Abstract
The use of lipidic nanoparticles can overcome the limitations of skin penetration of hydrophilic charged materials. Oleic acid as an ion pairing agent was used to enhance the encapsulation of positively charged arginine into lipophilic nanostructured lipid carrier (NLC). Histological study on the male hamsters was performed to assess the efficiency of drug delivery on hair growth. The accumulation of NLCs in the hair follicles was verified and in vivo studies showed more new hair follicle growth by utilizing arginine-loaded NLCs than arginine aqueous solution. It was concluded that employing ion pairing agent could enhance drug encapsulation into NLCs structure.
Graphical Abstract
Introduction
Hair loss, or alopecia, affects millions of men and women of all ages and frequently has significant social and psychological consequences. Alopecia decreases self-confidence and limits one’s ability to enjoy life, which leads to some degree of depression and even suicide (Aggarwal et al. Citation2010; Kaufman Citation2002; Pérez-Ornelas et al. Citation2005; Sintov et al. Citation2000). Recently, a newly-proposed formulation was patented based on arginine for topical application to promote hair growth (Cifra et al. Citation2004; Fossel Citation2009, Citation2013; Mészáros and Fuentes Citation2006; Taylor et al. Citation2011). Arginine is an amino acid which acts as the physiological precursor for nitric oxide (NO) synthesis in animal cells. In blood vessels, NO released by endothelial cells activates guanylyl cyclase in smooth muscle cells, thereby elevating cellular cGMP concentrations and causing smooth muscle relaxation. NO is of interest where hair loss and growth are concerned because of its role as vasodilator in the stimulation of hair growth. NO is also involved in opening potassium channels, the activity mechanism of minoxidil, the well-known FDA approved medicine administered in alopecia. Topical drug delivery is an essential objective for drugs used in alopecia to maximize treatment efficiency and minimize the associated side effects. However, penetration of the skin by charged and/or hydrophilic compounds is restricted. Obviously, the success of this strategy would depend on a suitable carrier, which could improve skin accumulation (Raj et al. Citation2015; Sintov et al. Citation2000; Tabbakhian et al.Citation 2006). Small particles are increasingly being employed in dermatological and cosmetic products that are applied to the skin. Since epidermal lipids are found in high amounts within the penetration barrier, lipid carriers such as liposomes, solid lipid nanoparticles (SLNs), and nanostructured lipid carriers (NLCs) appear to be promising carriers by attaching themselves to the skin surface and allowing lipid exchange between the outermost layers of the stratum corneum and the carrier (Agrawal et al. Citation2015; Alnasif et al. Citation2014; Lauterbach et al. Citation2014). NLCs have recently attracted serious focus as a novel colloidal drug vehicle, because it has several advantages over the other mentioned carriers, including satisfactory stability, drug loading, and skin permeation and accumulation (Cheng-Ying et al. Citation2015; Gupta and Trivedi Citation2015; Weber et al. Citation2014). However, due to its high hydrophilicity, encapsulating arginine into NLC formulations can be challenging. To overcome this issue, the pairing of ions with a lipophilic material (oleic acid) was designed. This novel concept enhanced the encapsulation of arginine into NLCs and was used for the follicular delivery of arginine for the treatment of alopecia.
Materials and methods
Materials
Arginine, o-phthaldialdehyde (OPA) and Poloxamer® 407 were supplied from Sigma-Aldrich Company (St. Louis, MO). Glyceryl palmtostearate (Precirol ATO-5®) was prepared from Gattefossé Company (Saint Priest, France). Oleic acid (Merck Chemicals, Darmstadt, Germany), and diethyl ether (KianKaveh Pharmaceutical and Chemical Company, Tehran, Iran) were used as received. Rhodamine B and 3-Mercaptopropionic acid were obtained from Merck Chemicals Company (Darmstadt, Germany).
Preparation of arginine-loaded NLC
Nanostructured lipid carriers formulations were prepared by the hot melt homogenization method as described previously (Hamishehkar et al. Citation2014). Briefly, Precirol® (800 mg) and oleic acid (200 mg) were melted at about 75 °C. Subsequently, arginine (100 mg) was dissolved in water (0.7 mL) and added dropwise into the oil phase under stirring at 20,000 rpm by homogenizer [DIAX 900, Heidolph, Germany) for 15 min]. Subsequently, aqueous phase (containing Poloxamer® 1.5% w/v, 30 mL) was dropped into the oil phase, keeping the temperature at 75 °C and the same stirring rate. Finally, after 15 min, the hot formulations were allowed to cool down at room temperature. Each formulation was prepared and characterized in triplicate (Pezeshki et al. Citation2014).
