Abstract
Background: The aim of this study was to evaluate the antimicrobial efficacy of liposomal meropenem formulation against clinical and laboratory isolates of Pseudomonas aeruginosa. P. aeruginosa isolates were used to determine the MIC and MBC of free and liposomal meropenem. As well as the effect of free and liposomes meropenem compare the minimum biofilm eradication concentration and inhibition of P. aeruginosa motility was assessed.
Results: In this study, the MICs and MBC of liposomal meropenem was more effective against all clinical and laboratory strains were significantly lower than those of free meropenem (6.25 μg/ml vs 100 μg/ml). Furthermore, liposomal meropenem (≥1.5 μg/ml) completely eradicated biofilm in all P. aeruginosa isolates. While the free form of drug could eradicate biofilm formation in most of isolates in ≥6.25 μg/ml concentration. In addition, lower concentrations of liposomal meropenem could inhibit the bacterial motility.
Conclusion: The results clearly indicate that the liposomal formulation of drug is more effective than meropenem alone against antibiotic resistant P. aeruginosa isolates.
Introduction
Pseudomonas aeruginosa is an opportunistic Gram-negative bacillus commonly associated with wide range of diseases. It typically infects the airway, urinary tract, burns, wounds, and also causes blood infections. In humans, these infections mostly happen in association with epithelial cell damage to the skin or eye or medical devices such as ventilators or catheters and in immunocompromised persons. P. aeruginosa lung infections are commonly seen in patients with chronic obstructive pulmonary disease (COPD), cystic fibrosis (CF), and ventilator-associated pneumonia (VAP) (Bazzi et al. Citation2013). The pathogenicity of P. aeruginosa depends on a large number of cell-associated and extracellular virulence factors such as, exotoxins, proteases, phospholipases, and lipopolysaccharide. These factors play an important role in the colonization, the survival of the microorganism and the invasion of tissues. Biofilm formation by bacteria allows it to adhere to epithelial host cells and thus help cause the disease. Also, they often cannot be treated efficiently with usual antibiotic therapy. It seems that the biofilm protects these bacteria from different environmental factors. This factor is also involved in establishment of the infection as shown by many studies (Alhariri et al. Citation2013, Smith Citation2005). So, the treatment of P. aeruginosa infections is very important. Carbapenems are a class of β-lactam antibiotics that has most effective antibacterial efficacy against P. aeruginosa. But resistance to these agents is a serious challenge to physicians (Lister et al. Citation2009, Smith Citation2005). Liposomes are extensively used as carriers of chemical substances in the research. Intensive research is being carried out on liposomal antibiotics to increase their antimicrobial activity and pharmacokinetic properties (Daraee et al. Citation2016). As Liposomal forms of the doxorubicin, vincristine, and the amphotericin B (Drulis-Kawa et al. Citation2006) are already successfully used in veterinary and human healthcare. Furthermore, we investigated the antibacterial activity of liposomal meropenem, ability to prevent biofilm formation, and motility of meropenem-resistant strains of P. aeruginosa.
Materials and methods
Chemicals and media
Meropenem was purchased from Jaber Ebne Hayyan Pharmaceutical Company (Tehran, Iran). Soybean phosphatidylcholine (SPC, Lipoid S 100, purity = 97.8%, the assumed average molecular weight ∼800 g/mol) was provided from Lipoid GmbH (Darmstadt, Germany). Cholesterol was supplied from Merck Chemicals (Darmstadt, Germany). Mueller–Hinton agar, Mueller–Hinton broth, and Tryptic soy broth were purchased from Merck company (Darmstadt, Germany) and were used for antibiotic susceptibility tests.
Microorganisms
Laboratory strains of P. aeruginosa (GIM1 and IPM2) and eight clinical isolates of P. aeruginosa were obtained from the Clinical Microbiology Laboratory of Tabriz Hospital (Sina, Tabriz, Iran), that each strain was isolated from one patient. All strains were stored at −80 °C in Tryptic soy broth supplemented with 15% glycerol.
Antimicrobial susceptibility testing
The isolated organisms were subjected to the disk diffusion agar method using disks containing meropenem, imipenem, ertapenem, and doripenem according by CLSI procedure (Wikler Citation2008). Pure colonies of P. aeruginosa were inoculated to tube containing normal saline sterile so that the turbidity of them reached 0.5 Mac Farland. Then using sterile swab, this bacterial suspension was cultured in Muller Hinton agar and the disks were placed in agar area. Also, modified Hodge test was used to detect carbapenemase producing strains in meropenem resistant isolates according to standard methods (Kafil and Mobarez Citation2015).
