have any reduction on ROS production of rat primary hepatocytes (). However, ROS production was significantly reduced when pre-incubated with 10 μL and 15 μL of Poly-[Hb-SOD-CAT-CA] (
Figure 3. Effects of Poly-[Hb-SOD-CAT-CA] on ROS generation in rat primary hepatocytes injured by alcohol (a). Rat primary hepatocytes were pre-incubated with 5 μL, 10 μL, or 15 μL of Poly-[Hb-SOD-CAT-CA] (pe) and PolyHb (p) for 2 h before treated with 200 mmol/L alcohol (a) for 12 h. *P < 0.05 versus 200 mm alcohol group.
![Figure 3. Effects of Poly-[Hb-SOD-CAT-CA] on ROS generation in rat primary hepatocytes injured by alcohol (a). Rat primary hepatocytes were pre-incubated with 5 μL, 10 μL, or 15 μL of Poly-[Hb-SOD-CAT-CA] (pe) and PolyHb (p) for 2 h before treated with 200 mmol/L alcohol (a) for 12 h. *P < 0.05 versus 200 mm alcohol group.](/cms/asset/4c5eb3d6-7a6f-4fdf-b953-6e930404726b/ianb_a_1191229_f0003_c.jpg)
Figure 4. ROS production was determined by CellRox Green and Hoechst 33342 staining in rat primary hepatocytes. Rat primary hepatocytes were pre-incubated with Poly-[Hb-SOD-CAT-CA] or PolySFHb for 2 h before treated with 200 mmol/L alcohol for 12 h. B: control, C: 200 mm alcohol, D: 5 μL PolyHb-SOD-CAT-CA +200 mm alcohol, E: 10 μL PolyHb-SOD-CAT-CA +200 mm alcohol, F: 15 μL PolyHb-SOD-CAT-CA +200 mm alcohol, G: 5 μL PolyHb +200 mm alcohol, H: 10 μL PolyHb +200 mm alcohol, I: 15 μL PolyHb +200 mm alcohol (arrow points an example of overlap of staining).
![Figure 4. ROS production was determined by CellRox Green and Hoechst 33342 staining in rat primary hepatocytes. Rat primary hepatocytes were pre-incubated with Poly-[Hb-SOD-CAT-CA] or PolySFHb for 2 h before treated with 200 mmol/L alcohol for 12 h. B: control, C: 200 mm alcohol, D: 5 μL PolyHb-SOD-CAT-CA +200 mm alcohol, E: 10 μL PolyHb-SOD-CAT-CA +200 mm alcohol, F: 15 μL PolyHb-SOD-CAT-CA +200 mm alcohol, G: 5 μL PolyHb +200 mm alcohol, H: 10 μL PolyHb +200 mm alcohol, I: 15 μL PolyHb +200 mm alcohol (arrow points an example of overlap of staining).](/cms/asset/c120bd39-9bec-413e-9c3e-a28019eb3b77/ianb_a_1191229_f0004_c.jpg)
Effects of PolyHb-SOD-CAT-CA on the viability of rat primary hepatocytes
The results of MTT assay () showed that 5, 10, 15 μL PolySFHb did not improve the viability of rat primary hepatocytes when compared with the group exposed to 200 mm alcohol for 12 h without pre-incubation with PolySFHb. On the other hand, Poly-[Hb-SOD-CAT-CA] significantly increased the viability of hepatocytes compared with the 200 mm alcohol group, but only at the higher dose of 15 μL.
Discussion
In the present study, the decrease of cell viability and the significant elevated activities of AST in the supernatants of the alcohol-induced hepatocytes indicated the cellular leakage and hepatocyte damage. Pretreatment of the hepatocytes with Poly-[Hb-SOD-CAT-CA] (15 μL group) significantly increased the cell viability and decreased the release of AST, indicating that Poly-[Hb-SOD-CAT-CA] could maintain the functional integrity of the hepatocyte membrane, and protect the hepatocytes against alcohol-mediated toxicity. Moreover, alcohol-mediated free radical formation has a great potential to react with lipid molecules, which leads to lipid peroxidation. During alcohol metabolism, alcohol oxidized into aldehydes especially acetaldehyde and malondialdehyde. MDA is a decomposition product of lipid hydroperoxide (Gutteridge Citation1995). It is widely used as a marker of lipid peroxidation (Mansour Citation2000) and its elevated level could reflect the degree of lipid peroxidation injury in hepatocytes (Hu et al. Citation2001). In the present study, MDA levels in the supernatant of alcohol-treated hepatocytes were significantly elevated, pretreatment of the hepatocytes with Poly-[Hb-SOD-CAT-CA] (15 μL group) significantly suppressed the elevation of MDA caused by alcohol, indicating the anti-lipid peroxidation effect of Poly-[Hb-SOD-CAT-CA].