Characterization of drug-loaded NLC
Particle size and zeta potential determination
Particle size and zeta potential of prepared NLCs formulations were analyzed by laser diffraction technique using a particle size analyzer (Nano ZS, Malvern, UK). Prior to measurement, NLCs formulation samples were diluted with double-distilled water. Each sample was measured in triplicate.
Scanning electron microscopy
The scanning electron microscopy (SEM) photographs of prepared NLCs formulations were obtained using scanning electron microscope MIRA3 TESCAN instrument (Tescan, Brno, Czech Republic) operating at 15 kV. The specimens were mounted on a metal stub with double-sided adhesive tape and coated under vacuum with gold in an argon atmosphere prior to observation using a direct current sputter technique (DST1, Nanostructured Coating Co., Tehran, Iran).
Encapsulation efficiency and loading capacity percent
The encapsulation efficiency percent (EE%) and loading capacity percent (LC%) values were expressed as the percentage of encapsulated drug to the added drug or to the used lipid, respectively. EE% was determined by first separation of the un-entrapped drug by centrifugation method using Amicon® Ultra-15 with the MWCO of 100 kDa (Millipore, Schwalbach, Germany) tubes. The formulation was added to the upper chamber of the Amicon® tube and then the tube was centrifuged (3K30, Sigma, Ostrode, Germany) at 5000 rpm for 5 min. The clear solution in the bottom of Amicon® tube was used for the determination of unloaded arginine (Mohammadi et al. Citation2014). The concentration of arginine was determined by measuring the concentration of unloaded drug, after conjugation with OPA, using UV/Visible spectrophotometer (1601, Shimadzu, Kyoto, Japan) (λmax = 336 nm, linear in the range of 2–30 μg/mL with R2 = 0.9995) (Kutlán et al. Citation2002). The EE% and LC% values of arginine-loaded NLCs formulations were calculated mathematically according to the following equation:
where, W(Initial drug) is the amount of initial added drug and W(Free drug) is the amount of unloaded drug detected in the lower chamber of Amicon® tubes after centrifugation of the NLCs formulations. Accordingly, W(Encapsulated drug) is the amount of loaded drug and W(Total lipid) is the amount of used lipid in the preparation process (Ghaderi et al. Citation2015). The formulations in the upper chamber of Amicon® tubes were rinsed five times by water to eliminate unloaded arginine. These rinsed formulations were used for the rest experiments.
X-ray diffraction study
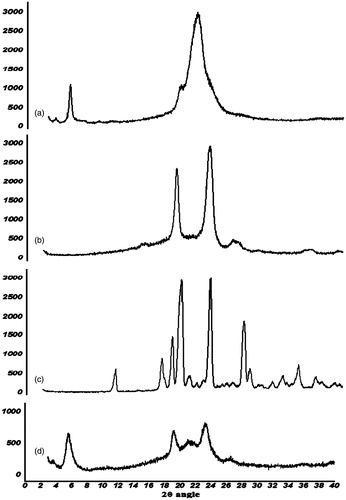
In order to evaluate the effect of NLCs preparation process on crystallographic patterns of arginine and lipids, X-ray diffraction (XRD) analysis was performed using an X-ray diffractometer (D-5000, Siemens, Karlsruhe, Germany, 2° to 40°) to assess the crystalline structures of Arginine, Precirol®, Poloxamer®, and vacuum oven-dried powder of optimized NLCs formulation. The diffraction pattern was measured using a Cu-Kα radiation source (30 mA and 40 kV).
