Abstract
AIDS is one of the global pandemic diseases that results from infection by HIV and was estimated 34.2 million people infected in 2011 by this virus. The investigators had previously shown that by early antiretroviral treatment, the risk of AIDS/HIV-related illness and transmission reduced significantly. Nanomaterials could be applied to improving the ability and sensitivity of sensors to detect serum biomarkers with low-sample volume. Moreover, results can be obtained faster. In this paper, we present a review of several experimental studies for HIV electrochemical detection based on nanomaterials. Furthermore, we explained each assay and detection limited.
Introduction
Nanomaterials are materials by nanoscale dimension (at least in one dimension) that preparation and synthesis by various methods (Farkhani and Valizadeh Citation2014, Valizadeh and Farkhani Citation2014, Valizadeh et al. Citation2016), and used in many different ways (Ajayan and Zhou Citation2001, Badrzadeh et al. Citation2016, Farkhani et al. Citation2014, Sohrabi et al. Citation2016, Valizadeh and Khosroushahi Citation2015, Valizadeh et al. Citation2012, Valizadeh Citation2015). Nanomaterials applied for detecting and diagnosis of different targets include (Valizadeh Citation2015):
Macromolecules such as:
Protein (Boeneman et al. Citation2010, Courty et al. Citation2006)
RNA and DNA (Cao et al. Citation2002)
Virus such as HIV (Kim et al. Citation2009), hepatitis B (Wang et al. Citation2010), influenza A (Cui et al. Citation2011) and etc.
Cells such as:
Bacteria such as E. coli (Hirschey et al. Citation2006), Salmonella Typhimurium (Yang and Li Citation2006), Staphylococcus aureus (Xue et al. Citation2009) and etc.
Eukaryotic cells such as human mesenchymal stem cells (Seleverstov et al. Citation2006), cancer cells (El-Sayed et al. Citation2005) and etc.
One of the global pandemic diseases is Acquired immunodeficiency syndrome (AIDS) that result from infection by the human immunodeficiency virus (HIV). It was estimated 34.2 million people infected by this virus in 2011 (Mohammed Fayaz et al. Citation2012, Valizadeh Citation2015). The HIV virus is one of the most variable viruses with a diameter of about 120 nm that can be seen as a biological nanostructure (Valizadeh Citation2015). HIV genetic material is RNA form and containing 9 genes that encoding 19 proteins (Valizadeh Citation2015). The investigators had previously shown that by early antiretroviral treatment, the risk of AIDS/HIV-related illness and transmission reduced to 96% (Cohen et al. Citation2011, Valizadeh Citation2015). Nanomaterials could be applied to achieve this goal by improving the ability to detect serum biomarkers of the blood-borne infectious diseases with low sample volume, more sensitivity, and rapidity in compare with currently methods (such as ELISA, Western Blotting assay, and particle agglutination assay and etc.) (Klostranec et al. Citation2007, Valizadeh Citation2015). The major viral markers of HIV-1 that have been used for detecting HIV-1 infection, monitoring disease progression, screening blood donors, and evaluating HIV-1 therapy are RNA, capsid protein (Tat, JCV, and p24 antigen), and anti-HIV antibodies (Fiebig et al. Citation2003, Petersen et al. Citation1994, Tang et al. Citation2007). Also, there are many studies about Nanomaterials that applied for HIV/AIDS treatment (Kumar et al. Citation2015). Here, we review several electrochemical detection methods that applying nanomaterials for improving sensitivity in 5 categories including: (1) Cyclic Voltammetry (CV), (2) Square Wave Voltammetry (SWV), (3) Electrochemical Impedance Spectroscopy (EIS), (4) Difierential Pulse Voltammetry (DPV), (5) Hybrid method.
Electrochemical detection methods
Various electrochemical biosensors design and fabricated for detection of targets (Anik et al. Citation2016, Dalkıran et al. Citation2016, Erdem et al. Citation2014). There are several electrochemical detection methods such as:
Cyclic voltammetry
The CV is a type of potentiodynamic electrochemical measurement that the working electrode potential is ramped linearly versus time. In a cyclic voltammogram, the current at the working electrode is plotted versus the applied voltage to give the cyclic voltammogram trace. The CV is commonly applied for detection and characterization of the electrochemical properties of an analyte in solution (Bard and Faulkner Citation1980, Nicholson and Shain Citation1964).
Square wave voltammetry
The Square Wave Voltammetry (SWV) is a form of linear potential sweep voltammetry. In this method, the current at a working electrode is measured, while the potential between a reference electrode and the working electrode is swept linearly in time (Mirceski et al. Citation2007, Osteryoung and Osteryoung Citation1985).
Electrochemical impedance spectroscopy
Electrochemical impedance Spectroscopy (EIS) or the Alternating Current (AC) impedance is a sensitive technique, which in the studied system monitors the electrical response after application of a periodic small amplitude AC signal. In this method, the system response provides information concerning the interface state (presence of adsorbed analyte or species) and can detect the occurrence of interfacial reactions (Hassen et al. Citation2008). Because of using electrochemically active labels, the electrochemical-based detection methods can be considered as one of the most sensitive tools for the detection of enzymes, antibody and nucleic acids (Laczka et al. Citation2009). An electrochemical biosensor array has many advantages such as low-detection limit, inexpensive instrument, and simplicity due to ease of obtaining electrical signal (Zhang et al. Citation2010). EIS as Nyquist plots were reported as an effective method to monitor the surface characteristics and thus allow an understanding of the chemical transformation and processes associated with the conductive surface of electrode at different modification steps (Rezaei et al. Citation2011).
Differential pulse voltammetry
Differential Pulse Voltammetry (DPV) is sensitive and extremely selective for measuring trace levels and the quantitative determination of analytes (Wang Citation2006). In this method, the potential is scanned with a series of pulses, and it can superior elimination of the capacitive/background current (Scholz Citation2010).
Hybrid method
In hybrid method, scientific used 2 or more electrochemical methods for analyte detection.
