Abstract
Autoimmunity arises when highly active immune responses are developed against the tissues or substances of one’s own body. It is one of the most prevalent disorders among the old-age population with prospects increasing with age. The major cause of autoimmunity and associated diseases is the dysregulation of host immune surveillance. Impaired repairment of immune system and apoptosis regulation can be seen as major landmarks in autoimmune disorders such as the mutation of p53 gene which results in rheumatoid arthritis, bowel disease which consequently lead to tissue destruction, inflammation and dysfunctioning of body organs. Cytokines mediated apoptosis and proliferation of cells plays a regulatory role in cell cycle and further in cancer development. Anti-TNF therapy, Treg therapy and stem cell therapy have been used for autoimmune diseases, however, with the increase in the use of immunomodulatory therapies and their development for autoimmune diseases and cancer, the understanding of human immune system tends to become an increasing requirement. Hence, the findings associated with the relationship between autoimmune diseases and cancer may prove to be beneficial for the improvement in the health of suffering patients. Here in, we are eliciting the underlying mechanisms which result in autoimmune disorders causing the onset of cancer, exploration of interactome to find the pathways which are mutual to both, and recognition of hotspots which might play important role in autoimmunity mediated therapeutics with different therapies such as anti-TNF therapy, Treg therapy and stem cell therapy.
Introduction
Autoimmune diseases are one of the important health problems over the globe with wide differences in incidence and severity [Citation1]. These diseases arise from an immune response against the substances, cells, tissues which are normally a part of the body. Autoimmune disease results when immune tolerance to some specific self-antigens gets broken. Primary immunodeficiencies (PIDs) are genetic disorders which make several infections, autoimmunity and cancer susceptible. The overall risk of development of cancer which is estimated to be 4–25%, increases with expanded life span of patients. The factors that play a role in the type of malignancy to be developed are the age of the patient, PID and viral infections possibly. More susceptibility to infection and greater incidence of malignancy are associated with PIDs.
There have been many experiments which indicate associations between malignancy and immunity and that the two processes have a vital relationship which needs to be elucidated. Autoimmunity and cancer are often more coincidental than what is usually appreciated and hence more interest in the relationship, and possible consequences between the onset of both have been raised. As autoimmunity at a high level is unhealthy, autoimmunity at a low level might prove to be of use. Autoimmune reactions can be considered as a process of defence which is carried out by the host against the tumour. It might also be possible that the anti-tumour immune response results in provoking auto-antibodies against different auto-antigens, which includes the antigens that are expressed in the tumour cells. Cancer forms simply because the immune system fails to control the growth of tumour cells and autoimmunity due to the dysregulation of autoreactive responses. If the immune system could be reprogrammed so that it does not require continuous treatment for the maintenance of homeostasis, it might prove to be a holy grail of treatments for such diseases. Conversely, the suppression of immune functions for the inhibition of the autoimmunity can allow the development of malignancy and can pose a risk to the resistance to infections.
There are some autoimmune diseases which are associated with the development of lymphoproliferative malignancies, such as Sjögren's syndrome, rheumatoid arthritis (RA) and systemic lupus erythematosus [Citation2]. Moreover, it has been found that in patients with solid tumours exists a group of auto-antibodies [Citation3]. Also, it has been seen, that the patients having dermatomyositis have a higher risk of developing solid-organ malignancies than the normal population. The relationship between autoimmunity and cancer is not exactly known. In autoimmune diseases, regeneration of damaged tissues occurs at the site of autoimmunity which is associated with fast cell division, and hence autoimmunity leads to cancer [Citation4]. This is a standard approach towards the explanation of the coincidence between cancer and autoimmunity. The association of autoimmunity with higher risks of development of malignancies connects to cancer. It has been suggested that in many autoimmune rheumatic diseases and cancer, the link may be bidirectional, i.e. the chronic inflammation and tissue damage from the autoimmune condition could lead to malignant transformation of the cells whereas immune responses against cancer cells result in autoimmunity [Citation5].
The study of mechanisms of autoimmunity, regulatory T (Treg) cells, dendritic cell (DC) activation and Toll-like receptors may help to generate new vaccines. These new vaccines may result in better clinical outcomes when used along with available standard adjuvant therapies. So, the knowledge of common targets is required to be explored for the research and the development of new therapies for the treatment of cancers. The relationship between autoimmunity was investigated while focusing on the immune system, apoptosis and therapeutic.
Autoimmunity, cancer and their interplay
Autoimmunity generally comes into picture when there seems a disorder of destructing oneself, but present scenario and evidence through many investigations prove it to be significantly associated with cancer under a specific set of conditions. Autoimmunity is reported to have some approved or constructive association with different cancer types, e.g. inducing lymphomas in immune distress conditions [Citation6,Citation7]. Autoimmunity and cancer both have got some common features like defects in repair mechanism as well as apoptosis pathways and the ultimate reason behind it is a mutation [Citation8]. Autoimmunity is reported to be involved in both preventions as well as the causation of cancer. Patients with immune compromised conditions are likely to be highly susceptible to cancer development [Citation9]. Certain immunogenic therapeutics and genetic mutations create such an environment that ultimately leads to cancer [Citation10,Citation11]. Cytokines, chemokines and free radicals are some pro-inflammatory molecules, which when activated ceaselessly, are shown to be responsible in the process of tissue demolition and this further leads to possibilities in triggering cancer [Citation12]. T-cells play an important role in tumour surveillance system when it comes into contact with tumour cells and their associated antigens, by releasing certain effector molecules whose deficiencies are reported to be associated with tumour initiation and progression which suggest their importance in tumour controlled mechanisms. The cytotoxic responses mediated by these effector molecules like IFN-γ, perforins and granzymes are crucial effector mechanisms in tumour regulation and control [Citation13–15].
Many studies so far have not been able to confirm the underlying mechanisms behind the generation of autoantibodies against the autologous antigens which certainly is the property of both autoimmune disorders as well cancer where these autoantibodies act as serum biomarkers. However, there are a set of logical reasons that have been hypothesized in order to explain the generation of autoantibodies against the autologous antigens which includes, transcriptional modifications like alternative splicing that modify the immunogenicity of some autoantigen transcripts by changing their structural motifs and thereby creates new epitopes that break the immune tolerance, some genetic instabilities like single nucleotide polymorphism and genetic mutations to these autoantigens, aberrant post-translational modifications, misfolding and overexpression of respective genes [Citation16–18].
Overall understandings give an idea that an immune response has potential in both combating as well in inducing cancer. Cancer combating immune response can be demonstrated as in the case of the paraneoplastic syndrome and tumour infiltrating lymphocytes (TILs) which further enhance prognosis of cancer. On the other hand, autoimmunity contributes in elevating the promotion of carcinogenesis as in the cases of certain infections and chronic inflammations which although results from autoimmune responses but their further stimulation can break the immune tolerance which ultimately aids in cancer development [Citation6].