Fourier transform infrared spectroscopy
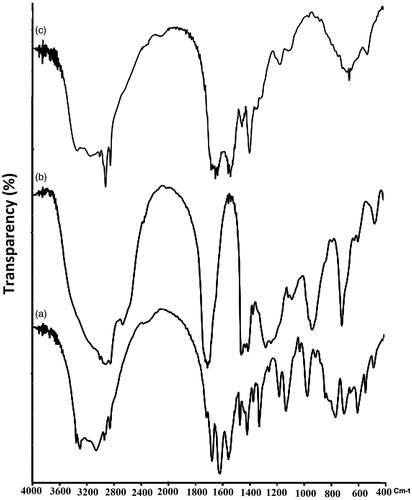
Fourier transform infrared spectroscopy (FTIR) spectra of pure drug, oleic acid and their mixture were obtained separately using FTIR spectrometer (8400S, Shimadzu, Kyoto, Japan). The potassium bromide discs were prepared by compressing the powders at pressure of 15 tons for 10 min in hydraulic press. Scans were obtained at a resolution of 2 cm−1, from 4000 to 400 cm−1. Arginine was dissolved in water and added to heated oleic acid under homogenizer (the same situation as preparation of NLCs excluding the solid lipid). The arginine-oleic acid mixture was used to investigate the possible ionic interaction by FTIR experiment.
In vitro drug release study
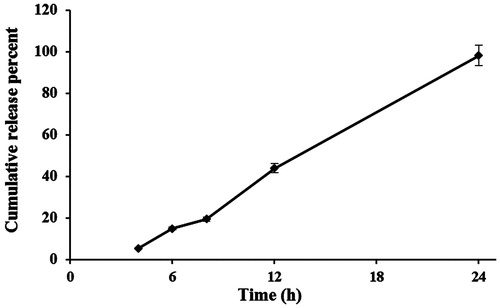
The drug release study was conducted immediately after separation of free arginine from NLCs formulations. Samples of optimized formulation (1.5 mL) were placed in the dialyzer and 120 mL of phosphate buffered saline (PBS) was used as dissolution medium (37 ± 2 °C). Samples from dissolution medium (2 mL) were collected at predetermined time intervals and replaced with equal volume of fresh medium and analyzed spectrophotometrically after derivatization at λ = 336 nm. Drug concentration was calculated using a standard calibration curve and expressed as cumulative released percent. The release study was performed in triplicates.
In vivo studies
The hamster flank organ has served as a model to study the therapeutic strategies against alopecia (Adachi and Kano Citation1972; Lucky et al. Citation1986). Rhodamine B-loaded NLCs formulation was contacted with hamster skin for 3 h and then hamster was scarified and skin was cut by a freeze-microtome (Frigocut® 2800 N, Leica, Bensheim, Germany) vertically in 10 μm slices. The dye distribution in the skin was investigated by fluorescence microscopic (BX50, Olympus Corp., Tokyo, Japan) (Babaie et al. Citation2015). The Rhodamine B solution in the same concentration with NLC formulation was applied on the other side of hamster skin as a control group. In the next step, 20 young male hamsters were used for in vivo experiment (weighing 70–120 g). The hamsters’ skin was shaved (1 cm2) on the dorsal side with the electrical razors. The hamsters were divided randomly into three groups (n = 6). In the first group, 200 μL of the arginine solution (the same arginine concentration in NLCs formulation) was applied on the left side flank organ of hamster twice a day for 8 weeks. In the second group, 200 μL of blank NLCs formulations, as a control, were applied twice a day on the left side of the treated skin and in the third group, arginine-loaded NLCs formulation was administrated. After 50 days, the animals were killed by over inhalation of anesthetic gases and the skin (1 cm2) was cut by a surgical blade. Samples were fixed in 10% phosphate buffer formalin for 24 h and then dehydrated in a descending series of ethyl alcohol, cleared in xylene and embedded in paraffin. Paraffin sections were cut vertically into slices (5 μm thickness) by a rotary microtome (Leitz, 1512, Wetzlar, Germany), mounted on slides, stained with hematoxylin-eosin (H&E staining) and examined under a light microscope (BX50, Olympus Corp., Tokyo, Japan) (Hamishehkar et al. Citation2014). The area of the projected image of hair follicles was determined by an expert pathologist with an image analyzer software (Scion Image, version Alpha 4.0.3.2, Scion Corporation, Frederick, MD). Evaluations were performed by two independent observations, with an agreement level of 92%. Data analysis was performed using Statistical Package for Social Sciences (SPSS) 20.0 (SPSS, Chicago, IL). Results were stated as the mean ± standard deviations. The independent-samples t-test was used to compare follicle areas between untreated and treated skins on both sides of hamsters in three studied groups.