Nanomaterials for electrochemical detection
Magnetic nanoparticles
Hassen et al. (Citation2008) developed a biosensor based on streptavidin functionalized magnetic NPs for HIV DNA detection. In this study, the magnetic layer is composed of the streptavidin functionalized magnetic NPs immobilized on surface of a gold electrode via a 300 mT magnet. Then, the biotinylated HIV DNA probes were linked through biotin–streptavidin interaction to magnetic layer, and DNA hybridization detection was conducted by impedimetric measurements. The CV measurements were performed in a 5 mM solution of redox couple [Fe(CN)6]4−/3− prepared in Phosphate-buffered saline (abbreviated PBS) buffer. Scanning potential was conducted between −600 mV and 600 mV, potential value of −300 mV and the frequency range between 100 mHz and 100 kHz. By this method, the lowest detection limit for HIV DNA target is obtained 160 pM. This approach can be considered as suitable method for PCR fragment detection whose length is often superior to 1000 bp (Hassen et al. Citation2008).
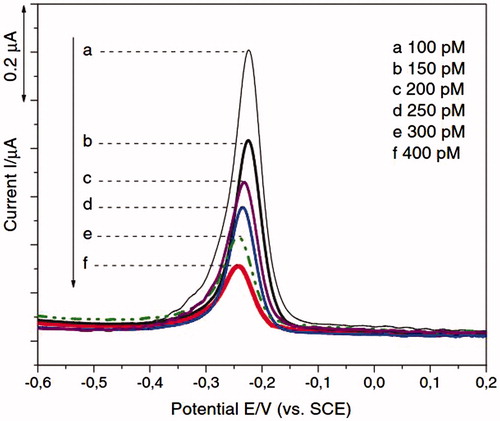
In Dai Tran and coworkers study, the detection of HIV-1 was demonstrated on chitosan/Fe3O4/GEM system with methylene blue (MB) Redox indicator, and using SWV and EIS techniques () (Dai Tran et al. Citation2011). GEM is abbreviation of 25-mer gene expression of modulator 91. The results suggest that the presence of Fe3O4 superparamagnetic NPs facilitates electron transfer. Thus, the current response and the sensitivity of the overall system showed enhancement. The scan rate 50 mV/s and potential range −0.5 V to +0.2 V vs. SCE (saturated calomel electrode) were the CV parameters. The frequency 12.5 Hz, the potential range from −600 to +400 mV, step 8 mV, amplitude 25 mV were optimized as SWV parameters. The frequency range from 200 kHz to 10 mHz using 5 mV alternating voltage superimposed on DC potential were optimized as EIS parameters. In this study, a detection limit is 50 pM (Dai Tran et al. Citation2011). The SWV response to different concentrations of the target DNA was displayed in . In this figure, the decrease in the magnitude of the voltammetric signal was due to the extent of the hybrid formation. EIS was also used to study the effect of hybridization on the chitosan/Fe3O4 interfacial platform of screen printed electrode (SPE) surface.
Figure 1. Schematic representation of HIV electrochemical detection on chitosan/Fe3O4 SPE, using MB as an intercalator (Reuse with permission from Elsevier) (Dai Tran et al. Citation2011).
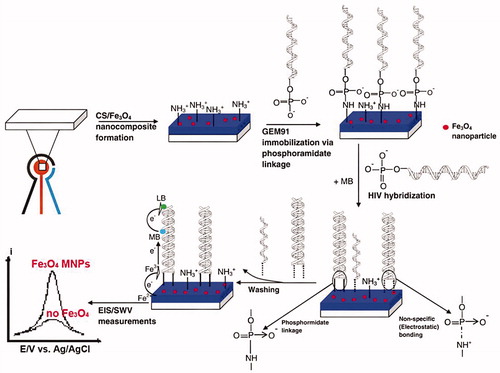
Figure 2. Quantitative detection of different target DNA concentrations by SWV is shown (Reuse with permission from Elsevier) (Dai Tran et al. Citation2011).
Gold-based nanomaterials
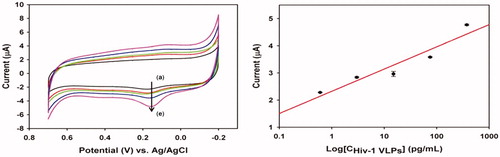
Lee et al. (Citation2013) proposed a method based on CV without the use of any mediators to detect direct electron transfer signal from HIV-1 virus. For improving the surface area, they fabricated AuNPs-modified Indium Tin Oxide (ITO) electrode by electrochemical deposition (Lee et al. Citation2013). Subsequently, antibody fragment was immobilized by self-assembly method and different concentrations of HIV-1 virus-like particles (VLPs) on ITO electrode surface. In this systems, HIV-1 VLPs were measured from 600 fg/mL to 375 pg/mL that shown in (Left) (Lee et al. Citation2013). In (Right), the calibration plot, acquired from oxidation peak of gp120 at ca. 0.153V shows a linear relation in a range from 600 fg/mL to 375 pg/mL (correlation coefficient was 0.958). This technique provided better electron-transfer kinetics and higher background charging current than current methods (Lee et al. Citation2013).
Figure 3. (Left) Cyclic voltammogram of different concentration of HIV-1 VLPs after immobilization of fragmented antibody (a) 600 fg/mL, (b) 3 pg/mL, (c) 15 pg/mL, (d) 75 pg/mL, and (e) 375 pg/m, respectively and (Right) linear plot of anodic current peak as a function of HIV-1 VLPs range from 600 fg/mL to 375 pg/mL (R = 0.958) (Reuse with permission from Elsevier) (Lee et al. Citation2013).