Mechanism of associations between autoimmunity and carcinogenesis
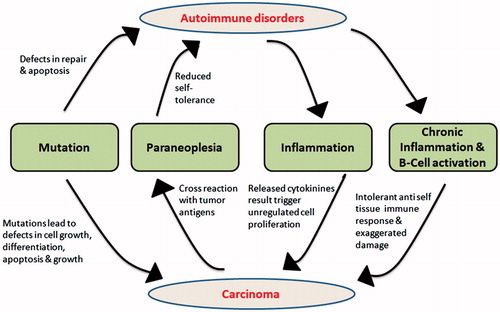
Autoimmunity tends to work against self-antigens by stimulating an immune response against it. Antibodies are released and perform against these self-antigens. As soon the self-antigen gets activated via immune response, this promotes ceaseless activation of B-cells as well as T-cell and thereby increases in their clone number which plays a major role in triggering the promotion of inflammation and at some set of situation even more severe, i.e. chronic inflammation [Citation19]. Cytokines produced during the process may be involved in activating these B cells and T cells. Among the recently discovered, IL-35 was found to be involved in autoimmune diseases and cancer by inhibiting T-effector cells and by activating B cells and T regulatory cells [Citation20]. Regulatory T-cells play a major role in maintaining immunological self-tolerance which can obstruct tumour immunity. This suggests that dysfunction or deficiency of Treg-cells is significantly associated with breakage of immune tolerance and hence leads to autoimmune response or disorders [Citation21]. Inflammation which comes into play as a consequence of autoimmunity, plays an important role in carcinogenesis as it involves cytokines which have a property of stimulating cell for their unchecked division and hence strengthen malignancies. Chronic inflammation also causes intolerant anti-self-tissue immune response and further provokes tissue destruction. Another mechanism where tumour antigens mimic the self-antigens and hence guide the formation of cross reactive antibodies is called as paraneoplasia syndrome that leads to autoimmunity [Citation22]. This way, with the reports and evidences in support of inter-relation of autoimmunity with carcinogenesis, we can even conclude them to be related in a cyclic pattern (). Balance of Wnt/β-catenin pathway signalling in DCs also regulates the occurrence of cancer transformation and autoimmune pathologies. Tumour cells signal DC cells to activate Wnt pathway due to which they would evade the anti-tumour immunity by upregulating T regulatory cells and inhibiting T effector cells. In contrast, DC cells activate Wnt pathway to suppress autoimmune pathologies. Therefore, downregulation of this pathway results in autoimmunity whereas upregulation in cancer [Citation23].
Figure 1. Autoimmune disorders and its interconnection with carcinogenesis in a cyclic pattern. Mutations in growth regulators, apoptotic proteins lead to both autoimmune disorder and cancer directly or indirectly. Autoimmune disorders caused due to deregulation of cell division proteins lead to inflammation, T-cell activation and B-cell activation which results in intolerance to self-tissues, i.e. immune responses are directed towards self-tissues which ultimately lead to cancer.
Factors underlying transformation of inflammation to B-cell lymphomagenesis
Although inflammation is generally a normal protective immune response by the vascular tissues of the body which come into play as a defence mechanism against harmful stimuli like any pathogen or some damaged tissue but under some conditions, it can get more fatal where some special factors provoke it to get transformed into some severe conditions like lymphoma. Following are some conditions where inflammation can undergo fatal transformations.
Genetic alterations
The main root causes behind the susceptibility towards autoimmunity and cancer are mutations [Citation24]. Loss of function and gain of function of genes like tumour suppressor genes and proto-oncogenes respectively are some of the major targets of mutation that leads to cancer generation [Citation25]. When some of the genes which are characterized by a distinctive role in maturation and proliferation of B-cells, undergoes mutations, than these alterations are reported to be associated with B-cell maturation and proliferation, it develops into B-cell lymphomas which have been a point of focus in research [Citation26,Citation27]. Gene polymorphism is another factor that can play a role in gene alterations which can lead to cancer. Gene polymorphism of genes, e.g. gene encoding TNF, gene encoding interleukin (IL)-10 are reported to be associated with genetic variations which are responsible for B-cell lymphomagenesis arising from autoimmunity. These polymorphisms at times recruit NF-κB pathways through the activation of TNF and also weaken the apoptotic mechanism which can lead to lymphomagenesis [Citation28–30].
Infections
Certain lymphomas transformations are reported to have a consistent pattern of significant positive association with viral infections due to human herpes virus 8, human T-lymphotropic virus type I. Conditions can get more severe due to infection with Plasmodium falciparum, Campylobacter jejuni, and with certain hepatitis virus like HBV, HCV. These infections can further cause autoimmune disorders which cause chronic immune stimulation that can lead to defective apoptotic mechanisms towards B-cells and hence cause lymphoma [Citation31]. Viruses that can cause lymphocyte infection are evidently associated with lymphoproliferative disorders and hence cause severe consequences like lymphomagenesis [Citation32]. Hence, it can be concluded that chronic infections, chronic inflammations and lymphomas are some severe consequences of autoimmune diseases [Citation33,Citation34].
Drugs against autoimmune disorders
In order to treat autoimmunity, certain anti-inflammatory drugs like corticosteroids are often involved to suppress the immune system and hence to avoid the generations of lymphomas. Although these drugs are successful in producing desired results but these medications carry a risk of getting lymphomas because suppressing the immune system leads to these immune compromised patients more susceptible to cancer formation because of having a number of unregulated and abnormal lymphocytes [Citation35,Citation36]. Certain more drugs like cyclophosphamide and azathioprine are reported to be more hazardous in causing such blood born malignancies, if used in an uncontrollable manner for a long period, e.g. SLE [Citation37].
Anti-TNF drugs
Anti-TNF drugs have been widely used against inflammatory disorders, but they come with a possible risk factor of lymphogenesis [Citation38]. In general, after receiving certain stimulus like TNF, EGF, insulin growth factor (IGF), inflammation by respective receptors, a particular signalling cascade mechanism comes into play which phosphorylates some protein kinases like PKC which further activate MAPK and PI3K pathways. MAPK activates ERK and JNK pathways which are responsible for cell division and proteasomal degradation mediated p53 dependent apoptosis, respectively [Citation39,Citation40]. On the other hand, PI3K activates AKT which has a major role in cell survival, differentiation, proliferation, migration, growth and also angiogenesis which can cause cancer generation [Citation41]. Modification by any means in some of these mediators – PI3K, AKT and mTOR, can cause the autoimmune disease progression as well as cancer, through cross-reactivity which takes place between tumour antigen and self-antigen [Citation42,Citation43]. On the other side of cascade mechanism, another receptor called death receptor (Fas/TNFR1) is reported to be involved in caspase dependent apoptosis which is mediated by mitochondria. Hence, any malfunction in this death receptor can lead to dysfunction of apoptotic mechanism and this can cause the patient more susceptible towards cancer [Citation44,Citation45]. This theory suggests that patients with autoimmune disorders are more vulnerable to cancer development, when treated with anti-TNF drugs [Citation46]. However, in a fairly recent study conducted on spondyloarthritis patients, TNF inhibitors did not show any increased risk of six common types of cancer cancer-prostate, lung, colorectal, breast, melanoma and lymphoma [Citation47]. NF-κB, which is another common mediator of all these pathways in relation to inflammation, further connects autoimmune disorders with carcinogenesis [Citation48]. Thus, a coherent molecular pathogenesis of autoimmunity and cancer can be deduced (). Putative hotspots for therapeutically targeting autoimmune diseases and cancer are mentioned in .
