?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Here, we investigated the effects of dual delivery of IGF-1R siRNA and doxorubicin by chitosan nanoparticles on viability of A549 lung cancer cells line by utilization of MTT and qRT-PCR. Furthermore apoptosis and migration of treated cells were assessed by Annexin-PI and wound healing assays, respectively. The chitosan nanoparticles had about 176 nm size with zeta potential and polydispersive index about 11 mV and 0.3, respectively. The IGF-1R siRNA had synergistic effect on DOX-induced cytotoxicity and apoptosis in tumour cells. In addition, siRNA/DOX-loaded chitosan nanoparticles could significantly decrease migration and expressions of mmp9, VEGF and STAT3 in A549 cells.
Introduction
Lung cancer is the most frequent cause of cancer mortality in the world. There are two forms of lung cancer, which are categorized by histological subtypes, including non-small cell lung cancer (NSCLC) and small-cell lung cancer (SCLC) [Citation1]. The former comprises about 85% of all lung cancers with a poor 5-year survival rate and prognosis [Citation2–6]. Despite significant advances in the therapeutic modalities including surgery, chemotherapy, radiotherapy and immunotherapy, NSCLC prognosis remains poor with short median survival time [Citation7]. The tendency of anticancer drugs in accumulation in normal tissues and consequent toxic side effects is one of the main reasons of unsuccessful outcome [Citation8]. In this regard, targeted therapy opens new insights for effective treatment of lung cancer. Drug delivery to the lungs provides many opportunities to improve drug therapies. In recent years, nanoparticles, as an efficient tumour-targeting drug delivery system, are considered as the hot topic in cancer research field. In this context, encapsulated anticancer drugs improve cellular uptake and increase drug concentration without dose-limiting toxicities (DLTs) in normal tissues, thus improved patient compliance [Citation9–12]. Despite advances in nanoparticle-based therapies for the treatment of NSCLC such as liposome, solid lipid nanoparticles (SLNs) and polymeric nanoparticles, few of them are successful in clinical results. It is a challenge to design suitable drug delivery systems for many of anticancer drugs with different physicochemical characteristics and efficient therapeutic activities in the physiologic condition [Citation13].
Polymeric nanoparticles for instance chitosan (Chi), a polysaccharide modified from chitin, have attracted much attention because of their unique characteristics like biocompatible, biodegradable, non-immunogenic, pH sensitive are appropriate vehicle for drug delivery that reduce nonspecific toxicity of anticancer drugs, prolong circulation time in blood and improve therapeutic efficiency [Citation14–19]. Mehrotra et al. [Citation20] demonstrated that the lomustine-loaded chitosan nanoparticles (ChiNPs) improved cytotoxicity in the L132 lung cancer cell line. Specific characters of modifying Chi has been introduced it as a potential tool for gene and drug delivery in combinational therapy setting [Citation13,Citation20].
Chemotherapy-resistant tumour cells are main challenge in clearing of tumours in patients. Thus, fighting against tumor cells with targeting different critical cancer hallmarks would improve the chance of successful cancer targeting therapy [Citation21]. Targeting essential and critical genes involving tumour development and growth such as insulin-like growth factor 1 receptor (IGF-1R) can be appropriate to accompany chemo drugs in combinational therapy.
The IGF-1R is a tetrameric transmembrane protein, which belongs to receptor tyrosine kinase family (RTKs). IGF-1R is activated by insulin-like growth factor 1 (IGF-1) and IGF-2 hormones. The activation of IGF-1R triggers tumour cell growth, resistance to apoptosis, angiogenesis, migration and invasion. Aberrant IGF-1R signalling has been reported in several tumour types, including colorectal and lung cancers [Citation22,Citation23]. IGF-1R is expressed in 39–84% of NSCLC patients [Citation24]. Moreover, several studies demonstrated that IGF-1R expression confers resistance to EGFR therapy in lung cancer [Citation25]. These findings show role of IGF-1R in numerous features of malignancy and suggesting this receptor could be a potential target for anticancer therapy. There are several therapeutic approaches to inhibit IGF-1R signalling such as using antibodies against IGF-IR or kinase inhibitors. Gene silencing potentially could be considered as another alternative in IGF-1R targeting in lung cancer [Citation26]. Thus, for the first time to our knowledge, we persuaded to investigate the efficiency of co-delivery of doxorubicin (DOX) and IGF-1R siRNA by ChiNP in A549 lung cancer cell line.
Material and methods
Preparation of chitosan nanoparticles (chi)
To obtain a 140 kDa molecular-weight ChiNP, 2 g of Chi (400 kDa, 87.3% deacetylation degree, Primex, Karma, Norway) was dissolved in acetic acid (6% v/v, Merck, Darmstadt, Germany) and then 10 ml NaNO2 (Merck, Darmstadt, Germany) at the concentration of 1 mg/ml was added and shaked for 2 h at room temperature. The depolymerized Chi was precipitated by adding NaOH (Merck, Darmstadt, Germany) 4 M at pH 9.0 and then was filtrated. Filtered Chi was then washed with acetone (Merck, Darmstadt, Germany) three times and dissolved in 40 ml of acetic acid (0.1 N). After dialysis (cut-off 12 kDa), ChiNP was lyophilized using freeze dryer (FD-10, Pishtaz Engineering Co, Tehran, Iran) and kept at 4 °C for further study.