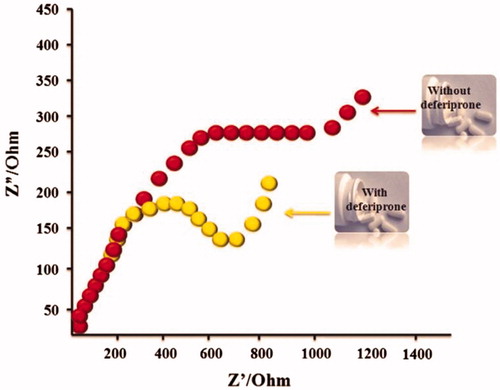
Recently, Narang and coworkers applied AuNRs for amplification of electrochemical sensing of anti-HIV replication drug, deferiprone, by the EIS method (Narang et al. Citation2015). In this study, electrochemical cell composed of: 1- Horse radish peroxidase/AuNRs/chitosan/pencil graphite electrode as working electrode, 2- Ag/AgCl as reference electrode, 3- and Pt wire as auxiliary electrode. It was recorded in PBS (50 mM, pH 6.5, 0.9% NaCl) containing 5 mM [Fe(CN)6]3-/4- and applied frequency range 0.01 Hz to10 KHz (Narang et al. Citation2015). Different concentrations of deferiprone drug were used into serum and urine samples, and after each addition, the potential between 0.0 and +1.75 V at a scan rate of 100 mV s−1 was recorded by cycling. The AuNRs sensor exhibits improved biosensing characteristics like fast response time of 15 s, linearity as 5 nM to 1000 μM, specific and anti-interferants. The response of AuNRs sensor with serum and urine samples showed its implications towards the development of biosensor for commercial drug monitoring device (Narang et al. Citation2015). In this study, a low detection limited 5 nM. showed Impedimetric response of AuNRs sensor for deferiprone.
Figure 4. Impedimetric responses of AuNRs sensor with deferiprone and without deferiprone were shown (Reuse with permission from Elsevier) (Narang et al. Citation2015).
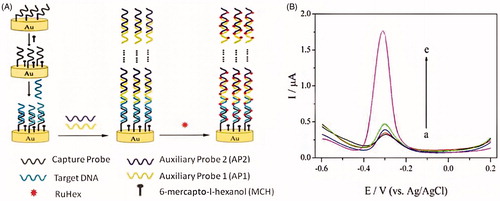
Chen et al. (Citation2012) fabricated a ultrasensitive electrochemical biosensor of DNA. In this method, long-range self-assembled DNA nanostructures were used as carriers for signal amplification. This method can achieve an impressive detection limit of 5 aM HIV DNA (38-base) even in complex biological samples such as cell lysates or human serum (Chen et al. Citation2012). In this work used a three electrode cell (capacity 10 mL; diameter 25 mm) consisting of Ag/AgCl reference electrode, working electrode (the assembled gold electrode, d = 2 mm), and platinum counter electrode was used for all electrochemical measurements. DPV was performed using a potential window of 0.2 to −0.6 V (versus Ag/AgCl) in 3 mL of RuHex solution ([Ru(NH3)6]3+, Hexaammineruthenium(III) chloride), which was degassed with nitrogen for 15 min (Chen et al. Citation2012). In this protocol, designed two auxiliary DNA probes by name auxiliary probe 1 (AP1) and auxiliary probe 2 (AP2). The capture probes (CP) were immobilized on the gold electrode by Au-S chemistry at the beginning of fabricating the electrochemical DNA biosensor, and after being hybridized CP with target DNA (TD), some more RuHex cations were binding to CP and TD. Then, the solution containing 1 μM of each AP1 and AP2 was dripped on the surface of the electrode, the DNA nanostructures self-assembled by numerous AP1 and AP2. So, AP1 and AP2 were firmly immobilized on the gold electrode via CP and TD. At result, the large number of RuHex can be binding to DNA nanostructures that produced a remarkable amplified electrochemical signal () (Chen et al. Citation2012).
Figure 5. (A) Schematic representation of the enzyme-free and label-free ultrasensitive electrochemical DNA biosensor based on long-range self-assembled DNA nanostructures. (B) DPV responses of the gold electrode modified with various oligonucleotides: (a) CP, (b) CP + TD, (c) CP + AP1 + AP2, (d) CP + TD + AP1, and (e) CP + TD + AP1 + AP2. The concentration of TD is 10 pM. The concentrations of AP1 and AP2 are both 1 μM (Reprinted with permission from American Chemical Society) (Chen et al. Citation2012).
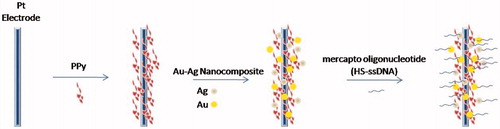
The immobilization of probe DNA molecules onto electrode surface is a common method in studies of the electrochemical DNA diagnostics. Fu et al. (Citation2006) immobilized a single strand DNA (ssDNA) on prefunctionalized platinum electrodes by combining Au–Ag nanoparticle composite and polymer polypyrrole (PPy) as shown in . The electrochemical characteristics of the modified electrode carried out by using CV and EIS. The CV was measured in 2.5 mM [Fe(CN)6]4-/3– (1:1) mixture as a redox probe in 0.1 M PBS, pH 7.0. EIS was measured in 0.3 M PBS, pH 7.0 at the frequency range from 10 to 105 Hz in a given open circuit voltage and amplitude was 5 mV. The detection limit is 0.5 pM (Fu et al. Citation2006).
Figure 6. A layer of PPy film was electrodeposited on the platinum electrode surface, and then the Au–Ag nanocomposite was bonded directly onto the surface of PPy, which is a layer of polycation. Finally, mercapto oligonucleotide was self-assembled onto the Au–Ag nanocomposite surface.
Labib et al. (Citation2011) used an organometallic peptide conjugate that is chemically linked to a nanostructured gold surface to the detection of the reverse transcriptase (RT) of HIV-1 in serum exploiting. The assay format is based on a thin film formation of a ferrocene-labeled lipoic acid onto an AuNPs-functionalized screen-printed carbon electrode (AuNPs-SPCE). Then, it modified with short peptide to bind to HIV-1 RT. This modified electrode is used to detect HIV-1 RT in human serum by 3 electrochemical forms, including CV (scan rate of 100 mV s−1 in the potential range from −100 to 350 mV), SWV (the potential range from −100 to 300 mV with a step potential of 5 mV, amplitude of 25 mV, and frequency of 10 Hz), and EIS (the frequency range of 100 kHz to 0.1 Hz, at a formal potential of 250 mV and AC amplitude of 5 mV) were performed. In this study, the SWV technique is rapid, sensitive, and capable of discriminating background capacitive currents. Also, it provides a two-dimensional robust measurement of RT. The AuNPs-SPCE with high surface-to-volume ratio can be considered as a nanodisc array that can enhance the electrode conductivity, and improve the detection limit of the target analyte (a detection limit of 0.8 pg/ml (0.7 fM) with a short response time) (Labib et al. Citation2011).