Figure 2. Stimulation of various membrane receptors by TNF, EGF, IGF leads to phosphorylation of protein kinase C which mediates the signal to the interior of the cell and activates MAPK and PI3-K dependent pathways. These pathways, in turn, regulate various cellular processes like cellular differentiation, cell division and apoptosis. Apoptosis is induced synergistically via both p53 dependent and caspase-dependent mechanisms. Deregulation in any signalling molecule or a mutation in the gene encoding these molecules of MAPK and PI3K pathways and apoptosis signalling leads to cellular transformation.
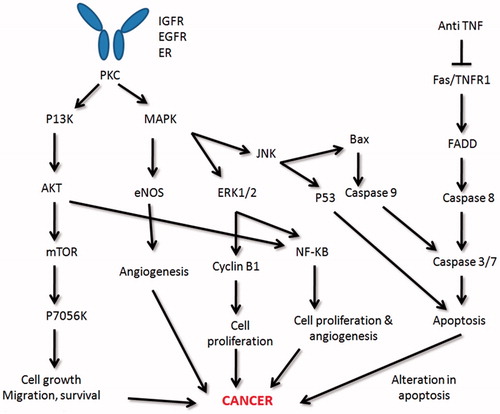
Table 1. Putative hotspots for therapeutically targeting autoimmune diseases and cancer.
TNF and NF-κB in cancer
Cytokines mediated signalling has various immunological responses, i.e. cell activation, inflammation, proliferation and apoptosis. Balance of proliferation and apoptosis of cells is the decisive feature among normal and cancer cells. Studies have been done to identify these cytokines signalling and use them for cancer treatment. Recently, studies have been focused on the response of tumour necrosis factors (TNF) on cancer cells. TNFs are a cytokine superfamily composed of 27 ligands. TNFs are produced by Natural Killer (NK) cells, macrophages, T-cells and some non-immune cells. TNF has dual role in cancer biology. On one hand, evidences have shown its cytotoxic role to tumour cells and promotion of anti-tumour response, but on the other hand, recent studies have suggested its tumour promoting and angiogenesis effect on tumour cells. This dual nature is generally governed by the amount of TNF present. Low amount of TNF in tumour environment promotes angiogenesis whereas higher concentration has antitumour effects.
TNF ligand expresses as trans-membrane protein, but after proteolytic processing extracellular domain becomes soluble ligand [Citation49]. TNF ligand binds to two distinct receptors – TNF receptor-1 (TNFR-1, p55 receptor) and TNF receptor-2 (TNFR-2, p75 receptor). TNFR-1 expresses ubiquitously, but TNFR-2 expresses mainly in immune cells. TNFR-1 acts as a transduction switch for both pro-apoptosis as well as pro-survival. Binding of TNF causes trimerization of TNFR-1 which further results in the release of silencer of death domain (SODD) from death domain. This allows the formation of complex 1 (receptor-interacting protein, TNFR-associated factor 2 (TRAF-2) TNFR-associated death domains (TRADD) and FAS-associated death domain, FADD) [Citation50].
Cell-death signal: FADD binds to procapase-8 which further induces DNA fragmentation and destruction of cellular proteins: TRAF-2 activates Jun N-terminal kinase (JNK), mitogen-activated protein kinase-kinase 4 (MEK4) and other activator proteins.
Cell survival signal: RIP of complex 1 activates inhibitor of κB (IκB) kinase (IKK). IKK further phosphorylates IκB on complex, which results in ubiquitination and then degradation in the proteasome. Then NFκB translocates to nucleus and promotes transcription of antiapoptotic genes such as cellular inhibitor of apoptosis protein (cIAP)1, B-cell lymphoma extra-large (Bcl-xL) [Citation51].
Constitutive expression of activated nuclear factor κB (NF-κB) in cancer cells has a critical role in their proliferation and apoptosis. Mechanism of activation of NF-κB depends on the down-regulated signalling of tumour necrosis factors receptors (TNFRs). Activated NF-κB and their end products in cancer cell enhance cell proliferation and invasion develops resistance to apoptosis.
Different studies have been done to identify the role of NF-κB in cancer cells. Various types of inhibition strategies were implemented. NF-κB inhibitors such as Bay 11-7082, IKK inhibitor VII and CDDO-Me were tested on the different cell line of thyroid cancer (papillary thyroid cancer (PTC) (BCPAP, TPC1) and anaplastic thyroid cancer (ATC)). Interestingly, the effects of these inhibitors were variable suggesting that these inhibitors may have off-target mechanisms of inhibitory effect [Citation52]. Previous studies on cancer model have also shown that NF-κB has role transcriptional regulation of cyclin E, cyclin D1, CDK2 and c-Myc [Citation53]. Blocking the transition of S to G1/M phase, the protein level of cyclin A remains unaffected by NF-κB inhibition [Citation54]. However, cyclin B1, which is required for entry into and progression through G2-phase and mitosis, and its protein levels were decreased following NF-κB inhibition [Citation52].
Therapies
Immunotherapy for autoimmune disease has undergone significant change since the first attempts to treat patients with autoimmune disease and possible innovation for betterment of therapy as first attempt therapy have shown capacity to treat the patient but not appropriate results as it requires some target-specific therapy which has specific biomarker and regulative way so that can modify the therapy using current approach to treating patient. Specifically, the group of treatments which inhibits or induces immune tolerance may represent therapy with avoiding severe adverse effects.
Anti-TNF therapy
TNF family members play a pivotal role in autoimmune diseases. Among all the members of the TNF super family, the role of TNF-α in autoimmune disease is maximum studied. TNF-α is macrophage activated member of cytokine group which is involved in regulative inflammation, cytotoxic enzymes and intracellular killing. Indeed, anti-TNF-α therapy using TNF-α blockers has been developed for Uveitis, multiple sclerosis (MS), systemic lupus, arthritis, psoriasis and Crohn’s disease, all are autoimmune diseases which are connected with dysregulation of various members of the TNF superfamily or their receptors [Citation55].