Preparation of pharmaceutics groups (chi/siRNA/DOX/CMD, chi/siRNA/CMD and chi/DOX/CMD)
To produce ChiNP solution with different concentrations of DOX (Pfizer, New York, NY) and IGF-1R siRNA (Santa Cruz Biotech, Dallas, TX), 100 μl carboxymethyl dextran (CMD) (Sigma Aldrich, St. Louis, MO) with concentration of 1 mg/ml in deionized water was added to 20, 10, 5, 2.5 and 1.25 μg/ml of DOX. Then, 5 μl of IGF-1 R siRNA (100 nM) was added to the prepared solution and mixed for 15 s by vortex. The mixture was slowly added to 1 ml of Chi solution (10 mg/ml in distilled water with pH 5.5) and was incubated for 30 min at room temperature. Other groups including ChiNP/CMD, ChiNP/DOX/CMD and ChiNP/siRNA/CMD were prepared as mentioned above except adding siRNA and DOX as described previously [Citation27]. Moreover, the complexes of Chi/siRNA/CMD were analysed by electrophoresis on a 2% agarose gel compared with a naked siRNA.
ChiNP morphology and characteristics
To study the morphology of ChiNP-loaded with DOX and IGF-1R specific siRNA, one drop of ChiNP/siRNA/DOX/CMD was dried on an aluminum disk coated with a layer of gold and then scanned by scanning electron microscopy (SEM) (XL 30; Philips, Eindhoven, The Netherlands). Furthermore, size, polydispersity index (PDI) and zeta potential of ChiNP with various pharmaceutics group including ChiNP/CMD, ChiNP/siRNA/DOX/CMD, ChiNP/siRNA/CMD and ChiNP/DOX/CMD were measured by dynamic light scattering and zeta sizer (Nano-ZS, Malvern, Worcestershire, UK). All measurements were repeated three times at 25 °C, wavelength of 633 nm and angle detection 90°.
Fourier transform infrared spectroscopy
To investigate the molecular structure of ChiNP/DOX/CMD and ChiNP/CMD, wavelengths emitted from amino group in Chi (NH3+), carboxyl group (COO-) in the CMD and C–C bond in DOX were measured by FTIR spectrometer (Magna IR 550; Madison, WI).
IGF-1R siRNA and DOX loading efficiency
The IGF-1R-specific siRNA loading efficiency (%) in nanoparticles was determined by investigating the free siRNA in the supernatant of ChiNP/siRNA/CMD solution by centrifugation (15,000g, 20 min). The optical densities (OD) of free siRNA and primary feeding content of siRNA, which are used in the preparation of ChiNP/siRNA/CMD were determined at the wavelength of 260 nm by spectrophotometry (Scinco, Seoul, Korea). Furthermore, the optical densities (OD) of the collected supernatant from unloaded ChiNP were used as a blank. According to this method, loading efficiency of DOX was calculated in the ChiNP/DOX/CMD solution through centrifugation (12,000g, 20 min) and OD absorbance of DOX at 480 nm.
Serum and heparin stability of siRNA encapsulated in ChiNP
To determine the stability of loaded siRNA against serum, 300 μl ChiNP/siRNA/CMD were incubated with 300 μl fetal bovine serum (FBS) (Gibco, New York, NY) at 37 °C. At different time points (4, 8, 12, 24, 36, 48 and 72 h), 40 μl of the incubated mixture was removed and stored at –20 °C as long as electrophoresis was performed (30 min, 110 V) on all samples. To determine stability of ChiNP encapsulated siRNA against heparin, various volumes (0, 0.6, 1.5 and 3 μl) of heparin (2 μg/ml) were incubated with 60 μl ChiNP/siRNA/CMD at 37 °C for 1 h, and then all samples were electrophoresed. Naked siRNA was considered as a control in both experiments.
In vitro IGF-1R siRNA and DOX releasing assay
To evaluate the percentage of the released siRNA, ChiNP/siRNA/CMD was dissolved in 4 ml phosphate-buffered saline (PBS, pH 5.5 and 7.4) and then dialysis (cut-off 12 kDa, Sigma Aldrich) was performed against PBS (50 ml, pH 5.5 and 7.4) in a shaker incubator (37 °C, 100 rpm) for 120 h. At each predetermined time (1, 3, 6, 12, 24, 48, 72, 96 and 120 h), 2 ml of PBS inside the container was taken out and replaced with fresh PBS. Subsequently siRNA released content was assessed by UV-Vis spectrometry (U.V-1601; Shimadzu, Kyoto, Japan) at 260 nm wavelength. Moreover, releasing of DOX was measured according to this method in ChiNP/DOX/CMD at 480 nm wavelength.
Cell line and culture
The A549 NSCLC cell line with overexpression of IGF-1R was obtained from the National Cell Bank of Iran (NCBI, Tehran, Iran). Cells were cultured in RPMI-1640 medium (Gibco, New York, NY) enriched with 10% FBS (Gibco, New York, NY), and antibiotics (streptomycin 100 ng/ml and penicillin 100 U/ml) at 37 °C in a humidified 5% CO2 atmosphere.
