Abstract
Nanoscale particles and molecules are a potential different for the treatment of disease because they have distinctive biologic property based on their structure and size, which is different from traditional small-molecule drugs. The antimicrobial mechanisms of silver nanoparticles include the formation of free radicals damaging the bacterial membranes, interactions with DNA, adhesion to cell surface altering the membrane properties, and enzyme damage. In this review, we focus on applications of silver nanoparticles in inhibition of herpes simplex virus.
Introduction
Nanoparticles are described as particles in the size range of 1–100 nm in diameter [Citation1]. With the small size, NPs can simply cooperate with biomolecules on the cell surface or inside cells, and carry genetic materials such as DNA, RNA, or small interfering RNA (siRNA) into target cells or tissues for gene expression [Citation2].
It is generally estimated that the use of nanoparticles will increase in the future. Presently, silver nanoparticles (Ag-NP) have one of the maximum degrees of commercialization among the nanomaterials, principally due to their characteristic antimicrobial properties [Citation3].
Nanoscale particles and molecules are a potential different for the treatment of disease because they have distinctive biologic property based on their structure and size, which is different from traditional small-molecule drugs [Citation4]. Nanoparticle systems can be engineered to have a number of attractive qualities for therapy, including (i) sustained and controlled release of drugs locally and (ii) deep tissue penetration due to their nanoscale size [Citation2].
According to the recent progress in nanotechnology, it is now possible to produce silver at the nanoscale [Citation5,Citation6]. The application of nanoparticles in biology and medicine is in fluorescent, biological labels, drug and gene delivery, bio detection of pathogens, detection of proteins, probing of DNA structure, tissue engineering, tumour destruction via heating (hyperthermia, separation, and purification of biological molecules and cells), MRI contrast enhancement, and phagokinetic studies [Citation6–13].
Preparation of silver nanoparticle
The most universal advance for synthesis of silver NPs is chemical reduction by organic and inorganic reducing agents. In general, different reducing agents such as sodium citrate, ascorbate, sodium borohydride (NaBH4), elemental hydrogen, polyol process, Tollens reagent, N,N-dimethylformamide (DMF), and poly (ethylene glycol)-block copolymers are used for the reduction of silver ions (Ag+) in aqueous or non-aqueous solutions. Scientist discovered that by using the boiling method at diverse citrate concentrations, AgNPs with a plasmon maximum absorbance at 420 nm were produced.
By increasing the relative concentration of sodium citrate to silver cation i.e. [citrate]/[Ag+] from 1 to 5 times [Citation14], the elapsed time for the formation of AgNP was reduced from 40 to 20 min, in that order, indicating that under equimolar conditions, a division of the Ag + was not reduced ().
Figure 1. Representation of the nucleation and growth mechanisms for AgNP obtained by the citrate method according to Ref. [Citation14].
![Figure 1. Representation of the nucleation and growth mechanisms for AgNP obtained by the citrate method according to Ref. [Citation14].](/cms/asset/15cf1c4a-4940-42a5-81da-a86d1e134283/ianb_a_1307208_f0001_c.jpg)
Antiviral properties of AgNPs
Resistance in human pathogens is a big challenge in fields like pharmaceutical and biomedicine [Citation15]. The biocidal activity of AgNPs depends on several morphological and physicochemical (e.g. size, shape, and surface) characteristics that influence directly in the success of these compounds as antimicrobial agents [Citation16]. The advantage of using silver nanoparticles for impregnation is that there is continuous release of silver ions enhancing its antimicrobial efficacy. The burn wounds treated with silver nanoparticles show better cosmetic appearance and scarless healing [Citation17]. Silver nanoparticles inhibit HSV-1 infections by blocking the attachment and thereby the entrance of the virus into the cells and/or by preventing the cell-to-cell spread of the virus [Citation18].
Viruses infect all cellular life forms: eukaryotes and prokaryotes. The infection of virus in prokaryotes is dominant in the form of bacteriophages. Viruses form the most considerable causes of disease and death in the world. Viruses are also found in all forms of life i.e. air, water, and predominantly in the soil [Citation19]. There is a greater need to progress new and distinctive treatment choices with antiviral agents, which can also overcome the problem of antiviral resistance [Citation18].
A variety of viruses, such as influenza, hepatitis, herpes simplex virus (HSV), and human immunodeficiency virus (HIV), must have been life threatening [Citation20]. AgNPs perform as a broad-spectrum cause against a range of viral strains and are not capable to increasing conflict [Citation21]. The antibacterial activities of the AgNP depend on many factors such as particle size, shape, absorption, and its aggregation/dissolution in specific growth media.