Preparation of meropenem-loaded liposomes
Meropenem-loaded liposomal formulations were prepared according to the modified ethanol injection method. Briefly, phosphatidylcholine, cholesterol, and meropenem were dissolved in ethanol and injected slowly into the 100 mL aqueous medium under mixing by homogenizer (DIAX 900, Heidolph, Schawabach, Germany) at 20000 rpm.
Characterization of meropenem-loaded liposomes
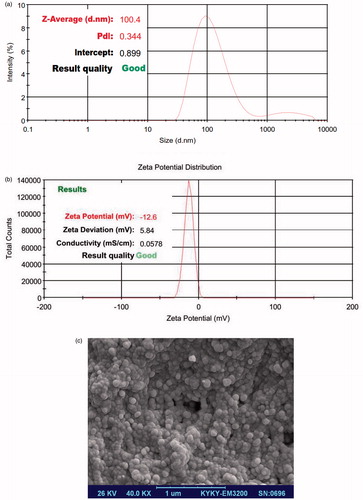
Particle size distribution of formulations was analyzed using dynamic light scattering (DLS) system and reported as intensity-weighted average (z average) and the polydispersity index (PDI), which quantifies size and distribution width, respectively. Zeta potential of prepared liposomes was also analyzed by the same system (Nano ZS, Malvern, Southborough, UK). Particles Morphology was investigated with scanning electron microscopy (SEM, VEGA TESCAN, Czech Republic). For this analysis, liposomal formulations were diluted in de-ionized water and few drops of this diluted liposome suspension were mounted on a glass lamella, air-dried and gold coated under vacuum.
Determination of entrapment efficiency (EE) and loading capacity (LC)
The EE (%) and LC (%) were expressed as the percentage of entrapped drug to the added drug or to the used lipid, respectively. EE was determined by first separation of the un-entrapped drug by centrifugation method using of Amicon® Ultra-15 (molecular weight cutoff of 100 kDa, Millipore, Germany) tube. The formulation was added to the upper chamber of the Amicon® tube and then the tube was centrifuged (Sigma 3K30, Germany) at 5000 rpm for 15 minutes. The clear solution in the bottom of Amicon® tube was used for Meropenem determination using a validated spectroscopy method in 300 nm and mathematically calculated according to the following equations:
where, W(Initial drug) is the amount of initial drug used and W(Free drug) is the amount of free drug detected in the lower chamber of Amicon® tube after centrifugation of the nanoethosomal formulations. Accordingly, W(Entrapped drug) is the amount of loaded drug and W(Total lipid) is the amount of used phospholipids and cholesterol in the preparation process. The formulations in the upper chamber of Amicon® tube were rinsed five times by hydro alcoholic (50%) solution to eliminate unloaded Meropenem. These rinsed formulations were used for the rest experiments.
Determination of minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC)
A broth micro-dilution method was used to determine the MICs of free and liposome-encapsulated meropenem. Overnight cultures of the clinical and standard strains of P. aeruginosa were diluted in sterile normal saline to achieve 0.5 McFarland standards. The bacterial cell populations were then exposed to several dilutions of free or liposome-encapsulated meropenem. The contents of the wells were cultured on the Mueller–Hinton agar plates and were incubated for 24 h at 37 °C. Broth medium alone and free meropenem bacterial cultures were used as negative and positive controls, respectively. The MBC was considered as the lowest concentration of free or liposomal antibiotic that caused in more than 99.9% reduction of the initial inoculum. Two separate experiments were done to confirm the results.