MTT assay
Cells viability were assayed by measuring blue formazan produced only in live cells and metabolized from 3–(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) by mitochondrial dehydrogenase. One day before treatment, 15 × 103 cells/well were seeded in 96-well transparent flat bottom plates (Nunc, Roskilde, Denmark). The cells were incubated for 24 and 48 h with pharmaceutics groups including ChiNP, ChiNP/siRNA/DOX/CMD, ChiNP/siRNA/CMD, ChiNP/DOX/CMD and free DOX (with various concentrations of DOX 20, 10, 5, 2.5 and 1.25 μg/ml and 100 nM siRNA in each well) and naked IGF-1R siRNA as controls. The supernatant of the wells were removed and 200 μl of MTT solution (5 mg/ml) was added to each well and incubated for 4 h at 37 °C in 5% CO2, then insoluble formazan crystals were dissolved in 100 μl of DMSO and read at 492 nm versus 690 nm reference wavelength by ELISA reader (Stat Fax 2100, Awareness Technology, Palm City, FL). All experiments were performed in triplicate.
Apoptosis assay
The percentages of cells undergoing apoptosis were assayed by Annexin V-FITC and PI (BD Pharmingen, San Diego, CA). A549 cells (200 × 103 cell/well) were seeded in 6-well plates and incubated with ChiNPs containing equivalent concentration of DOX IC50 (7.8 μg/ml) in various groups of ChiNP/siRNA/DOX/CMD, ChiNP/siRNA/CMD, ChiNP/DOX/CMD, free DOX for 24 h. Then, the cells were washed twice with PBS and resuspended in binding buffer. Five micro litres of Annexin V-FITC was added to the cells and then incubated for 15 min at room temperature in the dark. Finally, apoptotic cells were analysed by flow cytometry (Becton-Dickenson, Mountain View, CA, USA) and FlowJo software.
Cell migration and invasion
Scratch wound-healing method was applied to investigate migration and invasion capacity of A549 cells. A549 cells (200 × 103 cells/well) were seeded in 6-well plates and incubated for 24 h to become monolayer. A uniform scratch at the centre of each well was created by sterile micropipette tips. Then, cells were treated with pharmaceutics groups, ChiNP/siRNA/DOX/CMD, ChiNP/siRNA/CMD, and ChiNP/DOX/CMD, free DOX, naked IGF-1 R siRNA and blank ChiNP against untreated cells (control). Plates were visualized under ×10 magnification and photographs were taken of each well by inverted microscopes immediately after wounding and 12, 24 and 36 h after wounding.
RNA extraction and real-time PCR
The cells were treated with ChiNPs containing equivalent concentration of DOX IC50 (7.8 μg) in different groups for 24 and 48 h. Total mRNA was extracted using the TRIzol reagent (Invitrogen, Carlsbad, CA) according to the manufacturer’s instructions. Complementary DNA (cDNA) was synthesized using reverse transcriptase kit (TaKaRa Dalian Biotechnology Co., Ltd. Dalian, China). To investigate gene expression level, the RT-PCR technique by multi-color Real-time PCR detection system (Roche light cycler 96, Penzberg, Germany) and SYBR Premix Ex Taq TM II (TaKaRa Dalian Biotechnology Co., Ltd. Dalian, China) against β-actin as a housekeeping gene (Bioneer, Cheongwon, Korea) were used. The PCR reaction condition was done in 45 cycles, denaturation at 95 °C for 10 s, extension at 72 °C for 30 s, annealing at 59, 57 and 62 °C for 15 s using specific primers for MMP9, VEGF and STST3, respectively. Gene expression levels were normalized compared to β-actin at each corresponding sample. The sequences of PCR primers are presented as following: β-actin: forward 5'-AAGTTTATCTGTGTGCACCAACGA-3' and reverse 5'-CTTCACATTATTTCCAAACTGCAT-3', matrix metalloproteinase 9 (MMP-9): forward 5'-GGTTCTTCTGCGCTACTGCTG-3' and reverse 5'- GTCGTAGGGCTGCTGGAAGG-3', signal transducer and activator of transcription 3 (STAT3): forward 5'-AAGTTTATCTGTGTGCACCAACG-3' and reverse 5'-CTTCACCATTATTTCCAAACTGC-3', vascular endothelial growth factor (VEGF): forward 5'-TTGCTTGCCATTCCCCACTT-3' and reverse 5'-CCGAAGGCAGAACAGCCCAC-3'.
Statistical analysis
All data and results were statically analysed by Graph pad Prism v6.07 (Graph Pad Software, CA, USA). Non-parametric one-way ANOVA test were applied for different group’s analysis. Value with p < .05 was considered as significant level.
Result
Characteristics of nanoparticles
Dynamic light scattering was used to measure features of the generated ChiNP (140 kDa) including size, PDI and zeta potential. The results showed that in all groups containing ChiNP, PDI did not change, but size and zeta potential of ChiNP partially changed after the loading of CMD, IGF-1R siRNA and DOX (). In addition, SEM-based surface morphology analysis of ChiNP loaded with CMD, siRNA and DOX showed multifaceted surface with an irregular shape of ChiNP ().
Table 1. Physical characteristics of ChiNPs with different pharmaceutical groups.