In 1884, German obstetrician Crede introduced 1% silver nitrate (AgNO3) as an eye solution for avoidance of gonococcal ophthalmia neonatorum, that is too similar to the first scientific-documented medical use of silver [Citation22]. AgNPs take many important biological activities [Citation23]. Nano-silver have been used in the treatment of wounds, burns, in water-disinfecting systems, dental materials, and as antibacterials, antivirals, and anticancerous agents [Citation24–26]. Although, a comparative study of AgNPs, AgNO3, and silver chloride (AgCl) found that AgNPs have higher antibacterial properties than free silver ions [Citation26] ().
Figure 2. Mechanism of antiviral effect of AgNPs on different stages of virus replication: (1) interaction with viral surface, (2) interference with viral attachment, (3) inhibition of virus penetration into the cell, (4) interaction with viral genome, (5) inhibition of genome replication, (6) inhibition of protein synthesis, and (7) inhibition of assembly and release of virions.
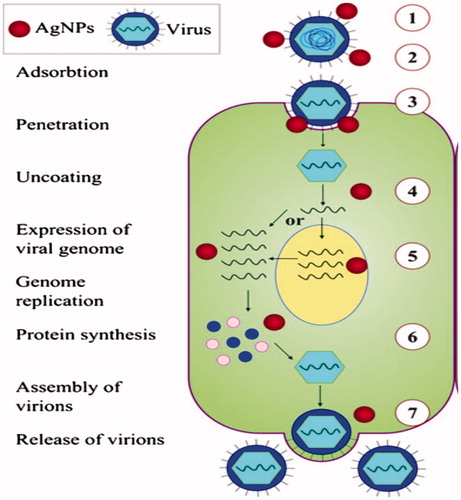
Viruses characterize one of the primary causes of disease and death worldwide. Virus effects are so detrimental that they can cause long-term suffering from persistent diseases that can lead to immunodeficiency, cancer, and are some of the most contagious diseases, infecting and killing as much as 40% of the population in one area [Citation27]. If the virus cannot attach to the healthy cell, it cannot do anymore damage [Citation28]. They are the most difficult infectious diseases to treat with present medicine because the virus is neither alive nor dead. They contain a nuclear capsid (microscopic cellular container) that consists of short incomplete sections of genetic material (DNA or RNA). Then viruses adhere to a healthy cell surface, fusion between the cell and virus occurs, after which the virus injects these incomplete segments of DNA or RNA into the cell where they bind with normal DNA/RNA and interfere with the normal replication of the cell [Citation28].
Prevention from viral trouble is far better to attempt to manage the virus after infection because there is so much DNA that has been changed [Citation29].
Viruses are the most complex infectious diseases to treat with present medicine because the virus is neither alive nor quiet. There are several ways to demolish, stop, or make immunity against a virus. Much effort has been expended in attempts to develop vaccines for these diseases [Citation28].
On one hand, some of the numerous diseases have been eradicated by vaccination programmes. Unless the patient have an abnormal immune response leading to persistent autoimmune syndromes like arthritis, inflammatory bowel, lupus, MS, paralysis, or even death. On the other hand, the virus may mutate and become a different virus describing that the vaccination cannot to give defence from disease. The most common non-approval way to stop viral attachment is the use of silver liquid and gel [Citation30].
The widespread use of broad-spectrum antibiotics has led to struggle to standard antimicrobial agents for many bacterial human pathogens and dead halt a major threat to the global health care.
Silver has been unavailable since time immemorial. However, the use of silver for medicine or as a local antibacterial agent was not known until the nineteenth century silver was used to pure water. Following penicillin introduced. In the 1940s, the use of silver for the management of bacterial infections is wide spread [Citation31]. Silver’s mode of action is supposed to be dependent on Ag + ions, which powerfully reduce bacterial growth through inhibition of respiratory enzymes and electron transport components and through interference with DNA functions [Citation32]. The benefit of using silver nanoparticles for impregnation is that there is permanent discharge of silver ions enhancing its antimicrobial value [Citation18].
Silver compounds have already been used clinically to decrease skin infections in the treatment of burns such as silver sulphadiazine and to prevent colonization of bacterial on different surfaces [Citation33].
They are among the emerging nanoproducts that have gained increasing interest in the field of nanomedicine due to their unique properties and obvious therapeutic potential in treating a variety of diseases, including retinal neovascularization, and acquired immunodeficiency syndrome due to human immunodeficiency virus [Citation33,Citation34]. The advantage of Ag-NP dramatically increased in the definite surface area for particles with a size in the range of a few nanometres [Citation35].
