Abstract
Propofol lipid emulsion (PLE) is a nanosized sedative, and it is used with a combination of salted antalgic prodrug, fentanyl citrate (FC). To illustrate the synergistic effect of mixing, we compared the sedation/analgesia resulting from simultaneous and sequential administration in surgically induced abortion (No. ChiCTR-IPC-15006153). Simultaneous group showed lower bispectral index, blood pressure, and heart rate, when cannula was inserted into the uterus. It also showed less frequency of hypertension, sinus tachycardia, movement, pain at the injection site, and additional FC. Therefore, premixing of PLE and FC enhanced the sedation and analgesia; stabilized the hemodynamics; lessened the incidence of movement and injection pain; and reduced the requirement of drugs.
Introduction
The procedure of surgical abortion commonly brings discomfort experience to women, while adequate sedation and sufficient analgesia may lessen the pain and anxiety, and support the surgery success (Ireland and Allen Citation2016). Propofol lipid emulsion (PLE) is one of the widely used nanosized sedative formulations in clinical anesthesiology with a particle size of 200–259 nm, and it is commonly used with a combination of salted antalgic prodrug, fentanyl citrate (FC) (Baker and Naguib Citation2005). Using such two hydrophilic formulations of hydrophobic drugs is one of the acceptable anesthetic strategies in surgical abortion because of the simple operation, rapid onset, and short action (Dean et al. Citation2011; Lindholm et al. Citation1994). However, the depth of sedation and analgesia is hard to balance in the short surgery. Deep anesthesia depresses the cardiovascular and respiratory, while inadequate anesthesia not only could not satisfy patients to effectively remove the pain and anxiety, but also could not satisfy surgeons to safe operation (Cohen et al. Citation2007; Horlocker et al. Citation2009). Stronger synergistic effect is needed because it allows the use of a low-dose drug that has few side effects.
The synergistic effects of drugs are determined by many factors, such as characteristics of drug, route of administration, and dosage form (Karande and Mitragotri Citation2009; Takasusuki et al. Citation2013). In clinic practice, PLE and FC are easy to mix together before administration or in blood circulation, thereby possibly altering their dosage forms. FC and PLE are physically compatible and chemically stable when mixed in a polypropylene syringe (Trissel et al. Citation1997). Single-syringe combination of ketamine and PLE apparently exerts minimal adverse hemodynamic effects and causes favorable characteristics to emerge (Grace et al. Citation2015; Ozgul et al. Citation2013). Single shot of PLE and rocuronium showed that the potency of rocuronium was significantly enhanced after PLE infusion for 30 min, compared with 2 min of PLE, which suggested that PLE increases the affinity of rocuronium for the receptor during steady-state condition (Stauble et al. Citation2015). These indicated that the mixing of PLE and FC might bring stronger synergistic effect. Our previous work in mouse has showed that the ED50 values of analgesia in the premixed group of PLE and FC decreased to half of that in the sequential group, which may be attributed to the structure of FC citrate (Gao et al. Citation2016). Nonetheless, few studies have reported that the premixing of PLE and FC results in stronger anesthetic effect and fewer side effects in patients compared with traditional sequential administration of FC and profopol. To our knowledge, most of the nanosized drugs were mainly tested in animals, and PLE rarely reported by the view of nano-field in humans.
In this prospective, randomized, single-blinded study of patients undergoing surgical termination of pregnancy, we compared intraoperative bispectral index (BIS) and hemodynamics, postoperative visual analogue scale (VAS), and side effects/complication of simultaneous versus sequential administration of FC-PLE. We hypothesized that simultaneous administration of FC-PLE resulted in more adequate sedation, more sufficient analgesia, more stable hemodynamics, and fewer side effects or complication.
Materials and methods
Study design
This study was approved by the ethics committee of the First Affiliated Hospital of Xi’an Jiaotong University (No. KYLLSL-2015-007-01). The protocol used in this clinical trial was registered at the Chinese Clinical Trial Registry (No. ChiCTR-IPC-15006153). PLE (10 mg/mL) and FC (50 μg/mL) were purchased from Astra Zeneca China Co., Ltd. (Shanghai, China) and Yichang Humanwell Pharmaceutical Co., Ltd. (Hubei, China), respectively.