Results and discussion
Characterization of arginine-loaded NLCs formulations
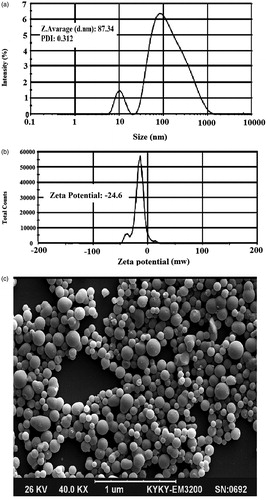
Arginine is not soluble in molten lipid, and its dispersion in molten lipid phase results in a low drug-loading capacity. Therefore, to maximize drug encapsulation and loading capacity into NLCs, drug was dissolved in the aqueous phase and added into the molten lipid phase to form the initial W/O emulsion. Then, the external aqueous phase containing polymeric surfactant was added to form the W/O/W emulsion. After cooling down the formulation, emulsion droplets were converted to nanoparticles. The formed nanoparticles were stabilized by the presence of a polymeric surfactant (Poloxamer®). The particle size distribution, SEM image, and zeta potential of the optimized formulation are shown in . The uniform distribution of NLC particles was clearly illustrated by SEM imaging (). The uniform size distribution enhances the predicted and reproducible skin penetration of drug following the administration of dosage form (Alomrani et al. Citation2015; Alvarez-Román et al. Citation2004; Dragicevic-Curic et al. 2004; Iannuccelli et al. Citation2014). EE% and LC% values of optimized NLC formulations were 68 ± 3.4% and 10 ± 1.2%, respectively. In the face of the high aqueous solubility of arginine (148.7 mg/mL), these high EE% and LC% values are important and valuable achievements.
Figure 1. Particle size distribution (a), zeta potential value (b), and scanning electron microscopy image of optimized formulation (c).
The crystalline state of the nanoparticles was analyzed using the X-ray diffraction method (). The diffraction pattern of arginine exhibited several sharp peaks, indicating the crystalline nature of arginine. The characteristic main peaks for arginine (2θ = 11.24, 17.15, 18.47, 19.52, 23.28, 27.53, 28.3, 32.47, and 34) were omitted from the XRD pattern of NLC formulations compared with pure arginine, suggesting that arginine was uniformly and molecularly distributed in the lipid matrix. The diffraction pattern of Precirol® in NLCs was much weaker than that of bulk lipid (2θ = 5.3, 2θ = 19.5, and 2θ = 21.5), which indicates that Precirol® in the arginine-loaded NLCs was partially recrystallized and formed less-ordered crystals. These types of less-crystalline structures would contribute to a higher drug-loading capacity. Both pieces of evidence regarding the conversion of crystalline drug to a molecularly distributed one and the reduction in the crystalline status of lipids interpret the findings of the current study in achieving high drug loading (10%) for the hydrophilic and ionic drug arginine into NLC formulations. In addition to these advantages, a decrease in lipid crystallinity may show a drawback in the gradual instability of NLC structures becoming a crystalline one during the storage period of the formulation. However, this procedure depends on the crystal change kinetic rate. Probably, the rate would be low enough to guarantee formulation stability during the shelf life of the product.
Figure 2. The X-ray diffractions pattern of precirol (a), Poloxamer® (b), arginine (c), and nanostructured lipid carrier formulation (d).
The FTIR experiment was carried out to investigate the possible ion-pair interaction between arginine and oleic acid (). The peak associated with the N-H stretch (3285 cm−1) in arginine was shifted to a higher value (3310 cm−1) in the mixture of arginine-oleic acid. Moreover, the peaks corresponding with the C = O O-H bends (carboxylic acid) at 1709 cm−1 and 949 cm−1 in oleic acid were shifted to 1695 cm−1 and 1012 cm−1, respectively, in the mixture of arginine-oleic acid. These changes indicate the interaction of arginine and oleic acid in the formulation. This finding explains how a hydrophilic material (such as arginine) can be uniformly distributed in the lipophilic (Precirol®) matrix as concluded from the XRD experiment. The high LC% value reported during the encapsulation study was also elucidated by the result of the FTIR experiment. Oleic acid is an oil phase emulsifier, which existed in melted lipid phase (20% of oil phase). The water droplets containing arginine was surrounded by oleic acid to make a primary w/o emulsion. This could also increase the chance of arginine-oleic acid interaction for observed elevated EE and LC values for arginine.