Effect of free or liposome-encapsulated meropenem against minimal biofilm eradication concentration (MBEC)
The 180 μl Trypticase soy broth containing 5.0% glucose and 20 μl of bacterial suspension equal 0.5 McFarland were added to each well. After incubation for 24 h at 37 °C, the contents of the wells was removed and washed three times with 300 μl of sterile phosphate buffer. Then 150 μl of methanol was added to the wells and were incubated for 20 min at the lab temperature. For Staining, 150 μl crystal violet was added and the resultant solution was incubated at room temperature for 15 minutes. The plates were washed and thoroughly dried by placing them upside down. Finally, 150 μl of 33% acetic acid was added to the wells and was placed 30 min at room temperature. The optical density of the contents of each well was measured at 570 nm by using a microtiter plate reader. Based on previous study the cut off optical density for biofilm formation was considered higher than OD570 = 0.524 (Abdi-Ali et al. Citation2006). Thus, the wells with optical density of higher than 0.524 indicated the formation of biofilms but those that had lower than 0.524 indicated inhibition of biofilm formation. In this way the biofilm-producing strains were selected for MBEC testing. Then different concentrations of antibiotics included liposomal formulation (0.75, 1.5, 3, 6.25, and 12.5 μg/ml) and free drug (0.75, 1.5, 3, 6.25, 12.5, 25, 50, 100, and 125 μg/ml) were used in MBEC assay. Such as biofilm formation assay after adding medium and bacterial suspension into the wells, desired concentration of two formulations of antibiotics were added to the wells and then incubated for 24 h at 37 °C. Wells without antibiotic and without bacterial cultures were considered as positive and negative controls, respectively.
Effect of free or liposome-encapsulated meropenem against bacterial motility
Free or liposomal meropenem was added to different percentages of agar plates at almost one eighth of the MIC, and motility was examined. As twitching (1% agar), swarming (0.5% agar), and swimming (0.3% agar) were used in this study. Overnight cultures were used for preparation of 0.5 McFarland suspension for all isolates, and 10 μl was inoculated onto a wells created on the surface of the plate. After 24 h of incubation at 37 °C, swimming and swarming diameters were measured. All experiments were performed in two independent experiments in duplicate.
Results
Antimicrobial susceptibility testing
All strains of our study were resistant to meropenem, imipenem, ertapenem, and doripenem.
Characterization of meropenem-loaded liposomes
The size of prepared Meropenem-loaded liposomes was analyzed and represented in . The particle size and PDI were in the range of 100.4 nm and 0.344, respectively. Also zeta potential distribution was assayed (). SEM image supported the reported size and narrow PDI (). The drug loading calculation indicated that the EE (%) and LC (%) values of liposomal formulations were 61.3% and 38.7%, respectively.
MIC and MBC
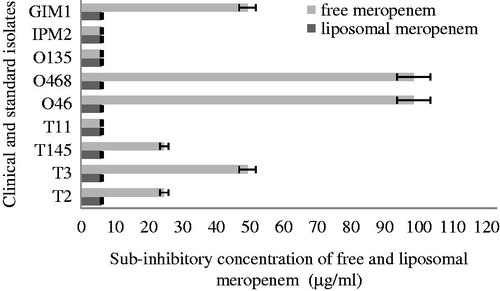
In our study, the MICs of liposome-encapsulated meropenem against all clinical and laboratory strains were significantly lower than those of free meropenem, as shown in . This result showed that the MBC of new drug formulations compared with free antibiotic has a greater effect on bacteria. The MICs of free meropenem for most P. aeruginosa isolates were >100 μg/ml compared to ≤6.25 μg/ml for liposomal meropenem.
Table 1. Antimicrobial activities of free and liposomal meropenem on clinical and laboratory strains of P. aeruginosa Strains.
Bactericidal of free and liposome-encapsulated meropenem activity on P. Aeruginosa biofilm
Our results were indicated that the liposomal meropenem (≥1.5 μg/ml) completely eradicated biofilm in all P. aeruginosa isolates. However the free form of drug could eradicate biofilm formation in most of isolates in ≥6.25 μg/ml concentration. In addition, eradicate biofilm formation in some of the tested isolates were inhibited at high concentrations with free drug.
Effect of free and liposomal meropene on P. aeruginosa motility
We examined bacterial motility, in the presence of sub-inhibitory concentrations of either free or liposomal meropenem. Concentrations of 12.5–125 μg/ml and 6.25–100 μg/ml of free and liposomal meropenem were used for detection of motility inhibition test, respectively. Our findings revealed that the concentrations of ≥6.25 μg/ml of liposomal meropenem were inhibiting the motility including twitching, swimming, and swarming in all isolates. However, the free form of the antibiotic in higher concentrations (12.5 μg/ml) could inhibit the bacterial motility. Surprisingly, in two clinical isolates that were resistant to all antibiotics tested in this study, no concentration of free form of drug could inhibit the bacterial motility. While the liposomal meropenem could inhibit the p. aeruginosa motility at a concentration of 6.25 μg/ml ().