This study recruited female patients who were scheduled for surgical abortion in the First Affiliated Hospital of Xi’an Jiaotong University between 1 April 2015 and 31 May 2015. The inclusion criteria were as follows: age, 18–40 years old; weight, 45–80 kg; height, 155–175 cm; American Society of Anesthesiologists classification Ι-II grade; and fetal age, 40–55 d. The informed consent was obtained from all patients. The exclusion criteria included the following: previous vaginal delivery; body temperature of >38 °C at surgery; taking β-blockers, anxiolytics, antidepressants, opioids, alcohol, or drug abuse; obesity (body mass index ≥30); having history of cardiac, neurological, or psychiatric disease; and enrollment in other clinical trials.
Study protocol
All patients who satisfied all of the inclusion criteria and none of the exclusion criteria were included and randomly allocated by GraphPad software (La Jolla, CA) into either the simultaneous group or the sequential group in a 1:1 ratio. A research nurse concealed the allocation code of each patient without indicating the grouping in a sealed envelope, which was sent to the anesthesiologist. Only the program analyst and the anesthesiologist were aware of the coding of the patients. Upon arrival in the operating room, the anesthesiologist opened the sealed envelope containing the allocation code of each patient. Both patients and the evaluators were blinded to their group assignment. Appearances of the testing drugs looked similar in both groups.
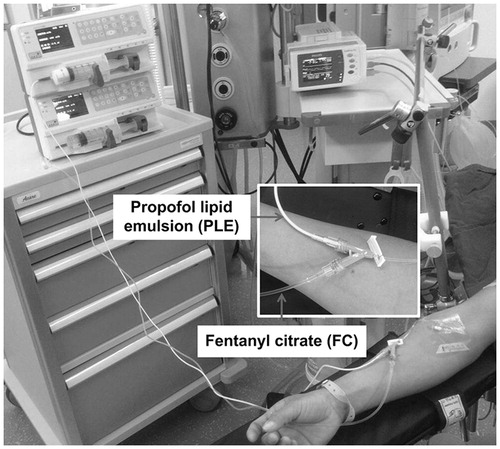
Patients were fasted for at least 6 h and were not provided with preoperative sedatives. All the patients were supported with 4 L/min nasal catheter oxygen inhalation. A 22-gauge intravenous indwelling needle was inserted in preoperative area and the connected Y-shaped cannula was used for administration of the testing drugs (). Manufactures recommend that PLE and FC should be only mixed via a Y-shaped cannula that is close to the patient due to possible instability of the lipid emulsion (Healy and Knight Citation2003; Najafabadi et al. Citation2015; Trissel et al. Citation1997). The main port of the Y-shaped cannula was connected to PLE and the side port was connected to FC. In the simultaneous group, the FC (2 mL; 50 μg/mL) was diluted to 20 mL (5 μg/mL) with saline so that the similar volume of PLE and FC will facilitate sufficient mixing during administration. In the sequential group, the FC (20 mL; 50 μg/mL) was not diluted. PLE was set at a constant rate at 2 mg/kg and FC at 1 μg/kg (CTN-TCI-VI, Beijing Eastern Chieftain Technology Co., Ltd, Beijing, China). After obtaining the baseline values for BP, HR, SpO2, and BIS using an electrocardiogram monitor (Philip, Eindhoven, Holland) and a BIS monitor (Vista, Norwood, MA), the anesthesiologist administered the drugs. In the simultaneous group, both PLE and FC were administered at 18 mL/kg/h within 40 s. In the sequential group, FC (14.4 mL/kg/h) was first administered within 5 s and then PLE (18 mL/kg/h) was administered within 40 s.
The primary outcomes were BIS values and the secondary outcomes were the values of systolic blood pressure (SBP), diastolic blood pressure (DBP), heart rate (HR), oxygen saturation (SpO2), the VAS scores of pain, and side effects. BIS, SBP, DBP, HR, and SpO2 were measured at different time points: before drug administration (a), when the cannula was inserted into the uterine (b), and at the moment of recovery (c). The VAS scores of pain (0: extremely anxious, 10: no anxiety) were measured at the moment of recovery (c), and 15 min after recovery. The eyelash reflex was used to confirm the effect of induction. Abortion was performed by vacuum aspiration with ultrasound monitoring (Belson, Wuxi, China). Dilation was conducted serially using metal dilators, and the surgeon used a cannula with a diameter of approximately 1–3 cm. When the cannula was removed from the uterus at the end of the procedure, an intravenous bolus of 10 U of oxytocin was administered over 10 s. To avoid the effect of oxytocin on hemodynamics, the side effects related to Bp and HR were recorded before administrating the oxytocin.