In addition, the strong toxicity that silver shows in various chemical forms to a wide range of micro-organisms is very well known, and AgNPs have recently been shown to be a capable antimicrobial material [Citation36]. A group of problems are related to herpes viruses; from shingles, genital herpes, chickenpox, infectious mononucleosis, equal to herpes keratitis, neonatal distributed infections, and viral Encephalitis [Citation37].
HSV is the name of viruses that is divided into two subgroups: herpes simplex virus type 1 (HSV-1) and herpes simplex virus type 2 (HSV-2). HSV is a double-stranded DNA virus having genome size 152 kbp, which is the classic clinical appearance of the primary. The connection and entrance of HSV-1 into cells contain of interaction between viral envelope glycoprotein and cell surface heparin sulphate [Citation38]. This is significant because of the damage that herpes virus can cause on a long-term basis. In addition, there is no cure for all herpes viruses [Citation39]. HSV-2 is an extremely widespread sexually transmitted infection [Citation40–42]. There are 11 known viral glycoproteins (B–M) and a 12th (gN) was forecasted [Citation43]. HSV-1 or HSV-2 genital infection is featured by mutual clusters of erythematous papules and vesicles on the external genitalia, usually 4–7 d after sexual exposure [Citation44].
HSV-1 is the most familiar infectious disease that occurs worldwide and infects humans. They have no animal vector, so spread is almost entirely person to person [Citation37]. They are the most serious human pathogens. HSV-1 is associated with orofacial infections and encephalitis, whereas HSV-2 generally causes genital infections and can be transmitted from infected mothers to neonates. HSV-2 is a kind of a virus that can cause a global trouble and the incidence of herpetic infection can considerably amplify an individual’s likelihood of becoming infected with human immunodeficiency virus (HIV)-1 [Citation45]. Both viruses found latent infections in sensory neurons and, leading reactivation, creating lesions at or near position of entry into the body. This latency increases the pathogenicity of HSV and will facilitate these viruses to be used as therapeutic tools [Citation46].
Epidemiology and pathogenesis of mucocutaneous HSV infection
There are more than 200 types of HPV. Based on the epidemiologic and molecular biological studies, there are 15 genital HPV types [Citation47,Citation48].
The herpes virus family consists of more than 100 double-stranded DNA viruses divided into α, β, and ɣ, subgroups. Symptomatic diseases caused by HSV-1 are commonly restricted to cold sores of the mouth and keratitis in the eyes, but HSV-1 is proficient of causing life-threatening diseases in immunodeficiency persons, including newborns, patients with HIV, or patients undergoing immunosuppressive treatment [Citation18]. HSV-1 adheres to and directly infects epithelial cells. As viral replication progress, cytoplasmic membranes get worse, and the damaged cells break up from one another and make a blister-like vesicle. HSV-1 is able to migrate through sensory nerve to the trigeminal cranial nerve ganglion in the brain and persists in absolutely in a quiescent state in the nuclei of the neurons [Citation48,Citation49].
The advantages of HSV-1 include capacity of contamination a extensive range of cells in both mitotic and non-mitotic phases [Citation50]. With regard to HSV-1, Ag-NPs are hypothesized to target the virus and to compete for its binding to cellular heparin sulphate through their sulphonate end groups, leading to the blockage of viral entry into the cell and the prevention of subsequent infection [Citation30]. Useful inhibition of HSV-1 infection in cell culture by the capped nanoparticles established that the use of the soluble surfactant with mercaptoethane sulphonate was unsuccessful [Citation51]. Ag-NPs were established to reduce the HSV-2-induced cytopathic result on cells [Citation27].
Conclusion
It is now clear that AgNPs possess a strong antibacterial and antiviral activity, highlighted by several studies. Nonetheless, for metal nanoparticles to be used in therapeutic or prophylactic treatment regimens, it is critical to understand the in vivo toxicity and potential for long-term sequelae associated with the exposure to these compounds. Additional research is needed to determine how to safely design, use, and dispose products containing metal nanomaterials without creating new risk to humans or the environment.
Disclosure statement
The authors report no declarations of interest. The authors alone are responsible for the content and writing of the paper.
Additional information
Funding
References
- Lövestam G, Rauscher H, Roebben G, et al. Considerations on a definition of nanomaterial for regulatory purposes. Luxembourg; Publications Office; 2010.
- Kafshdooz T, Kafshdooz L, Akbarzadeh A, et al. Applications of nanoparticle systems in gene delivery and gene therapy. Artif Cells Nanomed Biotechnol. 2016;44:581–587.