TNF blocks by two ways which are approved therapeutics, both are two types of antagonist – one may be a soluble receptor that binds to circulating TNF-α and preventing interaction with cell surface receptor and another by antibody development against TNF-α. Abatacept is a fusion protein composed of the extracellular binding domain of CTLA-4 fused to an Fc domain of human IgG. It was developed to block the binding of CD28 to B7. Abatacept was approved for the treatment of moderate to severe RA in the case of inadequate response to anti-TNFα therapy. Second antagonism via golimumab, which is fully humanized TNF-α antibody approved for RA, psoriasis. Similarly, certolizumabpegol is humanized, FDA approved antibody for osteoporosis [Citation56]. Since many drugs are developed and approved, show important role in curing autoimmune disease and cancer in many ways but therapies used for the patient have not shown appropriate results and it somewhere have shown side effects and toxicity, this requires some better and redefine approach to treat autoimmune disease [Citation57]. Co-stimulation possibly has therapeutics role using an inhibitory costimulatory molecule developed to block the binding of CD28 to B7, programmed death-1 (PD-1 or CD279), which is a inducible costimulator and also a member of the immunoglobulin superfamily of costimulatory molecules, blocking CD40-CD40L by using an anti-CD40L mAb [Citation56].
Current approaches using a combination of therapeutic agents might allow for fewer side-effects, lesser drug dose to the patient which possibly have less toxicity. Thus, the severe autoimmune side effects of treating cancer by using monoclonal antibody and a vaccine which is specific for cytotoxic T-lymphocyte antigen 4 (CTLA4) may be attenuated if it is used at lower doses and which will retain an amount of increase in immune responses which is desired while reducing the dose and duration of treatment. An antibody called sirukumab is under development for an alternative approach to anti-TNF therapy in RA patients. Sirukumab binds specifically to IL-7 and has shown promising effects in arthritis patients intolerant to conventional anti-TNF therapy [Citation58]. Other combination approaches include using abatacept, a CTLA4-immunoglobulin fusion protein, or rituximab, a B-cell-depleting agent combined with methotrexate, an immunosuppressive drug, for the treatment of RA which is resistant to blockade by TNF or drug. Advantages of combination therapy is lesser side effects, may have same effectiveness similar to that of TNF blockade alone.
In addition, many research groups have successfully designed and synthesized multivalent therapeutics using small molecules, peptides, mAbs or aptamers to increase binding affinity, avidity and selectivity to receptors [Citation59], with using specific drug-delivery systems such as nanoparticles, polymers, dendrimers and the like, are being explored as multivalent constructs and carriers of drugs. Association among various autoimmune diseases and cancer is depicted in .
Table 2. The association of different autoimmune diseases with the symptoms of cancer and the reason the development of malignancy.
T regulatory cell therapy
Tregs are a heterogeneous set of cells that consist of CD4+CD25+Foxp3+ cells, IL-10-producing CD4+ Tr1 cells, CD8+ cells, Th3 cells which produce – TGF-β, NK T cells and T (γδ) cells. Human and mouse models suggest pathology can develop Tregs dysfunction that occurs due to the continued lack of immune tolerance typically secreting IL-10. Treg cells are classified on the basis of their origin, generation and mechanisms of action. Natural (constitutive) and inducible (adaptive) CD4+ CD25+ Foxp3+ Treg cells are two classes based on their origin. Treg are involved in the control of immune self-tolerance, allograft rejection and also important in inhibiting the effector functions during infection and tumours. Natural CD4+ CD25+ Foxp3+ regulatory (or suppressor) Treg cells are the best-studied subset of suppressor CD4+ T cells [Citation69]. Tregs dysfunction or removal from normal rodents leads to the development of various autoimmune.
Nuclear transcription factor Foxp3 has been identified to be a highly specific marker for Tregs and is involved in function and development of Tregs. Many studies have shown that the numbers of CD4+CD25+ cells and CD4+FOXP3+ cells in patients with various autoimmune diseases are diminished and the Treg deficit is associated with disease activity [Citation70]. In many animal models, the adoptive transfer of natural Tregs can prevent development and appearance of autoimmune diseases. On the other hand, some studies demonstrate the unsatisfactory therapeutic effect of natural Tregs on diseases.
When Foxp3 was identified as Treg marker, many groups reported that TGF-β could induce Foxp3 expression in induced Tregs (iTregs), supported by in vivo TGF-β-dependent mechanism of Foxp3+ Tregs [Citation71].
Chemokines secreted by antigen-specific TGF-β iTregs regulate T cell trafficking and thereby suppress ongoing autoimmune and these iTregs were therapeutic in an ongoing autoimmune gastritis model [Citation72]. Other study revealed that only TGF-β-induced iTregs but not natural Tregs suppressed Th17-mediate disease, indicating iTregs as therapeutic potential in suppressing the established autoimmune diseases [Citation73].
A current study [Citation74] found therapeutics mechanism in combination with methotrexate and cyclophosphamide inhibits DCs maturity and downregulate the antigen presenting capacity of DC which is important in reestablishing balance of Th17 and Tregs. Co-transfer of CD4+ T-cells generated with TGF-β plus for RA can prolong the survival of diseased mice, indicating TGF-β is still able to induce human inducer Treg cell differentiation [Citation75]. More recently, combination of both TGF-β and rapamycin was able to induce human iTregs [Citation76]. In these studies, rapamycin alone was unable to induce human iTregs at RA, indicating that TGF-β is possibly a key factor mediating the differentiation of iTregs in human.
Some other therapies, Treg response augmented using ipilimumab monoclonal antibody towards the cytotoxic T-lymphocyte antigen (CTLA)-4 which bind B7 co-stimulatory molecules on antigen-presenting cells for the treatment of metastatic melanoma. In another study, ipilimumab and anti-PD-1 agent, nivolumab, were used together to prolong the effectiveness if the treatment was in metastatic melanoma [Citation77].
Stem cell therapy
Although, ADs are treated with immune suppressive agents, despite they are reported to cure the disease, however, associated with the risk of long-term adverse effects. Stem cell therapy has been demonstrated to have a therapeutic role for the various forms of autoimmune disease, due to their unique properties of self-renewability and differentiation to different lineages. Stem cells, specifically mesenchymal stem cells (MSCs), induce the production of Treg cells. MSCs are multipotent progenitor cells and they have properties to differentiate into cartilage cell lines, bone cells or fat cells. MSCs rarely express cell surface molecules, major histocompatibility complex-2 (MHC-2) and very low level of cell surface molecule MHC-1 [Citation78]. Furthermore, these characteristics allow being used as allogenic MSCs to express their effect on competent cells with no requirement of matching of host human leukocyte antigens (HLAs). Both autologous and allogeneic MSCs with their immunosuppressive properties have been tested in various number of autoimmune or other immune system mediated diseases [Citation79]. MSCs can be derived from various sources, including umbilical cord tissue which provides more number of cells.