The side effects or complication included inadequate sedation, excessive sedation, hypertension, hypotension, sinus tachycardia, sinus bradycardia, lachrymation and sweating, movement, respiratory depression, nausea and vomiting, postoperative agitation, and pain at injection site. Inadequate sedation was defined as BIS of >60, and 0.5 mg/kg PLE was administrated. The excessive sedation was defined as BIS of <40. Hypertension was defined as an SBP of >140 mm Hg or DBP of >90 mm Hg. Hypotension was defined as an SBP of <90 mm Hg or DBP of <60 mmHg. Sinus tachycardia was defined as sinus HR of >100 bpm, and sinus bradycardia was defined as sinus HR of <60 bpm. During inadequate analgesia, such as SBP of >140 mmHg or DBP of 90 > mmHg or HR of >100 mmHg or movement, an additional 0.5 μg/kg FC was administrated. Respiratory depression was defined as apnea for lasting more than 30 s or SpO2 less than 90%, positive pressure mask supplemental oxygen and assisted respiration were performed. Patients with missing data were excluded from analysis.
Morphology
To elucidate the potential mechanism of the interaction between PLE and FC, the morphological changes for mixing were analyzed via transmission electron micrographs (TEM, Hitachi, Japan). PLE (10 mg/mL), FC (50 μg/mL), and their mixture with volume ratio of 10:1 were dropped on a copper grid, air dried, and then stained with 3% phosphotungstic acid for TEM.
Statistical analysis
Initial power analysis suggested that a minimum of 45 patients in each group with β of 0.20 (0.80 power) and α of 0.05 is required, assuming an effect size of 0.6 in BIS (G-Power, version 3.1, Duesseldorf, Germany). To compensate for errors and dropouts, 50 patients were included in each group. Data were analyzed using SPSS 19.0 and expressed as mean ± SD (95% CI) or n, where appropriate. Student’s t-test was used to analyze parametric continuous variables. The difference between the groups in terms of the number of patients displaying side effects or complication was evaluated using χ2-test. A P value of <.05 was accepted as statistically significant.
Results
Baseline demographic characteristics
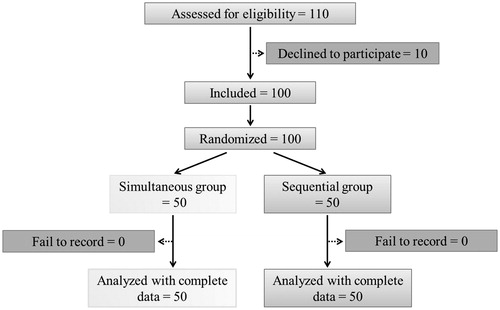
Of 110 patients who met inclusion criteria during the study period, 10 refused to participate in the study, leaving 100 patients enrolled in the study. A total of 50 patients were randomized in each group (). There were no significant differences regarding patients’ age, body weight, height, fetal age, and surgery time between the two groups ().
Table 1. Demographic characteristics at entry.
The performance of sedation and analgesia
The baseline SBP, DBP, HR, SpO2, and BIS were not significantly different between the two groups (). Moreover, none of the BIS, SBP, DBP, HR, and SpO2 values in the groups at the time of recovery showed significant differences. When the cannula was inserted into the uterus, BIS, SBP, DBP, and HR were significantly lower in the simultaneous group than in the sequential group, whereas the SpO2 values were not significantly different between the two groups.
Table 2. Comparison of the performance of sedation and analgesia between both groups.
BIS (the primary outcome) was significantly lower in the simultaneous group compared with the sequential group (50.9 ± 2.7 vs. 54.6 ± 3.3, P < .001; 95% CI: −4.9, −2.5). The secondary outcomes in the two groups, when the cannula was inserted into the uterus, were significantly different. The SBP was significantly lower in the simultaneous group compared with the sequential group (111.9 ± 10.1 mmHg vs. 118.1 ± 13.3 mmHg, P = .010; 95% CI: −10.9, −1.5). In addition, DBP was 78.7 ± 9.2 mmHg in sequential group, significantly higher compared with that in the simultaneous group (P = .029; 95% CI: −6.4, −0.3). HR was 6.5 bpm higher in the sequential group compared to the simultaneous group (P < .001; 95% CI: −9.8, −3.1). However, the VAS scores of pain of the two groups did not significantly differ in the arrival at the recovery room and 15 min after the arrival.