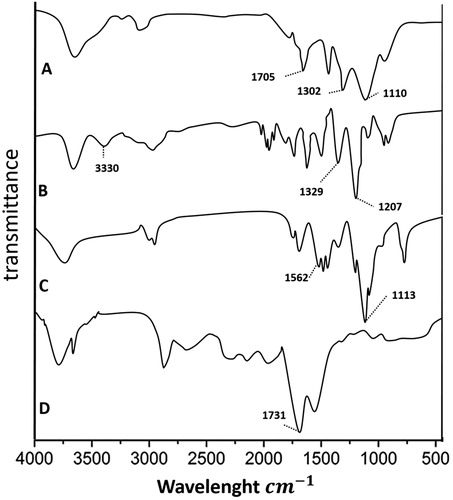
FTIR was used to approve appropriate conjugation of CMD, DOX with Chi using the specific wavelength of the carboxyl group in CMD (1731 cm−1), C–C linkage in DOX (1207 and 132 cm−1) and amino group in ChiNP (1562 and 1113 cm−1). Overlapping in wavelengths of ChiNP/DOX/CMD (amino group of Chi with 1110 cm−1, C–C linkage of DOX with 1302 cm−1 and carboxyl group of CMD with 1705 cm−1) indicated that the conjugation was accurately produced ().
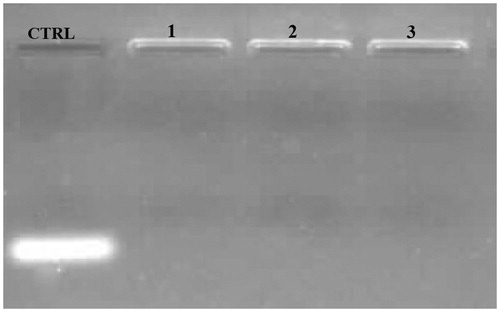
In the next step, IGF-1R siRNA loading capacity of ChiNP was detected using electrophoresis on a 2% agarose gel compared with a naked IGF-1R siRNA as control. Our results determined that siRNA was efficiently loaded on ChiNP (140 kDa) at 25, 50 and 100 nM of IGF-1R siRNA (). Furthermore, the siRNA encapsulation efficiency of ChiNP was measured through detection of the free siRNA absorbance in the ChiNP/siRNA/CMD solution by UV spectrometry and compared with the initial amount of siRNA (100 nM equal to 5 μl whit 10 μM/500 μl concentration) in this solution. Generated ChiNP with 140 kDa MW have 86% capacity for siRNA encapsulation. Moreover, encapsulation rate of DOX was 75%.
Serum and heparin stability of siRNA encapsulated in ChiNP
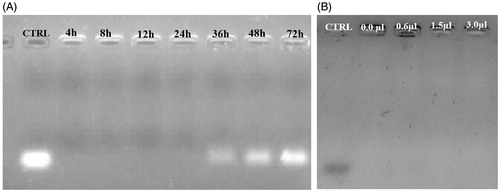
The siRNA-loaded ChiNP in serum was stable for 24 h (). However, siRNA was partially released from ChiNP after 36 h (). Comparison of siRNA band intensities showed that at 72 h time point, siRNA was completely released from ChiNP (). Although in serum environment encapsulated siRNA was released, heparin had no effect on the release of siRNA from ChiNP ().
Figure 4. The stability analysis of siRNA-loaded ChiNPs in serum and heparin challenges. (A) The stability of siRNA-loaded ChiNP/CMD against FBS after 72 h incubation. Naked siRNA was considered as control (CTRL). (B) The stability of siRNA-loaded ChiNP/CMD against different volumes of heparin (2 μg/mL) after 1 h incubation. Naked siRNA was considered as positive control (CTRL).
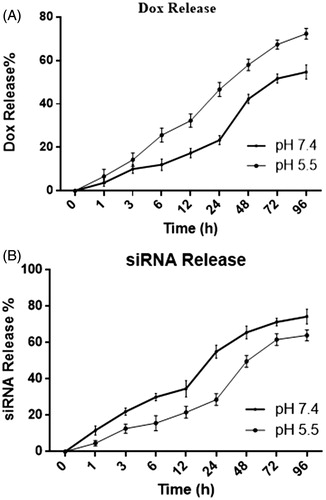
IGF-1R siRNA and DOX release rate was higher at low pH condition
Release curves of DOX and IGF-1R siRNA at 37 °C and different pH (7.4 and 5.5) are presented as . Our findings showed that siRNA and DOX release at higher rate at pH 5.5 compared to pH 7.4. IGF-1R siRNA release reached to 50% at 24 and 48 h at pH 5.5 and 7.4, respectively (). This releasing process continued until 96 h and reached the stationary phase. DOX release curve showed that DOX release reached to 50% at 24 h and 72 h at pH 5.5 and 7.4, respectively (). DOX release rate was slower at physiologic pH (7.4) compared to siRNA ().
