Abstract
The aim of this study was to fabricate propranolol hydrochloride (Prop. HCl) (as a water-soluble drug):Eudragit® RS100 (Eud) nanobeads and nanofibres applying the electrospraying method as an economical and one-step technique. Different ratios of Prop. HCl:Eud (i.e. 1:5 and 1:10) at total solution concentrations of 10–20% W/V were investigated. The FE-SEM studies revealed that morphology and size of the samples were highly affected by the solution concentration; so that, the nanobeads (a mean diameter of 82.9 nm) were formed in low concentration and at the highest concentration of the solution, nanofibres (a mean diameter of 232.3 nm) were resulted. Besides the morphological changes, the size of processed nanoformulations was increased with an increment of the solution concentrations. X-ray diffraction results as well as DSC thermograms clearly indicated that the drug crystallinity decreased in the electrosprayed samples. Furthermore, in vitro dissolution test showed that the electrosprayed samples had relatively slower release patterns toward the pure drug and physical mixtures, where the samples with the drug:polymer ratio of 1:10 indicated a faster release rate toward 1:5 ratio; nevertheless, the concentration of the injected formulations did not remarkably impressed the release behaviours. The current study established the suitability of electrospraying method in the fabrication of the water-soluble drugs nanobeads/nanofibres; however, in vivo effectiveness of the prepared nanoformulations should be meticulously considered.
Introduction
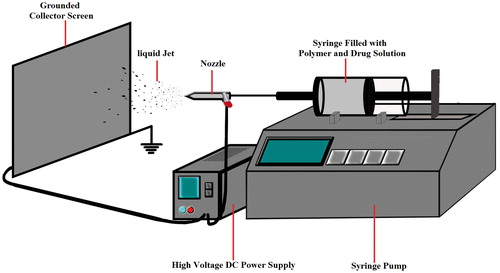
Recently, new drug delivery systems (DDSs) (such as nanodrugs) achieved tremendous advances in the field of medicine and treatment of the diseases [Citation1,Citation2]. Pharmaceutical industry based on the idea of delivering a sufficient dosage of a drug into the diseased organs and to minimize the associated side effects, needs appropriate carriers and formulations [Citation3]. Nanoparticles are one of the promising devices that can deliver a well-determined dose of a drug and act as a proper and controllable carrier [Citation4]. It is well known that the nanoparticles have a greater therapeutic effect [Citation5], lower toxicity and patient compliance due to the drug accumulation at the site of action [Citation6]. Moreover, it is reported that nanoparticles are effective in improving the physicochemical characteristics of poorly water-soluble drugs [Citation7]. A variety of organic and organometallic compounds as well as inorganic ingredients such as viruses, lipids and polymers can be used to design appropriate nanoparticles with improved function. One of the mostly used carriers in new DDSs is polymer-based nanoparticles (PBNPs) [Citation8]. Various methods have been developed in the manufacturing of PBNPs, including template synthesis [Citation9], supercritical fluid, aerosol flow reactor [Citation10], nanoprecipitation [Citation11], emulsification solvent evaporation and electrospraying [Citation12]. Among all, the electrospraying/electrohydrodynamic has much potential to be used for mass production or industry scale. It provides an outstanding method to fabricate submicron polymer-based structures [Citation12]. This technology uses an electrical force to atomize a mixture of a drug solution with a polymer. In other words, at high electrical force (about 20–30 kV), the liquid jet moves out towards the grounded collector screen through a capillary nozzle (syringe) (). Solvent evaporates during the liquid jet flying toward the collector screen, and based on the mixture viscosity, nanobeads or nanofibres are formed on the grounded collector. These nanoformulations contain the drug molecules that encapsulated into the nanobeads or nanofibres [Citation12,Citation13]. The system of electrospraying potentially has some advantageous such as controlling the morphology and the size distribution of droplets in order to fabricate nearly monodisperse particles in an economic and one-step manner at ambient pressure and temperature [Citation13]. The electrospraying method has been successfully used to fabricate submicron polymer-based structures such as thin film deposition [Citation14], capsule formation [Citation15] and particle production [Citation16]. The technique has various applications in microelectronics [Citation17], optical electronics, biotechnology, environmental engineering [Citation18], modern material and medical technologies, new DDSs and ingredients dosage fabrication in the food, cosmetic and medicine production industries [Citation19]. Some drugs such as insulin [Citation20], paclitaxel [Citation21], azithromycin [Citation22], triamcinolone acetonide [Citation23], methylprednisolone acetate [Citation24], ibuprofen [Citation25], naproxen [Citation26], etc. [Citation27–34] have been prepared by this method.