The side effect or complication
The number of side effects or complications, including inadequate sedation, excessive sedation, hypertension, hypotension, sinus tachycardia, sinus bradycardia, lachrymation and sweating, movement, respiratory depression, nausea and vomiting, postoperative agitation, pain at the injection site, additional positive pressure mask supplemental oxygen and assisted respiration, and additional FC and additional PLE were also compared in . Among these side effects or complications, the number of inadequate sedation in sequential group was 2 more than that in simultaneous group, followed with additional PLE, but did not show significant differences between the two groups. The frequencies of hypertension and sinus tachycardia were, respectively, 14% (7/50) and 16% (8/50) in sequential group, which were significantly higher than those in simultaneous group (P = .019, and P = .036). The movement was observed in 10% (5/50) of the patients in the simultaneous group versus the 28% (14/50) frequency in the sequential group (P = .041). Meanwhile, the additional FC administrated for the movement. The frequency of pain at the injection site was 12% (6/50) in the simultaneous group versus 32% (16/50) in the sequential group (P = .030). None of the other side effects/complications happened in both the groups.
Table 3. Comparison of the side effects or complications between both groups.
Morphology
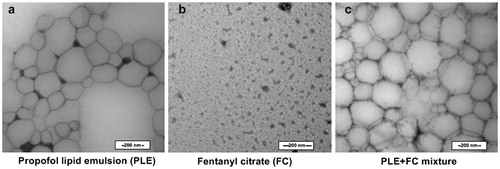
As shown in , the size of PLE was not homogenous (200–300 nm), and large area solution field presented in PLE. Black salt crystal appeared after drying of FC. Compared with PLE, the mixture of PLE and FC had more particles and less solution field with similar particle size, and showed fusiform borderline at the juncture of particles.
Discussion
There has been huge interest in applications of nanomaterials in biomedical science, including diagnosis, drug delivery, and development of human organs. Number of these nanomaterials has been already studied in human or at pre-clinical trial (Adabi et al. Citation2016). The interaction between nanomaterials and common molecular was important (Gao et al. Citation2015). Both the lipid emulsion of propofol and salted prodrug of fentanyl are the hydrophilic formulations of hydrophobic drugs. They are commonly administered in combination in anesthesia practice. These formulations tend to mix in vivo or sometimes in vitro. In our animal experiments, the premixed solution of PLE lipid emulsion and FC citrate at a volume ratio of 20: 2 enhanced synergic analgesia in twofold but did not influence acute toxicity and sedation, compared with individual injections (Gao et al. Citation2016). These results indicated that formulation interaction played an important role in the drug interaction. In clinical settings, not only the characteristics of drugs themselves, but also their formulation should be considered. However, few studies have investigated the effect of mixing on the sedative/analgesic properties of these drugs. In this study, we found that the BIS values in the simultaneous and sequential groups were 50.9 ± 2.7 and 54.6 ± 3.3 (P < .001), respectively (). Maintaining the BIS values between 40 and 60 reduces anesthesia awareness and provides appropriate sedation in general anesthesia (Ekman et al. Citation2004). No patient in both groups show excessive sedation, and there are no significant differences in the incidence of inadequate sedation during operation. These observations illustrated that the PLE and FC doses in our study for most patients resulted in adequate sedation. However, 2 patients in sequential group showed inadequate sedation, and the BIS values in sequential group were higher than that in simultaneous group. Such results illustrated that premixing of FC and PLE had slightly improved propofol to permeate BBB in human.
The signs of inadequate analgesia or nociception in response to surgery included tachycardia, hypertension, lachrymation, sweating, and movement (Ahonen et al. Citation2007). The values for HR, SBP, and DBP, and the incidence of hypertension, sinus tachycardia, and movement during surgery were lower in the simultaneous group than in the sequential group, thereby demonstrating that premixing of PLE and FC enhanced the fentanyl to penetrate the blood cerebrospinal barrier, compared with without premixing before entering body ( and ).
Moreover, the low incidence of movement in the simultaneous group reduced the risk of perforation of the uterus. VAS of pain was measured at the moment of recovery and 15 min after recovery, and the results revealed no significant differences between the two groups. This observation illustrated that the enhanced analgesia by simultaneous administration was not maintained up to the moment of recovery.