Discussion
The emergence of pathogens resistant to conventional antimicrobial agents and insufficient antibiotic access to infectious agents, have focused research to the design and optimization of new formulation drugs. In addition, various types of antibiotic are ineffective in penetrating bacterial biofilm colonies (Agarwal et al. Citation2005, Smith Citation2005). Inherent resistance bacterial biofilms to antimicrobial agents are responsible for long persistent and chronic bacterial infections (Halwani et al. Citation2008). The liposomal antibiotic delivery system seems to be an appealing strategy for penetrating biofilm barriers, modify the antibiotic time release and targeting the infection sites (Badrzadeh et al. Citation2016, Das et al. Citation2003, Kádár et al. Citation2010). In our study, the low (≤0.2) PDI value indicated the narrow size distribution of prepared formulations. Optimum formulation had z-average 98 ± 2 nm and PDI 0.341 ± 0.001 nm. Hopefully, the zeta potential value of optimized formulation () was less than −30 (−33.6 mV) providing the suitable situation for colloidal stability of suspended nano-vesicles. We determined that the liposome-encapsulated meropenem increase antimicrobial activity against a resistance clinical P. aeruginosa isolates. These results are in agreement with previous studies indicating that liposomal formulations are highly effective against bacteria compared to free drug (Das et al. Citation2003). We found that the MICs and MBCs of liposomal meropenem were less than those of free meropenem. As in most cases, the MIC of free and liposomal meropenem was 62.5 μg/ml and 6.25 μg/ml, respectively, which is consistent with a previous study (Gubernator et al. Citation2007). Also, in most isolates, the MBCs of liposomal formulation were 25 μg/ml, while the MBCs of free drug were 125 or 250 μg/ml. These suggest that the liposomal meropenem formulation when compared with free drug was highly effective against bacterial isolates, as reducing the MIC from a resistant to susceptible (256 μg/ml to 6.25 or 50 μg/ml). Similar results were acquired in previous studies, which showed that liposomal formulations revealed better antimicrobial efficiency against P. aeruginosa (Mugabe et al. Citation2006). Overall, this study and the results of other studies, it can be deduced that liposomal meropenem formulation may be involved in high fusion with bacterial cell membrane. In this research, we tested the effects of meropenem on the motility of bacteria. According with previous studies, we demonstrated that meropenem at sub-inhibitory concentrations inhibited bacterial motility, by participating in the prevention of biofilm formation, (Drulis-Kawa et al. Citation2006), thereby affecting decline of bacterial motility, such as twitching, swarming, and swimming. We revealed that liposomal meropenem caused in improving the efficacy of antibiotic in inhibiting P. aeruginosa motility. The enhanced activity of drug liposomal preparations is probably attributed to fusion of liposomes to the bacterial cell wall (Wozniak and Keyser Citation2004) and a high concentration of drug can be exported directly to the bacterial cytoplasm (Kawamura-Sato et al. Citation2000). Considering these findings, a combination of the anti-motility properties of meropenem, which might increase the bactericidal properties when encapsulated in liposomal preparations. As explained previously, the structure of bacterial biofilms is an impermeable barrier to access antibiotics in bacterial populations. Several studies have shown that liposomal drugs have the ability to penetrate into the biofilm layer (Abdi-Ali et al. Citation2006, Smith Citation2005, Wikler Citation2008). The results indicate that liposomal formulation drug ability penetrate into the biofilm layer and therefore can be used to overcome bacterial resistance to antibiotics. We have shown that liposomal meropenem formulation compared with free drug was highly effective against bacterial biofilm formation. In all of the tested isolates, it completely eradicates the biofilm formation at lower concentrations than the free drug.
Conclusion
We report a new strategy for liposomes, as a drug delivery system to eradicate antibiotic resistant bacterial infections. Our findings suggest that liposomal antibiotics formulation could be possibly used in local applications in the eradication of p. aeruginosa infections. Future investigation could examine the efficacy of this new formulation in the animal models.
Funding information
This work was financially supported by Drug Applied Research Center, Tabriz University of Medical Sciences, Tabriz, Iran (grant no.94/3) (project number 5/101699).
Acknowledgements
The authors wish to thank Dr. Saber Yousefi for providing the laboratory strains of p. aeruginosa.
Disclosure statement
The authors report no conflicts of interest.