Eudragit® RS100 (Eud) is a copolymer of poly (ethylacrylate, methyl-methacrylate) and chloro trimethyl-ammonium methyl methacrylate which contains some tetrad ammonium groups around 4.5–6.8%. Despite the insoluble nature of Eud at physiologic pH, its hydrophilic properties make it swellable at this pH. Besides, it can adhere to the negatively charged cells, so this polymer sounds to be an opportune carrier for localized and extended delivery of the proper drugs to the various physiologic fluids [Citation22–24]. There are some reports that show the application of Eud as a suitable carrier at delivery of triamcinolone acetonide [Citation23], methylprednisolone acetate [Citation24], azithromycine [Citation22] and some anti-inflammatory drugs to the eye [Citation12]. To the best of our knowledge, there is no report regarding the preparation of the water-soluble drug–Eud electrosprayed nanoformulations.
In the present study, Prop. HCl (water-soluble model drug)–Eud nanobeads and nanofibres in different ratios of drug to polymer (such as 1 to 5 and 1 to 10) were formulated using the electrospraying method and the morphological and physicochemical properties of the prepared nanoformulations were evaluated.
Methods and materials
Materials
Pure powders of propranolol and eudragit® RS100 were purchased from Iran Hormone (Iran) and Degussa (Darmstadt, Germany), respectively. Potassium phosphate monobasic, sodium hydroxide and ethanol were obtained from Merck (Darmstadt, Germany). All other chemical substances were of analytical grade.
Preparation of the electrospraying samples
Prop. HCl–Eud nanobeads and nanofibres were prepared by a custom-designed electrospraying apparatus (Fanavaran Nano-Meghyas, Tehran, Iran). Briefly, the drug and polymer were co-dissolved in ethanol by magnetic stirring at ambient temperature to prepare Prop. HCl–Eud solution with drug:polymer ratios of 1:5 and 1:10. The total concentrations of the drug:polymer solutions were regulated to be 10, 15 and 20% (w/v) ().
Table 1. Polymer: drug ratios of the prepared samples.
The prepared solutions were dropped (with a constant rate of 5 ml/h) to the grounded collector screen made of polytetrafluoroethylene through a ring-shaped polyethylene capillary cylinder with an internal diameter of 0.1 mm. The distance between the nozzle tip and the grounded collector screen was fixed at 10 cm and a voltage of 25 kV was utilized between them.
Morphological evaluation
The morphology of samples was investigated using scanning electron microscopy (FEG-SEM) operating at 20 kV (MIRA3, Tescan Company, Brno, Czech). The device was equipped with field emission and its low and high vacuum capability provided a good environment to study non-conductive materials. A thin gold layer (a thickness of 150°A) was coated on the samples using gold sputtering machine (Emitech K550, Kent, UK) before FEG-SEM evaluation.
Differential scanning calorimetry (DSC)
DSC 60 (Shimadzu, Kyoto, Japan) was benefitted to examine and realize the thermal attitude and thermograms of the samples. For this aim, test samples were meticulously weighed (5 mg) and then positioned in the sealed aluminium pans. A scanning rate of 20 °C per minute was used to evaluate the thermal attitude of the samples in the range of 25–220 °C. As a reference and a standard sample, the aluminium oxide and indium powders were employed, respectively. For final evaluation, TA60 software (SPSS Inc., Chicago, IL) was conducted to analyze the thermograms of samples.