Furthermore, no patient in both groups had experienced the SpO2 values lower than 90% and no patient showed apnea for more than 30 s during anesthesia, demonstrating that respiration was not significantly depressed in both groups. HR and BP during the operation fell within the acceptable range, and no patient displayed sinus bradycardia and hypotension, demonstrating that hemodynamics in both groups was not significantly depressed. Sinus tachycardia at the moment of recovery was mostly likely resulted from oxytocin (10 U) administration during surgery. Free propofol is currently regarded as one of the main causes of pain at injection sites (Ohmizo et al. Citation2005; Yamakage et al. Citation2005). FC pretreatment alleviates pain during PLE injection (Ahmad et al. Citation2008). FC-PLE mixture was more effective than FC pretreatment or a placebo in preventing PLE injection pain (Kizilcik et al. Citation2015). Compared with the traditional sequential administration, mixing PLE and FC through simultaneous administration induces low incidence of pain at the injection site, indicating that such mixing method does not cause leakage of higher amount of free propofol compared with sequential administration. The combination of PLE and ketamine results in less hypotension, better sedation, and improved patient comfort and safety, compared with the use of PLE alone (Phillips et al. Citation2010). Although the effect of mixing effect has been studied, the enhanced synergistic effect in the literature is mainly determined by the addition of ketamine, rather than mixing with ketamine.
To elucidate the mechanism of enhanced sedation and analgesia for mixing, the morphological changes of PLE, FC, and their mixture in TEM were compared. PLE consisted of propofol, soybean oil, egg phospholipids, glycerol, and sodium hydroxide. The structure of FC is similar to the cationic surfactant, and FC consisted of hydrophobic part and hydrophilic part with pKa value of 8.99. FC is located at the edge of particle for the amphipathicity and electrostatic adsorption with the negative charged emulsifier of egg phospholipid (Kawakami et al. Citation2006). Thus, the number of particles is increased and fusiform borderline present at the juncture of particles in the mixture of PLE and FC, compared with PLE. After mixing PLE and FC, fentanyl dissociated from citrate for sodium hydroxide or dilution in PLE, and then dissolved in the high hydrophobicity environment of soybean oil in PLE (Tsuji Citation2005). In brief, lipid emulsion in PLE increased the hydrophobicity of FC, which enhanced the analgesia of FC. Meanwhile, FC increased the number of particles in PLE and reduced propofol to release before reaching brain, which enhanced sedation of PLE.
Some unavoidable limitations were encountered in this study. Median effective dose (ED50) should be calculated to accurately compare sedation and analgesia, and the effect of mixing will become more obvious if PLE and FC are mixed in a single syringe, but these methods were abandoned in consideration of the safety of patients. In addition, the analgesia during operation cannot be evaluated by VAS score for sedation. Instead, clinical parameters, such as BIS, BP, HR, and SpO2, were evaluated. Moreover, PLE and FC were premixed by a Y-type venous cannula, which is consistent with the instruction of the use of PLE (Healy and Knight Citation2003). The induction times and recovery times of the groups were not compared because of the delay of manipulation in the sequential group.
In conclusion, compared with sequential injection, simultaneous PLE and FC by simultaneous using a Y-shaped cannula enhanced the analgesia and sedation, stabilized the hemodynamics, resulted in the lower incidence of pain at the injection site, and reduced the requirement of drugs. The simultaneous method in surgical abortion resulted in an appropriate sedation with adequate analgesia; it also caused minimum side effects/complications. Our findings support simultaneous administration of FC and PLE during surgical abortion.
Disclosure statement
The authors have no conflicts of interest to declare.
References
- Adabi M, Naghibzadeh M, Adabi M, Zarrinfard MA, Esnaashari SS, Seifalian AM, et al. 2016. Biocompatibility and nanostructured materials: applications in nanomedicine. Artif Cells Nanomed Biotechnol. 44:1049–1193.
- Ahmad N, Zanariah Y, Balan S. 2008. Fentanyl pre-treatment alleviates pain during injection of propofol-lipuro premixed with lignocaine. Med J Malaysia. 63:431–433.
- Ahonen J, Jokela R, Uutela K, Huiku M. 2007. Surgical stress index reflects surgical stress in gynaecological laparoscopic day-case surgery. Br J Anaesth. 98:456–461.
- Baker MT, Naguib M. 2005. Propofol: the challenges of formulation. Anesthesiology. 103:860–876.
- Cohen LB, Delegge MH, Aisenberg J, Brill JV, Inadomi JM, Kochman ML, Piorkowski JD Jr., Institute AGA. 2007. AGA Institute review of endoscopic sedation. Gastroenterology. 133:675–701.
- Dean G, Jacobs AR, Goldstein RC, Gevirtz CM, Paul ME. 2011. The safety of deep sedation without intubation for abortion in the outpatient setting. J Clin Anesth. 23:437–442.