References
- Abdi-Ali A, Mohammadi-Mehr M, Alaei YA. 2006. Bactericidal activity of various antibiotics against biofilm-producing Pseudomonas aeruginosa. Int J Antimicrob Agents. 27:196–200.
- Agarwal G, Kapil A, Kabra SK, Das BK, Dwivedi SN. 2005. In vitro efficacy of ciprofloxacin and gentamicin against a biofilm of pseudomonas aeruginosa and its free-living forms. Natl Med J India. 18:184–186.
- Alhariri M, Azghani A, Omri A. 2013. Liposomal antibiotics for the treatment of infectious diseases. Expert Opin Drug Deliv. 10:1515–1532.
- Badrzadeh F, Rahmati-Yamchi M, Badrzadeh K, Valizadeh A, Zarghami N, Farkhani SM, Akbarzadeh A. 2016. Drug delivery and nanodetection in lung cancer. Artif Cells Nanomed Biotechnol. 44:618–634.
- Bazzi W, Sabra A, Zahreddine L, Khairallah MT, Baroud M, Hadi U, Matar GM. 2013. The inhibitory effect of micafungin on biofilm formation by pseudomonas aeruginosa. Biofouling. 29:909–915.
- Daraee H, Etemadi A, Kouhi M, Alimirzalu S, Akbarzadeh A. 2016. Application of liposomes in medicine and drug delivery. Artif Cells Nanomed Biotechnol. 44:381–391.
- Das AR, Dattagupta N, Sridhar CN, Wu WK. 2003. A novel thiocationic liposomal formulation of antisense oligonucleotides with activity against mycobacterium tuberculosis. Scand J Infect Dis. 35:168–174.
- Drulis-Kawa Z, Gubernator J, Dorotkiewicz-Jach A, Doroszkiewicz W, Kozubek A. 2006. A comparison of the in vitro antimicrobial activity of liposomes containing meropenem and gentamicin. Cell Mol Biol Lett. 11:360–375.
- Gubernator J, Drulis-Kawa Z, Dorotkiewicz-Jach A, Doroszkiewicz W, Kozubek A. 2007. In vitro antimicrobial activity of liposomes containing ciprofloxacin, meropenem and gentamicin against gram-negative clinical bacterial strains. Lett Drug Des Discov. 4:297–304.
- Halwani M, Yebio B, Suntres ZE, Alipour M, Azghani AO, Omri A. 2008. Co-encapsulation of gallium with gentamicin in liposomes enhances antimicrobial activity of gentamicin against pseudomonas aeruginosa. J Antimicrob Chemother. 62:1291–1297.
- Kádár B, Szász M, Kristóf K, Pesti N, Krizsán G, Szentandrássy J, et al. 2010. In vitro activity of clarithromycin in combination with other antimicrobial agents against biofilm-forming pseudomonas aeruginosa strains. Acta Microbiol Immunol Hung. 57:235–245.
- Kafil HS, Mobarez AM. 2015. Assessment of biofilm formation by enterococci isolates from urinary tract infections with different virulence profiles. J King Saud Univ Sci. 27:312–317.
- Kawamura-Sato K, Iinuma Y, Hasegawa T, Horii T, Yamashino T, Ohta M. 2000. Effect of subinhibitory concentrations of macrolides on expression of flagellin in pseudomonas aeruginosa and Proteus mirabilis. Antimicrob Agents Chemother 44:2869–2872.
- Lister PD, Wolter DJ, Hanson ND. 2009. Antibacterial-resistant pseudomonas aeruginosa: clinical impact and complex regulation of chromosomally encoded resistance mechanisms. Clin Microbiol Rev. 22:582–610.
- Mugabe C, Azghani AO, Omri A. 2006. Preparation and characterization of dehydration–rehydration vesicles loaded with aminoglycoside and macrolide antibiotics. Int J Pharm. 307:244–250.
- Smith AW. 2005. Biofilms and antibiotic therapy: is there a role for combating bacterial resistance by the use of novel drug delivery systems? Adv Drug Deliv Rev. 57:1539–1550.
- Wikler MA. Performance standards for antimicrobial susceptibility testing: eighteenth informational supplement. 2008: Clinical and Laboratory Standards Institute (CLSI).
- Wozniak DJ, Keyser R. 2004. Effects of subinhibitory concentrations of macrolide antibiotics on Pseudomonas aeruginosa. Chest J. 125:62S–69S.