In vitro drug release
shows the release profile of arginine from the NLC formulation. The release profile indicates that arginine was released from the NLCs in an almost zero-order kinetic pattern after about 3 h of lag time. To analyze the release kinetics of arginine from NLCs, the data of release were subjected to the following equations: zero-order equation: Qt = K0.t, where Qt is the percentage of drug released at time t and k0 is the release rate constant; first-order equation: ln(100 - Qt)=ln100−k1.t where k1 is the release rate constant; Higuchi’s equation: Qt = KH .t1/2, where KH is the Higuchi release rate constant; Hixson-Crowell: (100−Qt)1/3 = 1001/3−kHc.t, where kHc is the Hixson-Crowell rate constant (Hamishehkar et al. Citation2015b). Mean percent error and R-squared values for zero-order, first-order, Higuchi, and Hixson-Crowell were 11.01 and 0.994, 212.46 and 0.932, 31.96 and 0.978, and 83.10 and 0.965 indicating the fit of zero-order kinetic to arginine release pattern. The release procedure was completed after 24 h (98.5% of loaded arginine).
In vivo studies
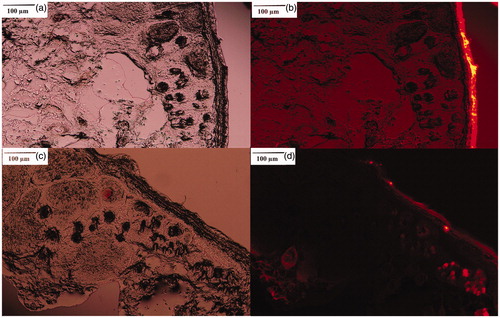
and shows the skin penetration capability of Rhodamine B solely in the solution after 3 h of administration on skin. The results illustrate that, although the physicochemical characteristics of Rhodamine B (MW of 422 and log P of 1.95) are suitable for skin penetration (MW <500 and log P of 1–4), no evidence of skin penetration of Rhodamine B was observed, even after 3 h. A minor penetration of Rhodamine B solution might be due to its close contact with the superficial junctions of the SC and the furrows between the corneocyte islands and hair follicles, allowing superficial spreading of the active agents (Ghanbarzadeh et al. Citation2015). However, the follicular accumulation of Rhodamine B-loaded NLCs ( and ) compared to unpenetrated Rhodamine B in solution proves the key role of NLCs in efficient follicular drug delivery.
Figure 5. Rhodamine B penetration into hamster skin: staining of hamster skin following the application of 0.001% Rhodamine B solution (a: light microscopic image, b: fluorescent microscopic image) and 0.001% Rhodamine B-loaded nanostructured lipid carrier (c: light microscopic image, d: fluorescent microscopic image).
The results of the histological studies for the aqueous solution of arginine, blank NLCs, and arginine-loaded NLCs, with respect to their ability to influence hair growth, are shown in . The histological qualitative () and quantitative () assessment demonstrated the enlargement of hair follicles area () than untreated skin () after 8 weeks. No significant change between untreated ( and ) and treated ( and () flanks in the skin histology of control groups (aqueous solution of arginine and blank NLCs) were observed (). The comparative results of and also verify the safety of the used NLC formulations for topical administration, at least for 8 weeks. The lipidic structure of the newly-developed nanocarriers might be responsible for their capability for safe action in topical administration.
Figure 6. Effect of aqueous solution of arginine (b), blank nanostructured lipid carrier (NLC) (d) and arginine-loaded NLC (f) on the hair follicles compared with untreated skin (a, c, and e, respectively).
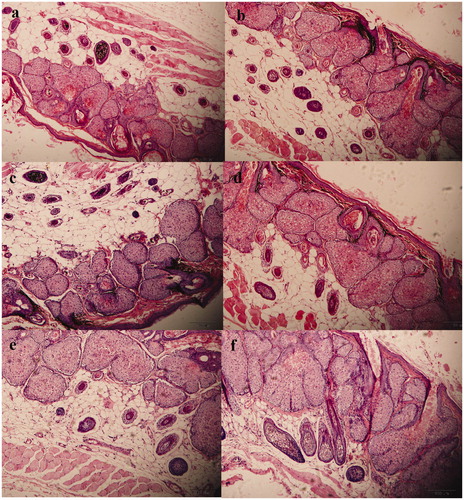
Table 1. The histological effect of arginine-loaded nanostructured lipid carrier (NLC) on hair follicles.