- Rai M, Kon K, Ingle A, et al. Broad-spectrum bioactivities of silver nanoparticles: the emerging trends and future prospects. Appl Microbiol Biotechnol. 2014;98:1951–1961.
- Yezhelyev MV, Gao X, Xing Y, et al. Emerging use of nanoparticles in diagnosis and treatment of breast cancer. Lancet Oncol. 2006;7:657–667.
- Sun Y, Mayers B, Xia Y. Transformation of silver nanospheres into nanobelts and triangular nanoplates through a thermal process. Nano Lett. 2003;3:675–679.
- Panáček A, Kvitek L, Prucek R, et al. Silver colloid nanoparticles: synthesis, characterization, and their antibacterial activity. J Phys Chem B. 2006;110:16248–16253.
- Chan WC, Nie S. Quantum dot bioconjugates for ultrasensitive nonisotopic detection. Science. 1998;281:2016–2018.
- Wang S, Mamedova N, Kotov NA, et al. Antigen/antibody immunocomplex from CdTe nanoparticle bioconjugates. Nano Lett. 2002;2:817–822.
- Mah C, Zolotukhin I, Fraites T, et al. Microsphere-mediated delivery of recombinant AAV vectors in vitro and in vivo. Mol Ther. 2000;1:S239.
- Pantarotto D, Partidos CD, Hoebeke J, et al. Immunization with peptide-functionalized carbon nanotubes enhances virus-specific neutralizing antibody responses. Chem Biol. 2003;10:961–966.
- Majidi S, Sehrig ZF, Farkhani SM, et al. Current methods for synthesis of magnetic nanoparticles. Artif Cells Nanomed Biotechnol. 2016;44:722–734.
- Nam J-M, Thaxton CS, Mirkin CA. Nanoparticle-based bio-bar codes for the ultrasensitive detection of proteins. Science. 2003;301:1884–1886.
- Mahtab R, Rogers JP, Murphy CJ. Protein-sized quantum dot luminescence can distinguish between” straight” bent”, and” kinked” oligonucleotides. J Am Chem Soc. 1995;117:9099–9100.
- Pacioni NL, Borsarelli CD, Rey V, et al. Synthetic routes for the preparation of silver nanoparticles. Silver nanoparticle applications. New York: Springer; 2015. p. 13–46.
- Tenover FC. Mechanisms of antimicrobial resistance in bacteria. Am J Med. 2006;119:S3–S10.
- Jana S, Pal T. Synthesis, characterization and catalytic application of silver nanoshell coated functionalized polystyrene beads. J Nanosci Nanotech. 2007;7:2151–2156.
- Chaloupka K, Malam Y, Seifalian AM. Nanosilver as a new generation of nanoproduct in biomedical applications. Trends Biotechnol. 2010;28:580–588.
- Galdiero S, Falanga A, Vitiello M, et al. Silver nanoparticles as potential antiviral agents. Molecules. 2011;16:8894–8918.
- Henderson DA. Principles and lessons from the smallpox eradication programme. Bull World Health Organ. 1987;65:535.
- Gaikwad S, Ingle A, Gade A, et al. Antiviral activity of mycosynthesized silver nanoparticles against herpes simplex virus and human parainfluenza virus type 3. Int J Nanomed. 2013;8:4303.
- Ge L, Li Q, Wang M, et al. Nanosilver particles in medical applications: synthesis, performance, and toxicity. Int J Nanomed. 2014;9:2399.
- Russell A, Path F, Sl FP, et al. Antimicrobial activity and action of silver. Prog Med Chem. 1994;31:351.
- Herizchi R, Abbasi E, Milani M, et al. Current methods for synthesis of gold nanoparticles. Artif Cells Nanomed Biotechnol. 2016;44:596–602.
- Elliott C. The effects of silver dressings on chronic and burns wound healing. Br J Nurs. 2010;19: S32–6.
- Zhang H, Smith JA, Oyanedel-Craver V. The effect of natural water conditions on the anti-bacterial performance and stability of silver nanoparticles capped with different polymers. Water Res. 2012;46:691–699.
- Kafshdooz T, Kafshdooz L, Akbarzadeh A, et al. Applications of nanoparticle systems in gene delivery and gene therapy. Artif Cells Nanomed Biotechnol, 2016;44:581–587.
- Hu R, Li S, Kong F, et al. Inhibition effect of silver nanoparticles on herpes simplex virus 2. Genet Mol Res. 2014;13:7022–7028.