Transplantation of MSCs has therapeutics role in autoimmune diseases with immunoregulatory and anti-inflammatory capabilities. The mechanisms behind MSCs’ immune modulation are still poorly understood, but MSCs possibly have favourable results in the cure of autoimmune disease as for rheumatic diseases for the setting of graft versus host disease [Citation80]. MSCs express an important immune modulator galectin-9 (Gal-9) which is strongly upregulated when cells are activated by interferon-γ. Gal-9 mediates the functional and anti-proliferative effects of MSCs on T cells and B cells. Gal-9 and activated MSCs contribute to the suppression of antigen triggered immunoglobulin release [Citation81]. To predict an immune modulatory potential of single cell preparations to be higher or lower, the Gal-9 expression could be used as a marker and therefore to distinguish the MSCs from different donors based on their therapeutic potency. The ability of MSCs to downregulate the proliferation of T cells was significantly weaker in diseases as compared to T cells of healthy individuals.
Combining therapy shows positive therapeutic role with paclitaxel plus trastuzumab as maintenance therapy for autologous HSC transplantation for patients with HER2-positive metastatic breast cancer [Citation82]. MSC-treated MS and myasthenia gravis lymphocytes were found to increase the number of IL-2 comparative to healthy individuals. MSC treatment in combination with neutralizing IL-2 antibodies results in similar inhibition levels as healthy controls [Citation83]. MSCs were also shown to down-regulate the IL-2 receptor expression presenting on lymphocyte surface. MSC-repressed lymphocytes through the addition of IL-2 restored proliferation which suggests that this cytokine plays a key role in the inhibitory mechanism.
Combination therapy
In recent times, apart from single drug approaches, combination therapy has shown promising effects. In this group of treatment, two or more therapies from different therapeutic categories with a different mechanism of actions have been used for the treatment of a particular disease. Recent applications of combination therapies against autoimmune diseases have proved that the approach is more efficient and less toxic than the single drug approach. The combination therapy works synergistically to decrease disease progression with each drug following its mechanism of action as also discussed in the previous section for autoimmune diseases including RA, MS and myasthenia gravis, have shown prominent results. Moreover, many other combination therapies have emerged to be promising a few of which will be discussed in this section.
Azathioprine is one of the drugs used against some of the autoimmune diseases like RA, Crohn’s disease, etc. It is an analogue of purine and thus disrupts the RNA or DNA synthesis. Infliximab also used against Crohn’s disease is a TNF-α antibody. Both the drugs individually induce and maintain remission in patients suffering in Crohn’s disease. In a study conducted over the period of 6 years, the two drugs administered together in a combination therapy showed the highest efficacy [Citation84].
In a relatively recent study concerning another major autoimmune disease MS, it was discovered that the atypical antipsychotic drugs (APP) like quietiapine, risperidone and clozapine reduce the cytokine production as well as the pathological demyelination and paralysis in experimental model encephalomyelitis (EAE) of autoimmune disease-MS. Glatiramer acetate is a FDA approved immunomodulator drug to decrease the relapse in the autoimmune disease MS. A combination therapy including the APP clozapine and immunomodulator GA decreases the pro-inflammatory responses and increased the anti-inflammatory response in vitro [Citation85].
In another study conducted in 2017, a combination of methotrexate and leflunomide was used to treat autoimmune disease. Combination therapy resulted in the reduction of IL-22 level in plasma with a correlation of circulating Th22 cells. Cytokine IL-22 activates the proliferative pathways and inhibits the apoptosis and controls tissue responses to inflammation. IL-2 are responsible for productions of inflammatory components, so inhibition of IL-22 indicates potential therapeutic target leflunomide is known to inhibit pyridine biosynthesis. The combination of methotrexate and leflunomide helped in the mechanistic understanding and for the treatment of RA [Citation86].
Even though many combination therapies have shown promising results, certain other therapies like, a TNF-α inhibitor and co-stimulation inhibitor, abatacept lack antigen specificity to attain the desired results. To overcome this, many combination therapies use auto-antigens or DNA coding for the antigen and an immunomodulator to enhance the efficacy of the treatment. This approach is called antigen-specific immunotherapy (ASIT) [Citation87]. Nanoparticles bound with the autoantigen complexed with MHC-II dampen the immune response in autoimmune diseases [Citation88]. This therapeutic method has been implied in vivo in rat models for RA, type 1 diabetes (T1D) and MS with successful depression of autoimmune response without affecting the normal immune response and induces tolerance to self-antigen/auto-antigen [Citation89]. The mechanism by which ASIT decreases autoimmune response is by converting T-cells intro T-regulatory cells of FOXP3+CD4+CD25+ Treg cells and FOXP3−CD4+CD25− TR1 type. Combination therapy can be made antigen-specific by combining ASIT (autoantigen) with the immunomodulator by co-administration or co-delivery. These are two distinct mechanisms applied to achieve antigen-specific combination therapy. Co-administration is the use of autoantigen with an immunosuppressant or biological molecule in the similar time frame but in different space, whereas in co-delivery, multiple components are delivered in the same time and space, i.e. in the same vehicle [Citation87].
Therefore, it is easy to conclude from the above-cited examples that various concoction of complementary drugs used in combination therapy against various autoimmune diseases works synergistically and complementary to increase efficacy and diminish toxicity. This therapeutic approach thus can bring about important changes in the future treatment, maintenance and prevention of the autoimmune diseases by modulation of the adaptive immunity. Moreover, ASIT combination therapy may even reduce the number of dosage as in the current immunosuppressant methods and could potentially cure the autoimmune disease instead of treating or preventing.
Discussion
Immune tolerance is the ability of a person to differentiate between self and non-self, while reacting against non-self. In various diseases such as (RA, lupus, MS, SLE, etc.) loss of immune tolerance occurs. This loss of immune tolerance is responsible for elicitation of specific and effective immune responses directed against self-determinants. Many observations suggest that the clones of lymphocytes that have bypassed the mechanisms of tolerance may play an important role in both malignancy and autoimmunity.