Recently, an electrochemistry biosensor based on graphene stabilized gold nanoclusters (GR/AuNCs) platform with exonuclease III-assisted DNA recycling amplification based on Cytosine-rich capture probe (Wang et al. Citation2015). The GR/AuNCs was prepared according to the one step ultrasonic method. Then, after fabrication and pretreatment of the bare GCE, GR/AuNCs dropped on its surface to construct GR/AuNCs-based biosensor. After dried of GR/AuNCs, capture probe was dropped onto GR/AuNCs modified electrode. Finally, the electrode was immersed in different concentration of target solution which contains exonuclease III (Wang et al. Citation2015). CV and EIS were performed in order to characterize the modification process of the biosensor. A detection limit of 30 aM (30 × 10−18) was recorded by this method (S/N = 3).
Carbon- based nanomaterials
Adam and colleagues used of paramagnetic microparticles with diameter of 2.8 μm that covered by streptavidin. Then this particle modified by an oligonucleotide probe with a specific viral sequence labeled by biotin to detect HIV (Adam et al. Citation2010). In this work, carbon nanotubes-based screen-printed electrodes were applied as the working electrode and SWV measurements were carried out (parameters: the potential step and the frequency were at 5 mV and 280 Hz, respectively). Target nucleic acids’ sequences gave an oxidation signal of adenine at 1.15 ± 0.05 V using carbon nanotubes-based screen-printed working electrodes, and the detection limits were estimated down to 0.1 pg/μL that were 15 times higher as compared to hanging mercury drop electrode (Adam et al. Citation2010).
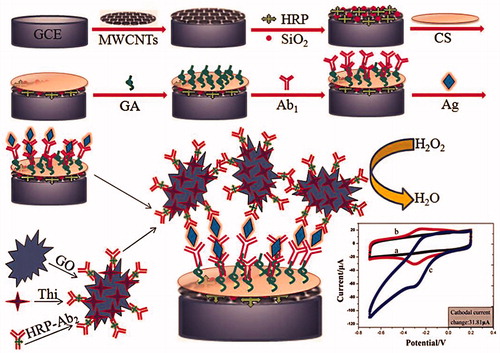
Recently, Fang and coworkers developed sandwich-type electrochemical immunoassay for the detection of HIV-p24 based on signal amplification strategy of multiple nanocomposites () (Fang et al. Citation2015). Nanocomposite made of enzyme encapsulated in carbon nanotubes-silica as a matrix and graphene oxide (GO) as a nanocarrier (Fang et al. Citation2015). The different concentrations of HIV-p24 or serum samples were dropped in the immunosensor array, and 10 μL of HRP-Ab2/TH/GO solution was deposited onto the electrode surface (HRP: horseradish peroxidase, TH: thionine). The electrode was recorded by CV from −200 to 600 mV, DPV from −100 to 600 mV with and without 3 mM H2O2 solution. EIS measurement were performed in 5.0 mM K3[Fe(CN)6]/K4[Fe(CN)6] containing 0.1 M KCl. This proposed method shows increase of response current to the HIV-p24 concentration in the range of 0.5 pg/mL to 8.5 ng/mL with the detection limit of 0.15 pg/mL, which was remarkably lower than other current techniques (Fang et al. Citation2015).
Figure 7. Schematic representation of preparation of immunosensor array and detection strategy by sandwich-type immunoassay (electrochemical voltammetry analysis) is shown. firstly, the immunosensors were fabricated by layer-by-layer coating MWCNTs, Si-HRP, Chitosan, glutaraldehyde composite on the working electrode; after that, the immunosensors were incubated with primary antibody 1 (Ab1); secondly, the primary Ab1 on the immunosensors were biorecognition with the corresponding antigens Ag; and thirdly, the immunocomplex-coated Ag were reacted with second antibodies labeled with TH/GO (Reprinted with permission from American Chemical Society) (Fang et al. Citation2015).
The DNA biosensor was fabricated by drop-coating graphene oxide (GO) on a glassy carbon (GC) electrode. Then, the designed single-stranded DNA probe covalently immobilizing onto GO using carbodiimide chemistry (Gong et al. Citation2015). The GO was later electrochemically reduced and applied to genosensing. In presence of target DNA, DNA double strand was formed at the electrode surface, and the negative charge in the electrode/electrolyte interface couple was changed. Also, the electron transfer resistance of the electrodes toward the [Fe(CN)6]3−/4− redox couple were changed. The detection limit of 3.0 × 10−13 M (S/N = 3) was recorded (Gong et al. Citation2015).
Hybrid nanomaterials
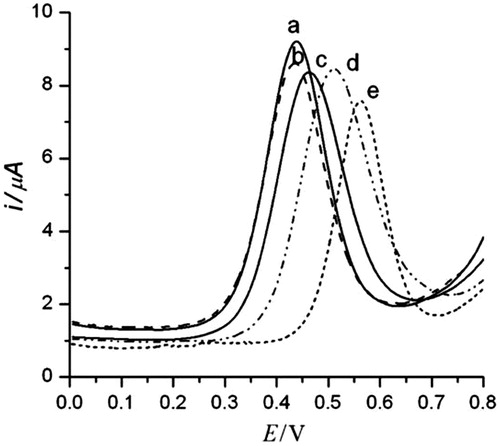
Mahmoud and Luong (Citation2010) developed an ultrasensitive electrochemical method for the detection of HIV-1 PR by modifying a disposable screen printed gold electrode (SPGE) with AuNPs-SWCNT. The monolayer of cystamine containing the Fc-pepstatin conjugate was formed on surface of the SWCNT/AuNP modified electrode, and then S-terminated Fc-pepstatin is self-assembled on such surfaces as the sensing probe. By SEM, AFM, and DPV, The interaction and binding between the Fc-pepstatin-modified substrates and HIV-1 PR is studied. CV, DPV, and amperometric measurements were performed using an electrochemical analyzer coupled with a picoamp booster and a Faraday cage. A platinum wire as counter electrode and Ag/AgCl as reference electrode were used. The electrodes were conditioned by CV between 0 and +1.4 V, and Ag/AgCl in 0.5 M H2SO4 at 100 mVs−1 until a stable CV profile was obtained. The process electrode preparation and surface recognition of HIV-1 PR was first confirmed by DPV as shown in . A strong potential shift of 98 mV was recorded in the presence of small viral amount (680 copies/mL). The peak shift (164 mV) was obtained at the highest viral replications of 1.14 × 106 copies/mL (Mahmoud and Luong Citation2010).