- Alkhouri N, Zein NN. Protease inhibitors: silver bullets for chronic hepatitis C infection. Cleve Clin J Med. 2012;79:213–222.
- Xiang D-x, Chen Q, Pang L, et al. Inhibitory effects of silver nanoparticles on H1N1 influenza A virus in vitro. J Virol Methods. 2011;178:137–142.
- Baram-Pinto D, Shukla S, Perkas N, et al. Inhibition of herpes simplex virus type 1 infection by silver nanoparticles capped with mercaptoethane sulfonate. Bioconjug Chem. 2009;20:1497–1502.
- Sohrabi N, Valizadeh A, Farkhani SM, et al. Basics of DNA biosensors and cancer diagnosis. Artif Cells Nanomed Biotechnol. 2016;44:654–663.
- Li Y, Leung P, Yao L, et al. Antimicrobial effect of surgical masks coated with nanoparticles. J Hosp Infect. 2006;62:58–63.
- Feng Q, Wu J, Chen G, et al. A mechanistic study of the antibacterial effect of silver ions on Escherichia coli and Staphylococcus aureus. J Biomed Mater Res. 2000;52:662–668.
- Kalishwaralal K, BarathManiKanth S, Pandian SRK, et al. Silver nano – a trove for retinal therapies. J Control Release. 2010;145:76–90.
- Alt V, Bechert T, Steinrücke P, et al. An in vitro assessment of the antibacterial properties and cytotoxicity of nanoparticulate silver bone cement. Biomaterials. 2004;25:4383–4391.
- Wei J, Khun E. Determination of critical coagulation concentration of silicon nanoparticles. Adv Mater Lett. 2014;5:2–5.
- Khandelwal N, Kaur G, Kumara N, et al. Application of silver nanoparticles in viral inhibition: a new hope for antivirals. Dig J Nanomater Biostruct. 2014;9:175.
- Gottesman R, Shukla S, Perkas N, et al. Sonochemical coating of paper by microbiocidal silver nanoparticles. Langmuir. 2010;27:720–726.
- Orlowski P, Tomaszewska E, Gniadek M, et al. Tannic acid modified silver nanoparticles show antiviral activity in herpes simplex virus type 2 infection. PLoS One. 2014;9:e104113.
- O'Farrell N. Increasing prevalence of genital herpes in developing countries: implications for heterosexual HIV transmission and STI control programmes. Sex Transm Infect. 1999;75:377–384.
- Whitley RJ, Kimberlin DW, Roizman B. Herpes simplex viruses. Clin Infect Dis. 1998;541–553.
- Weiss H. Epidemiology of herpes simplex virus type 2 infection in the developing world. Herpes: J IHMF. 2004;11:24A–35A.
- Forrester A, Farrell H, Wilkinson G, et al. Construction and properties of a mutant of herpes simplex virus type 1 with glycoprotein H coding sequences deleted. J Virol. 1992;66:341–348.
- Corey L, Adams HG, Brown ZA, et al. Genital herpes simplex virus infections: clinical manifestations, course, and complications. Ann Intern Med. 1983;98:958–972.
- Freeman EE, Weiss HA, Glynn JR, et al. Herpes simplex virus 2 infection increases HIV acquisition in men and women: systematic review and meta-analysis of longitudinal studies. AIDS. 2006;20:73–83.
- Abbasi E, Kafshdooz T, Bakhtiary M, et al. Biomedical and biological applications of quantum dots. Artif Cells Nanomed Biotechnol. 2016;44:885–891.
- Esmaeili M, Bonyadi M, Dastranj A, et al. HPV typing in women with cervical precancerous and cancerous lesions in northwestern Iran. Gynecol Obstet Invest. 2008;66:68–72.
- Muñoz N, Bosch FX, de Sanjosé S, et al. Epidemiologic classification of human papillomavirus types associated with cervical cancer. N Engl J Med. 2003;348:518–527.
- Anari E, Akbarzadeh A, Zarghami N. Chrysin-loaded PLGA-PEG nanoparticles designed for enhanced effect on the breast cancer cell line. Artif Cells Nanomed Biotechnol. 2016;44:1410–1416.
- Toda M, Rabkin SD, Martuza RL. Treatment of human breast cancer in a brain metastatic model by G207, a replication-competent multimutated herpes simplex virus 1. Hum Gene Ther. 1998;9:2177–2185.
- Gedanken A, editor. Antibacterial, antiviral, and antibiofilms nanoparticles. 2010 3rd International Nanoelectronics Conference (INEC); 2010.