Powder X-ray diffraction analyzing (PXRD)
PXRD measurements of the pure drug, polymer, physical mixture (PM) of the drug and polymer, nanofibres and nanobeads were made by X-ray diffraction (model D5000 X-ray diffractometer, Siemens, Munich, Germany) with a step size of 0.02°, at a scanning rate of 0.6°/min, using Cu Kα radiation (k = 1.5405 A°) from 5° to 40° working at 40 kV, 30 mA.
Fourier transform infrared spectroscopy (FTIR)
To validate the probability of chemical interaction between the drug and polymer, FTIR (43000 FTIR spectrophotometer, Shimadzu, Kyoto, Japan) analysis was used. To do this evaluation, the KBr disk method was applied to prepare compact disks of the Prop. HCl–Eud nanoformulations and their PMs. The scanning range, the resolution and average spectra were 600–4000 cm−1, 2 cm−1 and 32 scans, respectively.
In vitro drug release
The USP apparatus II-paddle stirrer was used to study the release profiles of the pure drug, PM and the electrosprayed nanosystems. The samples, all containing 20 mg of Prop. HCl, were located in the vessels containing 300 ml of phosphate buffer (pH 7.4) under rotational agitation of 50 rpm, at 37 ± 0.2 °C. At specified intervals, 3 ml of the prepared solutions was taken away and filtered via a cellulose acetate membrane with a pore diameter of 20 nm (Whatman, Kent, UK). Afterwards, an equal amount of the fresh buffer was added to the solution in order to retain a constant volume. To evaluate and record the cumulative drug release plot, UV spectrophotometer (Shimadzu, Kyoto, Japan) was conducted at the wavelength of 254 nm. For each of the diagrams, the data were attained from the average values of three measurements.
Results and discussion
Morphology and particle size characterization
The morphology and the size of electrosprayed formulations directly correlate with their effectiveness in improving the physicochemical and in vivo properties [Citation12]. The size and the morphology of Prop. HCl-Eud electrosprayed nanosystems were investigated using FE-SEM (). Several parameters such as nature of the polymer and its concentration, diffusion rate, chains intermolecular interlocking; solvent characteristic and coulomb forces as well as some operational factors, i.e. the feeding rate of the prepared solution; working voltage and distance between grounded collector screen and the nozzle are the important aspects which have a critical role in the particles size and morphology of the electrosprayed nanobeads and nanofibres [Citation13,Citation35,Citation36]. In this study, all of the mentioned variables were kept fixed except the polymer:drug ratios and the solution concentrations. According to , the nanobeads were resulted for the formulations F1 (a drug to polymer ratio of 1:5 and total solution concentrations of 10%), F2 (1:5, 15%), F4 (1:10, 10%) and F5 (1:10, 15%). The average beads size of these formulations was found to be 83, 105, 371 nm and 3.6 μm. Additionally, according to , the formulations with the total solution concentrations of 20% (F3 (1:5, 20%) and F6 (1:10, 20%)) led to the formation of nanofibres with the average diameters of about 232 and 148 nm, respectively.
Figure 2. FE-SEM images of Prop. HCl-eudragit® RS100 electrosprayed formulations with the polymer:drug ratios and solution concentrations of (a) 5:1–10% (w/v), (b) 5:1 – 15% (w/v), (c) 5:1 – 20% (w/v), (d) 10:1 – 10% (w/v), (e) 10:1 – 15% (w/v) and (f) 10:1 – 20% (w/v).