ChiNPs containing siRNA and DOX enhanced cytotoxicity and apoptosis in A549 cell line
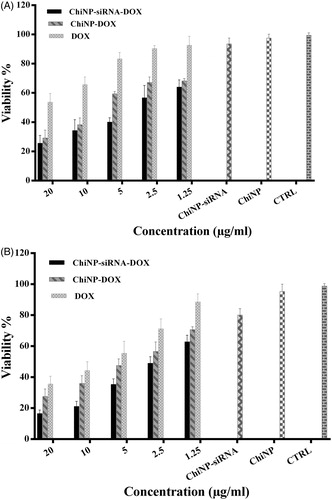
To finding out whether ChiNP containing siRNA and DOX can enhance the cytotoxicity compared with free DOX as well as ChiNP containing siRNA or DOX alone, A549 human lung cancer cell line was treated with different concentrations of various ChiNPs for 24 and 48 h. It was revealed that Chi/siRNA/DOX/CMD at concentrations of 20, 10 and 5 μg/ml significantly decreased cell viability in a time- and dose-dependent manner (p < .05) (). Moreover, compared with free DOX, ChiNP/DOX significantly enhanced drug cytotoxicity on A549 cells. In this regard, half maximal inhibitory concentration (IC50) of ChiNP/DOX was significantly lower than free DOX (p < .05) which implied on the potential application of ChiNP as a carrier in anticancer drugs delivery. It should be noted that there was no significant change on viability of cells treated with blank nanoparticles after 48 h ().
Figure 6. Combination of IGF-1R siRNA and DOX synergistically improved cytotoxicity in A549 human lung cancer cell line in a dose and time-dependent manner at 24 h (A) and 48 h (B). CTRL represents no treated cell control. Data shown as means ± SD of three independent experiments.
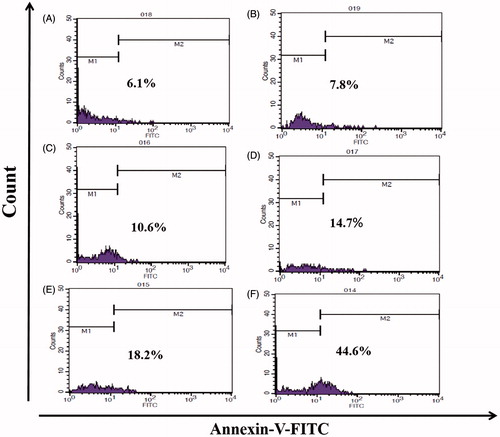
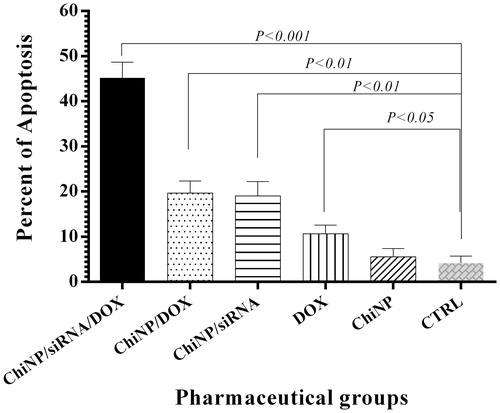
Effects of ChiNP/siRNA/DOX/CMD, ChiNP/DOX/CMD, ChiNP/siRNA/CMD and free DOX on the apoptosis of A549 cells were measured by flow cytometry (). Compared to controls, ChiNP/siRNA/DOX/CMD significantly induced apoptosis in 45% of treated cells after 24 h (p < .001) (). Furthermore, ChiNP/siRNA/CMD and ChiNP/DOX/CMD induced apoptosis (20%) lower than combinational formula of ChiNP/siRNA/DOX/CMD (45%) in treated cells. DOX and siRNA encapsulation in ChiNP was more efficient in triggering apoptosis pathways compared to free DOX (). Untreated cells and the blank nanoparticles have no significant effects on the apoptosis rate.
Combination of DOX and IGF-1R siRNA synergistically inhibited migration and invasion of A549 cells
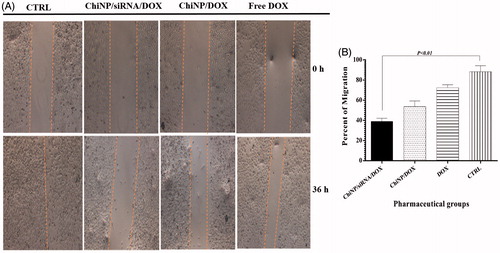
Because cell motility is an important factor in metastasis and invasion of tumor cells, the effect of DOX and IGF-1R siRNA on A549 cells migration was studied by wound healing assay, which measures the capability of cells to fill a wounded area. Photographic results showed that untreated cells were highly mobile and fill wounded area thoroughly after 36 h, whereas following ChiNP/siRNA/DOX/CMD treatment, migration into the wounded area was significantly decreased (p < .01) (). Free DOX alone weakly inhibited the migration of A549 cells compared with ChiNP/DOX/CMD. These results indicated that co-delivery of IGF-1R siRNA and DOX had greater effects on migration and invasion than DOX alone ().
Co-delivery of DOX and IGF-1R siRNA down-regulated MMP-9, VEGF and STAT3 expression levels in A549 cells
Previous studies have indicated that IGF-1R signalling induces overexpression and activation of invasion-related genes including MMP-9 and VEGF [Citation28,Citation29]. ChiNPs effects on MMP-9 and VEGF transcripts expression in A549 cells were analysed by quantitative RT-PCR. Our results showed that MMP-9 and VEGF mRNA expression levels were significantly down-regulated in cells treated with ChiNP/siRNA/DOX/CMD compared to untreated control cells (). Similar but less dramatic results were observed in the cells treated with ChiNP/siRNA/CMD group. Moreover, Chi/DOX or free DOX was not able to decrease MMP-9 and VEGF gene expression ().