Figure 8. DPV of SWCNT/AuNP modified gold electrodes modified with Fc-pepstatin conjugate in the presence of different concentrations of HIV-1 PR at: (a) 0 pM, (b) 5 pM, (c) 10 pM, (d) 100 pM, and (e) 1000 pM. Ag/AgCl was used as the reference electrode at 100 mV/s. The assay buffer consisted of 0.1 M sodium acetate, 2 M NaClO4, 1 mM EDTA, 1 mM DTT, 10% DMSO, pH 7.4. The E of the Fc/Fc+ couple under the experimental conditions is 448 (5) mV (Reprinted with permission from Taylor & Francis) (Mahmoud and Luong Citation2010).
Kheiri et al. immobilized a polyclonal antibody to HIV-1 p24 based on MWCNT, AuNPs and acetone-extracted propolis (AEP) to yield a hybrid film deposited on an Au electrode (Kheiri et al. Citation2011). AuNPs-MWCNTs and AuNPs-AEP film can enhance the conductivity of the propolis film. The cyclic voltammograms and electrochemical impedance spectra of different modified electrodes was carried out in the potential range from −0.2 to 0.4 V in the presence of 5 mM Fe(CN)6 − 3/Fe(CN)6 − 4 as a redox probe (0.1 M PBS, pH 7.0, 0.1 M KCl) and frequency range from 1.0 × 10−2 to 1.0 × 105 Hz at 25 °C with signal amplitude of 5 mV. The optimum ratio of signal-to-noise was obtained at −0.32 V (versus reference electrode), which was selected as the applied potential for the amperometric measurements, and each amperometric immunosensor exhibits a fast response time for the whole concentration range of p24 Ag ((between 8 and 18 s for 0.01–60.00 ng/mL) (Kheiri et al. Citation2011)).
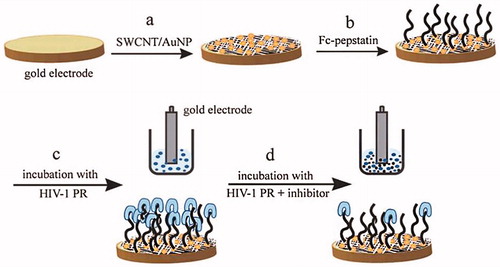
The HIV-1 virus encodes an aspartic protease (HIV-1 PR) that it is an essential enzyme for virion assembly and maturation. Methods for assaying HIV protease have been developed both in vitro and in vivo, and despite their advantages, such screening systems can only detect the inhibitor in the micromolar range, whereas only a nanomolar or lower range of such drugs can be used in HIV-1 therapy (Hu et al. Citation2005, Mahmoud and Luong Citation2008). So, there is a critical need for high throughput and sensitive protocols for detecting protease activity and/or screening novel protease inhibitors. Mahmoud and Luong demonstrated the use of nanomaterials in impedance spectroscopy for detecting HIV-1 protease and subsequent evaluation of the enzyme inhibitors at 10 pM levels. Thiolated single-walled carbon nanotubes beside AuNPs and a ferrocene-conjugate applied as sensitive enhancer tools in this assay format (Mahmoud and Luong Citation2008). The gold electrode surface is modified with thiolated single-walled carbon nanotube (SWCNT)/gold nanoparticle (AuNP) and thiol-terminated Fc-pepstatin is self-assembled on such surfaces as the sensing probe. The interaction and binding between HIV-1 PR and the Fc- pepstatin-modified substrates are studied by electrochemical impedance spectroscopy (EIS). Also, Differential Pulse Voltammetry (DPV) was performed to support the results obtained by EIS (Mahmoud and Luong Citation2008). is shown their protocol.
Figure 9. Schematic illustration protocol of the preparation of ferrocene- pepstatin conjugate/thiolated SWCNT and AuNP modified electrodes and their use for detecting HIV-1 protease and the subsequent assay of HIV-1 protease inhibitors. (Reproduced by permission of The American Chemical Society) (Mahmoud and Luong Citation2008).
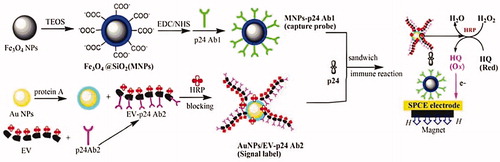
Gan et al. (Citation2013), has been developing an ultrasensitive portable electrochemical immunosensor that its detection sensitivity for HIV p24 was 1000 times higher than the ELISA method. In their study, a novel horseradish peroxidase (HRP) enzyme–antibody copolymer was synthesized and incubated with the secondary antibody of p24 (EV-p24 Ab2), then immobilized on AuNPs to fabricate a novel signal tag (AuNPs/EV-p24 Ab2). In the next step, a sandwich-type immunoreaction would take place between the capture probe (silicon dioxide-coated magnetic Fe3O4 NPs (MNPs) labeled with the primary p24 antibody (MNPs-p24 Ab1)), p24 and the signal tag (AuNPs/EV-p24 Ab2) to form the immunocomplex. In the final step, the immunocomplex was absorbed on the surface of SPCE by a magnet and immersed in the o-hydroxyl phenol (HQ) and H2O2, which induce an amplified reductive current when a large amounts of HRP on the signal tag catalyze the oxidation of HQ by H2O2 [56]. These process steps were shown in .
Figure 10. Demonstrated of the fabrication and detection procedure of the immunosensor (by permission from the authors (Gan et al. Citation2013)).