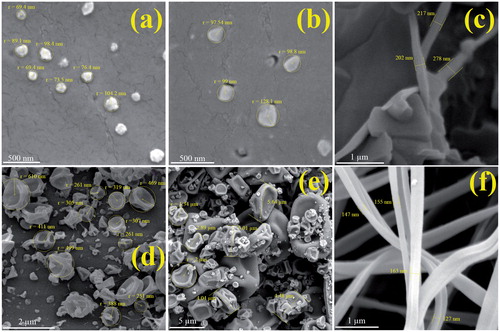
It can be concluded that the beads formation occurs in the low concentration of solutions (1:5 and 1:10 drug:polymer ratios). The solution at the low polymer concentrations has high surface tension which leads to the dispersion of liquid jet into the divided droplets; on the contrary, the fibre formation could be dominated at the high polymer concentrations because of the increasing in the viscoelastic forces [Citation12,Citation22–24,Citation37–39]. So, therefore, the formation of fibres or beads was highly affected by polymer solution concentrations; where the size of the prepared beads is increased by increasing the solution concentration and the fibres were produced at the highest solution concentration. The formation of larger beads by increasing the drug:polymer ratios could be attributed to the reduction in electrical conductivity of the solution at high ratios [Citation40].
DSC evaluation
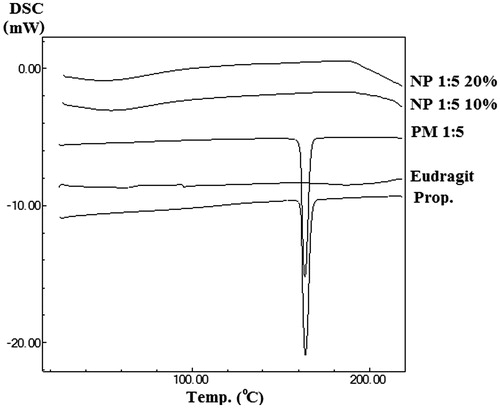
In order to estimate the physical characteristics of the samples, the pure drug, eudragit® RS100, PM and electrosprayed formulations were subjected to DSC analysis (). The Prop. HCl showed a sharp endothermic peak at 164.04 ± 0.2 °C related to its melting point, while the Eud exhibited an amorphous behaviour with a glass transition temperature (Tg) of 58.44 °C. Furthermore, the sharp endothermic peak of the drug was observable in the PM with a reduced intensity owing to the polymer dilution effect and/or solubilization of the drug in the melted polymer and/or heat induced interaction of the polymer and drug. Besides, DSC analysis of the electrosprayed formulations indicated the absence of any clear peak of Prop. HCl which might be related to heat induced interaction of the polymer and drug and/or solubilization of the Prop. HCl in the melted Eud and/or drug amorphization [Citation41,Citation42].
PXRD analysis
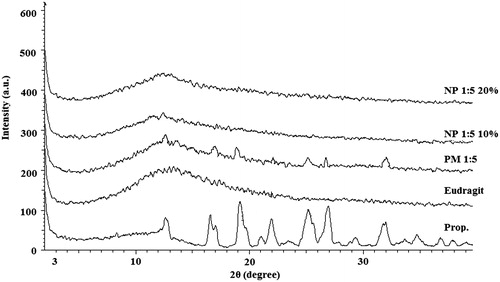
illustrates the PXRD patterns of the pure drug, the polymer, the PM and the electrosprayed samples. The dominant diffraction peaks at 2θ angles of 12.51, 16.73, 17.19, 19.76, 23.22, 25.08, 27.47 and 32 were the characteristic peaks of the Prop. HCl and were evident for its crystalline nature [Citation43]. The Eud did not reveal any characteristic peak in PXRD patterns, indicating its amorphous nature. The pattern of the PM had the distinct diffraction peaks of the drug; however, the intensity reduction of the peaks might be due to the dilution effect of the polymer. Additionally, no diffraction peaks were observed in the PXRD pattern of the electrosprayed samples which might be related to the amorphization of the Prop. HCl during the electrospraying procedure. These findings were in good agreement with DSC thermograms and similar previously reported results for the TA-PLGA, AZI-PLGA and MPA-Eud electrosprayed nanobeads [Citation22–24].