Figure 10. MMP-9, VEGF and STAT3 genes expression profiles in A549 human non-small cell lung cancer cell line treated with ChiNP with different pharmaceutical groups. (A) MMP-9; (B) VEGF; and (C) STAT3 gene expression alterations. Data shown as means ± SD of three independent experiments.
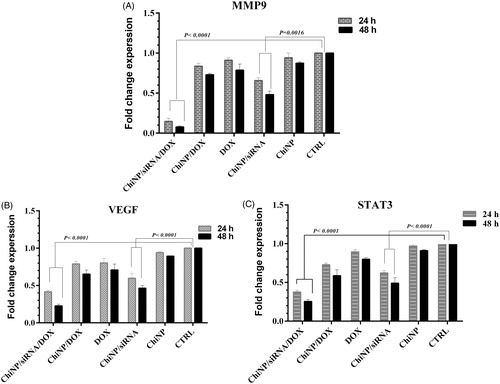
Moreover, STAT3 is one of the downstream adaptors of IGF-1R signalling pathway, which is associated with cellular angiogenesis and invasion. In order to better understand the effect of IGF-1R-specific siRNA on this signalling pathway, the total STAT3 mRNA levels in different treatment groups was investigated. Our results revealed that co-delivery of IGF-1R siRNA and DOX led to significant decrease of STAT3 mRNA expression compared with the other groups (). It has to be noted that the blank nanoparticles had no significant effect on gene expression profiles and these findings indicated that silencing efficiency of siRNA was augmented using ChiNP delivery compared to naked siRNA.
Discussion
The insulin-like growth factor-1 receptor (IGF-1R) is composed of two α subunits that bind to IGFs ligands and two intracellular β subunits with the tyrosine kinase activity [Citation30]. IGF-1R signalling is a complex pathway because activated receptor requite different adapter proteins and start a wide network of signalling pathways in cells such as two major cellular cascade, the RAS/RAF/MEK/MAPK and the PI3K/AKT/mTOR that mediate multiple mechanisms and implicate in numerous features in malignancy including tumor cell growth, resistance to apoptosis, angiogenesis also degradation of the extracellular matrix, and promote tumor migration and invasion [Citation22]. In addition, activation of IGF-1R is one of the mechanisms of resistance to anti-epidermal growth factor receptor (EGFR) therapy in several type of tumor including lung cancer [Citation31]. Numerous studies demonstrated that role of IGF-1R signalling in initiation and progression of lung cancer and has been reported that down-regulation of IGF-IR inhibits cell proliferation and increase response to chemotherapy and radiotherapy [Citation32–34]. These data suggested that IGF-1R is attractive therapeutic targets for NSCLC. On the basis of inhibition of IGF-1R, several therapeutic approaches including antibodies against IGF-IR, small-molecule inhibitors or siRNA used in different cancer cell line. In the current decade, because of the features of siRNA such as stability, versatility and specific gen silencing enhanced the potential for cancer treatment [Citation35–37]. On the other hand, targeted delivery of drugs or siRNA can increase the selective death of cancer cells.
In this study, we investigated combinational effects of encapsulated DOX and IGF-1R-specific siRNA by ChiNPs on A549 human lung cancer cell line. Due to the characteristics of ChiNP, loading CMD, siRNA and DOX can effect on the particles’ size and PDI so that ChiNP/siRNA/DOX/CMD had the largest size (176 nm) and PDI (0.3). ChiNP/CMD had smallest size (113 nm) among the other ChiNP groups (). PH-dependent nanoparticles such as ChiNPs are suitable drug carriers in cancer therapy. Because hypoxia and low number of vessels, high concentrations of lactic acid and carbonic acid result acidic pH in tumour microenvironment. DOX and siRNA release assay revealed that delivery of drug or siRNA at pH 5.5 was more efficient, which could increase drug and siRNA concentrations in the tumuor microenvironment compared to physiologic condition at pH 7.4 (). Likewise, our results showed that loaded ChiNP with acceptable high IGF-1R, siRNA loading capacity was stable against serum and heparin ().
It was also investigated whether DOX delivery by ChiNP increase drug concentration and cytotoxic effects on the cancer cell line. Interestingly, our result clearly indicated that growth inhibition significantly increased using ChiNP/DOX in comparison to free DOX (). According to previous reports, expression and activation of IGF-1R is essential for NSCLC proliferation and consequently IGF-1R inhibition by RNA interference has been reported to contribute to the cancer cell growth suppression [Citation38]. Therefore, we persuaded to determine whether co-delivery of siRNA and DOX may decrease in vitro viability of cells. Our findings showed that A549 cells treated with ChiNP/DOX/siRNA/CMD had significant differences in cell death compared to free DOX and also IGF-1R siRNA ( and ). In addition, siRNA inhibitory effects on cell growth time-dependently increased after 48 h (). It seems that capacity of nanoparticles in simultaneous delivery of several agents can open new insight to combinational and targeted therapies in cancer.