The merit of using lipid nanoparticles in follicular drug delivery has been previously reported (Puglia et al. Citation2008). The authors of the current report also had a successful experience on the delivery of flutamide to hair follicles by means of SLNs (Hamishehkar et al. Citation2015a).
Conclusions
Improving the skin penetration of hydrophilic and/or charged drugs by nanoparticular carrier systems has attracted increasing interest. The Precirol®-oleic acid based arginine-loaded NLC formulations showed appropriate drug EE% and LC% values. The importance of the ion-pair interaction between carrier composition and drug was verified by XRD and FTIR experiments. In vivo studies indicated the efficient performance of the developed NLC particles. The presented oleic acid-based NLC formulations may be introduced as a successful template carrier for dermal delivery of positively charged hydrophilic drug, especially amino acids and peptides which have recently become popular in cosmeceutical sciences.
Acknowledgements
This paper was extracted from Pharm.D. thesis (No. 3742, ethical code: TBZMED.REC.1394.896) submitted to the Faculty of Pharmacy of Tabriz University of Medical Sciences and financially supported by Drug Applied Research Center.
Disclosure statement
The authors report no conflicts of interest.
References
- Adachi K, Kano M. 1972. The role of receptor proteins in controlling androgen action in the sebaceous glands of hamsters. Steroids. 19:567–574.
- Aggarwal S, Thareja S, Verma A, Bhardwaj TR, Kumar M. 2010. An overview on 5α-reductase inhibitors. Steroids. 75:109–153.
- Agrawal U, Gupta M, Vyas S. 2015. Capsaicin delivery into the skin with lipidic nanoparticles for the treatment of psoriasis. Artif Cells Nanomed Biotechnol. 43:33–39.
- Alnasif N, Zoschke C, Fleige E, Brodwolf R, Boreham A, Rühl E, et al. 2014. Penetration of normal, damaged and diseased skin-an in vitro study on dendritic core-multishell nanotransporters. J Control Release. 185:45–50.
- Alomrani AH, Al-Agamy MH, Badran MM. 2015. In vitro skin penetration and antimycotic activity of itraconazole loaded niosomes: various non-ionic surfactants. J Drug Deliv Sci Technol. 28:37–45.
- Alvarez-Román R, Naik A, Kalia YN, Fessi H, Guy RH. 2004. Visualization of skin penetration using confocal laser scanning microscopy. Eur J Pharm Biopharm. 58:301–316.
- Babaie S, Ghanbarzadeh S, Davaran S, Kouhsoltani M, Hamishehkar H. 2015. Nanoethosomes for dermal delivery of lidocaine. Adv Pharm Bull. 5:549.
- Cheng-Ying S, Rui-Sheng L, Bao-De S, Gang S, Li-Qiang W, Juan Z, et al. 2015. Influence of drug physicochemical characteristics on in vitro transdermal absorption of hydrophobic drug nanosuspensions. Drug Dev Ind Pharm. 41:1997–2005.
- Cifra PN, Dake M, Elkins CJ, Waugh J. 2004. Cosmetic formulations containing L-arginine oligomers. US Patent Application No. 10/505,299.
- Dragicevic-Curic N, Gräfe S, Gitter B, Winter S, Fahr A. 2010. Surface charged temoporfin-loaded flexible vesicles: in vitro skin penetration studies and stability. Int J Pharm. 384:100–108.
- Fossel E. 2013. Topical delivery of L-arginine to cause beneficial effects. US Patent No. 8,603,519.
- Fossel ET. 2009. Topical delivery of L-arginine to cause beneficial effects. US Patent Application No. 12/608,169.
- Ghaderi S, Ghanbarzadeh S, Hamishehkar H. 2015. Evaluation of different methods to produce nanoparticle containing gammaoryzanol for potential use in food fortification. Pharm Sci. 20:130–134.
- Ghanbarzadeh S, Hariri R, Kouhsoltani M, Shokri J, Javadzadeh Y, Hamishehkar H. 2015. Enhanced stability and dermal delivery of hydroquinone using solid lipid nanoparticles. Colloids Surf B Biointerfaces. 136:1004–1010.
- Gupta V, Trivedi P. 2015. Ex vivo localization and permeation of cisplatin from novel topical formulations through excised pig, goat, and mice skin and in vitro characterization for effective management of skin-cited malignancies. Artif Cells Nanomed Biotechnol. 43:373–382.