- Ekman A, Lindholm ML, Lennmarken C, Sandin R. 2004. Reduction in the incidence of awareness using BIS monitoring. Acta Anaesthesiol Scand. 48:20–26.
- Gao W, Sha B, Fan Z, Liu Y, Shen X. 2016. Nanoformulation of premixing propofol lipid emulsion and fentanyl citrate and their effects on acute toxicity, sedation, and analgesia. J Nanomaterials. 2016:8.
- Gao W, Sha B, Liu Y, Wu D, Shen X, Jing G. 2015. The effect of cationic starch on hemoglobin, and the primary attempt to encapsulate hemoglobin. Artif Cells Nanomed Biotechnol. 43:196–202.
- Grace RF, Tang DW, Namel E. 2015. An audit of the haemodynamic and emergence characteristics of single-shot ‘ketofol’. Anaesth Intens Care. 43:503–505.
- Healy TE, Knight PR. 2003. Wylie Churchill-Davidson’s: a practice of anesthesia. 7th ed. New York: Oxford University Press.
- Horlocker TT, Burton AW, Connis RT, Hughes SC, Nickinovich DG, Palmer CM, et al. 2009. Practice guidelines for the prevention, detection, and management of respiratory depression associated with neuraxial opioid administration. Anesthesiology. 110:218–230.
- Ireland LD, Allen RH. 2016. Pain management for gynecologic procedures in the office. Obstet Gynecol Surv. 71:89–98.
- Karande P, Mitragotri S. 2009. Enhancement of transdermal drug delivery via synergistic action of chemicals. Biochim Biophys Acta. 1788:2362–2373.
- Kawakami K, Oda N, Miyoshi K, Funaki T, Ida Y. 2006. Solubilization behavior of a poorly soluble drug under combined use of surfactants and cosolvents. Eur J Pharm Sci. 28:7–14.
- Kizilcik N, Menda F, Bilgen S, Keskin O, Koner O. 2015. Effects of a fentanyl-propofol mixture on propofol injection pain: a randomized clinical trial. Korean J Anesthesiol. 68:556–560.
- Lindholm P, Helbo-Hansen HS, Jensen B, Bulow K, Nielsen TG. 1994. Effects of fentanyl or alfentanil as supplement propofol anaesthesia for termination of pregnancy. Acta Anaesthesiol Scand. 38:545–549.
- Najafabadi AH, Azodi-Deilami S, Abdouss M, Payravand H, Farzaneh S. 2015. Synthesis and evaluation of hydroponically alginate nanoparticles as novel carrier for intravenous delivery of propofol. J Mater Sci Mater Med. 26:145.
- Ohmizo H, Obara S, Iwama H. 2005. Mechanism of injection pain with long and long-medium chain triglyceride emulsive propofol. Can J Anaesth. 52:595–599.
- Ozgul U, Begec Z, Karahan K, Ali Erdogan M, Said Aydogan M, Colak C, Durmus M, Ozcan Ersoy M. 2013. Comparison of propofol and ketamine-propofol mixture (ketofol) on laryngeal tube-suction ii conditions and hemodynamics: a randomized, prospective, double-blind trial. Curr Therap Res Clin Exp. 75:39–43.
- Phillips W, Anderson A, Rosengreen M, Johnson J, Halpin J. 2010. Propofol versus propofol/ketamine for brief painful procedures in the emergency department: clinical and bispectral index scale comparison. J Pain Palliat Care Pharmacother. 24:349–355.
- Stauble CG, Stauble RB, Schaller SJ, Unterbuchner C, Fink H, Blobner M. 2015. Effects of single-shot and steady-state propofol anaesthesia on rocuronium dose-response relationship: a randomised trial. Acta Anaesthesiol Scand. 59:902–911.
- Takasusuki T, Yamaguchi S, Hamaguchi S, Yaksh TL. 2013. Effects of general anesthetics on substance P release and c-Fos expression in the spinal dorsal horn. Anesthesiology. 119:433–442.
- Trissel LA, Gilbert DL, Martinez JF. 1997. Compatibility of propofol injectable emulsion with selected drugs during simulated Y-site administration. AJHP. 54:1287–1292.
- Tsuji A. 2005. Small molecular drug transfer across the blood-brain barrier via carrier-mediated transport systems. NeuroRx. 2:54–62.
- Yamakage M, Iwasaki S, Satoh J, Namiki A. 2005. Changes in concentrations of free propofol by modification of the solution. Anesth Analges. 101:385–388.