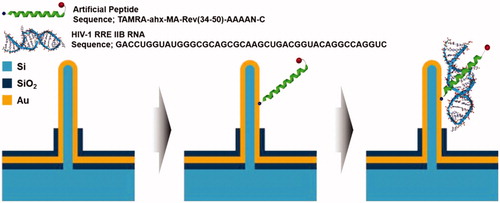
The vertical silicon nanowire electrode array (VSNEA) was fabricated and coated with thin layer of Au (Lee et al. Citation2016). Then, the peptide was immobilized on VSNEA surface by covalent interaction using cysteine () (Lee et al. Citation2016). Finally, CV and DPV measurements were performed for detection of HIV-1 Rev response element RNA. Potential range and the scan rate of CV were 0.8 to −0.8 V and 20 mV/s, respectively. DPV was carried out with pulse amplitude of 50 mV, a potential range from −0.2 to 0.6 V, and pulse width of 10 ms. By this method, it is possible to detect RNA concentrations of up to 1.513 fM and distinguish between wild-type RNA and mutant RNA (Lee et al. Citation2016).
Figure 11. Schematic illustration of principle of immunosensor using VSNEA. (Left) The VSNEA fabricated and coated by Au. (Center) Artificial peptide sequences attached on Au-coated VSNEA by covalent interaction. (Right) RNAs attached to peptides and current path is blocked reducing Fe(CN)63-/4- redox reaction (Lee et al. Citation2016).
Some of the experimental studies based on electrochemical detection of HIV were listed in .
Table 1. Some of the studies listed based on electrochemical detection methods.
Conclusion
In this paper, we demonstrated various electrochemical diagnosis methods based on nanomaterials for HIV detection, including CV, SWV, EIS, DPV, and hybrid methods. In each method, several examples were listed and explained, and noted its detection limit. Compared to other methods, such as optical biosensor array, electrochemical biosensor array has many advantages such as inexpensive instrument, low-detection limit and simplicity due to ease of obtaining electrical signal. So, in this paper, we tried to cover most experiments in this field.
Future prospects
In the future, by the development of new devices and materials in nanotechnology will be offered that it will bring a new revolution in the detection incurable diseases such as HIV/AIDS. These developments based on nanotechnology are:
The availability of sensitive and specific detection methods to identify HIV-1 using nanobiosensors
Prevention of sexual transmission of HIV by early diagnosis of infection with promotion sensitivity of methods
| Abbreviation | ||
| NP(s) | = | Nanoparticle(s) |
| AuNP(s) | = | Gold Nanoparticle(s) |
| AuNR(s) | = | Gold Nanorod(s) |
| AgNP(s) | = | Ag Nanoparticle(s) |
| AgNR(s) | = | Ag Nanorod(s) |
| QD(s) | = | Quantum Dot(s) |
| ELISA | = | Enzyme-Linked Immunosorbent Assay |
| aM | = | Attomolar, 10 − 18 mol/dm3 |
| MB | = | Methylene Blue |
| AIDS | = | Acquired immunodeficiency syndrome |
| HIV | = | human immunodeficiency virus |
| HIV-1 PR | = | HIV-1 protease |
| CV | = | Cyclic Voltammetry |
| SWV | = | Square Wave Voltammetry |
| EIS | = | Electrochemical Impedance Spectroscopy |
| DPV | = | Difierential Pulse Voltammetry |
| SWCNT | = | Single-Walled Carbon Nanotube |
| MWCNT | = | Multi-Walled Carbon Nanotubes |
| HIV-1 RT | = | HIV-1 Reverse Transcriptase |
| Ab | = | Antibody |
| SPCE | = | Screen-Printed Carbon Electrode |
| HQ | = | O-Hydroxyl Phenol |
| ITO | = | Indium Tin Oxide |
| VLPs | = | Virus like particles |
| AC impedance | = | Alternating Current impedance |
| PPy | = | polypyrrole |
| AP1 | = | auxiliary probe 1 |
| AP2 | = | auxiliary probe 2 |
| CP | = | capture probe |
| TD | = | target DNA |
| SPE | = | screen printed electrode |
| AuNPs-SPCE | = | AuNPs-Functionalized Screen-Printed Carbon Electrode |
| AEP | = | Acetone-Extracted Propolis |
| HRP | = | Horseradish Peroxidase |
| MNPs | = | Magnetic Nanoparticles |
| MNPs-p24 Ab1 | = | Magnetic NPs (MNPs) labeled with the primary p24 antibody |
| AuNPs/EV-p24 Ab2 | = | EV incubated with the secondary antibody of p24 that immobilized on AuNPs. The EnVision reagent (EV) is a kind of enzyme–polymer complex which contains about 100 molecules of HRP and 15 molecules of anti-IgG antibody connected in a poly-dextrin amine skeleton. |
| GO | = | Graphene Oxide |
| TH | = | Thionine |
| Si-HRP | = | silica matrix with horseradish peroxidase (HRP) entrapped |
Disclosure statement
The authors report no conflicts of interest in this work.
References
- Adam V, Huska D, Hubalek J, Kizek R. 2010. Easy to use and rapid isolation and detection of a viral nucleic acid by using paramagnetic microparticles and carbon nanotubes-based screen-printed electrodes. Microfluid Nanofluid. 8:329–339.
- Ajayan PM, Zhou OZ. 2001. Applications of carbon nanotubes. Carbon Nanotubes. Heidelberg (Germany): Springer, pp. 391–425.
- Anik Ü, Çubukçu M, Ertaş FN. 2016. An effective electrochemical biosensing platform for the detection of reduced glutathione. Artif Cells Nanomed Biotechnol. 44:971–977.
- Badrzadeh F, Rahmati-Yamchi M, Badrzadeh K, Valizadeh A, Zarghami N, Farkhani SM, Akbarzadeh A. 2016. Drug delivery and nanodetection in lung cancer. Artif Cells Nanomed Biotechnol. 44:618–634.
- Bard AJ, Faulkner LR. 1980. Electrochemical Methods: Fundamentals and Applications, vol. 2. New York: Wiley.
- Boeneman K, Delehanty JB, Susumu K, Stewart MH, Medintz IL. 2010. Intracellular bioconjugation of targeted proteins with semiconductor quantum dots. J Am Chem Soc. 132:5975–5977.