FT-IR spectroscopy
FT-IR spectroscopy was used to detect any probable chemical interactions between the drug and the polymer in the solid-state (. The FT-IR spectrum of the pure drug revealed characteristic bands at around 3280/cm (related to a secondary amine –NH stretch), 2964/cm (C–H stretch), 1579/cm (aryl C=C stretch), 1240/cm (aryl O–CH2 asymmetric stretch), 1030/cm (aryl O–CH2 symmetric stretch) and 798/cm (a peak due to alpha-substituted naphathalene) [Citation41]. Furthermore, the FTIR spectra of Eud showed the peaks at 2981/cm and 1724/cm due to CH aliphatic stretching and –C=O stretching, respectively [Citation23].
Figure 5. FT-IR spectrum of Prop. (Pure drug), Eudragit® RS100, PM (physical mixture) and NP (electrosprayed nanoformulations) with a polymer:drug ratio of 5:1 and the total solution concentrations of 10% and 20% (w/v).
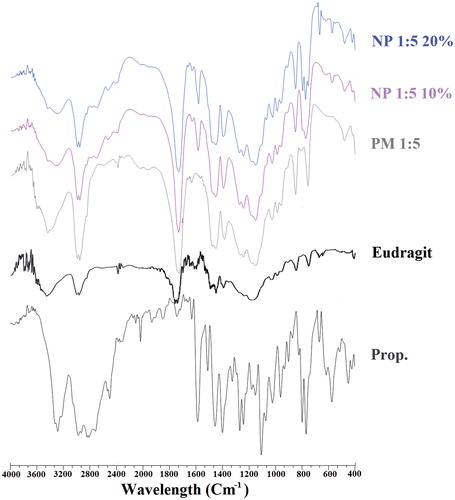
The normal bands of the drug and polymer were observable, whereas the reduced intensity of these spectra could be referred to the dilution effect of the polymer. Furthermore, it is obvious that the –C=O stretching bond of the Eud at 1724/cm disguised any probable new –C=O stretching bond at this wave number which could be due to the possible interactions of Prop. HCl with Eud. Consequently, the absence of any probable chemical interaction between Prop. HCl and Eud could not be absolutely realized from the FT-IR spectroscopy results [Citation22–24,Citation44].
In vitro drug dissolution study
In vitro dissolution tests were carried out to acquire the release behaviour of the Prop. HCl from the pure drug, PM and electrosprayed formulations (). The electrosprayed samples showed relatively slower release patterns compared with the pure drug and PMs at the same pH. The composition of the drug:polymer is one of the main parameters which could most likely affect the release rate of the drug from nanobeads/nanofibres; however, it should be remembered that a complicated phenomenon among the polymer chains and the drug may take place. Some of these complex interactions include the drug entrapment into the polymer molecules and the adhesion of the drug on the surface of polymeric structure due to the electrostatic forces [Citation12]. For instance, the drug dissolution process occurs through a swellable polymeric structure in the electrosprayed samples, which involves several mechanisms such as desorption of the adhered drug from the surface layer, diffusion of the drug over the polymeric matrix and the swelling of the hydrophilic polymer.
Figure 6. The release behaviour of the pure drug (Prop. HCl), PM (physical mixtures) and the electrosprayed nanoformulations. Drug:polymer ratios were indicated by 1:5 and 1:10. Solution concentrations were denoted by 10%, 15% and 20%. Each data show mean ± SD (n = 3).