Autoimmunity and cancer are interlinked in the interactome which aggravate each other in a cyclic fashion. Inflammation and paraneoplasia state serves as connecting link between them. Their molecular pathogenesis is dependent on the alteration of PI3K/AKT, ERK and JNK pathway. Molecular aberrations in PI3K, AKT, mTOR, p53 are found to be a causative agent who is responsible for their common onset. TNFR regulates cytokines mediated apoptosis in cells. Tumour necrosis factors signalling via NF-κB has role in the regulation of cyclin E, cyclin D1, CDK2 and c-Myc. Aberration in this signalling leads to the constitutive expression on NF-κB which results in a decrease in cell apoptosis. First attempt anti-TNF therapy has shown some side effects and toxicity, in combination anti-TNF drugs such as abatacept, golimumab, certolizumabpegol has shown appropriate results, curing strategies require new combination of drugs with immune-proteins, immunosuppressant and improved delivery systems. Foxp3 has been identified as specific marker for Tregs and critically involved in the development and function of Tregs. The diminished number of CD4+CD25+ cells and CD4+FOXP3+ cells on Treg is associated with various autoimmune diseases. The adoptive transfer of natural Tregs can prevent development and appearance of autoimmune diseases but not satisfactory on other way with iTreg in combination with TGF-β used in therapy. Combination therapies are found to have feasible results as in RA. Autoimmune diseases are treated with immune suppressive agents, although they are reported to cure the disease they possess the risk of long-term adverse effects. Overcoming the issues, stem cell therapy has been reported to cure autoimmune diseases with their unique properties including immune system modulation.
Mesenchymal stem cells have shown favourable results with unique properties including, do not require matching HLAs of the host. MSCs immune modulation progress can screen using therapy in combination with tracking biomarker for the cure of autoimmune disease as for rheumatic diseases.
There are many pieces of evidence which suggest that some parts of the immune system may act differently than other parts of the system, e.g. some parts may respond in an anticancer manner whereas the other parts may promote cancer. In perpetual activation, different immune mediators like cytokines, free radicals and chemokines may lead to chronic inflammation by causing damage to tissues. This inflammation may increase the risk of cancer. Additionally, the stimulation and hence, the fast proliferation of immune cells may further contribute to malignant lymphoproliferation. There are many other factors as well which may also strengthen the carcinogenic environment, like genetic mutations, immunomodulatory treatments and environmental conditions.
Conclusions
The relationship that exists between cancer and autoimmunity has been studied focusing on apoptosis, the implication of immune system, and new therapeutic treatments for autoimmune diseases. These autoimmune diseases which are characterized by a chronic state of inflammation with progressive antigenic simulation may help to cause haematological malignancies and development of solid tumours.
Extent of tissue damage and escalated inflammation are the major responsible factors which determine the pathogenesis of both autoimmune disorder and cancer in the same individual. Putative hotspots for therapeutically targeting autoimmunity and cancer are PI3K, AKT, mTOR and p53, growth receptors as well as death receptors of the diseased cell. Down-regulation signalling of TNFRs governs most of the pathways related to cells proliferation and apoptosis. Further NF-B constitutive expression affects cells cycle transition switches, which are critical for regulation of cell division. Therapies which are already in use (Treg and anti-TNF) have shown appropriate results but in some therapies may have side effects and some severe affects. Stem cell therapy using MSCs have found to possess potential therapeutic role in the curing of autoimmune diseases. Moreover, combination therapies in which two mechanisms are collectively used for autoimmune disease show good results in curing autoimmune disease.
Acknowledgements
We are grateful to the Council of Scientific and Industrial Research (CSIR) for providing financial assistance and necessary funds to Prof. Ramesh Chandra. We are also grateful to the University of Delhi for providing support and necessary facilities to carry out research work. Neeraj Kumar, Vartika Tomar and Ravi Tomar are thankful to Department of Biotechnology (DBT-JRF), Indian Council of Medical Research (ICMR-SRF) and Department of Science and Technology (DST) respectively.
Disclosure statement
The authors have no conflict of interest regarding the publication of the paper.
Additional information
Funding
References
- Silink M. Childhood diabetes: a global perspective. Horm Res Paediatr. 2002;57:1–5.
- Kiss E, Kovacs L, Szodoray P. Malignancies in systemic lupus erythematosus. Autoimmun Rev. 2010;9:195–199.
- Bei R, Masuelli L, Palumbo C, et al. A common repertoire of autoantibodies is shared by cancer and autoimmune disease patients: inflammation in their induction and impact on tumor growth. Cancer Lett. 2009;281:8–23.
- Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420:860–867.
- Egiziano G, Bernatsky S, Shah AA. Cancer and autoimmunity: harnessing longitudinal cohorts to probe the link. Best Pract Res Clin Rheumatol. 2016;30:53–62.
- Franks AL, Slansky JE. Multiple associations between a broad spectrum of autoimmune diseases, chronic inflammatory diseases and cancer. Anticancer Res. 2012;32:1119–1136.
- Alexander MJ, Teitelbaum H. Observation and analysis of a large amplitude mountain wave event over the Antarctic peninsula. J Geophys Res. 2007;112.
- Koike T. The new era of autoimmune disease research. Arthritis Res Ther. 2011;13:113.
- Brinckerhoff CE. Cancer stem cells (CSCs) in melanoma: there's smoke, but is there fire? J Cell Physiol. 2017;232:2674–2678.
- Barbaro G, Barbarini G. HIV infection and cancer in the era of highly active antiretroviral therapy. Oncol Rep. 2007;17:1121–1126.
- Moss SF, Blaser MJ. Mechanisms of disease: inflammation and the origins of cancer. Nat Clin Pract Oncol. 2005;2:90–97.
- Sansone F, Mencherini T, Picerno P, et al. Maltodextrin/pectin microparticles by spray drying as carrier for nutraceutical extracts. J Food Eng. 2011;105:468–476.
- Van den Broek MF, Kaegi D, Ossendorp F, et al. Decreased tumor surveillance in perforin-deficient mice. J Exp Med. 1996;184:1781–1790.
- Kaplan DH, Shankaran V, Dighe AS, et al. Demonstration of an interferon γ-dependent tumor surveillance system in immunocompetent mice. Proc Natl Acad Sci. 1998;95:7556–7561.
- Dunn GP, Old LJ, Schreiber RD. The immunobiology of cancer immunosurveillance and immunoediting. Immunity. 2004;21:137–148.
- Ng B, Yang F, Huston DP, et al. Increased noncanonical splicing of autoantigen transcripts provides the structural basis for expression of untolerized epitopes. J Allergy Clin Immunol. 2004;114:1463–1470.
- Tan HT, Low J, Lim SG, et al. Serum autoantibodies as biomarkers for early cancer detection. FEBS J. 2009;276:6880–6904.
- Anderson KS, LaBaer J. The sentinel within: exploiting the immune system for cancer biomarkers. J Proteome Res. 2005;4:1123–1133.
- Costa N, Pires AE, Gabriel AM, et al. Active regulatory T-cells contribute to broadened T-cell repertoire diversity in ivIg-treated SLE patients. J Transl Med. 2011;9:P6.
- Pylayeva-Gupta Y. Molecular pathways: interleukin-35 in autoimmunity and cancer. Clin Cancer Res. 2016;22:4973–4978.
- Barbi JJ, Vignali PD, Yu H, et al. The neurotrophic factor neuritin maintains and promotes the function of regulatory t cells in autoimmunity and cancer. J Immunol. 2016;196:58.12.