Various studies demonstrated that IGF-1R directly induces invasion and metastasis via MMPs, and the PI3K/AKT and RAS/ERK signalling pathways that are essential in MMP-9 expression regulation [Citation39]. Thus, in the next step, we focused on whether the IGF-1R downstream signalling pathway is affected by specific siRNA. Our results showed that IGF-1R siRNA decreased MMP-9 expression in ChiNP groups containing siRNA/CMD in contrast with free DOX and ChiNP/DOX/CMD (). In addition, in groups containing ChiNP/DOX/siRNA/CMD, MMP-9 expression was significantly down-regulated because of the synergic effect of siRNA and DOX on gene expression. Compatible with these findings, ChiNP/DOX/siRNA/CMD strongly inhibited invasion and metastasis of A549 cell line than the other groups (). Moreover, IGF-1R signalling is linked to angiogenesis through VEGF regulation in various tumours. IGF-1R activation recruits STAT3 independent of IRS1/2, constitutively activated STAT3 increases hypoxia-inducible factor 1α (HIF1α) expression. Under hypoxic conditions, HIF-1α is expressed and induces VEGF, which is essential in angiogenesis and tumour metastasis [Citation29,Citation40,Citation41]. The effects of IGF-1R down-regulation by specific siRNA with or without DOX were studied on STAT3 and VEGF expression levels. According to our results, IGF-1R down-regulation by ChiNP/siRNA/DOX/CMD and ChiNP/siRNA/CMD could lead to a significant decrease in the STAT3 and VEGF expression in the A549 cell line ().
Conclusions
In conclusion, ChiNPs as a dual agent carrier shows great promise for the efficient drug delivery to tumour cells. Our findings indicated that IGF-1R siRNA/DOX co-delivery system can be considered as an effective and potential approach in combinational targeted therapy of lung cancer that may be useful in preventing the metastatic spread and treatment of lung cancer. Finally, further researches especially in vivo animal models need to investigate the potential application of the presented ChiNPs containing siRNA and DOX in combinational therapy approaches.
Disclosure statement
There are no conflicts of interest to declare.
Additional information
Funding
References
- Janku F, Stewart D, Kurzrock R. Targeted therapy in non-small-cell lung cancer – is it becoming a reality? Nat Rev Clin Oncol. 2011;8:384.
- Edwards BK, Ward E, Kohler BA, et al. Annual report to the nation on the status of cancer, 1975-2006, featuring colorectal cancer trends and impact of interventions (risk factors, screening, and treatment) to reduce future rates. Cancer. 2010;116:544–573.
- Mitsudomi T, Morita S, Yatabe Y, et al. Gefitinib versus cisplatin plus docetaxel in patients with non-small-cell lung cancer harbouring mutations of the epidermal growth factor receptor (WJTOG3405): an open label, randomised phase 3 trial. Lancet Oncol. 2010;11:121–128.
- Lam WK, Watkins DN. Lung cancer: future directions. Respirology. 2007;12:471–477.
- Rami-Porta R, Crowley JJ, Goldstraw P. Review the revised TNM staging system for lung cancer. Ann Thorac Cardiovasc Surg. 2009;15:5.
- Fathi Karkan S, Mohammadhosseini M, Panahi Y, et al. Magnetic nanoparticles in cancer diagnosis and treatment: a review. Artif Cells, Nanomed Biotechnol. 2016.
- Schiller JH, Harrington D, Belani CP, et al. Comparison of four chemotherapy regimens for advanced non-small-cell lung cancer. N Engl J Med. 2002;346:92–98.
- Lu R-M, Chang Y-L, Chen M-S, et al. Single chain anti-c-Met antibody conjugated nanoparticles for in vivo tumor-targeted imaging and drug delivery. Biomaterials. 2011;32:3265–3274.
- Gualberto A, Karp DD. Development of the monoclonal antibody Figitumumab, targeting the insulin-like growth factor-1 receptor, for the treatment of patients with non–small-cell lung cancer. Clin Lung Cancer. 2009;10:273–280.
- Thundimadathil J. Cancer treatment using peptides: current therapies and future prospects. J Amino Acids. 2012 [Epub ahead of print]. DOI: 10.1155/2012/967347.
- Allen TM. Ligand-targeted therapeutics in anticancer therapy. Nat Rev Cancer. 2002;2:750–763.
- Badrzadeh F, Rahmati-Yamchi M, Badrzadeh K, et al. Drug delivery and nanodetection in lung cancer. Artif Cells Nanomed Biotechnol. 2016;44:618–634.
- Babu A, Templeton AK, Munshi A, et al. Nanoparticle-based drug delivery for therapy of lung cancer: progress and challenges. J Nanomater. 2013. Article ID 863951.
- des Rieux A, Fievez V, Garinot M, et al. Nanoparticles as potential oral delivery systems of proteins and vaccines: a mechanistic approach. J Control Release. 2006;116:1–27.
- Hosseini M, Haji-Fatahaliha M, Jadidi-Niaragh F, et al. The use of nanoparticles as a promising therapeutic approach in cancer immunotherapy. Artif Cells Nanomed Biotechnol. 2016;44:1051–1061.
- Kumari A, Yadav SK, Yadav SC. Biodegradable polymeric nanoparticles based drug delivery systems. ColloidSurf B Biointer 2010;75:1–18
- Siahmansouri H, Somi MH, Babaloo Z, et al. Effects of HMGA2 siRNA and doxorubicin dual delivery by chitosan nanoparticles on cytotoxicity and gene expression of HT‐29 colorectal cancer cell line. J Pharm Pharmacol 2016;68:1119–1130.