- Hamishehkar H, Ghanbarzadeh S, Sepehran S, Javadzadeh Y, Adib ZM, Kouhsoltani M. 2015a. Histological assessment of follicular delivery of flutamide by solid lipid nanoparticles: potential tool for the treatment of androgenic alopecia. Drug Dev Ind Pharm. 42:846–853.
- Hamishehkar H, Nokhodchi A, Ghanbarzadeh S, Kouhsoltani M. 2015b. Triamcinolone acetonide oromucoadhesive paste for treatment of aphthous stomatitis. Adv Pharm Bull. 5:277.
- Hamishehkar H, Shokri J, Fallahi S, Jahangiri A, Ghanbarzadeh S, Kouhsoltani M. 2014. Histopathological evaluation of caffeine-loaded solid lipid nanoparticles in efficient treatment of cellulite. Drug Dev Ind Pharm. 41:1640–1646.
- Iannuccelli V, Bertelli D, Romagnoli M, Scalia S, Maretti E, Sacchetti F, Leo E. 2014. In vivo penetration of bare and lipid-coated silica nanoparticles across the human stratum corneum. Colloids Surf B Biointerfaces. 122:653–661.
- Kaufman KD. 2002. Androgens and alopecia. Mol Cell Endocrinol. 198:89–95.
- Kutlán D, Presits P, Molnár-Perl I. 2002. Behavior and characteristics of amine derivatives obtained with o-phthaldialdehyde/3-mercaptopropionic acid and with o-phthaldialdehyde/N-acetyl-l-cysteine reagents. J Chromatogr. 949:235–248.
- Lauterbach A, Müller-Goymann CC. 2014. Comparison of rheological properties, follicular penetration, drug release, and permeation behavior of a novel topical drug delivery system and a conventional cream. Eur J Pharm Biopharm. 88:614–624.
- Lucky A, McGuire J, Nydorf E, Halpert G, Nuck B. 1986. Hair follicle response of the golden Syrian hamster flank organ to continuous testosterone stimulation using silastic capsules. J Invest Dermatol. 86:83–66.
- Mészáros GL, Fuentes J. 2006. Topical pharmaceutical compositions comprising L-arginine and uses thereof. EP1676570 A1.
- Mohammadi M, Ghanbarzadeh B, Hamishehkar H. 2014. Formulation of nanoliposomal vitamin D3 for potential application in beverage fortification. Adv Pharm Bull. 4:569.
- Pérez-Ornelas V, Cabeza M, Bratoeff E, Heuze I, Sánchez M, Ramírez E, Naranjo-Rodríguez E. 2005. New 5alpha-reductase inhibitors: in vitro and in vivo effects. Steroids. 70:217–224.
- Pezeshki A, Ghanbarzadeh B, Mohammadi M, Fathollahi I, Hamishehkar H. 2014. Encapsulation of vitamin A palmitate in nanostructured lipid carrier (NLC)-effect of surfactant concentration on the formulation properties. Adv Pharm Bull. 4:563.
- Puglia C, Blasi P, Rizza L, Schoubben A, Bonina F, Rossi C, Ricci M. 2008. Lipid nanoparticles for prolonged topical delivery: an in vitro and in vivo investigation. Int J Pharm. 357:295–304.
- Raj R, Mongia P, Ram A, Jain N. 2015. Enhanced skin delivery of aceclofenac via hydrogel-based solid lipid nanoparticles. Artif Cells Nanomed Biotechnol. 44:1434–1439.
- Sintov A, Serafimovich S, Gilhar A. 2000. New topical antiandrogenic formulations can stimulate hair growth in human bald scalp grafted onto mice. Int J Pharm. 194:125–134.
- Tabbakhian M, Tavakoli N, Jaafari MR, Daneshamouz S. 2006. Enhancement of follicular delivery of finasteride by liposomes and niosomes 1. In vitro permeation and in vivo deposition studies using hamster flank and ear models. Int J Pharm. 323:1–10.
- Taylor RP, Lalvani KS, Lalvani A. 2011. Composition for the treatment of hair loss and baldness. US Patent No. 8,883,131.
- Weber S, Zimmer A, Pardeike J. 2014. Solid lipid nanoparticles (SLN) and nanostructured lipid carriers (NLC) for pulmonary application: a review of the state of the art. Eur J Pharm Biopharm. 86:7–22.