- Getts M, Miller S. 99th Dahlem conference on infection, inflammation and chronic inflammatory disorders: triggering of autoimmune diseases by infections. Clin Exp Immunol. 2010;160:15–21.
- Suryawanshi A, Tadagavadi RK, Swafford D, et al. Modulation of inflammatory responses by Wnt/β-catenin signaling in dendritic cells: a novel immunotherapy target for autoimmunity and cancer. Front Immunol. 2016;7:460.
- Zenewicz LA, Abraham C, Flavell RA, et al. Unraveling the genetics of autoimmunity. Cell. 2010;140:791–797.
- Müllauer L, Gruber P, Sebinger D, et al. Mutations in apoptosis genes: a pathogenetic factor for human disease. Mutat Res/Rev Mutat Res. 2001;488:211–231.
- Morin RD, Mendez-Lago M, Mungall AJ, et al. Frequent mutation of histone-modifying genes in non-Hodgkin lymphoma. Nature. 2011;476:298–303.
- Rosebeck S, Lucas PC, McAllister-Lucas LM. Protease activity of the API2–MALT1 fusion oncoprotein in MALT lymphoma development and treatment. Future Oncol. 2011;7:613–617.
- Balkwill F, Coussens LM. Cancer: an inflammatory link. Nature. 2004;431:405–406.
- Wang SS, Cerhan JR, Hartge P, et al. Common genetic variants in proinflammatory and other immunoregulatory genes and risk for non-Hodgkin lymphoma. Cancer Res. 2006;66:9771–9780.
- Wang SS, Cozen W, Cerhan JR, et al. Immune mechanisms in non-Hodgkin lymphoma: joint effects of the TNF G308A and IL10 T3575A polymorphisms with non-Hodgkin lymphoma risk factors. Cancer Res. 2007;67:5042–5054.
- Kristinsson SY, Goldin LR, Björkholm M, et al. Genetic and immune-related factors in the pathogenesis of lymphoproliferative and plasma cell malignancies. Haematologica. 2009;94:1581–1589.
- Hjalgrim H, Engels E. Infectious aetiology of Hodgkin and non-Hodgkin lymphomas: a review of the epidemiological evidence. J Intern Med. 2008;264:537–548.
- van de Schans SA, van Spronsen DJ, Hooijkaas H, et al. Excess of autoimmune and chronic inflammatory disorders in patients with lymphoma compared with all cancer patients: a cancer registry-based analysis in the south of the Netherlands. Autoimmun Rev. 2011;10:228–234.
- Vanura K, Späth F, Gleiss A, et al. Prevalence and clinical impact of autoimmune diseases and chronic infections in malignant lymphomas at diagnosis. Ann Hematol. 2011;90:947–954.
- Bewtra M, Lewis JD. Update on the risk of lymphoma following immunosuppressive therapy for inflammatory bowel disease. Expert Rev Clin Immunol. 2010;6:621–631.
- Quartuccio L, De Re V, Fabris M, et al. Atypical lymphoproliferation progressing into B-cell lymphoma in rheumatoid arthritis treated with different biological agents: clinical course and molecular characterization. Haematologica. 2006;91:691–694.
- Bernatsky S, Joseph L, Boivin J, et al. The relationship between cancer and medication exposures in systemic lupus erythaematosus: a case-cohort study. Ann Rheum Dis. 2008;67:74–79.
- Mason M, Siegel CA. Do inflammatory bowel disease therapies cause cancer? Inflamm Bowel Dis. 2013;19:1306–1321.
- Geest CR, Coffer PJ. MAPK signaling pathways in the regulation of hematopoiesis. J Leukoc Biol. 2009;86:237–250.
- Kostenko S, Dumitriu G, Lægreid KJ, et al. Physiological roles of mitogen-activated-protein-kinase-activated p38-regulated/activated protein kinase. WJBC. 2011;2:73–89.
- Vara JÁF, Casado E, de Castro J, et al. PI3K/Akt signalling pathway and cancer. Cancer Treatment Rev. 2004;30:193–204.
- Martelli A, Tazzari P, Evangelisti C, et al. Targeting the phosphatidylinositol 3-kinase/Akt/mammalian target of rapamycin module for acute myelogenous leukemia therapy: from bench to bedside. CMC. 2007;14:2009–2023.
- Steelman L, Franklin R, Abrams S, et al. Roles of the Ras/Raf/MEK/ERK pathway in leukemia therapy. Leukemia. 2011;25:1080–1094.
- Cerutti E, Campagnoli MF, Ferretti M, et al. Co-inherited mutations of Fas and caspase-10 in development of the autoimmune lymphoproliferative syndrome. BMC Immunol. 2007;8:28.
- Ramenghi U, Bonissoni S, Migliaretti G, et al. Deficiency of the Fas apoptosis pathway without Fas gene mutations is a familial trait predisposing to development of autoimmune diseases and cancer. Blood. 2000;95:3176–3182.
- Setoguchi S, Solomon DH, Weinblatt ME, et al. Tumor necrosis factor α antagonist use and cancer in patients with rheumatoid arthritis. Arthritis Rheum. 2006;54:2757–2764.
- Hellgren K, Dreyer L, Arkema EV, et al. Cancer risk in patients with spondyloarthritis treated with TNF inhibitors: a collaborative study from the ARTIS and DANBIO registers. Ann Rheum Dis. 2017;76:105–111.
- Kundu JK, Surh Y-J. Inflammation: gearing the journey to cancer. Mutat Res. 2008;659:15–30.
- Naismith JH, Sprang SR. Modularity in the TNF-receptor family. Trends Biochem Sci. 1998;23:74–79.
- Wang X, Lin Y. Tumor necrosis factor and cancer, buddies or foes? 1. Acta Pharmacol Sin. 2008;29:1275–1288.
- Mocellin S, Rossi CR, Pilati P, et al. Tumor necrosis factor, cancer and anticancer therapy. Cytokine Growth Factor Rev. 2005;16:35–53.
- Bauerle KT, Schweppe RE, Haugen BR. Inhibition of nuclear factor-kappa B differentially affects thyroid cancer cell growth, apoptosis, and invasion. Mol Cancer. 2010;9:117.
- Naugler WE, Karin M. NF-kappaB and cancer-identifying targets and mechanisms. Curr Opin Genet Dev. 2008;18:19–26.
- Kaldis P, Aleem E. Cell cycle sibling rivalry: Cdc2 vs. Cdk2. Cell Cycle. 2005;4:1491–1494.
- Aggarwal BB, Gupta SC, Kim JH. Historical perspectives on tumor necrosis factor and its superfamily: twenty-five years later, a golden journey. Blood. 2011;119:651–665.
- Chittasupho C, Siahaan TJ, Vines CM, et al. Autoimmune therapies targeting costimulation and emerging trends in multivalent therapeutics. Ther Deliv. 2011;2:873–889.