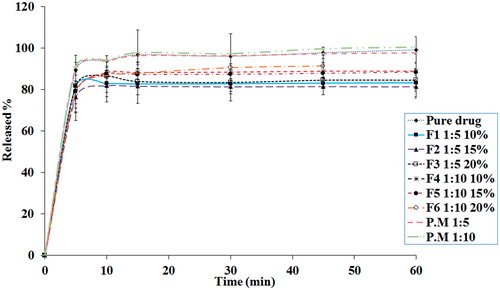
The graphs in imply that there was no consequential difference between all examined samples. The biphasic pattern was the predominant behaviour in all the samples so that a burst release followed by a plateau dissolution. The high surface area of the nanobeads and nanofibres along with the accumulation of drug molecules at the surface of the formed particles were the probable reasons for the observed burst behaviour. Therefore, dissolution and diffusion of the drug from the skin-deep layers attributed to the first phase, while the plateau phase connected to the drug dissolution from the core of nanobeads and nanofibres [Citation22].
Additionally, the electrosprayed samples with the drug:polymer ratio of 1:10 (F4–F6 formulations) indicated a comparatively faster release rate toward to the 1:5 ratio (F1–F3 formulations); nevertheless, the concentration of the injected formulations did not remarkably impressed the release behaviours. The enhanced released rate of 1:10 drug:polymer ratio could be related to the drug molecules homogenous and uniform dispersion at polymeric matrix, whereas the drug entrapment explained the slower release behaviour of the 1:5 ratio of drug:polymer [Citation12,Citation13].
Conclusions
Nanobeads and nanofibres of Prop. HCl (as a water-soluble drug) were successfully prepared by electrospraying method. FE-SEM results revealed that the formulations with the drug to polymer ratios of 1:5 and 1:10 at various total concentrations (10, 15 and 20% (w/v)) led to the formation of nanobeads and nanofibres so that the solution concentration enhancement increased the size of prepared beads and the fibres were formed at the highest solution concentrations. PXRD and DSC experiments obviously indicated that Prop. HCl crystallinity decreased in the electrosprayed samples. According to the in vitro drug dissolution results, the electrosprayed samples had just about slower release patterns toward the pure drug and physical mixtures which could be related to the hydrophilic nature of the polymer used in this study. To come to the point, the electrospraying method as an economic, surfactant-free and one-step straightforward process could be assumed as a potential technique to prepare water-soluble drug–polymer nanoformulations. Indubitably, it needs further in vitro and in vivo studies using different hydrophobic polymers such as poly (lactic-co-glycolic acid) (PLGA) or ethyl cellulose to prepare appropriate sustained release nanoformulations of water-soluble drugs.
Acknowledgements
The authors would like to thank the Drug Applied Research Center, Tabriz University of Medical Sciences, for financial support of this study. This article is a part of a thesis (No. 3848) submitted for the PharmD degree in the Faculty of Pharmacy, Tabriz University of Medical Sciences.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Demetzos C. 2016. Application of nanotechnology in modified release systems. In: Pharmaceutical nanotechnology. New York: Springer.
- Samiei M, Farjami A, Maleki Dizaj S, et al. Nanoparticles for antimicrobial purposes in Endodontics: a systematic review of in vitro studies. Mat Sci Eng C. 2016;58:1269–1278.
- Bhatia S. 2016. Nanotechnology and its drug delivery applications. In: Natural polymer drug delivery systems. New York: Springer.
- Wang A, Yang Yang Y, Qi W, et al. Li fabrication of mesoporous silica nanoparticle with well-defined multicompartment structure as efficient drug carrier for cancer therapy in vitro and in vivo. ACS Appl Mater Interfaces. 2016;8:8900–8907.
- Li Y, Wu Y, Huang L, et al. Sigma receptor-mediated targeted delivery of anti-angiogenic multifunctional nanodrugs for combination tumor therapy. J Control Release. 2016;228:107–119.
- Bottini M, Bhattacharya K, Fadeel B, et al. Nanodrugs to target articular cartilage: an emerging platform for osteoarthritis therapy. Nanomedicine. 2016;12:255–268.
- Patel HM, Patel BB, Shah CN. Nanosuspension: a novel approach to enhance solubility of poorly water soluble drugs: a review. IJAP. 2016;5:21–29.