ROS generated by alcohol metabolism is a putative mechanism underlying alcoholic liver diseases. In this study, ROS level of alcohol-treated hepatocytes was significantly elevated. Pretreatment of the hepatocytes with Poly-[Hb-SOD-CAT-CA] (10, 15 μL groups) significantly reduced the elevation of ROS caused by alcohol. Thus, Poly-[Hb-SOD-CAT-CA] treatment significantly prevented ethanol-induced intracellular ROS accumulation alcohol-treated hepatocytes.
In summary, the present findings demonstrated the hepatoprotective effect of Poly-[Hb-SOD-CAT-CA] against rat hepatocytes damage induced by alcohol. The hepatoprotective activity of Poly-[Hb-SOD-CAT-CA] may be attributed to the enhancement of the hepatic antioxidant system, such as down-regulating hepatic transaminase enzymes, MDA, and intracellular ROS generation. The result of our present study supports and explains the hepatoprotective effect of Poly-[Hb-SOD-CAT-CA] in our previous study, in a severe 90-min sustained hemorrhagic shock rat model (Bian and Chang Citation2015).
Disclosure statement
This laboratory is not connected to any commercial organizations. The research support for the study reported here comes from the Canadian Blood Service/Canadian Institutes of Health Research joint program that requires the authors to state that: “The opinion expressed in this article is the opinions of the investigators of this paper and not necessary those of the Canadian Blood Service nor of the Canadian Government”.
Dr. Wenhua Jiang came to this laboratory as a China Scholarship Council Visiting Scholar from the Department of Histology and Embryology, Norman Bethune Medical College of Jilin University, Changchun 130021, Jilin Province, China.
References
- Bian Y, Chang TMS. 2015. A novel nanobiotherapeutical poly-[hemoglobin-superoxide dismutase-catalase-carbonic anhydrase] with no cardiac toxicity for the resuscitation of a 90 min sustained severe hemorrhagic shock rat model with 2/3 blood volume loss. Artif Cells Nanomed Biotechnol. 43:1–9.
- Bian Y, Rong Z, Chang TMS. 2011. Polyhemoglobin-superoxide dismutase-catalase-carbonic anhydrase: a novel biotechnology-based blood substitute that transports both oxygen and carbon dioxide and also acts as an antioxidant. Artif Cells Blood Substit Immobil Biotechnol. 39:127–136.
- Chang TMS. 2007. Artificial Cells: Biotechnology, Nanotechnology, Blood Substitutes, Regenerative Medicine, Bioencapsulation, Cell/Stem Cell Therapy. Singapore: World Science Publishers/Imperial College Press. Available from: http://www.medicine.mcgill.ca/artcell/2007%20ebook%20artcell%20web.pdf.
- D’Agnillo F, Chang TMS. 1998. Polyhemoglobin-superoxide dismutase, catalase as a blood substitute with antioxidant properties. Nature Biotechnol. 16:667–671.
- Gutteridge J. 1995. Lipid peroxidation and antioxidants as biomarkers of tissue damage. Clin Chem. 41:1819.
- Hu CC, Chen WK, Liao PH, Yu WC, Lee YJ. 2001. Synergistic effect of cadmium chloride and acetaldehyde on cytotoxicity and its prevention by quercetin and glycyrrhizin. Mutat Res. 496:117–127.
- Ishii H, Kurose I, Kato S. 1997. Pathogenesis of alcoholic liver disease with particular emphasis on oxidative stress. J Gastroenterol Hepatol. 12:S272–S282.
- Mansour MA. 2000. Protective effects of thymoquinone and desferrioxamine against hepatotoxicity of carbon tetrachloride in mice. Life Sci. 66:2583–2591.
- Powanda D, Chang TMS. 2002. Cross-linked polyhemoglobin-superoxide dismutase-catalase supplies oxygen without causing blood brain barrier disruption or brain edema in a rat model of transient global brain ischemia-reperfusion. Artif Cells Blood Subst Biotechnol. 30:23–37.
- Seglen PQ. 1976. Preparation of isolated rat liver cells. Methods Cell Biol. 13:29–83.
- Sollinger D, Eißler R, Lorenz S. 2014. Damage-associated molecular pattern activated Toll-like receptor 4 signalling modulates blood pressure in L-NAME-induced hypertension. Cardiovasc Res. 101:464–472.
- Tome S, Lucey MR. 2004. Review article: current management of alcoholic liver disease. Aliment Pharmacol Ther. 19:707–714.
- Tronstad C, Pischke SE, Holhjem L, Tonnessen TI, Martinsen OG, Grimnes S. 2010. Early detection of cardiac ischemia using a conductometric pCO(2) sensor: real-time drift correction and parameterization. Physiol Meas. 31:1241–1255.


![Figure 2. Poly-[Hb-SOD-CAT-CA] down-regulates ethanol-induced MDA in rat primary hepatocytes. Rat primary hepatocytes were pre-incubated with Poly-[Hb-SOD-CAT-CA] (pe) PolySFHb (p) for 2 h before exposure to 200 mmol/L alcohol (a) for 12 h. *P < 0.05 versus 200 mm alcohol group.](/cms/asset/50bf411a-1c73-46f7-9447-d40f62c8d7e9/ianb_a_1191229_f0002_c.jpg)