- Cao YC, Jin R, Mirkin CA. 2002. Nanoparticles with Raman spectroscopic fingerprints for DNA and RNA detection. Science. 297:1536–1540.
- Chen X, Hong CY, Lin YH, Chen JH, Chen GN, Yang HH. 2012. Enzyme-free and label-free ultrasensitive electrochemical detection of human immunodeficiency virus DNA in biological samples based on long-range self-assembled DNA nanostructures. Anal Chem. 84:8277–8283.
- Cohen MS, Chen YQ, McCauley M, Gamble T, Hosseinipour MC, Kumarasamy N, et al. 2011. Prevention of HIV-1 infection with early antiretroviral therapy. N Engl J Med. 365:493–505.
- Courty S, Luccardini C, Bellaiche Y, Cappello G, Dahan M. 2006. Tracking individual kinesin motors in living cells using single quantum-dot imaging. Nano Lett. 6:1491–1495.
- Cui ZQ, Ren Q, Wei HP, Chen Z, Deng JY, Zhang ZP, Zhang XE. 2011. Quantum dot-aptamer nanoprobes for recognizing and labeling influenza A virus particles. Nanoscale. 3:2454–2457.
- Dai Tran L, Nguyen BH, Van Hieu N, Vinh Tran H, Le Nguyen H, Xuan Nguyen P. 2011. Electrochemical detection of short HIV sequences on chitosan/Fe 3 O 4 nanoparticle based screen printed electrodes. Mater Sci Eng C. 31:477–485.
- Dalkıran B, Erden PE, Kılıç E. 2016. Graphene and tricobalt tetraoxide nanoparticles based biosensor for electrochemical glutamate sensing. Artif Cells Nanomed Biotechnol. [Epub ahead of print]. DOI: 10.3109/21691401.2016.1153482
- El-Sayed IH, Huang X, El-Sayed MA. 2005. Surface plasmon resonance scattering and absorption of anti-EGFR antibody conjugated gold nanoparticles in cancer diagnostics: applications in oral cancer. Nano Lett. 5:829–834.
- Erdem C, Zeybek DK, Aydoğdu G, Zeybek B, Pekyardımcı S, Kılıç E. 2014. Electrochemical glucose biosensor based on nickel oxide nanoparticle-modified carbon paste electrode. Artif Cells Nanomed Biotechnol. 42:237–244.
- Fang YS, Huang XJ, Wang LS, Wang JF. 2015. An enhanced sensitive electrochemical immunosensor based on efficient encapsulation of enzyme in silica matrix for the detection of human immunodeficiency virus p24. Biosens Bioelectron. 64:324–332.
- Farkhani SM, Valizadeh A, Karami H, Mohammadi S, Sohrabi N, Badrzadeh F. 2014. Cell penetrating peptides: efficient vectors for delivery of nanoparticles, nanocarriers, therapeutic and diagnostic molecules. Peptides. 57:78–94.
- Farkhani SM, Valizadeh A. 2014. Review: three synthesis methods of CdX (X = Se, S or Te) quantum dots. IET Nanobiotechnology. 8:59–76.
- Fiebig EW, Wright DJ, Rawal BD, Garrett PE, Schumacher RT, Peddada L, et al. 2003. Dynamics of HIV viremia and antibody seroconversion in plasma donors: implications for diagnosis and staging of primary HIV infection. Aids. 17:1871–1879.
- Fu Y, Chai Y, Zhou L, Zhang Y. 2006. Coupling of a reagentless electrochemical DNA biosensor with conducting polymer film and nanocomposite as matrices for the detection of the HIV DNA sequences. Anal Lett. 39:467–482.
- Gan N, Du X, Cao Y, Hu F, Li T, Jiang Q. 2013. An ultrasensitive electrochemical immunosensor for HIV p24 based on Fe3O4@ SiO2 nanomagnetic probes and nanogold colloid-labeled enzyme: antibody copolymer as signal tag. Materials. 6:1255–1269.
- Gong Q, Yang H, Dong Y, Zhang W. 2015. A sensitive impedimetric DNA biosensor for the determination of the HIV gene based on electrochemically reduced graphene oxide. Anal Methods. 7:2554–2562.
- Hassen WM, Chaix C, Abdelghani A, Bessueille F, Leonard D, Jaffrezic-Renault N. 2008. An impedimetric DNA sensor based on functionalized magnetic nanoparticles for HIV and HBV detection. Sens Actuators B Chem. 134:755–760.
- Hirschey MD, Han YJ, Stucky GD, Butler A. 2006. Imaging Escherichia coli using functionalized core/shell CdSe/CdS quantum dots. J Biol Inorg Chem. 11:663–669.
- Hu K, Clément JF, Abrahamyan L, Strebel K, Bouvier M, Kleiman L, Mouland AJ. 2005. A human immunodeficiency virus type 1 protease biosensor assay using bioluminescence resonance energy transfer. J Virol Methods. 128:93–103.
- Kheiri F, Sabzi RE, Jannatdoust E, Shojaeefar E, Sedghi H. 2011. A novel amperometric immunosensor based on acetone-extracted propolis for the detection of the HIV-1 p24 antigen. Biosens Bioelectron. 26:4457–4463.
- Kim YG, Moon S, Kuritzkes DR, Demirci U. 2009. Quantum dot-based HIV capture and imaging in a microfluidic channel. Biosens Bioelectron. 25:253–258.
- Klostranec JM, Xiang Q, Farcas GA, Lee JA, Rhee A, Lafferty EI, et al. 2007. Convergence of quantum dot barcodes with microfluidics and signal processing for multiplexed high-throughput infectious disease diagnostics. Nano Lett. 7:2812–2818.
- Kumar L, Verma S, Prasad DN, Bhardwaj A, Vaidya B, Jain AK. 2015. Nanotechnology: a magic bullet for HIV AIDS treatment. Artif Cells Nanomed Biotechnol. 43:71–86.
- Labib M, Martić S, Shipman PO, Kraatz HB. 2011. Electrochemical analysis of HIV-1 reverse transcriptase serum level: exploiting protein binding to a functionalized nanostructured surface. Talanta. 85:770–778.