- Dass CR, Choong PF. The use of chitosan formulations in cancer therapy. J Microencapsul. 2008;25:275–279.
- Wang F, Wang Y, Ma Q, et al. Development and characterization of folic acid-conjugated chitosan nanoparticles for targeted and controlled delivery of gemcitabinein lung cancer therapeutics. Artif Cells Nanomed Biotechnol 2016 [Epub ahead of print]. DOI: 10.1080/21691401.2016.1260578
- Mehrotra A, Nagarwal RC, Pandit JK. Lomustine loaded chitosan nanoparticles: characterization and in-vitro cytotoxicity on human lung cancer cell line L132. Chem Pharm Bull. 2011;59:315–320.
- Denduluri SK, Idowu O, Wang Z, et al. Insulin-like growth factor (IGF) signaling in tumorigenesis and the development of cancer drug resistance. Genes Dis. 2015;2:13–25.
- Shali H, Ahmadi M, Kafil HS, et al. IGF1R and c-met as therapeutic targets for colorectal cancer. Biomed Pharmacother. 2016;82:528–536.
- Scagliotti GV, Novello S. The role of the insulin-like growth factor signaling pathway in non-small cell lung cancer and other solid tumors. Cancer Treat Rev. 2012;38:292–302.
- Fidler MJ, Shersher DD, Borgia JA, et al. Targeting the insulin-like growth factor receptor pathway in lung cancer: problems and pitfalls. Ther Adv Med Oncol. 2012;4:51–60.
- Lee Y, Wang Y, James M, et al. Inhibition of IGF1R signaling abrogates resistance to afatinib (BIBW2992) in EGFR T790M mutant lung cancer cells. Molec Carcinogenesis. 2016;55:991–1001.
- Qian J, Dong A, Kong M, et al. Suppression of type 1 Insulin-like growth factor receptor expression by small interfering RNA inhibits A549 human lung cancer cell invasion in vitro and metastasis in xenograft nude mice. Acta Biochim Biophys Sinica. 2007;39:137–147.
- Jadidi-Niaragh F, Atyabi F, Rastegari A, et al. Downregulation of CD73 in 4T1 breast cancer cells through siRNA-loaded chitosan-lactate nanoparticles. Tumor Biol. 2016;37:8403–8412.
- Zhang D, Samani A, Brodt P. The role of the IGF-I receptor in the regulation of matrix metalloproteinases, tumor invasion and metastasis. Hormone Metab Res. 2003;35:802–808.
- King ER, Wong K-K. Insulin-like growth factor: current concepts and new developments in cancer therapy. Recent Pat Anticancer Drug Discov. 2012;7:14–30.
- Riedemann J, Macaulay V. IGF1R signalling and its inhibition. Endocrine-Related Cancer. 2006;13(Supplement 1):S33–S43.
- Morgillo F, Della Corte CM, Fasano M, et al. Mechanisms of resistance to EGFR-targeted drugs: lung cancer. ESMO Open. 2016;1:e000060.
- Linnerth NM, Siwicky MD, Campbell CI, et al. Type I insulin-like growth factor receptor induces pulmonary tumorigenesis. Neoplasia. 2009;11:672–682.
- Goetsch L, Gonzalez A, Leger O, et al. A recombinant humanized anti-insulin-like growth factor receptor type I antibody (h7C10) enhances the antitumor activity of vinorelbine and anti-epidermal growth factor receptor therapy against human cancer xenografts. Int J Cancer. 2005;113:316–328.
- Cosaceanu D, Carapancea M, Castro J, et al. Modulation of response to radiation of human lung cancer cells following insulin-like growth factor 1 receptor inactivation. Cancer Lett. 2005;222:173–181.
- Uprichard SL. The therapeutic potential of RNA interference. FEBS Lett. 2005;579:5996–6007.
- Miyagishi M, Taira K. U6 promoter–driven siRNAs with four uridine 3′ overhangs efficiently suppress targeted gene expression in mammalian cells. Nat Biotechnol. 2002;20:497–500.
- Wang C, Ding C, Kong M, et al. Tumor-targeting magnetic lipoplex delivery of short hairpin RNA suppresses IGF-1R overexpression of lung adenocarcinoma A549 cells in vitro and in vivo. Biochem Biophys Res Commun. 2011;410:537–542.
- Yin M, Guan X, Liao Z, et al. Insulin-like growth factor-1 receptor-targeted therapy for non-small cell lung cancer: a mini review. Am J Transl Res. 2009;1:101–114.
- Nian W, Ao X, Wu Y, et al. miR-223 functions as a potent tumor suppressor of the Lewis lung carcinoma cell line by targeting insulin-like growth factor-1 receptor and cyclin-dependent kinase 2. Oncol Lett. 2013;6:359–366.
- Gariboldi MB, Ravizza R, Monti E. The IGFR1 inhibitor NVP-AEW541 disrupts a pro-survival and pro-angiogenic IGF-STAT3-HIF1 pathway in human glioblastoma cells. Biochem Pharmacol. 2010;80:455–462.
- Zhang W, Zong CS, Hermanto U, et al. RACK1 recruits STAT3 specifically to insulin and insulin-like growth factor 1 receptors for activation, which is important for regulating anchorage-independent growth. Molec Cell Biol. 2006;26:413–424.