- Smedby KE, Baecklund E, Askling J. Malignant lymphomas in autoimmunity and inflammation: a review of risks, risk factors, and lymphoma characteristics. Cancer Epidemiol Prev Biomark. 2006;15:2069–2077.
- Aletaha D, Bingham CO, Tanaka Y, et al. Efficacy and safety of sirukumab in patients with active rheumatoid arthritis refractory to anti-TNF therapy (SIRROUND-T): a randomised, double-blind, placebo-controlled, parallel-group, multinational, phase 3 study. Lancet. 2017;389:1206–1217.
- Chittasupho C. Multivalent ligand: design principle for targeted therapeutic delivery approach. Ther Deliv. 2012;3:1171–1187.
- Chen YJ, Chang YT, Wang CB, et al. The risk of cancer in patients with rheumatoid arthritis: a nationwide cohort study in Taiwan. Arthritis Rheum. 2011;63:352–358.
- Hill CL, Zhang Y, Sigurgeirsson B, et al. Frequency of specific cancer types in dermatomyositis and polymyositis: a population-based study. Lancet. 2001;357:96–100.
- Sigurgeirsson B, Lindelöf B, Edhag O, et al. Risk of cancer in patients with dermatomyositis or polymyositis. A population-based study. N Engl J Med. 1992;326:363–367.
- Kovács L, Szodoray P, Kiss E. Secondary tumours in Sjögren's syndrome. Autoimmun Rev. 2010;9:203–206.
- Mayer L. Evolving paradigms in the pathogenesis of IBD. J Gastroenterol. 2010;45:9–16.
- Frohman EM, Racke MK, Raine CS. Multiple sclerosis—the plaque and its pathogenesis. N Engl J Med. 2006;354:942–955.
- Daniels CE, Jett JR. Does interstitial lung disease predispose to lung cancer? Curr Opin Pulm Med. 2005;11:431–437.
- Catassi C, Bearzi I, Holmes GK. Association of celiac disease and intestinal lymphomas and other cancers. Gastroenterology. 2005;128:S79–S86.
- Delahunt B, Abernethy D, Johnson C, et al. Prostate carcinoma and the Lambert–Eaton myasthenic syndrome. J Urol. 2003;169:278–279.
- Horwitz DA, Zheng SG, Gray JD. Natural and TGF-β-induced Foxp3+ CD4+ CD25+ regulatory T cells are not mirror images of each other. Trends Immunol. 2008;29:429–435.
- Lan Q, Fan H, Quesniaux V, et al. Induced Foxp3+ regulatory T cells: a potential new weapon to treat autoimmune and inflammatory diseases? J Mol Cell Biol. 2012;4:22–28.
- Chen Q, Kim YC, Laurence A, et al. IL-2 controls the stability of Foxp3 expression in TGF-beta-induced Foxp3+ T cells in vivo. J Immunol. 2011;186:6329–6337.
- Nguyen T-LM, Sullivan NL, Ebel M, et al. Antigen-specific TGF-β-induced regulatory T cells secrete chemokines, regulate T cell trafficking, and suppress ongoing autoimmunity. J Immunol. 2011;187:1745–1753.
- Huter EN, Stummvoll GH, DiPaolo RJ, et al. Cutting edge: antigen-specific TGF beta-induced regulatory T cells suppress Th17-mediated autoimmune disease. J Immunol. 2008;181:8209–8213.
- Yu X, Wang C, Luo J, et al. Combination with methotrexate and cyclophosphamide attenuated maturation of dendritic cells: inducing Treg skewing and Th17 suppression in vivo. Clin Dev Immunol. 2013;2013:238035.
- Lu L, Zhou X, Wang J, et al. Characterization of protective human CD4CD25 FOXP3 regulatory T cells generated with IL-2, TGF-β and retinoic acid. PLoS One. 2010;5:e15150.
- Qian X, Wang K, Wang X, et al. Generation of human regulatory T cells de novo with suppressive function prevent xenogeneic graft versus host disease. Int Immunopharmacol. 2011;11:630–637.
- Loo K, Daud AI. Inhibitors of cytotoxic T lymphocyte antigen 4 and programmed death 1/programmed death 1 ligand for metastatic melanoma, dual versus monotherapy—summary of advances and future directions for studying these drugs. Cancer J. 2017;23:3–9.
- Chamberlain G, Fox J, Ashton B, et al. Concise review: mesenchymal stem cells: their phenotype, differentiation capacity, immunological features, and potential for homing. Stem Cells. 2007;25:2739–2749.
- Bai L, Lennon DP, Eaton V, et al. Human bone marrow‐derived mesenchymal stem cells induce Th2‐polarized immune response and promote endogenous repair in animal models of multiple sclerosis. Glia. 2009;57:1192–1203.
- Scherer HU, van Pel M, Toes RE. Mesenchymal stem cells in autoimmune diseases: hype or hope? Arthritis Res Ther. 2010;12:126.
- Ungerer C, Quade-Lyssy P, Radeke HH, et al. Galectin-9 is a suppressor of T and B cells and predicts the immune modulatory potential of mesenchymal stromal cell preparations. Stem Cells Dev. 2013;23:755–766.
- Cheng YC, Rondón G, Anderlini P, et al. Paclitaxel and trastuzumab as maintenance therapy in patients with HER2-positive metastatic breast cancer who underwent high-dose chemotherapy and autologous hematopoietic stem cell transplantation. J Cancer. 2013;4:679.
- Ben-Ami E, Miller A, Berrih-Aknin S. T cells from autoimmune patients display reduced sensitivity to immunoregulation by mesenchymal stem cells: role of IL-2. Autoimmun Rev. 2014;13:187–196.
- Colombel JF, Sandborn WJ, Reinisch W, et al. Infliximab, azathioprine, or combination therapy for Crohn's disease. N Engl J Med. 2010;362:1383–1395.
- Green LK, Zareie P, Templeton N, et al. Enhanced disease reduction using clozapine, an atypical antipsychotic agent, and glatiramer acetate combination therapy in experimental autoimmune encephalomyelitis. Mult Scler J – Exp Transl Clin. 2017;3:2055217317698724.
- Zhong W, Zhao L, Liu T, et al. IL-22-producing CD4+ T cells in the treatment response of rheumatoid arthritis to combination therapy with methotrexate and leflunomide. Sci Rep. 2017;7.
- Northrup L, Christopher MA, Sullivan BP, et al. Combining antigen and immunomodulators: emerging trends in antigen-specific immunotherapy for autoimmunity. Adv Drug Deliv Rev. 2016;98:86–98.
- Wraith D. Autoimmunity: antigen-specific immunotherapy. Nature. 2016;530:422.
- Flemming A. Autoimmunity: nanoparticles engineered for antigen-specific immunotherapy. Nat Rev Immunol. 2016;16:204–205.