- Laczka O, Ferraz RM, Ferrer-Miralles N, Villaverde A, Muñoz FX, del Campo FJ. 2009. Fast electrochemical detection of anti-HIV antibodies: coupling allosteric enzymes and disk microelectrode arrays. Analytica Chimica Acta. 641:1–6.
- Lee J, Hong MH, Han S, Na J, Kim I, Kwon YJ, Lim YB, Choi HJ. 2016. Sensitive and selective detection of HIV-1 RRE RNA using vertical silicon nanowire electrode array. Nanoscale Res Lett. 11:341.
- Lee JH, Oh BK, Choi JW. 2013. Electrochemical sensor based on direct electron transfer of HIV-1 Virus at Au nanoparticle modified ITO electrode. Biosens Bioelectron. 49:531–535.
- Mahmoud KA, Luong JH. 2010. A sensitive electrochemical assay for early detection of HIV-1 protease using ferrocene-peptide conjugate/Au nanoparticle/single walled carbon nanotube modified electrode. Anal Lett. 43:1680–1687.
- Mahmoud KA, Luong JHT. 2008. Impedance method for detecting HIV-1 protease and screening for its inhibitors using ferrocene: peptide conjugate/Au nanoparticle/single-walled carbon nanotube modified electrode. Anal Chem. 80:7056–7062.
- Mirceski V, Komorsky-Lovric S, Lovric M. 2007. Square-Wave Voltammetry: Theory and Application. Springer Science & Business Media.
- Mohammed Fayaz A, Ao Z, Girilal M, Chen L, Xiao X, Kalaichelvan P, Yao X. 2012. Inactivation of microbial infectiousness by silver nanoparticles-coated condom: a new approach to inhibit HIV-and HSV-transmitted infection. Int J Nanomed. 7:5007.
- Narang J, Malhotra N, Singh G, Pundir CS. 2015. Electrochemical impediometric detection of anti-HIV drug taking gold nanorods as a sensing interface. Biosens Bioelectron. 66:332–337.
- Nicholson RS, Shain I. 1964. Theory of stationary electrode polarography. Single scan and cyclic methods applied to reversible, irreversible, and kinetic systems. Anal Chem. 36:706–723.
- Osteryoung JG, Osteryoung RA. 1985. Square wave voltammetry. Anal Chem. 57:101A–110A.
- Petersen L, Satten GA, Dodd R, Busch M, Kleinman S, Grindon A, Lenes B. 1994. Duration of time from onset of human immunodeficiency virus type 1 infectiousness to development of detectable antibody. Transfusion. 34:283–289.
- Rezaei B, Majidi N, Rahmani H, Khayamian T. 2011. Electrochemical impedimetric immunosensor for insulin like growth factor-1 using specific monoclonal antibody-nanogold modified electrode. Biosens Bioelectronics 26:2130–2134.
- Scholz F. 2010. Electroanalytical Methods, vol. 1. Berlin-Heidelberg: Springer.
- Seleverstov O, Zabirnyk O, Zscharnack M, Bulavina L, Nowicki M, et al. 2006. Quantum dots for human mesenchymal stem cells labeling. A size-dependent autophagy activation. Nano Lett. 6:2826–2832.
- Sohrabi N, Valizadeh A, Farkhani SM, Akbarzadeh A. 2016. Basics of DNA biosensors and cancer diagnosis. Artif Cells Nanomed Biotechnol. 44:654–663.
- Tang S, Zhao J, Storhoff JJ, Norris PJ, Little RF, Yarchoan R, et al. 2007. Nanoparticle-based biobarcode amplification assay (BCA) for sensitive and early detection of human immunodeficiency type 1 capsid (p24) antigen. J AIDS. 46:231–237.
- Valizadeh A, Bakhtiary M, Akbarzadeh A, Salehi R, Frakhani SM, Ebrahimi O, Rahmati-yamchi M, Davaran S. 2016. Preparation and characterization of novel electrospun poly (ε-caprolactone)-based nanofibrous scaffolds. Artif Cells Nanomed Biotechnol. 44:504–509.
- Valizadeh A, Farkhani SM. 2014. Electrospinning and electrospun nanofibres. IET Nanobiotechnol. 8:83–92.
- Valizadeh A, Khosroushahi AY. 2015. Single-cell analysis based on lab on a chip fluidic system. Anal Methods. 7:8524–8533.
- Valizadeh A, Mikaeili H, Samiei M, Farkhani SM, Zarghami N, Kouhi M, Akbarzadeh A, Davaran S. 2012. Quantum dots: synthesis, bioapplications, and toxicity. Nanoscale Res Lett. 7:480.
- Valizadeh A. 2015. Nanomaterials and optical diagnosis of HIV. Artif Cells Nanomed Biotechnol. [Epub ahead-of-print].
- Wang J. 2006. Analytical electrochemistry. John Wiley & Sons.
- Wang X, Lou X, Wang Y, Guo Q, Fang Z, Zhong X, et al. 2010. QDs-DNA nanosensor for the detection of hepatitis B virus DNA and the single-base mutants. Biosens Bioelectron. 25:1934–1940.
- Wang Y, Bai X, Wen W, Zhang X, Wang S. 2015. Ultrasensitive electrochemical biosensor for HIV gene detection based on graphene stabilized gold nanoclusters with exonuclease amplification. ACS Appl Mater Interfaces. 7:18872–18879.
- Xue X, Pan J, Xie H, Wang J, Zhang S. 2009. Fluorescence detection of total count of Escherichia coli and Staphylococcus aureus on water-soluble CdSe quantum dots coupled with bacteria. Talanta. 77:1808–1813.
- Yang L, Li Y. 2006. Simultaneous detection of Escherichia coli O157 : H7 and Salmonella Typhimurium using quantum dots as fluorescence labels. Analyst. 131:394–401.
- Zhang D, Peng Y, Qi H, Gao Q, Zhang C. 2010. Label-free electrochemical DNA biosensor array for simultaneous detection of the HIV-1 and HIV-2 oligonucleotides incorporating different hairpin-DNA probes and redox indicator. Biosens Bioelectron. 25:1088–1094.