- Allen TM, Cullis PR. Drug delivery systems: entering the mainstream. Science. 2004;303:1818–1822.
- Tang Z, He C, Tian H, et al. Polymeric nanostructured materials for biomedical applications. Prog Polym Sci. 2016;60:86–128.
- Ding S, Anton N, Vandamme TF, et al. Microfluidic nanoprecipitation systems for preparing pure drug or polymeric drug loaded nanoparticles: an overview. Expert Opin Drug Deliv. 2016;13:1447–1460.
- Saad WS, Prud’homme RK. Principles of nanoparticle formation by flash nanoprecipitation. Nano Today. 2016;11:212–227.
- Jahangiri A, Davaran S, Fayyazi B, et al. Application of electrospraying as a one-step method for the fabrication of triamcinolone acetonide-PLGA nanofibers and nanobeads. Colloids Surf B Biointerfaces. 2014;123:219–224.
- Nguyen DN, Clasen C, Van den Mooter G. Pharmaceutical applications of electrospraying. J Pharm Sci. 2016;105:2601–2620.
- Oliveira MB, Hatami J, Mano JF. Coating strategies using layer-by-layer deposition for cell encapsulation. Chem Asian J. 2016;11:1753–1764.
- Coghetto CC, Brinques GB, Siqueira NM, et al. Electrospraying microencapsulation of Lactobacillus plantarum enhances cell viability under refrigeration storage and simulated gastric and intestinal fluids. J Funct Foods. 2016;24:316–326.
- Fung WY, Liong MT, Yuen KH. Preparation, in-vitro and in-vivo characterisation of CoQ10 microparticles: electrospraying-enhanced bioavailability. J Pharm Pharmacol. 2016;68:159–169.
- Mokhtari F, Latifi M, Shamshirsaz M. Electrospinning/electrospray of polyvinylidene fluoride (PVDF): piezoelectric nanofibers. J Text I. 2016;107:1037–1055.
- Grande D, Ramier J, Versace DL, et al. Design of functionalized biodegradable PHA-based electrospun scaffolds meant for tissue engineering applications. N Biotechnol. 2016;37:129–137.
- Eltayeb M, Stride E, Edirisinghe M, et al. Electrosprayed nanoparticle delivery system for controlled release. Mater Sci Eng C Mater Biol Appl. 2016;66:138–146.
- Vellayappan MV, Venugopal JR, Ramakrishna S, et al. Electrospinning applications from diagnosis to treatment of diabetes. RSC Adv. 2016;6:83638–83655.
- Roman JA, Reucroft I, Martin RA, et al. Local release of paclitaxel from aligned, electrospun microfibers promotes axonal extension. Adv Healthc Mater. 2016;5:2628–2635.
- Payab S, Jafari-Aghdam N, Barzegar-Jalali M, et al. Preparation and physicochemical characterization of the azithromycin-Eudragit RS100 nanobeads and nanofibers using electrospinning method. J Drug Deliv Sci Technol. 2014;24:585–590.
- Payab S, Davaran S, Tanhaei A, et al. Triamcinolone acetonide-Eudragit(®) RS100 nanofibers and nanobeads: morphological and physicochemical characterization. Artif Cells Nanomed Biotechnol. 2016;44:362–369.
- Jafari-Aghdam N, Adibkia K, Payab S, et al. Methylprednisolone acetate-Eudragit® RS100 electrospuns: preparation and physicochemical characterization. Artif Cells Nanomed Biotechnol. 2016;44:497–503.
- Panah NG, Alizadeh P, Yekta BE, et al. Preparation and in-vitro characterization of electrospun bioactive glass nanotubes as mesoporous carriers for ibuprofen. Ceram Int. 2016;42:10935–10942.
- Akduman C, Özgüney I, Kumbasar EPA. Preparation and characterization of naproxen-loaded electrospun thermoplastic polyurethane nanofibers as a drug delivery system. Mater Sci Eng C. 2016;64:383–390.
- Jia L-n, Zhang X, Xu H-Y, et al. Development of a doxycycline hydrochloride-loaded electrospun nanofibrous membrane for GTR/GBR applications. J Nanomater. 2016;2016:1–10.
- Chen J, Wang X, Zhang W, et al. A novel application of electrospinning technique in sublingual membrane: characterization, permeation and in vivo study. Drug Dev Ind Pharm. 2016;42:1365–1374.
- Illangakoon UE. 2016. Advanced drug delivery systems prepared by electrospinning. London: UCL (University College London).
- Barry M, Pearce H, Cross L, et al. Advances in nanotechnology for the treatment of osteoporosis. Curr Osteoporos Rep. 2016;14:87–94.
- El-Newehy MH, El-Naggar ME, Alotaiby S, et al. Preparation of biocompatible system based on electrospun CMC/PVA nanofibers as controlled release carrier of diclofenac sodium. J Macromol Sci Pure Appl Chem. 2016;53:566–573.
- Zhang X, Tang K, Zheng X. Electrospinning and crosslinking of COL/PVA nanofiber-microsphere containing salicylic acid for drug delivery. J Bionic Eng. 2016;13:143–149.
- Chou Y-C, Cheng Y-S, Hsu Y-H, et al. Biodegradable nanofiber-membrane for sustainable release of lidocaine at the femoral fracture site as a periosteal block: in vitro and in vivo studies in a rabbit model. Colloids Surf B Biointerfaces. 2016;140:332–341.
- Mendes AC, Gorzelanny C, Halter N, et al. Hybrid electrospun chitosan-phospholipids nanofibers for transdermal drug delivery. Int J Pharm. 2016;510:48–56.
- Bhardwaj N, Kundu SC. Electrospinning: a fascinating fiber fabrication technique. Biotechnol Adv. 2010;28:325–347.
- Almería B, Deng W, Fahmy TM, et al. Controlling the morphology of electrospray-generated PLGA microparticles for drug delivery. J Colloid Interface Sci. 2010;343:125–133.
- Kim HS, Kim K, Jin HJ, et al. Morphological characterization of electrospun nano‐fibrous membranes of biodegradable poly (l‐lactide) and poly (lactide‐co‐glycolide). Macromol Symp. 2005;224:145–154 [in Wiley Online Library]
- Liu H, Hsieh Y‐L. Ultrafine fibrous cellulose membranes from electrospinning of cellulose acetate. J Polym Sci B Polym Phys. 2002;40:2119–2129.
- Jian H, Yu SV, Fridrikh GC, et al. The role of elasticity in the formation of electrospun fibers. Polymer 2006;47:4789–4797.
- Midhun BT, Shalumon KT, Manzoor K, et al. Preparation of budesonide-loaded polycaprolactone nanobeads by electrospraying for controlled drug release. J Biomater Sci Polym Ed. 2011;22:2431–2444.
- Jana U, Mohanty AK, Manna PK, et al. Preparation and characterization of nebivolol nanoparticles using Eudragit® RS100. Colloids Surf B Biointerfaces. 2014;113:269–275.
- Srikanth MV, Sreenivasa Rao N, Sunil SA, et al. Statistical design and evaluation of a propranolol HCl gastric floating tablet. APSB. 2012;2:60–69.
- Javadzadeh Y, Musaalrezaei L, Nokhodchi A. Liquisolid technique as a new approach to sustain propranolol hydrochloride release from tablet matrices. Int J Pharm. 2008;362:102–108.
- Barzegar-Jalali M, Alaei-Beirami M, Javadzadeh Y, et al. Comparison of physicochemical characteristics and drug release of diclofenac sodium–eudragit® RS100 nanoparticles and solid dispersions. Powder Technol. 2012;219:211–216.